Abstract
Reaction time to a visual event can be dramatically reduced if the visual stimulus is accompanied by a startling sound. The mechanism may involve a motor programme being stored and triggered early by the sound. However, in a choice reaction task the required response is not known in advance, and so cannot be stored. In this case startling sound does not usually speed up the reaction and may even be detrimental to performance. Here we show that the reaction time of a special type of visually evoked movement can be substantially reduced by startling sound, even though the movement requires choice. The task involved stepping onto an illuminated target that sometimes moved mid-step left or right, requiring a foot trajectory adjustment. These adjustments occur at much shorter latency than conventional visuomotor reaction tasks and are thought to involve subcortical brain areas. The presence of the sound, which carried no information, shortened the already fast mean response time of 134 ms by ∼20 ms. We attribute this to auditory–visual interaction since sound alone had no effect. Although we observed startle responses, the quickening effect was not contingent upon their presence. Given minimum motor and sensory conduction time, we estimate that the loud sound reduced the central visuomotor processing time by at least 30%.
Reaction time (RT) can be reduced when stimuli from multiple modalities are presented simultaneously, compared with any one stimulus alone. This intersensory facilitation effect has long been demonstrated for simple RT tasks where the required response is known in advance (Todd, 1912). It also occurs for choice RT tasks where the required response depends upon the stimulus, and so cannot be fully prepared. For example, orienting behaviour (Stein & Meredith, 1993) and object recognition (Giard & Peronnet, 1999) happen faster and with less error when both acoustic and visual stimuli simultaneously provide information regarding the required response. However, even stimuli which carry no information about the required response can also reduce choice RT when presented around the same time as an imperative stimulus (e.g. Bernstein et al. 1969; Posner et al. 1976; Hackley & Valle-Inclán, 1999).
In recent years a strand of research has examined the facilitatory effect of startling sound upon RT. Valls-Sole et al. (1995) employed a simple wrist flexion paradigm for this purpose. They reported that when a startling sound is presented at the same time as a visual imperative stimulus, RT is reduced from 177 ms to 80 ms. This cannot be attributed to a non-specific manifestation of the generalized startle response, because the characteristic tri-phasic agonist/antagonist muscle pattern of voluntary movement remains intact (Valls-Sole et al. 1999). The mechanism, however, may not be the same as that underlying the intersensory facilitatory effects described above. This is because in a similar paradigm a marked RT reduction was also reported when sound was used as both the imperative and startling stimulus (Carlsen et al. 2003). The effect was dependent on the presence of an overt startle response as determined by short-latency neck muscle bursts, and so was not merely an effect of high stimulus intensity (Carlsen et al. 2006; although see Valls-Sole et al. 2005). It is known that the acoustic startle response is mediated by brainstem regions (Brown et al. 1991b). Furthermore, Valls-Sole et al. (1995) concluded that the fastest RT that they observed was too fast for the cerebral cortex to be involved. This has led to the theory that startle-evoked reductions in simple RT are due to an interaction of the brainstem-mediated startle response with a motor programme stored subcortically, thus causing its early release (Rothwell, 2006). Consistent with this theory is the observation that startle-evoked facilitation of RT is not observed for choice reaction tasks, in which the required movement is not known in advance and so cannot be subcortically stored (Carlsen et al. 2004a). Indeed, in these experiments startling sound was found only to interfere with performance of the task.
Here we examine the effect of startling sound upon a special type of choice reaction task. This category of reactive movement is observed when reaching or stepping towards a target that unpredictably moves to a new location as the limb approaches (Day & Lyon, 2000; Reynolds & Day, 2005). In response, appropriate trajectory adjustments are generated at remarkably short latency even though the occurrence and the direction of the target jump are unpredictable. Thus, the task constitutes a type of choice reaction task in which the required movement is not known in advance, but depends upon the visual stimulus. Such tasks are special because they are thought to engage a subcortical visuomotor process (Day & Brown, 2001). We considered that subcortical visuomotor processing, even if it requires a response choice being made, may be influenced by a startling sound which also engages subcortical circuitry. We therefore sought to determine the effect of startling sound on the latency of these visually guided limb adjustments. We used a stepping paradigm in which a floor-mounted target suddenly shifted position mid-step. We have previously reported that step adjustments are seen within ∼120 ms of the target jump, and that these very fast reactions are not affected by the balance constraints associated with upright stance (Reynolds & Day, 2005). The results show that these reactions, which ordinarily are very fast, can be made even faster by the presence of simultaneous startling sound, suggesting a reduced visuomotor processing time.
Methods
Twenty-three subjects (12 men; 23–36 year) gave informed consent to participate. They had no reported neurological disorder or hearing loss. The experiments were approved by the local ethics committee and were performed in accordance with the Declaration of Helsinki.
Protocol
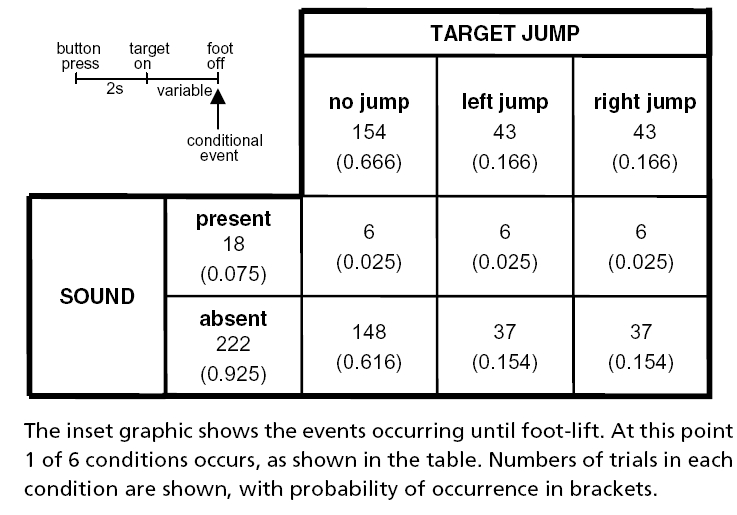
Seventeen subjects participated in the first experiment. Two seconds after pressing a button to initiate the trial, a target was illuminated in front of the right foot. The subject was instructed to step onto this with the right foot, and to bring the trailing left foot along side. The position of the trailing foot was not specified. In 1/3rd of 240 trials the target was randomly made to jump 21 cm to the left or right at the time of foot-lift. The subject was told to attempt to place the right foot upon the new target location if this happened. Once the foot had landed no further corrections were permitted. Startling acoustic stimuli were occasionally given at exactly the same time as foot-lift. There were 18 stimuli in total: six were given during control (no jump) trials, and six were given during each of the two target jump conditions (medial and lateral jumps). These were interspersed randomly, but with a minimum separation of eight trials between each startle trial (see Table 1 for conditions).
Table 1.
Experimental conditions
Six subjects participated in the second experiment, which was designed to see if the effect of the loud sound upon reaction time was dependent on the presence of an overt startle response. Each subject performed on two sessions, separated by at least 4 days. One session was exactly the same as in experiment 1 described above. In the other session subjects received a clear warning sound at the beginning of each trial in which a startling stimulus would occur. The purpose of this was to attenuate or abolish the startle response, while maintaining the presence of the loud sound. The warning sound was given immediately after the button was pressed to initiate the trial. This was then followed by the initial target being illuminated 2 s after the button press, as usual. To further reduce the probability of startle responses during the warned session, subjects were exposed to three paired acoustic stimuli (warning stimulus followed by startling stimulus) immediately before the start of the experiment to habituate them to the sound. The order of the two sessions, warned and unwarned, was counterbalanced between subjects.
Apparatus
Subjects stepped barefoot to 27 × 14 cm floor mounted targets made from electroluminescent paper which illuminates in response to an applied voltage (Pacel, Poole, UK). A central target was placed directly ahead of the right foot with a gap of ∼5 cm. This could be extinguished as one of two secondary targets were simultaneously illuminated 21 cm to the left or right, giving the appearance of a jumped target. Foot-lift timing was precisely measured by passing a small current (∼20 μA) through the subject, forming a circuit with conductive material underfoot. Lifting the foot breaks the circuit, creating a recordable voltage change which was used to trigger the onset of the target jump and acoustic stimulus.
Foot trajectory was measured using two infrared markers, placed on the head of the first metatarsal and heel. These were sampled at 400 Hz using three codamotion mpx30 cameras (Charnwood Dynamics, Leicestershire, UK). To evaluate startle responses, EMG activity was recorded from the sternocleidomastoid (SCM) and obicularis oculi (OO) muscles using an MT8 telemetry unit (MIE Medical Research, Leeds, UK). Square adhesive electrodes of 1 cm2 were placed approximately 3 cm apart along the belly of the SCM on both sides of the neck. A third reference electrode was placed to the side, to which a 1000× preamplifier was attached. The signal was further amplified by 2 times, if necessary. To measure OO activity, electrodes were placed directly above and below the eye, with the reference electrode placed laterally to the eye. EMG was sampled at 2 kHz.
Acoustic stimuli were delivered binaurally through headphones with good passive noise cancelling properties (Sennheiser HD 205). To provide a startling stimulus, we used a 120 dB (SPL) 750 Hz sine wave tone lasting 50 ms. To provide a warning stimulus for the second experiment, a 750 Hz, 120 dB, 1.5 s sine wave was multiplied by a Gaussian window. This produced a sound with a very gradual onset, intended to be loud but not startling.
Analysis
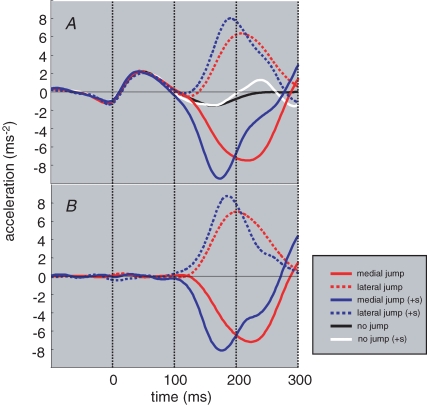
Average foot placement at the end of the trial was measured for each condition and subject. Each subject's footprint was chalk-marked and digitized by tracing around it with an infrared marker at the beginning of the experiment. The footprint was registered with the positions of the two lights on the feet. Thus it could be reconstructed wherever the foot landed, given the positions of the two lights. The lateral displacement of the footprint centroid was measured. Control (no jump) data were subtracted from target jump conditions (see Fig. 4A and B). A two-factor repeated-measures ANOVA was used to analyse this data, with jump direction (medial and lateral) and sound (on and off) as factors.
Figure 4.
Mean foot acceleration A, medio-lateral foot acceleration. B, the same data with matching control data subtracted, i.e. ‘no jump’ data were subtracted from both target jump conditions, and ‘no jump (+ s)’ was subtracted from both target jump (+ s) conditions.
The latency of responses to target jumps was derived from the kinematic data obtained from the infrared marker on the head of the first metatarsal of the right foot. Lateral position data were low-pass filtered below 15 Hz (2nd order, zero phase lag butterworth filter). This was then differentiated twice to derive lateral acceleration. To determine the response latency for each subject we first subtracted the mean control (no jump) data from the mean jump data, for both lateral and medial conditions (see Fig. 4A and B). After reversing the sign of the medial trace, we added the two together. The response latency was taken when the summed trace exceeded 2 standard deviations of a baseline period, defined as 300 ms before foot-off. Even though the foot had not fully left contact with the floor during this baseline period, it had clearly begun to move and the step was fully underway.
We also compared acceleration magnitude at the same points in time. An earlier response induced by the sound should manifest itself as a larger acceleration of the foot. Mean control data were subtracted, but in this case they came from the condition that had no target jump but did include a sound. Thus any non-specific effects of the startling sound were removed. Any remaining effect could only be attributed to an interaction of the sound and target jump. A two-factor repeated measures ANOVA was used to determine any significant effects upon acceleration. The two factors were target jump direction (medial and lateral) and sound (on and off). As in our previous work, we also determined latencies visually for comparison (Reynolds & Day, 2005).
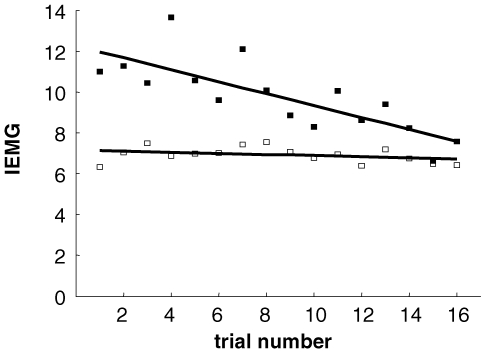
To determine if the startle response habituated, a correlation analysis was performed between EMG responses and trial order, for trials with sound. Startle magnitude was taken as the integrated normalized EMG signal 40–250 ms post-stimulus for SCM and 30–250 ms for OO. Although each subject experienced 18 trials, only 16 were included for this analysis because some trials were excluded due to poor signal quality.
The purpose of the second experiment was to determine if any effect of the loud sound upon response latency was contingent upon the presence of an overt startle response. We therefore measured the magnitude of the startle response in terms of sternocleidomastoid EMG. This was rectified, then filtered (60 Hz low-pass, zero-lag, 2nd order butterworth) In order to make comparisons between the two experimental sessions we normalized the EMG with respect to a functional task; subjects lay horizontally on their back with the head unsupported, and were asked to hold their head against gravity in line with their body. EMG was then given as a percentage of this response. This normalization procedure was intended to reduce differences between sessions due to factors such as altered electrode placement or skin conductivity. Startle magnitude was taken as the integrated normalized EMG signal as described above. Since the standard deviation of EMG responses differed ∼20 times between conditions, a log10 transform was performed in order to make the distributions similar (a requisite of parametric statistics). After ensuring that there were no significant differences in EMG during trials without any sound between the two sessions (t test), we collapsed these data. We then performed a one-factor repeated-measures ANOVA, with three levels (no sound, startle alone, startle + warning). We used planned simple contrasts to compare startle alone and startle + warning trials against trials with no sound.
Results
Responses to target jumps
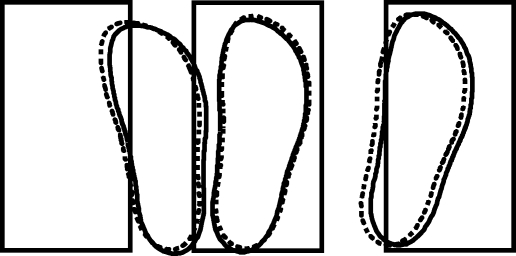
When the target jumped, subjects altered their foot trajectory appropriately, bringing the foot toward the new target location (Fig. 1). There was a tendency to undershoot the target, with responses to medial jumps being less successful than to lateral jumps (foot displacement after control subtraction: medial 10.8 cm; lateral 17.2 cm; F1,16= 133; P < 0.001). The mean latency of these corrective adjustments was 134 ± 14 ms (mean ±s.d.; see Table 2). These results confirm our previous findings demonstrating the existence of a fast visuomotor process guiding foot trajectory during a step (Reynolds & Day, 2005).
Figure 1.
Average foot placement Target areas are shown for control trials (central target) and trials which included a medial or lateral target jump. Targets are 27 cm × 14 cm. Dotted lines represent trials with sound.
Table 2.
Response times (±s.d.)
| No sound | Sound | |||
|---|---|---|---|---|
| Medial | Lateral | Medial | Lateral | |
| Statistical | 153±16 | 144±13 | 122±30 | 131±28 |
| Visual | 135±16 | 132±12 | 117±15 | 126±13 |
The effect of sound
The 120 dB sound generated a startle response in all subjects. This consisted of an EMG burst in obicularis oculi (OO) at a latency of 30 ± 7 ms. The sternocleidomastoid (SCM) burst occurred at 48 ± 11 ms. The SCM response habituated over the course of the experiment. This resulted in a significant inverse correlation between trial number and peak EMG magnitude (r =−0.74, P = 0.001; see Fig. 2). In contrast, the OO response remained constant (r = 0.085, P = 0.75). This is consistent with previous research showing that the auditory blink reflex does not habituate (Brown et al. 1991a).
Figure 2.
Startle responses IEMG responses are plotted for successive trials with sound. SCM and OO are represented by filled and open squares, respectively. Lines show linear regression lines.
There was no main effect of the sound upon final foot placement (F1,16= 0.11; P = 0.75; see Fig. 1). However there was a tendency for the sound to cause the foot to land slightly more medially during both medial and lateral target jumps (mean difference after control subtraction = 1.2 cm). This resulted in a significant direction–sound interaction (F1,16= 8.2; P < 0.011). Nevertheless this effect was small, and subjects were still capable of performing the task even in the presence of the startling stimulus. Therefore, purely in terms of final foot placement, the sound did not interfere with (or improve) task performance. This is consistent with the findings of Nieuwenhuijzen et al. (2000) showing that startle responses had only a minimal effect upon locomotion, being well integrated into the step cycle.
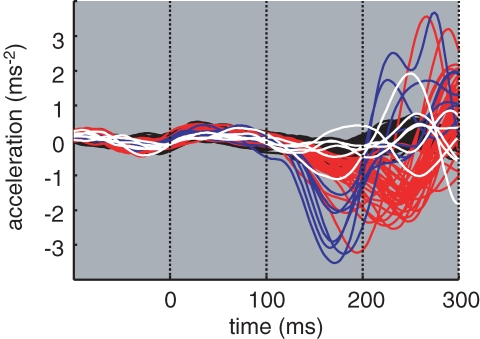
The primary question of the study is whether mid-step foot trajectory adjustments can be made faster by the addition of a simultaneous startling sound. Figure 3 shows single trial acceleration data for the control and medial target jump conditions. When the sound is present the foot appears to move in the medial direction earlier than with the target jump alone. In contrast, when the startle is given alone there is no consistent effect upon foot trajectory. This quickening effect occurs for the lateral direction also, and can be seen clearly on the group mean acceleration data in Fig. 4A. In trials with sound alone (white trace), the acceleration profile was very similar to the equivalent control condition without sound (black trace). The effect of the sound is therefore specific for the direction of target jump. Further analysis was performed after control data were subtracted, as shown in Fig. 4B.
Figure 3.
Foot acceleration Medio-lateral acceleration of the big toe marker. Individual trials are shown from a representative subject for trials with no target jumps and with medial (leftward) jumps. Target jumps occur at the same time as foot-off, at time zero. Black = control trials; white = control trials + sound; red = medial target jumps; blue = medial target jumps + sound.
Mean response latencies were determined both statistically and by visual inspection, and are presented in Table 2. The former technique resulted in longer latencies but has the advantage of being entirely objective. The latter produces more accurate times but is open to subjective distortion. However, in both cases ANOVA revealed a significant main effect of sound (F1,16 > 12; P < 0.003). This confirms that the presence of the startling stimulus did indeed reduce response latency. This made the response up to 31 ms faster (18 ms when measured by visual inspection).
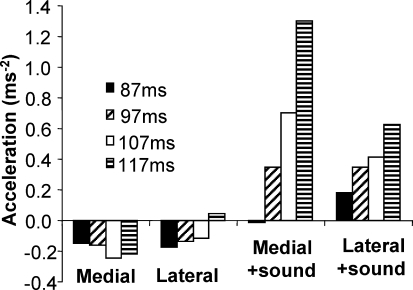
To support the latency analysis, we compared acceleration magnitude between conditions at various points in time. Control trial data were first subtracted. For each subject, we then measured acceleration at 30, 40, 50 and 60 ms prior to the first statistically detected response seen in control trials. This corresponds to average times of 117, 107, 97 and 87 ± 14 ms, respectively. The result is shown in Fig. 5. At 87 ms after the target jump, acceleration is close to zero for all conditions. At 97 ms there is a significant main effect of sound (F1,16= 12.3; P < 0.011). This analysis therefore provides an earlier estimate of the time at which the first response was initiated due to sound. Later, at 117 ms, there is a interaction between sound and direction (F1,16= 7.1; P < 0.017). This is due to a greater facilitating effect of sound for the medial direction.
Figure 5.
Acceleration at different time points Acceleration in the direction of the target is shown for target jump conditions with and without sound at various times. Mean data from control trials were subtracted first.
The relationship between startle magnitude and effect size
Once we had established that the loud sound caused a reduction in response latency, we sought to determine if this effect was contingent upon the existence of an overt startle response. Since we had observed habituation of the startle response, we examined the relationship between the magnitude of the neck muscle response and the magnitude of the quickening effect. We performed a correlation analysis of startle magnitude, as assessed by the integrated SCM response, and effect magnitude, as assessed by acceleration magnitude. There was no correlation, either for peak acceleration values or acceleration at response onset (r < 0.075, P > 0.32).
To further examine whether the quickening effect of the sound was contingent upon the presence of an overt startle, we designed an experiment in which a warning sound was used to attenuate the startle response. The warning sound was loud (120 dB peak SPL) but had a gradual onset so as not to be startling. One of the six subjects in this experiment exhibited SCM responses to the warning signal and so was excluded from further analysis. In the remaining subjects, the warning sound proved very effective in attenuating startle responses to the subsequent sound: SCM bursts were minimal or absent in all cases. As reported previously, the OO response was persistent and only showed minimal habituation, due to the acoustic blink response (Brown et al. 1991a). To quantify the startle response we integrated the normalized SCM activity (IEMG) over a time window of 40–250 ms after foot-off/sound. There was no difference in IEMG values during trials without sound between the two conditions (t = 1.086; P = 0.34). We therefore collapsed these data across sessions. In the absence of sound, IEMG values (in arbitrary units) were 6.11 ± 3.23. When the startling sound was introduced without warning IEMG rose to 45.42 ± 60.64, but when the warning was present IEMG remained low at 6.36 ± 2.86. There was a significant main effect of sound (F2,8= 11.04; P = 0.005; log10 transformed data). Simple contrast analysis revealed that when the startle was given alone IEMG was significantly greater than when no sound was given (F1,4= 13.75; P = 0.021). When the warning sound was present there was no such difference (F1,4= 1.02; P = 0.37). So subjects were exposed to a sound capable of evoking a startle response, but this response was minimal or absent when this sound was preceded by a warning sound. However, when we examined the effect of startle upon acceleration values, there was no obvious pattern. In just one subject was the quickening effect clearly contingent on the presence of the startle; both medial and lateral responses were faster only in the presence of a sound which produced clear SCM responses. However, this pattern was not repeated throughout the group. Two subjects showed the opposite pattern, with a clear quickening effect of sound only seen during the warned condition. The remaining subjects showed mixed responses. There was a clear overall effect of the startle sound (F1,4= 45.6; P = 0.003), but there was no interaction between the warning sound and startle sound (F1,4= 0.046; P = 0.084). Hence, although subjects' behaviour was not entirely consistent within the group, when combined with the previous experiment the results suggest that the facilitation effect is not strictly related to, nor dependent upon, an overt startle response.
Discussion
A loud (120 dB) tone sounded at the same moment as a floor-mounted target jumped left or right caused the foot to be accelerated in the direction of the new target location earlier than would have normally occurred. Response times were shortened by up to 31 ms when measured statistically, and 18 ms when measured visually. This was confirmed by a comparison of lateral foot acceleration with and without sound at the same point in time. In the absence of sound, the response to the target jump had not yet been initiated at 97 ms, whereas it was well underway in the presence of the sound.
Choice versus simple reaction
Previous research has shown that auditory startle can massively reduce simple reaction time (Valls-Sole et al. 1995; Carlsen et al. 2004b). For example, when a wrist flexion is demanded in response to a visual stimulus, reaction time can be more than halved in the presence of a startling sound, from 177 to 80 ms (Valls-Sole et al. 1995). It has been theorized that during such a task the motor programme can be prepared in advance and stored subcortically. This would allow for an interaction of the startle response and the stored motor programme at a subcortical level, causing its early release (Valls-Sole et al. 1999; Carlsen et al. 2004a; Rothwell, 2006). In our experiment this explanation cannot apply because the direction of the upcoming target jump was unpredictable, thus constituting a choice reaction task. The location of the visual stimulus must first be processed before the appropriate movement can be prepared and generated.
It has long been known that sound presented at the same time as a visual imperative stimulus can improve choice reaction time, even if the sound carries no information (Bernstein et al. 1969; Posner et al. 1976; Hackley & Valle-Inclán, 1999). However, startling sound has previously only been shown to interfere with choice RT. Carlsen et al. (2004a) specifically investigated the effect of startling sound upon choice reaction time but their results differed from ours. After replicating previous findings regarding simple reaction tasks, they examined a choice condition where either a flexion or extension of the wrist was required depending on the visual stimulus. In this case not only did the startling sound fail to facilitate response time, it actually resulted in more movement errors. This contrasts with our result which shows a shortening of reaction time without any increase in movement error. This discrepancy suggests a fundamental difference in the two tasks.
Shortening of reaction time is not contingent upon startle
Although we often observed a clear startle response, the shortening of reaction time cannot be attributed to non-specific effects of the startle since the movement of the foot was always appropriate for the direction of the jumped target. This is further supported by the absence of any clear effect of the startle upon foot trajectory when given without a target jump. Furthermore, two other findings showed a dissociation between startle and the reaction-time shortening. The loud sound evoked short-latency responses in both the SCM and OO muscles. The SCM response, which we used as a startle indicator, habituated over the course of the experiment, while the OO response did not. This is consistent with previous research describing a persistent auditory blink reflex, independent of startle (Brown et al. 1991a). We found no correlation between startle magnitude and foot acceleration. In a further experiment we abolished the startle response with a warning sound and the quickening effect still occurred in some subjects. Taken together these results suggest that the facilitatory effect of loud sound upon reaction time is not contingent upon an overt startle response. This does not exclude the possibility that the same neural pathway involved in generating the startle response may be involved. It is conceivable that even after the startle response itself habituates, this pathway could mediate the facilitatory effect of the loud sound upon reaction time.
Two recent studies disagree on whether the same is true for simple reaction tasks. Valls-Sole et al. (2005) abolished startle responses with a small electrical stimulus applied to the skin 100 ms before sounding a 130 dB acoustic stimulus (‘prepulse inhibition’). The sound reduced reaction time irrespective of startle, although there exists a potential confound of the electrical stimulus rendering the imperative stimulus predictable. In contrast, Carlsen et al. (2006) found a clear relationship between startle magnitude and reaction time. Reaction times were fastest when both SCM and OO bursts occurred, and slowest when no muscle responses were seen. Intermediate response times occurred for OO bursts alone. Additionally, there was an independent effect of sound intensity: louder sounds shortened reaction time even in the absence of startle. However, their paradigm differed from that of Valls-Sole et al. (and our own), since they used sound as both the imperative and startling stimulus.
We deliberately used a low number of trials with sound to minimize habituation of the startle response. This raises the question of whether the quickening effect of sound would persist with more frequent presentation. We saw no clear relationship between startle responses and RT, suggesting that habituation of the startle response caused by more frequent presentation would not in itself reduce the facilitatory effect of sound. However, it is possible that the quickening effect itself could habituate, irrespective of startle.
Although we have shown that sound improves visual RT, the possibility that intersensory facilitation per se may not underlie the RT reduction cannot be excluded. It is conceivable that combined visual stimuli, or a stronger stimulus might have a similar effect. The effect of startling stimuli upon simple RT can occur when the imperative and startling stimuli are both acoustic (Carlsen et al. 2003). In our paradigm, sound was presented simultaneously with the imperative visual stimulus during trials with target jumps. The extent to which the quickening effect is dependent on synchronicity is unknown. Valls-Sole et al. (1995) reported that the facilitatory effect of startling sound upon simple RT was greatest when the sound and visual imperative stimulus were synchronous. Facilitation waned with increasing delay between the two, disappearing completely when the sound came 100 ms later. Whether this is true of our paradigm is unknown.
Sound affects visuo-motor processing
In the present study, the sound carried no information regarding the likelihood or direction of the target jump. This precludes the possibility that the reduced reaction time resulted from prediction. Rather, the sound interacted with the visual stimulus to cause a reduction in visuo-motor processing time. We measured this reduction at 18–31 ms. This may seem modest in absolute terms, but it actually constitutes a large proportion of the processing time, given that visually guided step adjustments are already very fast (Reynolds & Day, 2005). Although here we measured foot kinematics, we have previously shown that the first leg muscle responses occur at around 100 ms after the target jump, with approximately 20 ms consumed by electro-mechanical delay. Neural transmission of motor output from the brain to the upper leg takes at least 20 ms (Mrachacz-Kersting et al. 2006). The time taken for sensory transmission to processing centres is unknown but presumably takes at least 24 ms, the latency at which subcortical neurons first respond to light in the monkey (Bair et al. 2002). Processing time must therefore constitute a maximum of 60 ms out of the total response time of 100 ms. Our most conservative estimate of the reduction in processing time induced by the loud sound is therefore 30% (18 in 60 ms). Higher sensory and motor transmission times would merely serve to increase this estimate.
Moving the foot rapidly in response to visual information is important to maintain balance, when avoiding obstacles for example. This raises possible ethological significance of the current finding. Obstacle avoidance and target interception reactions are empirically similar. They both have short latencies which remain short even in situations requiring choice responses (Weerdesteyn et al. 2004). For example, in a situation where an obstacle can be avoided through either stride lengthening or shortening, the latency of the response remains the same as when no such choice exists. These similarities raise the possibility that obstacle avoidance could be facilitated by loud sound in the same way as target jump responses.
A possible subcortical interaction between sound and vision
As argued above, for strong theoretical reasons our task cannot be compared to a simple reaction task in which the upcoming movement is known in advance. We have also presented evidence that suggests our task differs fundamentally from conventional choice reaction tasks. Generally, conventional choice reaction tasks involve arbitrary visuo-motor associations and typically have reaction times greater than 200 ms (van der Molen & Keuss, 1979; Hackley & Valle-Inclán, 1998; Schluter et al. 1998; Carlsen et al. 2004a). Trajectory adjustments of limbs reaching for real visual targets that unpredictably move are much faster, between 100 and 150 ms (Carlton, 1981; Soechting & Lacquaniti, 1983; Day & Lyon, 2000). Arbitrary visuo-motor associations involve discrimination and classification of visual stimuli such as shapes or letters, which determines the required movement. This type of visual processing probably depends upon the cerebral cortex (Schluter et al. 1998; Schluter et al. 2001). Fast trajectory adjustments of the moving limb towards jumping targets may not be dependent upon the cerebral cortex in the same way. Supporting evidence comes from a patient with a congenital absence of a corpus callosum. In this patient, when responding with an arbitrary movement of the right hand to a visual stimulus presented in the left visual field, reaction time was much longer than if reacting with the left hand, and vice versa. This prolongation was not observed when the arm was used to reach for a target that jumped into the left or right hemifield, suggesting that the relevant sensorimotor pathway bypasses the cerebral cortex (Day & Brown, 2001). The step adjustment behaviour we have studied here has similarly fast response times to these upper limb reach adjustments, suggesting that the neural pathways are similar (Reynolds & Day, 2005). This raises the possibility that the quickening effect observed here is due to a facilitation of loud sound on subcortical visuo-motor processing.
The superior colliculus is a potential site for this facilitation. It has fast output pathways for controlling limb movement (Courjon et al. 2004), and with appropriate cortical input (Wallace & Stein, 1994) its neurons can integrate multimodal sensory input synergistically (Stein & Meredith, 1993). However, synergy is strongest in response to weak sensory stimuli emanating from the same spatial location (Stanford et al. 2005). This contrasts with our study, in which the acoustic stimulus is very loud, and carries no information regarding the direction of the target jump. The startle response itself displays cross-modal facilitation (Yeomans et al. 2002). This raises the question of whether the brainstem regions which subserve startle, such as the caudal pontine reticular formation (Yeomans & Frankland, 1995), can subserve intersensory facilitation for other behaviours.
References
- Bair W, Cavanaugh JR, Smith MA, Movshon JA. The timing of response onset and offset in macaque visual neurons. J Neurosci. 2002;22:3189–3205. doi: 10.1523/JNEUROSCI.22-08-03189.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IH, Clark MH, Edelstein BA. Intermodal effects in choice reaction time. J Exp Psychol. 1969;81:405–407. [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991a;114:1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. The hyperekplexias and their relationship to the normal startle reflex. Brain. 1991b;114:1903–1928. doi: 10.1093/brain/114.4.1903. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Startle response is dishabituated during a reaction time task. Exp Brain Res. 2003;152:510–518. doi: 10.1007/s00221-003-1575-5. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–309. doi: 10.1007/s00221-004-1924-z. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004b;36:253–264. doi: 10.3200/JMBR.36.3.253-264. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res. 2006;176:199–205. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carlton LG. Processing visual feedback information for movement control. J Exp Psychol Hum Percept Perform. 1981;7:1019–1030. doi: 10.1037//0096-1523.7.5.1019. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Olivier E, Pelisson D. Direct evidence for the contribution of the superior colliculus in the control of visually guided reaching movements in the cat. J Physiol. 2004;556:675–681. doi: 10.1113/jphysiol.2004.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Brown P. Evidence for subcortical involvement in the visual control of human reaching. Brain. 2001;124:1832–1840. doi: 10.1093/brain/124.9.1832. [DOI] [PubMed] [Google Scholar]
- Day BL, Lyon IN. Voluntary modification of automatic arm movements evoked by motion of a visual target. Exp Brain Res. 2000;130:159–168. doi: 10.1007/s002219900218. [DOI] [PubMed] [Google Scholar]
- Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci. 1999;11:473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclán F. Automatic alerting does not speed late motoric processes in a reaction-time task. Nature. 1998;391:786–788. doi: 10.1038/35849. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclán F. Accessory stimulus effects on response selection: does arousal speed decision making? J Cogn Neurosci. 1999;11:321–329. doi: 10.1162/089892999563427. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Grey MJ, Sinkjaer T. Evidence for a supraspinal contribution to the human quadriceps long-latency stretch reflex. Exp Brain Res. 2006;168:529–540. doi: 10.1007/s00221-005-0120-0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijzen PH, Schillings AM, van Galen GP, Duysens J. Modulation of the startle response during human gait. J Neurophysiol. 2000;84:65–74. doi: 10.1152/jn.2000.84.1.65. [DOI] [PubMed] [Google Scholar]
- Posner MI, Nissen MJ, Klein RM. Visual dominance: an information-processing account of its origins and significance. Psychol Rev. 1976;83:157–171. [PubMed] [Google Scholar]
- Reynolds RF, Day BL. Rapid visuo-motor processes drive the leg regardless of balance constraints. Curr Biol. 2005;15:R48–R49. doi: 10.1016/j.cub.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. The startle reflex, voluntary movement, and the reticulospinal tract. Clin Neurophysiol. 2006;58(Suppl):223–231. doi: 10.1016/s1567-424x(09)70071-6. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Lacquaniti F. Modification of trajectory of a pointing movement in response to a change in target location. J Neurophysiol. 1983;49:548–564. doi: 10.1152/jn.1983.49.2.548. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci. 2005;25:6499–6508. doi: 10.1523/JNEUROSCI.5095-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA, USA: MIT Press; 1993. [Google Scholar]
- Todd JW. Reaction to multiple stimuli. Arch Psychol. 1912;3:1–65. [Google Scholar]
- Valls-Sole J, Kofler M, Kumru H, Castellote JM, Sanegre MT. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res. 2005;165:541–548. doi: 10.1007/s00221-005-2332-8. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Sole A, Valldeoriola F, Munoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett. 1995;195:97–100. doi: 10.1016/0304-3940(94)11790-p. [DOI] [PubMed] [Google Scholar]
- van der Molen MW, Keuss PJ. The relationship between reaction time and intensity in discrete auditory tasks. Q J Exp Psychol. 1979;31:95–102. doi: 10.1080/14640747908400709. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. Cross-modal synthesis in the midbrain depends on input from cortex. J Neurophysiol. 1994;71:429–432. doi: 10.1152/jn.1994.71.1.429. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Nienhuis B, Hampsink B, Duysens J. Gait adjustments in response to an obstacle are faster than voluntary reactions. Hum Mov Sci. 2004;23:351–363. doi: 10.1016/j.humov.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev. 1995;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]