Abstract
We tested the hypothesis that mechanical deformation of forearm blood vessels via acute increases in extravascular pressure elicits rapid vasodilatation in humans. In healthy adults, we measured forearm blood flow (Doppler ultrasound) and calculated forearm vascular conductance (FVC) responses to whole forearm compressions and isometric muscle contractions with the arm above heart level. We used several experimental protocols to gain insight into how mechanical factors contribute to contraction-induced rapid vasodilatation. The findings from the present study clearly indicate that acute increases in extravascular pressure (200 mmHg for 2 s) elicit a significant rapid vasodilatation in the human forearm (peak ΔFVC∼155%). Brief, 6 s sustained compressions evoked the greatest vasodilatation (ΔFVC∼260%), whereas the responses to single (2 s) and repeated compressions (five repeated 2 s compressions) were not significantly different (ΔFVC∼155%versus∼115%, respectively). This mechanically induced vasodilatation peaks within 1–2 cardiac cycles, and thus is dissociated from the temporal pattern normally observed in response to brief muscle contractions (∼4–7 cardiac cycles). A non-linear relation was found between graded increases in extravascular pressure and both the immediate and peak rapid vasodilatory response, such that the responses increased sharply from 25 to 100 mmHg, with no significant further dilatation until 300 mmHg (maximal ΔFVC∼185%). This was in contrast to the linear intensity-dependent relation observed with muscle contractions. Our collective findings indicate that mechanical influences contribute largely to the immediate vasodilatation (first cardiac cycle) observed in response to a brief, single contraction. However, it is clear that there are additional mechanisms related to muscle activation that continue to cause and sustain vasodilatation for several more cardiac cycles after contraction. Additionally, the potential contribution of mechanical influences to the total contraction-induced hyperaemia appears greatest for low to moderate intensity single muscle contractions, and this contribution becomes less significant for sustained and repeated contractions. Nevertheless, this mechanically induced vasodilatation could serve as a feedforward mechanism to increase muscle blood flow at the onset of exercise, as well as in response to changes in contraction intensity, prior to alterations in local vasodilating substances that influence vascular tone.
Brief muscle contractions elicit a significant increase in skeletal muscle blood flow which is proportional to the strength of contraction and can be detected immediately post contraction (Corcondilas et al. 1964; Lind & Williams, 1979; Tschakovsky et al. 1996, 2004; Naik et al. 1999). In humans, the observed exercise-induced hyperaemia following a single muscle contraction is typically characterized by a monophasic pattern and appears to peak within approximately 4–5 s (∼4–6 cardiac cycles after contraction) (Corcondilas et al. 1964; Tschakovsky et al. 1996, 2004). Although some have suggested that this rapid elevation in blood flow might be attributed to the skeletal muscle pump (widening of the arteriovenous pressure gradient) (Laughlin, 1987; Sheriff et al. 1993), recent experimental evidence clearly indicates that a rapid vasodilatation is obligatory for the rapid hyperaemic response to brief muscle contractions (Naik et al. 1999; Hamann et al. 2004; Mihok & Murrant, 2004; Tschakovsky et al. 2004; VanTeeffelen & Segal, 2006).
To date, the mechanisms underlying contraction-induced rapid vasodilatation are not fully understood. Data derived from studies in the human forearm and the canine hindlimb indicate that neural mechanisms do not contribute to this vasodilatation (Corcondilas et al. 1964; Brock et al. 1998; Dyke et al. 1998; Buckwalter & Clifford, 1999; Naik et al. 1999). Additionally, it has been suggested that the release of, and responsiveness to, local vasodilators is too sluggish to account for this response (Wunsch et al. 2000). Indeed, studies employing a variety of experimental approaches have found that independent or combined inhibition of nitric oxide and prostaglandins do not impact on rapid vasodilatation in humans (Shoemaker et al. 1996; Brock et al. 1998; Saunders et al. 2005). Together, the mechanisms involved in contraction-induced rapid vasodilatation remain unclear.
It is well known that during muscle contraction intramuscular pressure increases in proportion to contraction intensity (Sadamoto et al. 1983; Sejersted et al. 1984), thus causing a subsequent elevation in extravascular pressure and a decrease in transmural pressure. As originally proposed by Bayliss (1902) and later demonstrated experimentally by Mohrman & Sparks (1974) in a canine hindlimb preparation, this decrease in transmural pressure may stimulate the relaxation of resistance vessels and therefore contribute to exercise hyperaemia. Further, in addition to blood vessel distortion observed during contraction (Gray et al. 1967), a transfiguration of the vascular network during direct mechanical modification of skeletal muscle length may also occur (Nakao & Segal, 1995). Together, these mechanical effects of muscle contraction have been postulated to evoke vasodilatation and contribute to the hyperaemic response.
Recently, Clifford et al. (2006) demonstrated that acute mechanical deformation of rat skeletal muscle feed arteries via extravascular pressure in vitro elicits a rapid vasodilatation that peaks in ∼4–5 s, which is strikingly similar to the contraction-induced vasodilatation in vivo (Tschakovsky et al. 1996). These investigators also demonstrated that the mechanically evoked vasodilatation was greatest when the vessel was repeatedly compressed, as compared with the responses to a single 1 s compression or a sustained compression (5 s). Thus, a local mechanical effect of muscle contraction on extravascular pressure could potentially explain this rapid vasodilatation independent of neural and/or metabolic factors. To date, however, the effects of acute increases in extravascular pressure (independent of the muscle pump) on limb vascular tone in humans are not clear. Further, it is unclear how mechanical influences on skeletal muscle vascular tone potentially contribute to contraction-induced rapid vasodilatation in humans.
Therefore, we tested the general hypothesis that mechanical deformation of forearm blood vessels via acute increases in extravascular pressure elicits a rapid vasodilatation in the human forearm. We used a variety of experimental approaches to determine (1) the temporal pattern of vasodilatation, (2) whether the vasodilatory responses were more sensitive to repeated versus sustained pressure, (3) whether the vasodilatation was linear across a wide range of extravascular pressure, and (4) whether a normal muscle recruitment pattern was obligatory to exhibit the temporal pattern of rapid vasodilatation observed in response to brief contractions.
Methods
Subjects
A total of 17 young healthy adults (12 men, five women; age, 25 ± 1 year; weight, 68 ± 3 kg; height, 176 ± 2 cm; body mass index, 22 ± 0.5 kg m−2; means ±s.e.m.) participated in the present study. Seven of the 17 subjects (total n = 24) participated in all experimental protocols (see below). All subjects were non-smokers, non-obese, normotensive, and not taking any medications. Because the current study utilized within subject analysis, experimental sessions involving female volunteers were not adjusted to account for phase within the menstrual cycle, as any influence of their menstrual cycle on the primary outcome variables would be expected to be the same throughout the entire study session. Studies were performed in a temperature-controlled environment (20–22°C) after a minimum of 4 h fasting. The subjects abstained from caffeine and exercise on the day of the study, and all studies were performed with the subjects in the supine position. This research was approved by the Human Research Committee of Colorado State University. All subjects gave their written informed consent to participate, and all studies were performed according to the Declaration of Helsinki.
Arterial blood pressure and heart rate
Resting arterial blood pressure was measured non-invasively over the brachial artery of the control arm (right) after 30 min of supine rest before any experimental trials, and just prior to each experimental trial after the study began (Cardiocap/5, Datex-Ohmeda Louisville, CO, USA). Beat-by-beat arterial blood pressure was measured at heart level by finger photoplethysmography (Finapres, Ohmeda) on the middle finger of the control hand during all experimental trials. Heart rate was determined using a three-lead ECG (Cardiocap/5, Datex-Ohmeda Louisville).
Forearm blood flow and vascular conductance
A 4 MHz pulsed Doppler probe (Model 500V, Multigon Industries, Mt Vernon, NY, USA) was used to measure brachial artery mean blood velocity (MBV) with the probe securely fixed to the skin over the brachial artery as we have previously described (Dinenno & Joyner, 2003, 2004). The probe insonation angle was 45 deg. Pilot studies indicated no change in brachial artery diameter during all trials and conditions, therefore brachial artery diameter was measured in triplicate at the end of the study for calculation of forearm blood flow. To do so, a linear 7.0 MHz echo Doppler ultrasound probe (Hewlett-Packard Sonos 4500, Andover, MA, USA) was placed over the skin where the velocity probe was secured in order to measure brachial artery diameter. Forearm blood flow (FBF) was calculated as:
where FBF is in ml min−1, the MBV is in cm s−1, the brachial diameter is in cm, and 60 is used to convert from ml s−1 to ml min−1.
Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100, and expressed as ml min−1 (100 mmHg)−1, where MAP is mean arterial pressure.
Forearm position
The subject lay supine with the experimental (left) arm abducted 90 deg and elevated ∼20 cm above heart level upon a tilt-adjustable table. All measurements and procedures were performed in this manner in order to empty forearm veins and minimize the influence of the muscle pump on forearm haemodynamics (Folkow et al. 1971; Tschakovsky et al. 1996, 2004; Shoemaker et al. 1998). Additionally, a fan was directed towards the experimental arm to minimize the contribution of skin blood flow to forearm haemodynamics.
Forearm compressions (extravascular pressure)
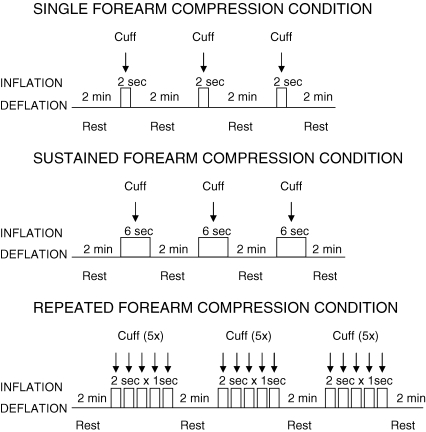
Acute increases in extravascular pressure were achieved by instrumenting the subject with a custom-designed tapered blood pressure cuff (length, ∼30 cm) that was wrapped snugly around the entire experimental forearm and rapidly inflated to the desired pressure (mmHg) using a rapid cuff inflation unit (Hokanson E20, Bellevue, WA, USA) as we have previously described (Kirby et al. 2005). Depending on the experimental protocol, forearm compressions were performed in any of the following ways: (1) single compression – inflation for 2 s followed by complete deflation; (2) sustained compression – inflation for 6 s followed by complete deflation; and (3) repeated compression – inflation for 2 s followed by 1 s of deflation repeated over five cycles. To most closely mimic brief muscle contractions (see below), all forearm compression conditions included an additional second more than contraction conditions to allow ∼0.5 s for complete cuff inflation, and another ∼0.5 s for transmission of this pressure through the forearm tissue. Pilot studies in our laboratory using forearm compression times of 1 or 2 s resulted in similar temporal vasodilator responses, thus we chose to use 2 s to allow adequate time for pressure transmission.
Isometric handgrip exercise
Maximum voluntary contraction (MVC) for each subject was determined for the experimental arm as the average of three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL, USA) that were within 3% of each other. Isometric handgrip exercise was performed in a similar manner as the conditions described below for forearm compressions to the desired target workload (% MVC; see Protocols 1 and 2). All contractions were 1 s less than for forearm compression as explained above (single, 1 s; sustained, 5 s; repeated, 1 s/1 s × 5). Isometric handgrip exercise was chosen as the mode of exercise because intramuscular pressure has been shown to correlate linearly with both isometric contraction force over a wide range of forces including maximal contraction (Sejersted et al. 1984), and with pressure applied externally by a cuff (Sadamoto et al. 1983).
Electrical stimulation
Single isometric muscle contractions of the experimental arm were produced by electrical stimulation in two manners: (1) direct stimulation of the forearm muscle belly; and (2) stimulation of the median nerve. To prevent movement during electrical stimulation, the forearm and hand were secured to the table using adjustable straps. For muscle stimulation, two electrodes (5 cm diameter) were placed on the anterior side of the forearm muscle belly, one just distal and slightly medial to the antecubital fossa and the other placed 2.5cm in distal and lateral to the first electrode in an effort to cover a large surface area of the muscle belly. Nerve stimulation was performed topically utilizing a blunt stimulating probe placed directly over median nerve ∼1 in distal to the antecubital fossa; the ground electrode was placed on the posterior side of the experimental hand just below the first digit. An electrical stimulus (3 ms pulse duration at 100 Hz for a total duration of 1 s; see Protocol 3) was delivered to the muscle and nerve via a S48 Stimulator (Astro-Medical Inc., GRASS Instrument Division, W. Warwick, RI, USA). Based on pilot studies in our laboratory, a voltage (60–70 V) was chosen that produced modest vasodilatation yet minimized subject discomfort.
Experimental protocols
Protocol 1: effect of acute increases in extravascular pressure on forearm vasodilator responses and its temporal association to contraction-induced rapid vasodilatation
The purpose of this protocol was to determine (1) whether acute increases in extravascular pressure evoke a rapid vasodilatation in the human forearm and, if so, (2) the temporal pattern of the observed vasodilator response. In the first group of subjects (n = 12; 10 men, two women), the forearm vasodilator responses to acute increases in extravascular pressure were determined immediately in response to the following conditions: (1) single forearm compressions at 100 and 200 mmHg; (2) sustained forearm compressions at 100 and 200 mmHg; and (3) repeated forearm compressions at 100 and 200 mmHg. In order to initially determine the effects of forearm compression on mechanically induced vasodilatation, two different cuff pressures were utilized for all conditions. As previously described, externally applied cuff pressures of 100 and 200 mmHg translate well to intramuscular pressure of the same magnitude (Sadamoto et al. 1983). Based on the findings of Clifford et al. (2006), we hypothesized that repeated forearm compressions would evoke the greatest vasodilatation, whereas the responses to single and sustained compressions would be similar.
To determine whether the temporal pattern of mechanically induced vasodilatation is similar to that observed in response to forearm muscle contractions, subjects performed isometric handgrip exercise in a similar fashion to forearm compressions. In each subject, we attempted to match the peak vasodilatation observed in condition 1 in response to 200 mmHg of forearm compressions with that of a single contraction for simple comparisons of the temporal response. This corresponded to a workload of ∼20% MVC.
For each subject, forearm compression conditions were performed in triplicate with all three trials of a given condition separated by 2 min, and the remaining two conditions followed in the same manner. After 15 min of quiet rest, isometric contraction conditions were performed in triplicate in the same manner as compression conditions. One half of the subjects underwent forearm compressions first, and the other half performed contractions first. The order of the compression or contraction conditions was randomized and counterbalanced across subjects to eliminate any potential order effect. The timeline for the experimental conditions and trials in Protocol 1 are shown in Fig. 1.
Figure 1.
Schematic timeline for Protocol 1 Acute increases in extravascular pressure were acheived via whole forearm compression with a custom-made cuff utilizing pressures of 100 and 200 mmHg. Single compressions were performed with cuff inflated for 2 s, sustained compressions were performed with cuff inflated for 6 s, repeated compressions were performed with cuff inflated for 2 s followed by 1 s of deflation for 5 cycles. Each compression trial was preceded and followed by 2 min of rest. Isometric handgrip was performed at ∼20% maximum voluntary contraction in a similar fashion. (See Methods for more detail).
Protocol 2: effect of acute graded increases in extravascular pressure on forearm vasodilator responses and its relation to contraction-intensity dependent vasodilatation
In light of our findings from Protocol 1 (see Results), the purpose of Protocol 2 was to determine (1) the relation between graded increases in acute extravascular pressure via whole forearm compression and rapid vasodilatation, and (2) the relation between graded increases in muscle contraction intensity and rapid vasodilatation. Therefore, in the second group of subjects (n = 12; nine men, three women), the forearm vasodilator responses were determined in response to single forearm compressions over the following range of pressures: 25, 50, 75, 100, 150, 200, 250 and 300 mmHg. To determine the relation between graded increases in exercise intensity and rapid vasodilatation, a range of workloads was also used: 10, 25, 50, 75 and ∼100% MVC.
As in Protocol 1, all conditions included 2 min of rest between forearm compressions and muscle contractions and were performed in triplicate. The compression and contraction trials were separated by 15 min of rest. One half of the subjects underwent forearm compressions first, and the other half performed contractions first. The order of the compression or contraction conditions were randomized and counterbalanced across subjects to eliminate any potential order effect. The timeline for the experimental trials in Protocol 2 are similar to that shown in Fig. 1 (single condition), with the addition of several more trials.
Protocol 3: effect of electrical stimulation on forearm vasodilator responses and its temporal association to mechanical and voluntary contraction-induced vasodilatation
The purpose of Protocol 3 was to determine whether normal muscle recruitment patterns were obligatory to produce the observed temporal pattern of contraction-induced rapid vasodilatation. We acknowledge that use of an external compression cuff might not perfectly mimic the manner in which voluntary muscle contractions increase intramuscular pressure and cause subsequent deformation/distortion of muscle resistance vessels. Therefore, in four subjects who participated in Protocol 2 (three men, one woman), we performed direct muscle and nerve stimulation to evoke forearm contractions. Electrical stimulation alters the normal muscle recruitment pattern and causes the muscles to contract simultaneously, in contrast to the typical sequential and finely controlled contraction pattern during voluntary contractions (Burke, 1981; Laughlin, 1987; Naamani et al. 1995). In this context, we reasoned that if the temporal pattern of mechanically induced vasodilatation differed from contractions, electrical stimulation would provide insight into whether this was due to any condition-related differences in mechanical effects on forearm vessels (i.e. simultaneous versus sequential compression). Muscle and nerve stimulation were performed in triplicate with a timeline similar to those of Protocols 1 and 2. Two subjects received muscle stimulation first, whereas the other two subjects received nerve stimulation first. In these subjects, we compared the forearm vasodilator responses to muscle and nerve stimulation with their matched responses obtained for voluntary contractions and forearm compressions from Protocol 2.
Data acquisition and analysis
Data were collected and stored on a computer at 250 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). Baseline FBF and MAP represent an average of the last 10 s of the resting time period. The hyperaemic values were calculated for each cardiac cycle beginning with the first unimpeded cardiac cycle after forearm compression and muscle contraction, and were averaged for the three trials across all subjects. The percentage increase in FVC after forearm compression or muscle contraction was calculated as: ((FVC post compression – FVC pre compression)/(FVC pre compression)) × 100. We used percentage increase in FVC as our standard index of forearm vasodilatation in all experimental protocols.
Statistics
All values are reported as mean ±s.e.m. Specific hypothesis testing for each protocol was performed using repeated measures ANOVA. In the case of a significant F value, Newman–Keuls method for multiple comparisons was used to determine where differences occurred. Statistical significance was set a priori at P < 0.05.
Results
Protocol 1
Forearm vasodilator responses to acute increases in extravascular pressure
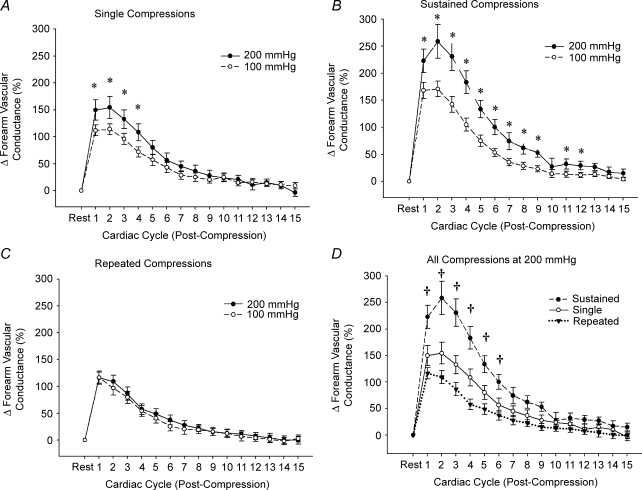
Baseline forearm haemodynamics and MAP for all experimental conditions in Protocol 1 are presented in Table 1A. In general, all trials regardless of the cuff inflation pressure or compression mode (single, sustained or repeated) resulted in significant forearm vasodilatation (Fig. 2A–C; P < 0.05). In condition 1, brief single forearm compressions of 100 and 200 mmHg evoked a significant and graded vasodilatation (peak ΔFVC, ∼110% and ∼155%, respectively; P < 0.05; Fig. 2A). In contrast to the results expected from our hypothesis, sustained forearm compressions (condition 2) evoked the greatest vasodilatation (ΔFVC, ∼170% and ∼260% for 100 and 200 mmHg, respectively; P < 0.05; Fig. 2B), whereas repeated forearm compressions (condition 3) resulted in ∼115% increase in FVC for both 100 mmHg and 200 mmHg (P < 0.05; Fig. 2C). Thus, the following order of vasodilator sensitivity to acute increases in extravascular pressure was observed: sustained > single = repeated (Fig. 2D). Heart rate and MAP for all trials was not significantly different at baseline and after compressions.
Table 1.
Baseline forearm haemodynamics for Protocol 1 and Protocol 2
| Baseline | Heart rate (beats min−1) | Mean arterial pressure (mmHg) | Forearm blood flow (ml min−1) | Forearm vascular conductance (ml min−1 mmHg−1) |
|---|---|---|---|---|
| A. Baseline forearm haemodynamics for Protocol 1 | ||||
| Compression conditions | 53 ± 3 | 89 ± 2 | 24 ± 4 | 27 ± 4 |
| Contraction conditions | 54 ± 3 | 89 ± 2 | 25 ± 3 | 30 ± 3 |
| B. Baseline forearm haemodynamics for Protocol 2 | ||||
| Compression conditions | 53 ± 3 | 89 ± 3 | 25 ± 4 | 28 ± 4 |
| Contraction conditions | 53 ± 2 | 89 ± 3 | 23 ± 2 | 27 ± 2 |
Figure 2.
Rapid vasodilatory responses to acute increases in extravascular pressure Significant forearm vasodilatation (P < 0.05) was observed for all compression conditions at 100 and 200 mmHg of cuff pressure (A–C). A brief, 6 s sustained compression at 200 mmHg evoked the largest rapid vasodilatory response when compared with single or repeated compressions (D). *P < 0.05 versus 100 mmHg cuff pressure within specific condition; †P < 0.05 versus single and repeated compressions.
Forearm vasodilator responses to isometric handgrip exercise at ∼20% MVC
Isometric contractions performed at ∼20% MVC evoked significant forearm vasodilatation for all conditions (see Fig. 3A–C). In contrast to the vasodilatation seen with forearm compressions, repeated isometric contractions produced the greatest vasodilatation of all exercise conditions (ΔFVC, ∼470%; P < 0.05), followed by sustained isometric contractions (∼375%; P < 0.05) and single contractions (∼160%; P < 0.05).
Figure 3.
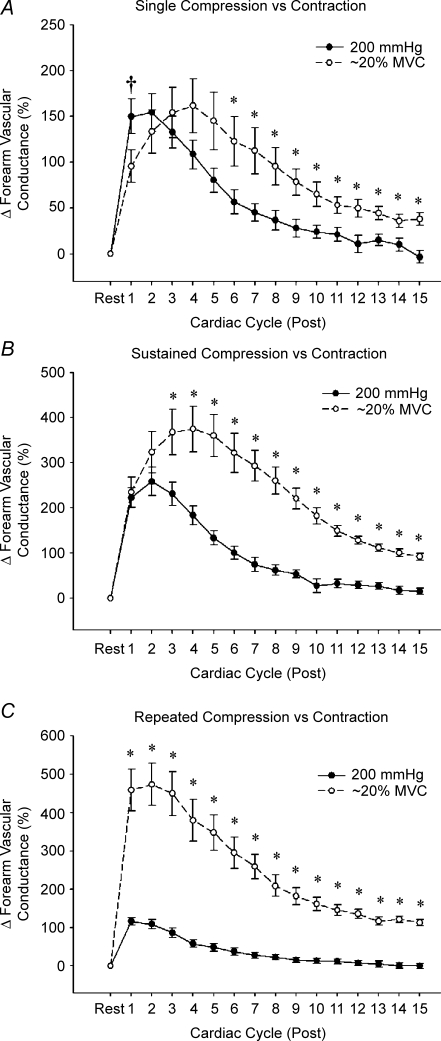
Temporal association between mechanically and contraction-induced rapid vasodilatation Peak vasodilator responses to mechanically induced vasodilatation occurred within 1–2 cardiac cycles, whereas the peak responses to isometric contractions (∼20% maximum voluntary contraction) typically occurred in ∼4 cardiac cycles (A and B). Further, the peak and total vasodilatation for sustained (B) and repeated (C) contractions were significantly greater than the values observed for compressions. In general, mechanically induced vasodilatation was temporally dissociated from vasodilatation observed in response to muscle contractions. *P < 0.05 versus compression; †P < 0.05 versus contraction.
Temporal association between mechanical and contraction-induced rapid vasodilatation
Single isometric contractions at ∼20% MVC resulted in peak vasodilatation post contraction in ∼4 cardiac cycles (Fig. 3A). Further, sustained isometric contractions also reached peak vasodilatation in ∼4 cardiac cycles (Fig. 3B), whereas the peak vasodilatory response to repeated isometric contractions occurred within 2 cardiac cycles (Fig. 3C). By contrast, the peak vasodilatation for all forearm compression trials always occurred within 1–2 cardiac cycles following cuff deflation, dissociating the temporal pattern of mechanically induced rapid vasodilatation from contraction-induced rapid vasodilatation (Fig. 3A–C). As planned, the peak vasodilatation in response to a single contraction was similar to that in the single 200 mmHg compression trial (Fig. 3A). However, the magnitude of peak vasodilatation was significantly greater for both sustained (Fig. 3B) and repeated (Fig. 3C) contractions compared with compressions (P < 0.001). Finally, FVC was still significantly elevated above baseline 15 cardiac cycles after contraction for all exercise conditions, whereas FVC had returned to baseline after all forearm compression conditions (Fig. 3A–C).
Protocol 2
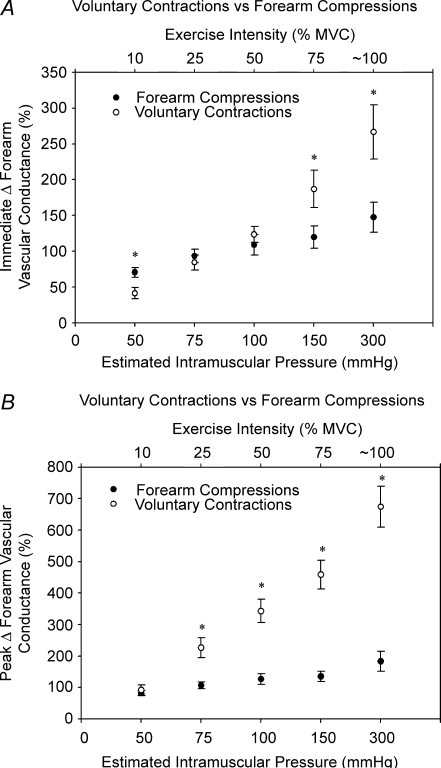
Baseline forearm haemodynamics and MAP for all experimental conditions in Protocol 2 are presented in Table 1B. In general, all cuff inflation pressures (25–300 mmHg) resulted in significant forearm vasodilatation (P < 0.05; Fig. 4A–C). Interestingly, a non-linear relation is demonstrated between increases in cuff pressure and both the immediate (first cardiac cycle post) and peak rapid vasodilatory response, such that the responses increased sharply from 25 to 100 mmHg, with no significant further dilatation until 300 mmHg (Fig. 4B and C). This pattern is in contrast to the linear relation observed with graded increases in contraction intensity (Fig. 4E and F). As in Protocol 1, the peak vasodilatation for all forearm cuff pressures occurred within 1–2 cardiac cycles following cuff deflation, whereas single isometric contractions produced a peak vasodilatory response in ∼4–7 cardiac cycles. Time-to-peak vasodilatation tended to increase with increasing exercise intensity, while time-to-peak vasodilatation for mechanical compressions was not different over the range of cuff pressures (Fig. 4A and D). Further, FVC was still significantly elevated above baseline 15 cardiac cycles after contraction for all exercise conditions, whereas FVC had returned to baseline after all forearm compression conditions. When comparing the vasodilator responses over a range of estimated intramuscular pressures (Sadamoto et al. 1983), it is clear that mechanically induced vasodilatation contributes largely to the immediate contraction-induced vasodilatation over a wide range of contraction intensities (Fig. 5A). However, the potential contribution of mechanically induced vasodilatation to the peak contraction-induced vasodilatation appears to become progressively less with increasing contraction intensity (Fig. 5B). Heart rate and MAP for all trials were not significantly different between baseline and post compression/contraction.
Figure 4.
Effects of graded extravascular pressure and contraction intensity on rapid vasodilatation All cuff compressions and muscle contractions elicited rapid vasodilatation (A and D). A non-linear relation was found between forearm cuff pressure and the immediate (first cardiac cycle post; B) and peak rapid vasodilatation (C), whereas the relation was linear with respect to contraction intensity (E and F). Subjects achieved ∼95% maximum voluntary contraction for the ‘∼100%’ trial. *P < 0.05 versus zero; †P < 0.05 versus 25 mmHg; ‡P < 0.05 versus ≤ 50 mmHg; #P < 0.05 versus ≤ 75 mmHg; ##P < 0.05 versus ≤ 200 mmHg.
Figure 5.
Relation between estimated intramuscular pressure and rapid vasodilatation for compressions and contractions Based on findings from Sadamoto et al. (1983), we conservatively estimated intramuscular pressure for the various isometric contraction intensities and compared the immediate (first cardiac cycle post) and peak vasodilatation observed in response to contractions and cuff compressions at these pressures. This figure illustrates that mechanically induced vasodilatation contributes largely to the immediate response (A), and that the mechanical contribution to the peak contraction-induced vasodilatation (B) is greatest for low levels of contraction intensity, and becomes progressively less as intensity increases. *P < 0.05 versus compressions at same estimated intramuscular pressure.
Protocol 3
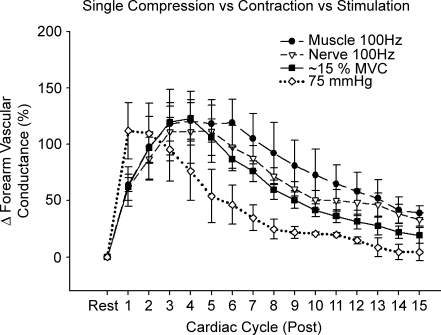
Electrical stimulation of forearm muscle and the median nerve evoked significant forearm vasodilatation (ΔFVC, ∼120%; P < 0.05; Fig. 6). It is interesting that peak vasodilatation occurred in ∼4 cardiac cycles after stimulation, similar to voluntary contractions that evoke the same peak response (Fig. 6). When comparing the mechanically induced vasodilatation with that observed in response to all types of muscle contraction (voluntary, muscle stimulation and nerve stimulation), it is clear that mechanical compression per se elicits a vasodilator response that is temporally dissociated from the vasodilator response to muscle contraction (Fig. 6). Again, FVC was still significantly elevated above baseline 15 cardiac cycles after contraction for all exercise conditions, whereas FVC had returned to baseline after forearm compression.
Figure 6.
Effects of muscle and nerve stimulation on forearm vasodilator responses and the temporal association with mechanically and voluntary contraction-induced rapid vasodilatation Electrical stimulation of both the forearm muscle belly and median nerve evoked a significant vasodilatation (P < 0.05). Of importance, when these responses were compared with those following voluntary contractions, the temporal pattern was similar. By contrast, the temporal pattern of the mechanically induced vasodilatation was dissociated from all muscle contraction conditions (n = 4.) Peak vasodilator responses for both forearm compressions and voluntary contractions were matched to those observed in response to electrical stimulation. Maximum voluntary contraction, 10% for three subjects and 25% for one subject.
Discussion
The primary new findings from this study are as follows. Firstly, mechanical deformation of forearm blood vessels via acute increases in extravascular pressure elicits a rapid vasodilatation in humans, and this appears to be more sensitive to sustained than repeated vascular deformation in vivo. Importantly, this mechanically induced rapid vasodilatation exhibits a temporal pattern that reaches its peak within 1–2 cardiac cycles, and thus is dissociated from that generally observed in response to brief muscle contractions (∼4–7 cardiac cycles; Protocols 1 and 2). Secondly, a non-linear relation was demonstrated between increases in extravascular pressure and both the immediate and peak rapid vasodilatory response, such that the responses increased sharply from 25 to 100 mmHg, with no significant further dilatation until 300 mmHg. This was in contrast to the linear intensity-dependent relation observed with muscle contractions (Protocol 2). Finally, single electrically evoked forearm muscle contractions (via muscle or nerve stimulation) result in rapid vasodilatation in humans that resembles the temporal pattern of voluntary contractions, indicating that normal sequential muscle fibre recruitment is not obligatory to observe the typical rapid vasodilatory response (Protocol 3).
Acute increases in extravascular pressure and rapid vasodilatation
In a recent study, mechanical compression of rat skeletal muscle feed arteries via extravascular pressure in vitro was demonstrated to produce significant vasodilatation with a time course similar to the increase in blood flow following a brief muscle contraction, indicating that mechanical influences could potentially explain contraction-induced rapid vasodilatation (Clifford et al. 2006). Additionally, these investigators found that the peak vasodilatation was more sensitive to repeated compressions, than to single and sustained compressions. These findings prompted us to test the general hypothesis that mechanical deformation of forearm blood vessels via acute increases in extravascular pressure elicits rapid vasodilatation in humans.
Previous studies in humans indicate either minimal or no effect of acute increases in extravascular pressure on limb haemodynamics when the influence of the muscle pump is minimized (i.e. arm at heart level or above). Lind & Williams (1979) found that FBF was not significantly elevated after acute inflation of a blood pressure cuff over the forearm. In two of their subjects, venous occlusion plethysmography was used to measure FBF 2 s after cuff release, and the slope of the plethysmogram was determined over the next 6 cardiac cycles. Thus, given that the peak response occurs within 2 cardiac cycles and decreases rapidly thereafter, the resolution and timing of their measurements could potentially explain their negative findings. Additionally, use of a normal blood pressure cuff that compresses a smaller portion of the forearm muscle mass than in the present study could have also contributed. Employing similar techniques as in the present study, Tschakovsky et al. (1996) found that the mechanical effect of cuff inflations (using 100 mmHg cuff pressure) on FBF is much greater with the limb in the dependent position, leading many to conclude that this was solely due to a muscle pump effect. However, in closely reviewing their data obtained when the arm was above heart level, it does appear that there is a rapid vasodilatory response. In the present investigation, we provide the first clear evidence that acute increases in extravascular pressure elicit a significant hyperaemic response in the human forearm. It is important to note that given that the arm was above heart level during our experiments, we believe that any muscle pump contribution was minimized (if not eliminated) and therefore this indicates a mechanically induced rapid vasodilatation.
We next sought to determine whether the forearm vasodilator responses were greater for repeated versus sustained compression/deformation as demonstrated in vitro by Clifford et al. (2006). In contrast to the data generated in the soleus feed artery, we found that sustained compressions evoked a much greater vasodilatory response compared with repeated compressions (Fig. 2D). We believe these discrepant findings could reflect fundamental differences between the in vitro versus in vivo conditions. For example, in our study, the sustained forearm compression condition using pressures of both 100 and 200 mmHg caused brief and graded levels of tissue ischaemia. Although this would only be for ∼5 s, an accumulation of metabolites acting in concert with a mechanical effect could potentially explain the greater vasodilatation we observed in vivo. Likewise, for the repeated compressions in our study, it is quite possible that local washout of metabolites occurred from the increase in blood flow over the duration of the repeated compressions (∼15 s total), and that this interaction with the mechanical influences resulted in a lower observed peak vasodilatory response. Nonetheless, isolated vessels not exposed to changes in local concentrations of vasoactive substances might indeed exhibit greater vasodilator sensitivity in response to repeated versus sustained compressions, but this was not demonstrated in vivo.
Mechanical versus contraction-induced rapid vasodilatation
In Protocol 1, we matched peak vasodilatation observed in response to single forearm compressions (200 mmHg) with that of a single muscle contraction (∼20% MVC) to gain insight into the temporal pattern of the responses. Although we found evidence for a mechanically induced rapid vasodilatation, our data clearly indicate that the temporal pattern of the response was dissociated from that observed in response to a single brief contraction (Fig. 3; also see Fig. 4A and D). We interpret these data to indicate that there is a role for mechanically induced vasodilatation, but its maximum independent contribution to contraction-induced rapid vasodilatation is within 1–2 cardiac cycles. Additionally, we believe these data indicate that some other mechanism(s) related to muscle activation must be involved in the response that continues to cause and sustain vasodilatation for several more cardiac cycles (Murrant & Sarelius, 2002; Mihok & Murrant, 2004; VanTeeffelen & Segal, 2006).
An interesting observation in Protocol 1 was that the magnitude of vasodilatation in response to repeated and sustained contractions was substantially greater than that observed in response to forearm compressions performed in a similar manner (Fig. 3B and C). This was true for both peak and sustained vasodilatation. Thus, we speculate that the potential mechanical contribution to contraction-induced vasodilatation is less significant for repeated and sustained contractions, than for a brief, single contraction. Further, these data clearly emphasize that greater levels of muscle activation, evoked by either increasing the number of contractions (i.e. repeated condition) or increasing contraction duration (i.e. sustained condition), cause a significantly greater hyperaemic response, which cannot be accounted for by an augmented mechanically induced vasodilatation. As such, we believe these findings again indicate that other factors associated with contracting muscle are intimately involved in determining the rapid peak vasodilatory response, as well as the sustained vasodilatation in response to brief contractions.
Mechanically induced rapid vasodilatation and graded extravascular pressure
In Protocol 1, the effects of acute increases in extravascular pressure appeared to be graded with the level of cuff pressure used. This stimulated us to determine the level of pressure required to initiate forearm vasodilatation, as well as to determine whether increasing cuff pressures above 200 mmHg evoke an even greater response. The data generated in Protocol 2 indicate a non-linear relation between extravascular pressure and rapid vasodilatation. We found that cuff pressures as low as 25 mmHg were capable of causing a rapid vasodilatation of ∼20%, and the magnitude of vasodilatation sharply increased up to 100 mmHg (∼110–125%). Further elevations in cuff pressure resulted in only mild additional vasodilatation, with the greatest response observed at 300 mmHg (∼150–185%; Fig. 4B and C). Although the greatest vasodilatation was observed at 300 mmHg, it is tempting to speculate that extravascular pressures of 100 mmHg could result in near maximal forearm resistance vessel compression, which might explain why the ‘gain’ in mechanically induced vasodilatation at higher levels of extravascular pressure is relatively modest.
As shown by others (Corcondilas et al. 1964; Tschakovsky et al. 2004), the rapid vasodilator responses to muscle contractions increased linearly with exercise intensity from 10% MVC to near maximal levels (∼95% MVC; Fig. 4E and F). This clearly contrasts with the relation previously described between extravascular pressure and rapid vasodilatation. Integrating these observations, we suggest that mechanical influences contribute largely to the immediate contraction-induced vasodilatation across a wide range of exercise intensities. However, it appears that the potential contribution of mechanically induced vasodilatation to the peak contraction-induced vasodilatation is greatest for low to moderate intensity contractions and becomes progressively less with increasing contraction intensities. This is illustrated in Fig. 5, where we compared the immediate and peak vasodilator responses to selected cuff pressures that we conservatively estimated to match the intramuscular pressure produced by the isometric contractions (Sadamoto et al. 1983). Although we recognize the limitation of estimating intramuscular pressure, we believe this illustrates an important point related to the potential contribution of mechanically induced vasodilatation to the immediate and peak response of a contraction as exercise intensity increases.
Our findings from Protocol 2 also indicate that time-to-peak vasodilatation tended to increase at high contraction intensities, whereas the peak response always occurred within 1–2 cardiac cycles for the forearm compressions (Fig. 4A). It is also clear that contractions elicited a much greater sustained vasodilatation compared with compressions, and this was greater with increasing contraction intensities but not extravascular pressure. This again indicates that other factors associated with a single brief muscle contraction, independent of mechanical influences, contribute significantly to the peak and total hyperaemic response (Murrant & Sarelius, 2002; Mihok & Murrant, 2004; VanTeeffelen & Segal, 2006).
Electrical stimulation and rapid vasodilatation
Direct electrical stimulation of the forearm muscle belly and median nerve was used to determine whether a normal muscle recruitment pattern was obligatory to observe the typical pattern of rapid vasodilatation that occurs in response to voluntary contractions. We recognize that increasing extravascular pressure via forearm cuff might not perfectly mimic the manner in which a normal muscle contraction causes mechanical deformation of blood vessels. Interestingly, it has been demonstrated that electrical stimulation alters the normal pattern of muscle fibre recruitment and evokes simultaneous (non-sequential) muscle contraction (Burke, 1981; Laughlin, 1987; Naamani et al. 1995). Thus, we reasoned that the mechanical effect of forearm compressions might more closely resemble the mechanical effect of contractions evoked via electrical stimulation, and that perhaps this ‘irregular’ (non-sequential) mechanical stimulus might explain the observed differences in the temporal pattern of vasodilatation. Our data from Protocol 3 clearly indicate that, no matter how muscle contraction was evoked (voluntary contraction versus muscle/nerve stimulation), the time-to-peak vasodilatation and pattern of the responses were nearly identical (Fig. 6). Further, the temporal pattern of rapid vasodilatation due to forearm compressions was again dissociated from that due to muscle contraction. Taken together, we believe that normal muscle recruitment patterns are not obligatory to observe the typical pattern of vasodilatation, and that mechanisms related to muscle activation are requisite to observe this response.
Experimental limitations and considerations
One limitation of the present study is the lack of intramuscular pressure measurements in our subjects, which limits the ability to make direct comparisons regarding the mechanical stimulus between forearm compression and muscle contraction conditions. For example, in Protocol 1, the immediate vasodilatation observed within the first cardiac cycle was greater for single compressions compared with single contractions. From the available data, we believe that forearm intramuscular pressure for isometric contractions at 20% MVC is much less (< 75 mmHg) than during forearm compressions at 200 mmHg (Sadamoto et al. 1983). Thus, in this protocol, the mechanical stimulus for vasodilatation would clearly be much greater for the compression condition. This would also be the case for the repeated and sustained conditions. As such, caution is warranted when interpreting these data with respect to quantifying the contribution of mechanical vasodilatation to that observed in response to contractions in Protocol 1. We would like to point out, however, that one purpose of this protocol was to simply compare the temporal pattern of the responses, and we chose to match peak vasodilatation for the single compression condition (200 mmHg) with that of a single contraction in an attempt to facilitate this initial comparison.
Although direct measures of intramuscular pressure appear to be ideal, most studies in humans only measure intramuscular pressure at one site of the muscle, and it is well documented that these pressures can vary markedly depending on the site of measurement (Saltin et al. 1981; Sejersted et al. 1984; Nakhostine et al. 1993). Further, we still would not know the exact impact this increase in intramuscular pressure would have on blood vessel deformation across all conditions and within different muscles of the forearm. Thus, although we recognize this potential limitation, we believe our findings are novel and provide the first clear evidence for mechanically induced rapid vasodilatation in humans. We also believe our findings provide unique insight into contraction-induced vasodilatation in response to various types of brief contractions (single, repeated and sustained) over a wide range of exercise intensities. Nevertheless, future studies utilizing isolated arteries and arterioles will be required to determine several characteristics of mechanically induced vasodilatation including how extravascular pressure translates to vessel deformation, what level of deformation is required to initiate vasodilatation, and what level evokes maximal vasodilatation.
Another potential limitation relates to whether the use of an external blood pressure cuff to compress the forearm resistance vessels causes the same distortion/deformation as occurs during muscle contractions. As already discussed, we employed the use of electrical stimulation of the median nerve and forearm muscle belly in an attempt to address this concern. Although the pattern of rapid vasodilatation in response to electrically evoked contractions was similar that in response to voluntary contractions, and thus temporally dissociated from mechanically induced vasodilatation, we still do not know whether the mechanical effect on forearm blood vessels was similar under all conditions. Thus, the possibility exists that our data underestimate the relative contribution of mechanical effects to contraction-induced rapid vasodilatation in humans. In this context, caution is again warranted when interpreting these data.
In the present study, 300 mmHg was our maximal level of cuff pressure used for mechanical stimulation. In Protocol 2, it appeared that the rapid vasodilator response might continue to increase in magnitude if we were to utilize greater pressures. However, with respect to intramuscular pressures observed during contractions of muscles of the upper extremity, most studies report that intramuscular pressure rarely exceeds 300 mmHg (Sadamoto et al. 1983; Sjogaard et al. 2004). Therefore, although we are unsure whether a maximal vasodilatation was evoked with this level of cuff pressure, we believe our data do provide insight into how mechanical influences contribute to low- and high-intensity brief muscle contractions.
Perspectives
We believe that our findings provide important insight with regard to how mechanically induced vasodilatation contributes to exercise hyperaemia in humans. First, at the onset of exercise, this mechanical effect on vascular tone could serve as a feedforward mechanism to increase blood flow to contracting muscle prior to the release of, and response to, local vasodilating substances. This feedfoward mechanism could also be important in response to alterations in contraction intensity, as the immediate blood flow response appears to be similar in magnitude during rapid increases and decreases in exercise intensity (Rogers et al. 2006). Second, as contractions persist at a given intensity (sustained or repeated), the relative contribution of mechanically induced vasodilatation to the total vasodilatation is reduced as the contribution of other local vasodilating factors (metabolic and/or shear-mediated) increase. Finally, we speculate that the mechanically induced vasodilatation largely contributes to the immediate vasodilatation across a wide range of exercise intensities, whereas the potential contribution of mechanical influences to the total contraction-induced hyperaemia appears greatest for low to moderate intensity single muscle contractions.
Although we have demonstrated that acute increases in extravascular pressure elicit a rapid vasodilatation in the human forearm, the temporal pattern and magnitude of the response was dissociated from isometric contractions in many instances. Thus, mechanical influences on skeletal muscle vascular tone do not completely explain contraction-induced rapid vasodilatation. Data derived from humans and the canine hindlimb argue against a significant role for neural mechanisms (Brock et al. 1998; Dyke et al. 1998; Buckwalter & Clifford, 1999; Naik et al. 1999), and acute inhibition of certain local endothelium-dependent vasodilators (e.g. nitric oxide and prostaglandins) does not impact on rapid vasodilatation (Shoemaker et al. 1996; Brock et al. 1998; Saunders et al. 2005). However, recent data clearly indicate that vasodilatation is obligatory to observe the rapid hyperaemia in response to a single muscle contraction (Hamann et al. 2004). Thus, we suggest that future studies should be aimed at determining which substance(s) can be released from the contracting myocyte or the vascular endothelium that are capable of evoking smooth muscle cell relaxation within a time frame consistent with rapid vasodilatation.
Conclusions
The findings from the present study indicate that acute increases in extravascular pressure elicit a rapid vasodilatation in the human forearm. This mechanically induced vasodilatation peaks within 1–2 cardiac cycles, and thus is dissociated from that normally observed in response to brief muscle contractions. Our collective findings indicate that mechanical influences contribute largely to the immediate vasodilatation (first cardiac cycle) observed in response to a brief, single contraction. However, it is clear that there are additional mechanisms related to muscle activation that continue to cause and sustain vasodilatation for several more cardiac cycles after contraction. Additionally, the potential contribution of mechanical influences to the total contraction-induced hyperaemia appears greatest for low to moderate intensity single muscle contractions, and this contribution becomes less significant for sustained and repeated contractions. Nevertheless, this mechanically induced vasodilatation could serve as a feedforward mechanism to increase muscle blood flow at the onset of exercise, as well as in response to changes in contraction intensity, prior to alterations in local vasodilating substances that influence vascular tone.
Acknowledgments
We would like to thank the subjects who volunteered for this study. This research was supported by National Institutes of Health awards AG022337 and AG027150 (F.A.D.).
References
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol. 1998;85:2249–2254. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol Heart Circ Physiol. 1999;277:H1872–H1877. doi: 10.1152/ajpheart.1999.277.5.H1872. [DOI] [PubMed] [Google Scholar]
- Burke RE. Handbook of Physiology. The Nervous System. Bethesda: American Physiological Society; 1981. Motor units: anatomy, physiology, and functional organization; pp. 345–422. [Google Scholar]
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–567. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol. 1964;19:142–146. doi: 10.1152/jappl.1964.19.1.142. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Dietz NM, Lennon RL, Warner DO, Joyner MJ. Forearm blood flow responses to handgripping after local neuromuscular blockade. J Appl Physiol. 1998;84:754–758. doi: 10.1152/jappl.1998.84.2.754. [DOI] [PubMed] [Google Scholar]
- Folkow B, Haglund U, Jodal M, Lundgren O. Blood flow in the calf muscle of man during heavy rhythmic exercise. Acta Physiol Scand. 1971;81:157–163. doi: 10.1111/j.1748-1716.1971.tb04887.x. [DOI] [PubMed] [Google Scholar]
- Gray SD, Carlsson E, Staub NC. Site of increased vascular resistance during isometric muscle contraction. Am J Physiol. 1967;213:683–689. doi: 10.1152/ajplegacy.1967.213.3.683. [DOI] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol. 2004;557:1013–1020. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Markwald RR, Smith EG, Dinenno FA. Mechanical effects of muscle contraction do not blunt sympathetic vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2005;289:H1610–H1617. doi: 10.1152/ajpheart.00391.2005. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol. 1987;253:H993–H1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Lind AR, Williams CA. The control of blood flow through human forearm muscles following brief isometric contractions. J Physiol. 1979;288:529–547. [PMC free article] [PubMed] [Google Scholar]
- Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol. 2004;82:282–287. doi: 10.1139/y04-016. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol. 1974;227:531–535. doi: 10.1152/ajplegacy.1974.227.3.531. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Regul Integr Comp Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- Naamani R, Hussain SN, Magder S. The mechanical effects of contractions on blood flow to the muscle. Eur J Appl Physiol Occup Physiol. 1995;71:102–112. doi: 10.1007/BF00854966. [DOI] [PubMed] [Google Scholar]
- Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87:1741–1746. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- Nakao M, Segal SS. Muscle length alters geometry of arterioles and venules in hamster retractor. Am J Physiol Heart Circ Physiol. 1995;268:H336–H344. doi: 10.1152/ajpheart.1995.268.1.H336. [DOI] [PubMed] [Google Scholar]
- Nakhostine M, Styf JR, Van Leuven S, Hargens AR, Gershuni DH. Intramuscular pressure varies with depth. The tibialis anterior muscle studied in 12 volunteers. Acta Orthop Scand. 1993;64:377–381. doi: 10.3109/17453679308993649. [DOI] [PubMed] [Google Scholar]
- Rogers AM, Saunders NR, Pyke KE, Tschakovsky ME. Rapid vasoregulatory mechanisms in exercising human skeletal muscle: dynamic response to repeated changes in contraction intensity. Am J Physiol Heart Circ Physiol. 2006;291:H1065–H1073. doi: 10.1152/ajpheart.00368.2006. [DOI] [PubMed] [Google Scholar]
- Sadamoto T, Bonde-Petersen F, Suzuki Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur J Appl Physiol Occup Physiol. 1983;51:395–408. doi: 10.1007/BF00429076. [DOI] [PubMed] [Google Scholar]
- Saltin B, Sjogaard G, Gaffney FA, Rowell LB. Potassium, lactate, and water fluxes in human quadriceps muscle during static contractions. Circ Res. 1981;48:I18–I24. [PubMed] [Google Scholar]
- Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol. 2005;288:H214–H220. doi: 10.1152/ajpheart.00762.2004. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. 1984;56:287–295. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol. 1996;81:1516–1521. doi: 10.1152/jappl.1996.81.4.1516. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol. 1998;76:418–427. doi: 10.1139/cjpp-76-4-418. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Jensen BR, Hargens AR, Sogaard K. Intramuscular pressure and EMG relate during static contractions but dissociate with movement and fatigue. J Appl Physiol. 2004;96:1522–1529. doi: 10.1152/japplphysiol.00687.2003. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Van Teeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–H127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Wunsch SA, Muller-Delp J, Delp MD. Time course of vasodilatory responses in skeletal muscle arterioles: role in hyperemia at onset of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1715–H1723. doi: 10.1152/ajpheart.2000.279.4.H1715. [DOI] [PubMed] [Google Scholar]