Abstract
Caffeine (CAF) impedes insulin-mediated glucose disposal (IMGD) and increases plasma adrenaline concentrations ([ADR]; 0.6 nm). While the antagonism of ADR abolishes the CAF effect, infusion of ADR (0.75 nm) has no effect on IMGD. We have now examined CAF and ADR in concert to determine whether or not they elicit an additive response on IMGD. We hypothesized that CAF + ADR would elicit a greater effect than either CAF or ADR alone (i.e. that CAF effects would not be solely attributed to ADR). Subjects (n = 8) completed four trials in a randomized manner. An isoglycaemic–hyperinsulinaemic clamp was performed 30 min after the following treatments were administered: (1) placebo capsules and saline infusion ([ADR]= 0.29 nm) (PL trial), (2) CAF capsules (dose = 5 mg kg−1) and saline infusion ([ADR]= 0.62 nm) (CAF trial), (3) PL capsules and ADR infusion ([ADR]= 1.19 nm) (ADR trial), and (4) CAF capsules (dose = 5 mg kg−1) and ADR infusion ([ADR]= 0.93 nm) (CAF + ADR trial). As expected, CAF, ADR and CAF + ADR decreased (P ≤ 0.05) IMGD compared to PL. CAF + ADR resulted in a more pronounced decrease in IMGD versus PL (42%) compared to CAF (26%) or ADR (24%) alone; however, the effect was not fully additive (P = 0.08). Furthermore, CAF decreased IMGD to a similar magnitude as ADR despite a 50% lower [ADR]. In summary, while ADR contributes to the CAF-induced impairment in IMGD, it is not solely responsible for caffeine's effects.
Adrenaline is a potent inhibitor of insulin action. Studies employing the euglycaemic–hyperinsulinaemic clamp have reported a 40–50% decline in whole body insulin-mediated glucose disposal at plasma adrenaline concentrations of 2–4 nm (Deibert & DeFronzo, 1980; Baron et al. 1987; Laurent et al. 1998). While the exact mechanism has yet to be elucidated, most evidence suggests a direct impairment of insulin action within skeletal muscle (Lee et al. 1997). Although some controversy remains as to the exact insulin signalling molecules affected (e.g. PI3K, PDK, Akt), the negative effect on GLUT4 transporters has consistently been reported. Studies conducted on rodent skeletal muscle have reported decreases in both the number and activity (i.e. ability of transporter to transport glucose) of GLUT4 transporters in the cell membrane (Han et al. 1998; Han & Bonen, 1998).
Interestingly, the effects of acute caffeine ingestion on glucose metabolism resemble most of those observed with adrenaline. Caffeine ingestion results in a 20–25% impairment in whole body insulin-mediated glucose disposal (Greer et al. 2001; Keijzers et al. 2002; Thong et al. 2002; Lee et al. 2005; Battram et al. 2005). This is likely to be predominantly attributed to a 50% decrease in skeletal muscle glucose uptake (Thong et al. 2002). Similar to adrenaline, the exact mechanism(s) by which caffeine elicits these effects are unknown. While caffeine has been reported to decrease the activity of a number of insulin signalling intermediates (e.g. PI3K and Akt), without affecting insulin binding within adipose tissue (Green, 1987; Akiba et al. 2004), this does not seem to occur in human skeletal muscle (Thong et al. 2002). Nevertheless as observed with adrenaline, a decrease in both the number and accessibility in GLUT4 transporters has been documented in both adipose (Steinfelder & Petho-Schramm, 1990; Akiba et al. 2004) and skeletal muscle tissues (Han et al. 1998).
The ingestion of 350 mg of caffeine results in an increase in plasma adrenaline concentrations from a resting concentration of 0.3 nm to 0.6 nm (Graham et al. 2001; Thong & Graham, 2002; Petrie et al. 2004; Robinson et al. 2004; Battram et al. 2005). While adrenaline does not appear to contribute significantly to the blood pressure and heart rate effects of caffeine (Smits et al. 1990; Smits et al. 1991), its potential role in caffeine's metabolic effects remains unclear. Due to the potency of adrenaline in its antagonism of insulin, it was speculated that the caffeine-induced increase in plasma adrenaline concentrations may mediate the inhibitory effect on insulin action. This has been investigated by simultaneously administering caffeine with propranolol (a non-selective β-adrenergic receptor antagonist). While caffeine ingestion alone induced the characteristic glucose intolerance, the effect was abolished in the presence of propranolol suggesting that caffeine exerts its effect indirectly via adrenaline (Thong & Graham, 2002). These findings were recently confirmed in persons with tetraplegia who upon caffeine ingestion do not demonstrate either the characteristic rise in adrenaline concentrations (Van Soeren et al. 1996) or the decline in glucose tolerance (Battram et al. 2007). In direct contrast to these findings, the infusion of adrenaline to a level similar to that observed following caffeine ingestion (0.75 nm) demonstrated no effect on whole body insulin-mediated glucose disposal (Battram et al. 2005). Taken together, these observations suggest that while the presence of adrenaline is necessary for the caffeine-induced impairment in glucose disposal, it cannot account for the entire caffeine effect and another mechanism(s) must be involved.
Therefore, the purpose of the present study was to examine the effects of caffeine and adrenaline in concert to determine (1) if their effects would be additive with respect to their impairment on whole body insulin-mediated glucose disposal and therefore (2) to elucidate the role of adrenaline in caffeine's actions. We hypothesized that the simultaneous administration of caffeine and adrenaline would result in a more pronounced, but non-additive effect on insulin-mediated glucose disposal compared to caffeine and adrenaline alone.
Methods
Subjects
All experimental procedures in this study were performed in accordance with the Declaration of Helsinki and approved by the local ethical committee for Copenhagen. Potential subjects were recruited from the University of Copenhagen community, and eight healthy, male subjects were chosen to participate in the study after providing written and informed consent. Subject characteristics are presented in Table 1. All subjects were non-smokers and regular caffeine users. Regular caffeine use was defined as ≥ 2 cups of coffee/tea and/or ≥ 5 caffeine-containing soft drinks per week.
Table 1.
Subject characteristics
| Parameter | Value |
|---|---|
| Age (years) | 25 ± 2 |
| Weight (kg) | 81 ± 3 |
| Height (cm) | 182 ± 1 |
| BMI (kg m−2) | 24 ± 1 |
| Body fatness (%) | 19 ± 2 |
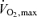 (ml min−1 kg−1) (ml min−1 kg−1) |
44.9 ± 1.7 |
| Glucose (mm) | 5.3 ± 0.1 |
| Insulin (pm) | 34 ± 5 |
| Adrenaline (nm) | 0.28 ± 0.07 |
| Caffeine (μm) | 0.22 ± 0.09 |
Data are means ±s.e.m. Blood chemistry values are in the fasting state measured from arterialized blood. BMI, body mass index. 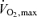 , maximal oxygen consumption.
, maximal oxygen consumption.
Experimental procedures
Prior to the commencement of the experiment, subjects underwent both a dual-energy X-ray absorptiometry (DEXA; LUNAR DPX-IQ, Lunar Corp., Madison, WI, USA) scan and a 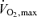 test (Oxycon Pro: Jaeger, Germany) for the determination of percentage body fatness and lean tissue mass and maximal oxygen consumption, respectively. In order to achieve similar muscle and liver glycogen stores prior to each trial, subjects were required to consume a diet containing at least 250 g of carbohydrate per day and to maintain similar activity patterns for 3 days prior to each experiment. In addition, 24 h before entering the laboratory, subjects were required to abstain from any strenuous activity. Furthermore, subjects were required to abstain from any caffeinated beverages and/or products and to avoid alcohol consumption for 48 hours prior to each experimental day.
test (Oxycon Pro: Jaeger, Germany) for the determination of percentage body fatness and lean tissue mass and maximal oxygen consumption, respectively. In order to achieve similar muscle and liver glycogen stores prior to each trial, subjects were required to consume a diet containing at least 250 g of carbohydrate per day and to maintain similar activity patterns for 3 days prior to each experiment. In addition, 24 h before entering the laboratory, subjects were required to abstain from any strenuous activity. Furthermore, subjects were required to abstain from any caffeinated beverages and/or products and to avoid alcohol consumption for 48 hours prior to each experimental day.
This study involved 4 days of investigation and included the following treatments: (1) placebo (gelatin; 5 mg (kg body weight)−1) capsules and saline infusion (PL trial), (2) caffeine (alkaloid caffeine; dose = 5 mg kg−1) capsules and saline infusion (CAF trial), (3) placebo capsules and adrenaline infusion (rate = 0.006 μg min−1 kg−1) (ADR trial), and (4) caffeine capsules and adrenaline infusion (rate = 0.0025 μg min−1 kg−1) (CAF + ADR trial). These adrenaline infusion rates were designed to achieve a 1.2 nm total adrenaline concentration in both adrenaline infusion trials. All subjects were first randomized, in a double blinded manner, to trials 1 and 2 to ensure that CAF ingestion resulted in a decrease in glucose disposal compared to PL. In previous work, our laboratory has reported that some subjects respond in a paradoxical manner to both CAF and adrenaline (i.e. an increase in whole body glucose disposal) (Battram et al. 2005). Due to the fact that the purpose of the present study was to examine the role of adrenaline in caffeine's impairment of glucose disposal, those subjects that responded to CAF with an increase in glucose disposal were excluded from the study. Those subjects eligible to continue (i.e. those subjects that responded to caffeine with a decrease in glucose disposal) then completed trials 3 and 4 in a blinded, randomized fashion. The adrenaline infusions were prepared from a 1 mg ml−1 stock solution and isotonic saline. To prevent the oxidation of adrenaline by daylight, all infusion syringes and lines were covered with aluminium foil. The rate of saline infusion during trials 1 and 2 were identical to that calculated for adrenaline in trial 3 (i.e. this achieved an identical infusion volume in all trials).
Each experimental day was as follows. Upon entering the laboratory, both height and weight were measured. Subjects then rested and two catheters were placed in antecubital veins for infusions of 20% glucose, insulin, and saline or adrenaline. A third catheter was placed in the dorsal hand-vein in a retrograde position for all blood sampling. This hand was kept in a heating pad throughout the experiment to arterialize the blood. Following an approximate 5 min rest period, a baseline (t = 0 min) blood sample, blood pressure and heart rate was taken. This was immediately followed by the consumption of capsules (PL or CAF) with 200 ml of water and the initiation of either saline or adrenaline infusions. Following 30 min of infusions, a one step isoglycaemic–hyperinsulinaemic clamp was initiated (t = 30–150 min). For each experiment, the insulin infusate was prepared using 2.5 ml of the subject's plasma collected at baseline and isotonic saline. A 2 ml priming dose of insulin was administered, followed immediately by a constant infusion at a rate of 40 μIU ml−1 m−2. To maintain isoglycaemia throughout the clamp, arterialized blood samples were collected every 5 min for the measurement of blood glucose concentration using an automatic analyser (ABL 700, Radiometer, Copenhagen, Denmark) and the glucose infusion rate was then adjusted accordingly. Blood samples were collected at time points indicated for the measurement of hormones and metabolites. In addition, blood pressure and heart rate were measured every 10 min during the pre-clamp period and every 30 min thereafter (i.e. clamp period).
For the determination of blood glucose and lactate concentrations, blood samples were collected in pre-heparinized tubes (PICO 50, Radiometer, Copenhagen, Denmark) and immediately analysed by an automatic analyser (ABL 700, Radiometer, Copenhagen, Denmark). For the determination of insulin, 1 ml of blood was stabilized with 500 Kalikrenin inhibitory units aprotinin (Trasylol) and 1.5 mg EDTA. The sample was then centrifuged and the plasma stored at −20°C for later analysis by sandwich ELISA (DakoCytomatics, Glostrup, Denmark). For the determination of adrenaline, 1.5 ml of blood was stabilized with 7.5 μmol ethylene glycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) and 6 μmol reduced glutathione in 30 μl 0.6 n sodium hydroxide. This sample was then centrifuged and the plasma stored at −80°C for later analysis by radioimmunoassay (Adrenaline Radioimmunoassay, Immunobiological Laboratories, Hamburg, Germany). For the determination of caffeine and FFA, 1 ml of blood for each was stabilized with 10 μl of heparin. Each sample was centrifuged and the plasma stored at −80°C until analysis could be performed. Plasma FFA analysis was performed by an enzyme colour assay (ACS-ACOD, Wako, Glostrup, Denmark). Plasma caffeine concentrations were determined by high-performance liquid chromatography as previously described (Aldridge et al. 1979).
Calculations and statistics
The GIR was averaged during the final 30 min of the isoglycaemic–hyperinsulinaemic clamp (steady state) and corrected for the corresponding insulin concentration (insulin-corrected GIR). The average adrenaline concentration was calculated from t = 60–150 min
All data is presented as means ±s.e.m. To elucidate time and treatment effects a two-way ANOVA for repeated measures was employed for plasma glucose, caffeine, adrenaline, insulin and FFA concentrations, in addition to blood pressure (SBP and DBP) and heart rate. A one-way ANOVA for repeated measures was used to determine differences in treatment for the average insulin-corrected GIR during the final 30 min of the clamp, the average adrenaline concentrations during the clamp and the change in FFA concentrations during the pre-clamp period (t = 0–30 min). Tukey's post hoc test was used to locate differences when necessary. Differences were considered significant at a P ≤ 0.05 in two-tailed testing.
Results
Subjects
Of the 11 potential subjects recruited from the University of Copenhagen community, three (27%) responded to CAF with an increase in glucose disposal and were therefore excluded from continuing in the experiment. While the reason for this response is currently unknown and being examined further in our laboratory, it is noteworthy that this rate of occurrence is similar to that observed previously (3 out of 12 subjects or 25%; Battram et al. 2005).
Caffeine, insulin and adrenaline concentrations
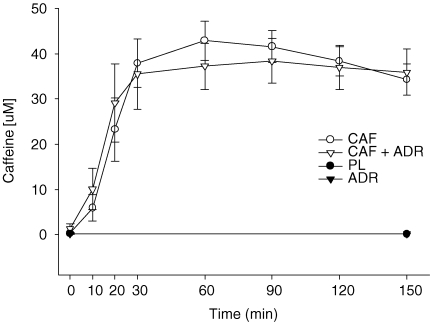
At baseline, plasma caffeine concentrations were similar between all treatments (Fig. 1). Within both the PL and ADR trials, plasma caffeine concentrations remained unchanged (P > 0.05) from baseline throughout the experiment. Following caffeine ingestion (CAF and CAF + ADR), plasma caffeine concentrations increased steadily such that levels achieved at 20 min were above (P ≤ 0.05) those observed at baseline. Plasma caffeine levels remained constant throughout the clamp (time 30–150 min) and were not different (P > 0.05) between CAF and CAF + ADR treatments. As expected, plasma caffeine concentrations in the CAF and CAF + ADR treatments were higher (P ≤ 0.05) than those observed with PL and ADR.
Figure 1.
Plasma caffeine concentrations during the pre-clamp period (t = 0–30 min) and during the isoglycaemic–hyperinsulinaemic clamp (t = 30–150 min) for the PL (•), CAF (○), ADR (▾) and CAF + ADR (▿) treatments As expected, CAF and CAF + ADR resulted in higher levels (P ≤ 0.05) than PL and ADR and were not different (P > 0.05) from one another. Values are means ±s.e.m.
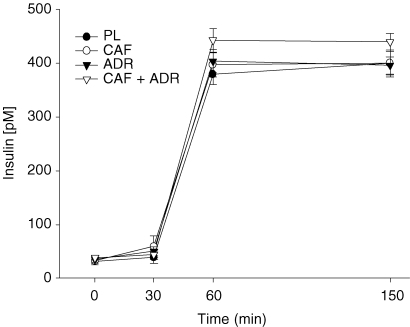
At baseline, insulin concentrations were similar (P > 0.05) between treatments and remained unchanged throughout the pre-clamp (t = 0–30 min) period (Fig. 2). As expected, upon initiation of the isoglycaemic–hyperinsulinaemic clamp, insulin concentrations increased (P ≤ 0.05) above pre-clamp concentrations. These levels remained elevated and stable throughout the clamp period. Furthermore, insulin concentrations were similar between all treatments with one exception; CAF + ADR resulted in higher insulin concentration (442 ± 13 versus 384 ± 14 pm) compared to PL (see Discussion).
Figure 2.
Plasma insulin concentrations during the pre-clamp period (t = 0–30 min) and during the isoglycaemic–hyperinsulinaemic clamp (t = 30–150 min) for PL (•), CAF (○), ADR (▾) and CAF + ADR (▿) treatments As expected, insulin concentrations increased upon initiation of the clamp (t = 30 min). All treatments were similar to one another with one exception CAF + ADR resulted in a higher (P ≤ 0.05) insulin concentration than PL. Values are means ±s.e.m.
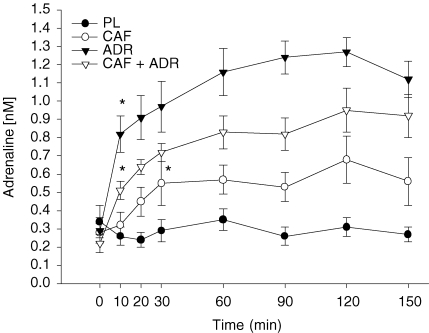
Basal plasma adrenaline concentrations were similar between treatments (Fig. 3). No changes in adrenaline levels were observed throughout the PL trial (P > 0.05; Fig. 3). Within the CAF trial, adrenaline levels increased to 0.55 ± 0.05 nm by 30 min post-capsule ingestion (t = 30 min) and remained stable throughout the remainder of the trial. Within the adrenaline infusion trials (ADR and CAF + ADR), adrenaline concentrations were elevated above baseline within 10 min of infusion. During the final 30 min of the clamp when GIR was calculated, adrenaline concentrations were stable at 0.29 ± 0.05, 0.62 ± 0.12, 1.19 ± 0.08 and 0.93 ± 0.11 nm for the PL, CAF, ADR and CAF + ADR trials, respectively. All treatments (P ≤ 0.05) were different from one another.
Figure 3.
Plasma adrenaline concentrations during the pre-clamp period (t = 0–30 min) and during the isoglycaemic–hyperinsulinaemic clamp (t = 30–150 min) for the PL (•), CAF (○), ADR (▾) and CAF + ADR (▿) treatments All treatments were different (P ≤ 0.05) from one another. The asterisk indicates the first time point at which adrenaline concentrations were higher than baseline within a trial. Values are means ±s.e.m.
Isoglycaemic–hyperinsulinaemic clamp
Glucose concentrations were similar (P > 0.05) between treatments at baseline and remained unchanged throughout both the pre-clamp (t = 0–30 min) and clamp (t = 30–150 min) periods (data not shown). The coefficient of variation during the clamps was 4.0 ± 0.4%.
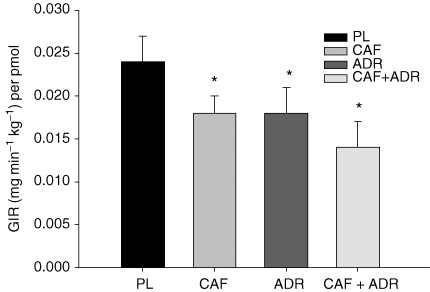
As expected, CAF, ADR and CAF + ADR resulted in decreases (P ≤ 0.05) in insulin-corrected GIR compared to PL (Fig. 4). These differences were evident within 40 min of the initiation of the clamp. The relative differences calculated during the final 30 min of the clamp were 26, 24 and 42% for the CAF, ADR and CAF + ADR treatments (versus PL), respectively. The decrease in insulin-corrected GIR with CAF + ADR resulted in a more pronounced effect compared to either CAF or ADR alone and approached significance (P = 0.08). The insulin-corrected GIR was used instead of GIR alone to ensure that the higher insulin concentration observed in CAF + ADR (versus PL; Fig. 2) did not lead to erroneous conclusions.
Figure 4.
Insulin-corrected glucose infusion rates (GIR per pmol) averaged during the final 30 min of the isoglycaemic–hyperinsulinaemic clamp CAF, ADR and CAF + ADR decreased glucose disposal (P ≤ 0.05) by 26, 24 and 42%, respectively, compared to PL, as indicated by an asterisk. Values are means ±s.e.m.
Plasma FFA
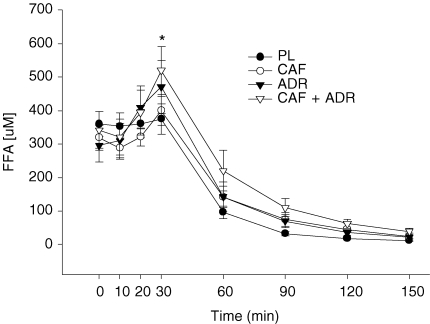
At baseline, FFA concentrations were similar (P > 0.05) among treatments (Fig. 5). During the pre-clamp period, there was an overall time effect – an increase in FFA concentrations. However, this increase in FFA levels reflects the responses that occurred within both of the adrenaline infusion treatments (ADR and CAF + ADR versus PL). Therefore, we examined the change in FFA concentrations (FFA concentration at t = 30 min – FFA concentration at t = 0 min) during the pre-clamp period for all treatments. This analysis revealed no effect (P > 0.05) of PL (+14 ± 20 μm) and CAF (+81 ± 56 μm) on FFA concentrations and a higher (P ≤ 0.05) FFA response in both the ADR (+ 175 ± 47 μm) and CAF + ADR (+ 178 ± 34 μm) trials compared to PL (Fig. 5). As expected, with hyperinsulinemia FFA levels decreased such that by 30 min postinsulin infusion FFA levels were lower (P ≤ 0.05) than baseline values. These levels remained low throughout the remainder of the experiment.
Figure 5.
Plasma FFA concentrations during the pre-clamp period (t = 0–30 min) and during the isoglycaemic–hyperinsulinaemic clamp (t = 30–150 min) for PL (•), CAF (○), ADR (▾) and CAF + ADR (▿) treatments The asterisk indicates the time in which both ADR trials (ADR and CAF + ADR) resulted in higher FFA response (P ≤ 0.05) than PL. Values are means ±s.e.m.
Blood pressure and heart rate
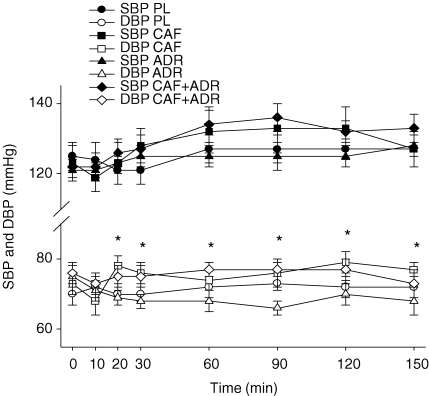
Systolic (SBP) and diastolic blood pressure (DBP) and heart rate were similar (P > 0.05) at baseline between treatments (Fig. 6). While no treatment effect was observed with respect to SBP, there was an increase in SBP from the pre-clamp to clamp periods (time effect). In contrast to SBP, DBP was higher (P ≤ 0.05) in both caffeine trials (CAF and CAF + ADR) compared to ADR – an effect observed within 20 min of treatment administration. No effect was observed on heart rate during either the pre-clamp or clamp periods (Table 2).
Figure 6.
Systolic (SBP) and diastolic blood pressure (DBP) for PL (circles), CAF (squares), ADR (triangles) and CAF + ADR (diamonds) during the pre-clamp period (t = 0–30 min) and during the isoglycaemic–hyperinsulinaemic clamp (t = 30 to 150 min) Both CAF (□) and CAF + ADR (⋄) treatments resulted in higher (P ≤ 0.05) DBP than ADR (▿). The asterisks represent the time points at which CAF and CAF + ADR elicited higher DBP compared to ADR. Values are means ±s.e.m.
Table 2.
Heart rate during both the pre-clamp and clamp periods
| Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | 0 | 10 | 20 | 30 | 60 | 90 | 120 | 150 |
| PL | 60 ± 6 | 61 ± 5 | 59 ± 5 | 60 ± 5 | 59 ± 4 | 62 ± 2 | 60 ± 5 | 64 ± 5 |
| CAF | 60 ± 3 | 59 ± 3 | 59 ± 4 | 64 ± 4 | 61 ± 4 | 64 ± 5 | 64 ± 7 | 66 ± 5 |
| ADR | 57 ± 4 | 64 ± 4 | 62 ± 4 | 62 ± 4 | 63 ± 4 | 63 ± 6 | 63 ± 5 | 62 ± 4 |
| CAF + ADR | 63 ± 4 | 57 ± 3 | 61 ± 4 | 60 ± 6 | 61 ± 5 | 62 ± 5 | 64 ± 5 | 64 ± 5 |
Data are means ±s.e.m. Heart rate is given at baseline (0 min), during the pre-clamp period (10, 20 and 30 min) and clamp periods (60–150 min). No treatment effect was observed.
Discussion
This study examined the role of adrenaline in caffeine's impairment of whole body insulin-mediated glucose disposal in humans. To directly compare the effects of CAF + ADR to either CAF or ADR alone, the combined treatment was designed to achieve similar caffeine concentrations to the CAF trial (39.3 ± 1.8 versus 37.2 ± 2.4 μm for CAF and CAF + ADR, respectively) and similar adrenaline concentrations to the ADR trial (0.93 versus 1.19 nm). While the simultaneous administration of CAF + ADR resulted in a more pronounced reduction in whole body glucose disposal (42%versus PL; P ≤ 0.05) compared to either CAF (26%) or ADR (24%) alone, the effect was not fully additive (i.e. the reduction in GIR by CAF + ADR ≠ reduction by CAF + reduction by ADR; Fig. 4). While the mean data create the impression of an additive effect, close examination of the individual data fails to support such a conclusion. The difference in reduction only approached statistical significance (P = 0.08). Our interpretations of the data are that the mechanisms by which CAF and ADR impair insulin action while not identical are likely to be closely related and share some common pathways, for if they were acting via independent mechanisms a fully additive effect on whole body glucose disposal would have been observed.
We have demonstrated that both caffeine and adrenaline independently elicit similar relative reductions (26 and 24%versus PL, respectively) in whole body glucose disposal, in accordance with previous findings (Greer et al. 2001; Keijzers et al. 2002; Lee et al. 2005; Battram et al. 2005). Interestingly, however, while the relative magnitude of reduction in glucose disposal was identical between these two treatments, the plasma adrenaline concentrations achieved in each treatment were significantly different. While CAF ingestion resulted in a plasma adrenaline concentration of 0.62 nm, this concentration was 50% that achieved in the ADR trial (1.2 nm). Due to the fact that adrenaline impairs whole body glucose disposal in a dose-dependent manner (Deibert & DeFronzo, 1980; Baron et al. 1987; Laurent et al. 1998), if adrenaline was solely responsible for caffeine's effects, one would expect a lesser response with CAF compared to ADR rather than a similar response. These findings suggest that the actions of caffeine on insulin sensitivity are not solely governed by an indirect action of adrenaline and that additional mechanism(s) are likely to be involved – a finding confirmed by our earlier work (Battram et al. 2005).
Although the protocol was designed to elicit similar insulin concentrations during all experiments, the insulin concentrations achieved in the CAF + ADR trial was unexpectedly higher (P ≤ 0.05) than that achieved in the PL trial. The reason for this difference is not readily obvious as the insulin infusion was prepared and administered in a similar manner in each experiment. Furthermore, close examination of the raw data reveals no unusual responses (Fig. 2). Regardless, to examine the potential impact of these different insulin concentrations, we corrected for the different insulin levels by dividing the GIR by the accompanying insulin concentration for each treatment (insulin corrected GIR). This manoeuvre is of course based on the presumption that there is linearity in insulin action in this range of insulin concentrations. Dose–response studies of insulin action in young, healthy men support this belief (Mikines et al. 1991).
In addition to the differences in insulin concentrations, the adrenaline concentrations achieved in the CAF + ADR treatment were lower (P ≤ 0.05; 0.93 versus 1.19 nm) than that achieved in the ADR treatment. Due to the fact that adrenaline decreases glucose disposal in a dose-dependent manner (Deibert & DeFronzo, 1980; Baron et al. 1987; Laurent et al. 1998), the lower adrenaline concentration in the CAF + ADR trial (0.93 versus 1.19 nm ADR alone) would be likely to result in a less inhibitory effect of adrenaline within the combined treatment compared to ADR alone. Taken together, it is likely that both a higher insulin and lower adrenaline concentration within the CAF + ADR trial resulted in an underestimation of the CAF + ADR effect on glucose disposal. These considerations only support our interpretation of the data showing an enhanced effect of the combined CAF + ADR trial, even though the P-value only approached significance (see above).
A lack of a fully additive effect on whole body glucose disposal by the CAF + ADR treatment is not surprising as some of caffeine's effects are attributed to adrenaline. Studies conducted on both rodents and humans have demonstrated an abolition of caffeine's effects on glucose metabolism when either the caffeine-induced release of adrenaline (via adrenalectomy or due to tetraplegia) is prevented or the actions of adrenaline on peripheral tissues is antagonized by adrenergic receptor antagonists (Strubelt, 1969; Sacca et al. 1975; Thong & Graham, 2002; Battram et al. 2007). It is likely then that a portion of the 26% decrease in whole body glucose disposal by CAF is attributed to adrenaline. Theoretically, if a 1.2 nm dose of adrenaline decreases whole body glucose disposal by 24% (versus PL), then a 0.93 nm dose of adrenaline (as achieved in the CAF + ADR trial) should result in a similar or slightly lesser decrease (≤ 24%) in glucose disposal compared to ADR. This decrease would account for only some of the observed decrease in disposal by CAF + ADR (42%) and suggests that caffeine must elicit some adrenaline-independent effects on whole body glucose disposal.
With the present dose of caffeine, adenosine receptor antagonism is suggested to be the predominant mechanism by which caffeine elicits its effects (Fredholm, 1995). The role of adenosine in glucose metabolism, and in particular skeletal muscle glucose metabolism remains controversial. Within this tissue, adenosine receptor antagonism has demonstrated increases (Espinal et al. 1983; Leighton et al. 1988), decreases (Han et al. 1998) and no changes (Vergauwen et al. 1994) in insulin action. Whether or not the antagonism of adenosine receptors contributes to the observed enhancement of caffeine's effects compared to adrenaline alone in the present study is unknown, but within isolated rodent adipocytes the removal of adenosine has been reported to enhance the inhibitory effects of isoproterenol (a β-adrenergic receptor agonist) on insulin-mediated glucose uptake (Joost et al. 1986; Vannucci et al. 1992). Therefore in humans it is possible that the simultaneous rise in plasma adrenaline concentrations and the antagonism of adenosine receptors is contributing to the enhanced caffeine effect.
Plasma FFA concentrations are used as an indicator of adipose tissue lipolysis. Due to the potency of adrenaline as a lipolytic agent (Galster et al. 1981) it is not surprising that we observed an increase (P ≤ 0.05) in plasma FFA concentrations during both adrenaline treatments (CAF + ADR and ADR). While caffeine ingestion is known to induce a similar lipolytic action (Graham et al. 2001; Keijzers et al. 2002), we did not observe this in the present study. This lack of increase in FFA response is likely to be due to the fact that the isoglycaemic–hyperinsulinaemic clamp was initiated 30 min after caffeine was ingested. At this point both adrenaline and caffeine concentrations had just reached high levels. Therefore before these levels could exert their lipolytic effects insulin was administered, thereby attenuating the stimuli for lipolysis (Burns et al. 1979). Regardless, while FFAs are known inhibitors of glucose disposal (Kruszynska et al. 2002), this probably did not contribute significantly to the observed reductions in glucose disposal within ADR and CAF + ADR as the infusion of insulin resulted in a rapid decline in FFA levels which remained below baseline values throughout the last 90 min of the clamp period.
Both caffeine treatments (CAF and CAF + ADR) resulted in higher DBP compared to ADR (Fig. 6). This elevation in DBP occurred despite different adrenaline concentrations (0.62 versus 0.93 nm). These findings suggest an independent action of caffeine and confirm the results of Smits et al. (1991) who report that the haemodynamic effects of caffeine are via an adrenaline-independent mechanism (Smits et al. 1983), specifically via the inhibition of adenosine-induced vasodilation (Smits et al. 1990).
Although in the present study's acute situation caffeine elicits an impairment in insulin-mediated whole body glucose disposal, the chronic ingestion of coffee (both decaffeinated and caffeinated) is protective against type 2 diabetes development (Salazar-Martinez et al. 2004; van Dam et al. 2004; Wu et al. 2005). Due to the fact that the presence of caffeine does not appear to impede the protective effect of coffee, it is possible that a tolerance to caffeine's acute effects develops over time. We have demonstrated that the investigation of this tolerance needs to involve not only caffeine's adrenaline-dependent mechanism, but its adrenaline-independent mechanism (i.e. adenosine receptor antagonism) as well. Furthermore, we have demonstrated that a subset of subjects respond to caffeine and adrenaline in an uncharacteristic manner – a rise in insulin-mediated glucose disposal. Interestingly, Martin et al. (2006a, b) have also observed a lack of response of both adenosine and isoproterenol on blood flow in a subset of their subjects. While the reasons for these similar and uncharacteristic effects of adenosine/caffeine and isoproterenol/adrenaline are at present unknown, Cornelis et al. (2006) have demonstrated that the elevated risk of heart disease with chronic caffeinated coffee ingestion is dependent on an inherent ability to metabolize caffeine, with slow-metabolizers demonstrating an increased risk of heart disease with chronic coffee ingestion. Whether or not this inherent difference in caffeine metabolism contributes to the uncharacteristic response to caffeine and adrenaline in our subjects is currently unknown and being investigated in our laboratory.
In summary, we have demonstrated that caffeine is eliciting its effects on whole body insulin-mediated glucose disposal via both an indirect action of adrenaline and an additional mechanism, likely the antagonism of adenosine receptors. We have demonstrated that while the combined CAF + ADR treatment resulted in a more pronounced effect on glucose disposal, this did not result in a classical additive response. These findings suggest that while not identical, the mechanisms involved in caffeine's actions are likely to be closely related, possibly by eliciting their effects on common insulin-mediated pathways.
Acknowledgments
We wish to thank the subjects for their time and commitment to this project. We also wish to thank Regitze Kransøe, Jeppe Bach, Thomas Beck, Premila Sathasivam, Rasmus Rabøl, Mette Sonne and Bente Stallknecht for their technical assistance. The financial assistance of the Natural Science and Engineering Research Council (NSERC), Aase og Ejnar Danielsens Foundation, the Danish Diabetes Foundation, the Novo Nordic Foundation, the Lundbeck Foundation and the Simon Founger Hartmanns Family is greatly appreciated.
References
- Akiba T, Yaguchi K, Tsutsumi K, Nishioka T, Koyama I, Nomura M, Yokogawa K, Moritani S, Miyamoto K. Inhibitory mechanism of caffeine on insulin-stimulated glucose uptake in adipose cells. Biochem Pharmacol. 2004;68:1929–1937. doi: 10.1016/j.bcp.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Aldridge A, Aranda JV, Neims AH. Caffeine metabolism in the newborn. Clin Pharmacol Ther. 1979;25:447–453. doi: 10.1002/cpt1979254447. [DOI] [PubMed] [Google Scholar]
- Baron AD, Wallace P, Olefsky JM. In vivo regulation of non-insulin-mediated and insulin-mediated glucose uptake by epinephrine. J Clin Endocrinol Metab. 1987;64:889–895. doi: 10.1210/jcem-64-5-889. [DOI] [PubMed] [Google Scholar]
- Battram DS, Bugaresti J, Gusba J, Graham TE. Acute caffeine ingestion does not impair glucose tolerance in persons with tetraplegia. J Appl Physiol. 2007;102:374–381. doi: 10.1152/japplphysiol.00901.2006. [DOI] [PubMed] [Google Scholar]
- Battram DS, Graham TE, Richter EA, Dela F. The effect of caffeine on glucose kinetics in humans – influence of adrenaline. J Physiol. 2005;569:347–355. doi: 10.1113/jphysiol.2005.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns TW, Terry BE, Langley PE, Robison GA. Insulin inhibition of lipolysis of human adipocytes: the role of cyclic adenosine monophosphate. Diabetes. 1979;28:957–961. doi: 10.2337/diab.28.11.957. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65:717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal J, Challiss RA, Newsholme EA. Effect of adenosine deaminase and an adenosine analogue on insulin sensitivity in soleus muscle of the rat. FEBS Lett. 1983;158:103–106. doi: 10.1016/0014-5793(83)80685-1. [DOI] [PubMed] [Google Scholar]
- Fredholm BB. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Galster AD, Clutter WE, Cryer PE, Collins JA, Bier DM. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981;67:1729–1738. doi: 10.1172/JCI110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TE, Sathasivam P, Rowland M, Marko N, Greer F, Battram D. Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Can J Physiol Pharmacol. 2001;79:559–565. [PubMed] [Google Scholar]
- Green A. Chronic administration of theophylline to rats induces a post-insulin binding defect in adipocyte glucose transport. Diabetologia. 1987;30:188–192. doi: 10.1007/BF00274226. [DOI] [PubMed] [Google Scholar]
- Greer F, Hudson R, Ross R, Graham T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes. 2001;50:2349–2354. doi: 10.2337/diabetes.50.10.2349. [DOI] [PubMed] [Google Scholar]
- Han XX, Bonen A. Epinephrine translocates GLUT-4 but inhibits insulin-stimulated glucose transport in rat muscle. Am J Physiol Endocrinol Metab. 1998;274:E700–E707. doi: 10.1152/ajpendo.1998.274.4.E700. [DOI] [PubMed] [Google Scholar]
- Han DH, Hansen PA, Nolte LA, Holloszy JO. Removal of adenosine decreases the responsiveness of muscle glucose transport to insulin and contractions. Diabetes. 1998;47:1671–1675. doi: 10.2337/diabetes.47.11.1671. [DOI] [PubMed] [Google Scholar]
- Joost HG, Weber TM, Cushman SW, Simpson IA. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986;261:10033–10036. [PubMed] [Google Scholar]
- Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25:364–369. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab. 2002;87:226–234. doi: 10.1210/jcem.87.1.8187. [DOI] [PubMed] [Google Scholar]
- Laurent D, Petersen KF, Russell RR, Cline GW, Shulman GI. Effect of epinephrine on muscle glycogenolysis and insulin-stimulated muscle glycogen synthesis in humans. Am J Physiol Endocrinol Metab. 1998;274:E130–E138. doi: 10.1152/ajpendo.1998.274.1.E130. [DOI] [PubMed] [Google Scholar]
- Lee AD, Hansen PA, Schluter J, Gulve EA, Gao J, Holloszy JO. Effects of epinephrine on insulin-stimulated glucose uptake and GLUT-4 phosphorylation in muscle. Am J Physiol Cell Physiol. 1997;273:C1082–C1087. doi: 10.1152/ajpcell.1997.273.3.C1082. [DOI] [PubMed] [Google Scholar]
- Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R. Caffeine ingestion is associated with reductions in glucose uptake independent of obesity and type 2 diabetes before and after exercise training. Diabetes Care. 2005;28:566–572. doi: 10.2337/diacare.28.3.566. [DOI] [PubMed] [Google Scholar]
- Leighton B, Lozeman FJ, Vlachonikolis IG, Challiss RA, Pitcher JA, Newsholme EA. Effects of adenosine deaminase on the sensitivity of glucose transport, glycolysis and glycogen synthesis to insulin in muscles of the rat. Int J Biochem. 1988;20:23–27. doi: 10.1016/0020-711x(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol. 2006a;101:492–499. doi: 10.1152/japplphysiol.00684.2005. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol. 2006b;101:1678–1684. doi: 10.1152/japplphysiol.00546.2006. [DOI] [PubMed] [Google Scholar]
- Mikines KJ, Richter EA, Dela F, Galbo H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J Appl Physiol. 1991;70:1245–1254. doi: 10.1152/jappl.1991.70.3.1245. [DOI] [PubMed] [Google Scholar]
- Petrie HJ, Chown SE, Belfie LM, Duncan AM, McLaren DH, Conquer JA, Graham TE. Caffeine ingestion increases the insulin response to an oral-glucose-tolerance test in obese men before and after weight loss. Am J Clin Nutr. 2004;80:22–28. doi: 10.1093/ajcn/80.1.22. [DOI] [PubMed] [Google Scholar]
- Robinson LE, Savani S, Battram DS, McLaren DH, Sathasivam P, Graham TE. Caffeine ingestion before an oral glucose tolerance test impairs blood glucose management in men with type 2 diabetes. J Nutr. 2004;134:2528–2533. doi: 10.1093/jn/134.10.2528. [DOI] [PubMed] [Google Scholar]
- Sacca L, Perez G, Rengo F, Pascucci I, Condorelli M. Effects of theophylline on glucose kinetics in normal and sympathectomized rats. Diabetes. 1975;24:249–256. doi: 10.2337/diab.24.3.249. [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004;140:1–8. doi: 10.7326/0003-4819-140-1-200401060-00005. [DOI] [PubMed] [Google Scholar]
- Smits P, Hoffmann H, Thien T, Houben H, van't Laar A. Hemodynamic and humoral effects of coffee after beta 1-selective and nonselective beta-blockade. Clin Pharmacol Ther. 1983;34:153–158. doi: 10.1038/clpt.1983.145. [DOI] [PubMed] [Google Scholar]
- Smits P, Lenders JW, Thien T. Caffeine and theophylline attenuate adenosine-induced vasodilation in humans. Clin Pharmacol Ther. 1990;48:410–418. doi: 10.1038/clpt.1990.169. [DOI] [PubMed] [Google Scholar]
- Smits P, Straatman C, Pijpers E, Thien T. Dose-dependent inhibition of the hemodynamic response to dipyridamole by caffeine. Clin Pharmacol Ther. 1991;50:529–537. doi: 10.1038/clpt.1991.178. [DOI] [PubMed] [Google Scholar]
- Steinfelder HJ, Petho-Schramm S. Methylxanthines inhibit glucose transport in rat adipocytes by two independent mechanisms. Biochem Pharmacol. 1990;40:1154–1157. doi: 10.1016/0006-2952(90)90508-i. [DOI] [PubMed] [Google Scholar]
- Strubelt O. The influence of reserpine, propranolol, and adrenal medullectomy on the hyperglycemic actions of theophylline and caffeine. Arch Int Pharmacodyn Ther. 1969;179:215–224. [PubMed] [Google Scholar]
- Thong FS, Derave W, Kiens B, Graham TE, Urso B, Wojtaszewski JF, Hansen BF, Richter EA. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes. 2002;51:583–590. doi: 10.2337/diabetes.51.3.583. [DOI] [PubMed] [Google Scholar]
- Thong FS, Graham TE. Caffeine-induced impairment of glucose tolerance is abolished by beta-adrenergic receptor blockade in humans. J Appl Physiol. 2002;92:2347–2352. doi: 10.1152/japplphysiol.01229.2001. [DOI] [PubMed] [Google Scholar]
- van Dam RM, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: the Hoorn Study. Diabetologia. 2004;47:2152–2159. doi: 10.1007/s00125-004-1573-6. [DOI] [PubMed] [Google Scholar]
- Van Soeren M, Mohr T, Kjaer M, Graham TE. Acute effects of caffeine ingestion at rest in humans with impaired epinephrine responses. J Appl Physiol. 1996;80:999–1005. doi: 10.1152/jappl.1996.80.3.999. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Nishimura H, Satoh S, Cushman SW, Holman GD, Simpson IA. Cell surface accessibility of GLUT4 glucose transporters in insulin-stimulated rat adipose cells. Modulation by isoprenaline and adenosine. Biochem J. 1992;288:325–330. doi: 10.1042/bj2880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergauwen L, Hespel P, Richter EA. Adenosine receptors mediate synergistic stimulation of glucose uptake and transport by insulin and by contractions in rat skeletal muscle. J Clin Invest. 1994;93:974–981. doi: 10.1172/JCI117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Willett WC, Hankinson SE, Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-Peptide levels, a marker of insulin secretion, in U.S. Women. Diabetes Care. 2005;28:1390–1396. doi: 10.2337/diacare.28.6.1390. [DOI] [PubMed] [Google Scholar]