Abstract
The role of adenosine in exercise hyperaemia has been controversial. Accumulating evidence now demonstrates that adenosine is released into the venous efflux of exercising muscle and that adenosine is responsible for 20–40% of the maintained phase of the muscle vasodilatation that accompanies submaximal and maximal contractions. This adenosine is mainly generated from AMP that is released from the skeletal muscle fibres and dephosphorylated by ecto 5′nucleotidase bound to the sarcolemma. During exercise, the concentration of ecto 5′nucleotidase may be increased by translocation from the cytosol, while release of AMP and affinity of ecto 5′nucleotidase for AMP are increased by acidosis. The adenosine so formed, acts on extraluminal A2A receptors on the vascular smooth muscle. In addition, ATP is released from red blood cells into the plasma during exercise, in association with the unloading of O2 from haemoglobin, while ATP and adenosine may be released from endothelium as a consequence of local hypoxia. It is unlikely that this intraluminal ATP, or adenosine, contributes significantly to exercise hyperaemia, for muscle vasodilatation induced by intraluminal ATP or adenosine is strongly nitric oxide dependent, while vasodilatation induced by adenosine in hypoxia is mediated by A1 receptors. Neither is a recognized feature of exercise hyperaemia.
The idea that adenosine might contribute to exercise hyperaemia in skeletal muscle stems from the ‘adenosine hypothesis’ originally put forward for the coronary circulation by Berne (1963). The hypothesis was that blood flow is regulated by interstitial adenosine, released from cardiac or skeletal muscle fibres when there is a mismatch between O2 supply and O2 demand. When there is insufficient O2 to regenerate ATP, ADP and AMP accumulate leading to adenosine generation. By causing vasodilatation, adenosine helps to restore the O2 delivery, reversing the mismatch and allowing ATP to be regenerated. The idea that ATP might contribute to exercise hyperaemia in skeletal muscle can also be traced back to the 1960s when Forrester & Lind (1969) reported that ATP was released into venous efflux of the forearm during sustained contraction and showed that infusion of ATP into the resting forearm caused vasodilatation.
Within this review, consideration is given to the evidence for and against adenosine or ATP contributing to exercise hyperaemia, the mechanisms by which they may be released or generated, and the mechanisms by which they may act.
Historical perspective
Early studies of Berne's group showed accumulation of adenosine, ADP and AMP in biopsies taken from dog hind limb muscle during contraction under ischaemic conditions (Berne et al. 1971; Dobson et al. 1971) and constant blood flow (Bockman et al. 1976). Adenosine concentration in the venous efflux also increased during contraction of dog muscle perfused at low, or resting blood flow (Bockman et al. 1975; Belloni et al. 1979).
However, release of adenosine into venous efflux was soon shown for dog muscle contracting with constant high flow (Ballard et al. 1987; see below) and during free flow (Fuchs et al. 1986; Karim et al. 1988). The positive outcomes of these studies may be explained by improvements in the sensitivity of the assay methods. Moreover, greater understanding of the rapidity with which adenosine is cleared from plasma led to improvement in the collection of samples for assay and in experimental design (see Ballard et al. 1987; Poucher et al. 1990 and below).
It also emerged that cat soleus, a slow oxidative (red) muscle produces more adenosine during contraction than the gracilis, a fast-twitch, glycolytic (white) muscle. Indeed, the cat soleus has a two-fold higher activity than the gracilis of 5′nucleotidase (5′N), which dephosphorylates AMP to adenosine, whereas the gracilis has a three-fold higher activity than the soleus, of AMP deaminase, which degrades AMP to inosine monophosphate (Rubio et al. 1973; Bockman & McKenzie, 1983). Significantly, the 5′N was shown to be widely distributed across soleus muscle, whereas in gracilis it was concentrated in the borders of muscle fibres adjacent to blood vessels and in vascular endothelium, the density increasing towards the capillaries (Rubio et al. 1973).
Results obtained with the aid of pharmacological antagonists intended to test the role of adenosine in exercise hyperaemia were far more equivocal. Thus, adenosine deaminase (ADA) reduced exercise hyperaemia by 30–40% in the highly oxidative, gracilis muscle of dogs (Kille & Klabunde, 1984) and in the soleus, but not gracilis of cats (Schwartz & McKenzie, 1990). Addition of ADA to the superfusate also reduced the arteriolar dilatation evoked in hamster cremaster by graded muscle contractions (Proctor, 1984). However, ADA reduced exercise hyperaemia in dog gracilis muscle by only ∼11% (Karim & Goonewardene, 1989), and did not affect that induced in the rat by low-speed treadmill exercise (Klabunde et al. 1988).
Further, dipyridamole, which blocks the equilibrative transporter for adenosine, enhanced the hyperaemia evoked in various muscles of pigs performing maximum treadmill exercise (Laughlin et al. 1989), and that evoked by maximal contraction in soleus muscle of dogs (Kille & Klabunde, 1984), but not that evoked by submaximal contractions in dog gracilis (Klabunde, 1986).
Finally, studies involving the most selective adenosine receptor antagonist available at the time, and still the only one available for use in humans, theophylline (aminophylline), achieved mixed success. For example, it reduced exercise hyperaemia in dog gracilis (Tabaie et al. 1977), hamster cremaster (Proctor, 1984) and in dogs during treadmill exercise (Metting et al. 1986). By contrast, theophylline did not affect exercise hyperaemia in dog gracilis muscle (Honig & Frierson, 1980), or in dogs during treadmill exercise (Koch et al. 1990).
Even pharmacological findings that apparently support a role for adenosine in exercise hyperaemia, must be treated with caution. The breakdown product of ADA, inosine, is a vasodilator in its own right. Further, there is doubt over the extent to which ADA and dipyridamole can pass from the bloodstream into the interstitial space (see Poucher et al. 1990; Schwartz & McKenzie, 1990; Martin et al. 2007). This is important if most of the adenosine generated during muscle contraction is released into interstitium (see below). Moreover, dipyridamole stimulates prostacyclin production and inhibits phosphodiesterase activity (see Martin et al. 2007), and although theophylline is a relatively potent adenosine receptor antagonist (pA2 5), it inhibits phosphodiesterase activity (see Poucher et al. 1990). Such non-selective effects probably explain why both antagonists tend to cause resting vasodilatation, making their effects on exercise hyperaemia difficult to interpret.
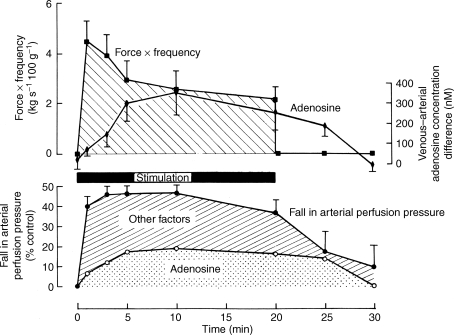
Against this background, the study that is a landmark in the field is that of Ballard et al. (1987). In dog gracilis muscle perfused at a constant high flow rate equivalent to that recorded during contraction, arterial and venous samples were collected for assay of adenosine before, during and after contraction at 4 Hz for 20 min. The high blood flow avoided fatigue, and meant that the released adenosine was not diluted by an increase in blood flow, and was exposed to uptake and degradation at a constant rate. Subsequently, adenosine was infused into the muscle at a rate that achieved the venous concentration reached during contraction, and the magnitude of dilatation was measured. These results clearly showed release of adenosine into the venous efflux, reaching a peak around the 10th minute of contraction and returning to baseline 5–10 min after contraction (Fig. 1). Comparison of the contraction-induced vasodilatation and that evoked by infused adenosine indicated that adenosine contributed ∼15% of the total vasodilatation in the first minute of contraction, ∼40% between the 5th and 20th minute and ∼80% for 5 min after contraction (Fig. 1).
Figure 1.
Comparison between time course of the venous-arterial differences in plasma adenosine concentration and the force frequency of contractions (above) and the change in arterial perfusion pressure (below), during and after contractions of the constant-flow perfused gracilis muscle for 20 min In the graph below, the proportion of the total vasodilatation that could be attributed to the released adenosine, is shown by the stippled area. All recorded data are shown as mean ±s.e.m. Reproduced from Ballard et al. (1987) with permission from Blackwell Publishing Ltd.
Similar conclusions were drawn from the first experiments with 8-phenyltheophylline (8-PT), a potent specific adenosine receptor antagonist (PA2 6.4) that lacks phosphodiesterase activity. 8-PT reduced exercise hyperaemia in cat gracilis muscle, by ∼40% from the third minute of contraction (see Poucher et al. 1990). More recently, theophylline reduced by ∼20%, hyperaemia induced by submaximal knee extensor exercise (Radegren & Calbet, 2001).
Taken together, these results indicate that adenosine makes a substantial contribution to the maintained phase of exercise hyperaemia even at submaximal workloads and in muscle that is mainly glycolytic. Thus, even though total 5′N activity is low in cat gracilis, its localization (see above) apparently enables vasoactive concentrations of adenosine to be produced close to resistance arterioles. Confirmatory evidence that adenosine is an essential contributor to exercise hyperaemia was provided by Murrant & Sarelius (2002). A characteristic of exercise hyperaemia is that vasodilatation initiated in terminal arterioles propagates retrogradely towards proximal arterioles. By applying xanthine amine congener, another specific adenosine receptor antagonist, to hamster cremaster muscle, they showed that adenosine contributed ∼30–40% of the local and remote vasodilatation evoked by muscle contraction.
Cellular origins of adenosine and adenine nucleotides
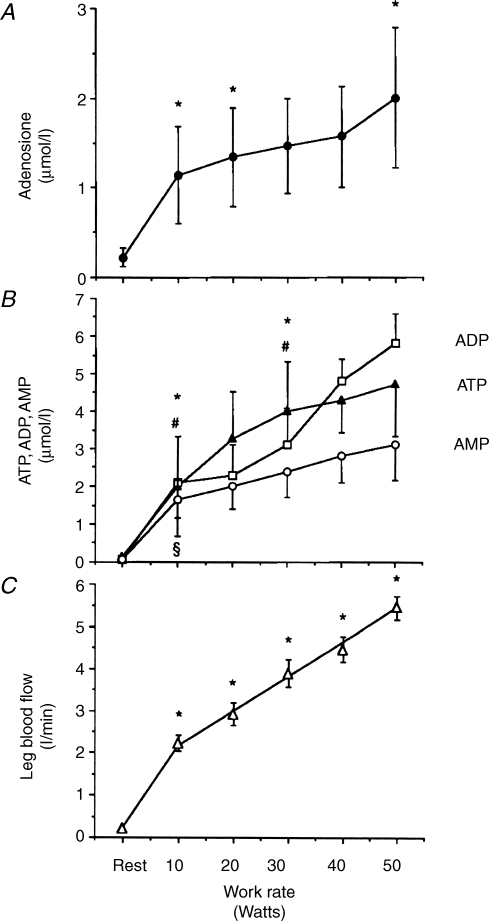
The assumption in many of the earlier studies was that adenosine that appears in venous efflux during contraction is generated within the skeletal fibres and released into the interstitial fluid. Certainly, Hellsten et al. (1998), who made the first measurements of adenosine in the interstitium by using microdialysis, showed that the interstitial adenosine concentration in vastus lateralis muscle increased progressively during graded knee extensor exercise. Further, the increase in interstitial adenosine was highly correlated with the increase in muscle blood flow, as would be expected if adenosine acts on receptors on the extraluminal surface of vascular smooth muscle to cause vasodilatation (Fig. 2). Similarly, interstitial adenosine concentration increased during contraction in dog gracilis and in the oxidative soleus and more glycolytic, extensor digitorum longus muscles of rats (Lo et al. 2001; Mo & Ballard, 2001).
Figure 2.
Measurements made at rest and during graded dynamic knee extensor exercise A, concentration of adenosine; B, concentrations of ATP, ADP and AMP in human skeletal muscle interstitial fluid, measured in the dialysate; C, femoral arterial blood flow. Data are mean ±s.e.m., n = 5–7 in each case. *Significant increases in adenosine, ATP and blood flow, other symbols indicate significant increases in AMP or ADP: P < 0.05 in each case. Reproduced from Hellsten et al. (1998) with permission from Lippincott, Williams & Wilkins.
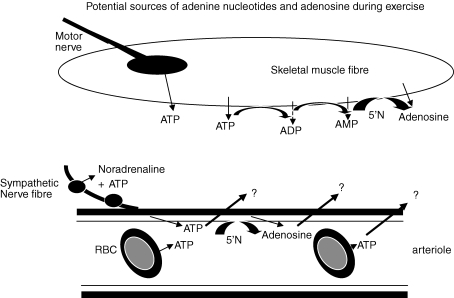
However, in human thigh and dog gracilis muscles the interstitial concentrations of AMP, ADP and even ATP, also increased during muscle contraction (Fig. 2, Hellsten et al. 1998; Mo & Ballard, 2001). This brings into question the mechanisms of release and cellular sources of adenosine and adenine nucleotides. Firstly, it seems unlikely that skeletal muscle fibres release ATP, the high-energy phosphate fuel during contractions. Thus, other sources of ATP need to be considered. ATP is released from motor nerves when they are activated (Cunha & Sebastiao, 1993) and it is coreleased with noradrenaline from sympathetic nerve varicosities (Burnstock, 2006). Further, ATP can be released from red blood cells (RBCs, Sprague et al. 2001; Jagger et al. 2001), and ATP and adenosine can be released from endothelial cells (Deussen et al. 1986; Burnstock, 2006): either might diffuse across blood vessel walls into the interstitium (Fig. 3). Secondly, even exhaustive exercise that causes pronounced ATP degradation, does not lead to an increase in adenosine content within skeletal muscle fibres (Tullson et al. 1995). Thus, it is a reasonable assumption that interstitial adenosine is mainly generated extracellularly by phosphatases and/or by ecto 5′N.
Figure 3.
Schematic showing potential sources of adenosine and adenine nucleotides in skeletal muscle during exercise Adenine nucleotides and adenosine might be released as such into interstitial fluid from skeletal muscle fibres, or formed after metabolism by ecto-phosphatases or ecto 5′nucleotidase (5′N). ATP may be released from motor and sympathetic neurons during their activation. ATP released from RBCs into plasma and ATP and adenosine released as such from endothelium, or generated by ectoenzymes, may diffuse into interstitial fluid. For further discussion see text.
Release from skeletal muscle fibres
During spontaneous contractions of rat primary skeletal muscle cells in culture, the concentrations of adenine nucleotides and adenosine gradually increased in the medium (Hellsten & Frandsen, 1997). When the muscle cells were made to contract at different frequencies, there was a graded increase in adenosine in the medium, but no further accumulation of nucleotides. Nevertheless, maximal stimulation led to an 18% reduction in the intracellular concentrations of ATP and a trend for intracellular concentrations of ADP and AMP to increase (Hellsten & Frandsen, 1997).
The muscle cells were shown to have substantial extracellular ATPase, ADPase and AMP 5′N activities, although the ATPase and 5′N activities were only about one-quarter and one-sixtieth, respectively, of those measured in cultured microvascular endothelial cells. Nevertheless, the ecto 5′N activity of muscle cells was increased by ∼60% during contraction, and they generated far more adenosine when AMP rather than ATP was provided as substrate (Hellsten & Frandsen, 1997). Further, addition of medium from stimulated muscle cells to non-stimulated muscle cells caused a 20% increase in ecto 5′N activity. This suggested that contracting cells release a substance that enhances ecto 5′N activity. Addition of K+, lactate, or nitric oxide (NO), substances that might be released by muscle contraction, were not able to reproduce this effect. However, evidence was obtained that the increase in ecto 5′N activity might be partly explained by translocation of cytosolic vesicles containing 5′N to the sarcolemma (see Hellsten, 1999).
Another mechanism that may increase adenosine production via ecto 5′N was uncovered by Cheng et al. (2000). When the pH of buffer perfusing rat gracilis muscle at rest was reduced, both adenosine and AMP were released into the perfusate. Further, the affinity of ecto 5′N for AMP was increased when pH was decreased. Indeed, the combined effect of increase in substrate and increase in affinity of 5′N was estimated as a 10–20% increase in the ability of 5′N to generate adenosine for every 0.5 unit decrease in pH (Cheng et al. 2000). These findings agree with the linear relationship between venous adenosine concentration and decrease in venous pH during muscle contraction (Achike & Ballard, 1993).
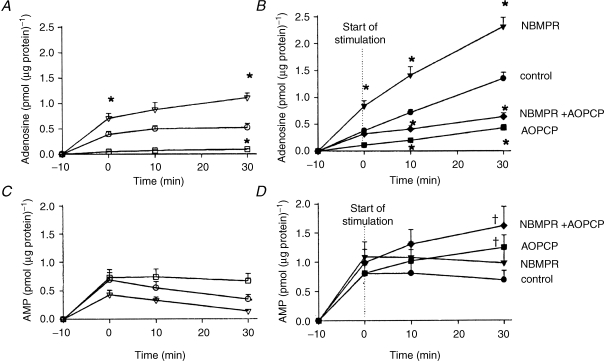
That adenosine is mainly generated extracellularly during muscle contraction was confirmed by Lynge et al. (2001). When rat skeletal muscle cells in culture contracted in the presence of AOPCP (αβ methylene ADP), which inhibits ecto 5′N, adenosine accumulation in the medium was virtually attenuated (Fig. 3). By contrast, NBMPR (nitrobenzylthioinosine), which inhibits the equilibrative adenosine transporter, accentuated adenosine accumulation (Fig. 3). Thus the transporter is largely involved in the uptake of adenosine, rather than release of preformed adenosine. Importantly, in the presence of AOPCP, there was greater release of AMP (Fig. 3, Lynge et al. 2001).
Thus, these results demonstrated that during contraction, AMP is released from the skeletal muscle fibres into the interstitium and metabolized by muscle ecto 5′N to adenosine. Release of AMP and the affinity of ecto 5′N for AMP increases when acidosis accompanies muscle contraction, while the concentration of ecto 5′N may be increased by translocation from intracellular stores. The adenosine so generated may be taken up again by the equilibrative transporters on the muscle cells. The enzymes that metabolize adenosine, ADA and adenosine kinase, are mainly intracellular (see Cheng et al. 2000).
Release from motor and sympathetic nerves
The very fact ATP was not released from cultured muscle cells when they were made to contract (Hellsten & Frandsen, 1997), but did appear in the interstitium of human muscles during exercise and in dog muscle made to contract by stimulation of its mixed nerve supply (Hellsten et al. 1998; Mo & Ballard, 2001), is consistent with ATP being released from motor and/or sympathetic nerves. It is unlikely that ATP diffuses from the blood into the interstitial fluid during contraction, for ATP infused intra-arterially into dog gracilis muscle did not appear in the interstitium until the arterial concentration was >50 times higher than in the interstitium (Mo & Ballard, 2001). Similar observations were made for ADP, AMP and adenosine (Mo & Ballard, 2001). These findings are consistent with evidence that endothelium has an avid transport mechanism for adenosine and high activities of enzymes that metabolize adenine nucleotides and adenosine (see Hellsten & Frandsen, 1997).
Release from red blood cells and endothelium
Even if ATP and adenosine generated within the blood stream do not reach the interstitial fluid during contraction, it is still reasonable to question whether either contribute to exercise hyperaemia.
ATP is released from RBCs by mechanical deformation (Sprague et al. 2001) and during haemoglobin deoxygenation (Jagger et al. 2001). ATP is also released from endothelium in response to shear and hypoxia (Deussen et al. 1986; Burnstock, 2006). Clearly, mechanical compression and shear rate are likely to increase during muscle contraction. In resting muscle, O2 diffuses across arterial walls, such that two-thirds of the O2 is lost before the capillaries. Thus, when muscle O2 consumption increases during exercise and O2 is downloaded from haemoglobin, this longitudinal diffusion gradient increases (Pittman, 2000). Consequently, blood and haemoglobin O2 saturation must fall, providing potential stimuli for ATP release from RBCs and endothelium, at all sites from proximal arterioles to venules.
Certainly, consistent with the findings of Forrester & Lind (1969) on the forearm, ATP was released in a graded manner into venous efflux during graded knee-extensor exercise. Further, the ATP release was accentuated during exercise performed under hypoxic conditions, but reduced when exercise was performed during hyperoxia, or when 1% CO was added to the inspirate (González-Alonso et al. 2002). These results accord with evidence that ATP is released from RBCs when the haemoglobin molecule undergoes the conformational change from its oxygenated, to its deoxygenated state: the high-affinity binding of CO prevents this change (Jagger et al. 2001). Importantly, González-Alonso et al. 2002) confirmed that ATP infused intra-arterially under resting conditions in normoxia, can reproduce the muscle vasodilatation of exercise.
Actions of intraluminal ATP and adenosine
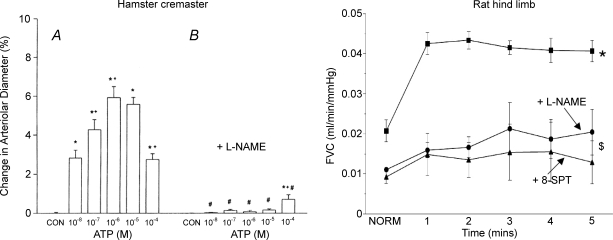
Application of ATP extraluminally to arterioles in hamster cheek pouch evoked vasoconstriction via P2X receptors (McCullough et al. 1997), as does ATP released from sympathetic nerve fibres in muscle (Johnson et al. 2001). However, intraluminal ATP evoked vasodilatation (McCullough et al. 1997) that was attributable to stimulation of endothelial P2Y receptors (Burnstock, 2006). Moreover, dilatation evoked by intraluminal application of ATP to arterioles was propagated retrogradely to proximal arterioles (McCullough et al. 1997; Fig. 5), while that evoked by intraluminal application to venules was conducted across the capillaries to arterioles (Collins et al. 1998). Thus, in these respects, ATP released intraluminally fits the criteria required of a candidate for exercise hyperaemia.
Figure 5.
Effects of NO synthase inhibition on conducted vasodilator responses evoked in arterioles of hamster cremaster muscle by intraluminal application of ATP (left) and on vasodilatation evoked in rat hindlimb muscle by intra-arterial infusion of ATP (right) Left, change in arteriolar diameter after intraluminal application of control vehicle (CON) and graded concentrations of ATP alone (A) and after (B) systemic administration of l-NAME. Arteriolar diameters were measured 150 ± 52 μm upstream from the site of application of ATP. All values are shown as mean ±s.e.m.*Significant change in diameter; +significantly different from change induced by next lower concentration, #significant difference between before versus after l-NAME. Reproduced from McCullough et al. (1997) with permission from The American Physiological Society. Right, femoral vascular conductance (femoral blood flow/arterial blood pressure), before (time 0) and during intra-arterial infusion of ATP, before and after systemic administration of l-NAME, and then, after subsequent administration of the adenosine receptor antagonist 8-SPT. All values are shown as mean ±s.e.m.*P < 0.05 before versus after L-NAME, $P < 0.05 after l-NAME versus after 8-SPT. Drawn from data included in Skinner & Marshall (1996).
Vasodilator responses evoked by intraluminal ATP in hamster cheek pouch arterioles were attenuated by the NO synthesis (NOS) inhibitor nitro-l-arginine methyl ester (l-NAME, McCullough et al. 1997; see Fig. 5), as might be expected if ATP acts on endothelial P2Y receptors. Similarly, ATP infused intra-arterially into rat hindlimb muscle could reproduce the vasodilatation evoked by systemic hypoxia, and this response was greatly reduced by l-NAME. However, the remaining dilatation was attenuated by the adenosine receptor antagonist, 8-sulphophenytheophylline (8-SPT, Skinner & Marshall, 1996; see Fig. 5). Muscle vasodilatation evoked by intra-arterial ATP was also reduced by AOPCP (Skinner & Marshall, 1996). Thus, vasodilatation induced by intraluminal ATP is largely NO dependent, and partly mediated by adenosine. This is not surprising given the extremely high ecto-phosphatase and ecto 5′N activity of endothelial cells (see Hellsten & Frandsen, 1997). Unfortunately, the contribution of ATP acting on P2Y receptors to exercise hyperaemia or hypoxia-induced vasodilatation cannot be tested because no selective P2Y receptor antagonist is available for use in vivo.
Turning to adenosine, at least 50% of vasodilatation evoked in rat hind limb muscle by systemic hypoxia is mediated by adenosine and attenuated by 8-PT or 8-SPT (see Marshall, 2000). A similar finding was made in human forearm muscle by using aminophylline (Leuenberger et al. 1999). It seems likely that in systemic hypoxia, adenosine itself is released, rather than being generated extracellularly, for hypoxia-induced muscle vasodilatation was not affected by AOPCP (Skinner & Marshall, 1996). Further, the available evidence indicates that during hypoxia, adenosine is largely released by endothelial, rather than extravascular cells. (see Marshall, 2000). Notably, adenosine was released from coronary endothelium during hypoxic perfusion (Deussen et al. 1986). Moreover, systemic hypoxia released adenosine into the venous efflux of dog skeletal muscle, but had no effect on venous concentrations of ATP, ADP or AMP, or on interstitial concentrations of adenine nucleotides or adenosine (Mo & Ballard, 2001).
Figure 4.
Adenosine and AMP accumulation in the medium of non-stimulated and electrostimulated primary skeletal muscle cells: effect of NBMPR and AOPCP Muscle cells were incubated with medium alone (control) or in solution containing either NBMPR or AOPCP, or both. This was performed without electrostimulation (A and C, open symbols) or with electrostimulation (B and D, closed symbols). Values are mean ±s.e.m. of 6–17 cell dishes. *Significantly different from control value at same point in time: P < 0.05. †Significantly different from values measured without electrostimulation: P < 0.05. Reproduced from Lynge et al. (2001) with permission from Blackwell Publishing Ltd.
In human skeletal muscle, adenosine A1, A2A and A2B receptors are present on endothelium and vascular smooth muscle (Lynge & Hellsten, 2000). Accordingly, adenosine infused intra-arterially into rat hindlimb muscle produces vasodilatation by stimulating A1 and A2A receptors (Bryan & Marshall, 1999). However, muscle vasodilatation induced by systemic hypoxia was attenuated only by the selective A1 receptor antagonist DPCPX, and not by the selective A2A receptor antagonist, ZM241385 (Bryan & Marshall, 1999). Further, muscle vasodilatation evoked by hypoxia and by infused adenosine was largely attenuated by l-NAME. Moreover, hypoxia-induced dilatation was restored when the tonic level of NO was restored by infusion of NO donor, and this dilatation was also mediated by A1 receptors (Edmunds et al. 2003). Thus, the adenosine component of hypoxia-induced vasodilatation is mediated by A1 receptors and is NO dependent. When the tonic level of NO is restored after NOS inhibition, then adenosine released by hypoxia presumably acts on A1 receptors on vascular smooth muscle (Edmunds et al. 2003).
There is direct evidence that adenosine releases NO from the endothelium of rat aorta and iliac arteries, by stimulating A1 or A2A receptors (Ray et al. 2002). Moreover, systemic hypoxia releases NO into the venous efflux of rat hindlimb muscle by stimulating A1 receptors (Ray & Marshall, 2005). Thus, it can be concluded that systemic, and local hypoxia causes endothelial cells to release adenosine, which acts back on endothelial A1 receptors to induce muscle vasodilatation. Smits et al. (1995), who similarly restored vasodilator tone after NOS inhibition with NO donor, showed that vasodilatation evoked in human forearm by intra-arterial adenosine is partly mediated by NO.
Exercise hyperaemia versus intraluminally acting adenosine and ATP
Putting the results discussed in the section above together, a key question arises: does vasodilatation induced by adenosine generated from ATP, or released into the bloodstream by the relatively hypoxic conditions of exercise, or by intra-arterially infused adenosine, show similar characteristics to the contribution adenosine makes to exercise hyperaemia? This is considered below.
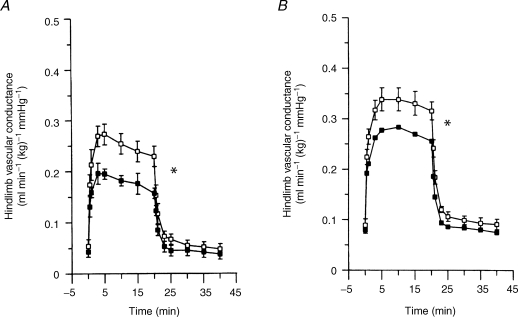
(i) The availability of the highly selective A2A receptor antagonist ZM24138, allowed Poucher (1996) to show it had an exactly comparable effect to 8-PT (Fig. 6), reducing exercise hyperaemia in cat gracilis muscle by ∼40%. Thus, in the cat, exercise hyperaemia is mediated by A2A and not by A1 or A2B receptors. In our recent experiments, ZM241385 reduced by ∼30%, vasodilatation evoked in rat hind limb by contractions at 4 Hz for 5 min. Moreover, subsequent administration of 8-SPT, which is non-selective between adenosine receptor subtypes, had no further effect (Ray & Marshall, 2007, unpublished findings). Thus, in the rat also, the adenosine component of exercise hyperaemia is attributable to A2A receptors, even though A1 receptors are responsible for vasodilatation evoked by systemic hypoxia (Bryan & Marshall, 1999).
Figure 6.
Effects of the non-selective adenosine receptor antagonist 8-phenyltheophylline (8-PT) and the selective A1 receptor antagonist ZM 241385 on vasodilatation evoked in cat hindlimb muscle by contraction Graphs show hindlimb vascular conductance calculated as hindlimb blood flow/arterial blood pressure, before (time 0) and during contraction at 3 Hz for 20 min. A, before and after 8-PT; B, before and after ZM241385. In each case, closed symbols show values after antagonist. All values are mean ±s.e.m.*P < 0.05, before versus after antagonist. Reproduced from Poucher (1996) with permission from Blackwell Publishing Ltd.
(ii) There have been conflicting reports on whether or not NO contributes to exercise hyperaemia (see Radegren & Hellsten, 2000; Clifford & Hellsten, 2004 for reviews). When differences in methodologies are taken into account, the consensus seems to be that NO plays little role in maintaining exercise hyperaemia, but may contribute during recovery. Even in studies in which NOS inhibition did reduce exercise hyperaemia, this was largely attributable to its effect on baseline vascular tone, rather than on the change in blood flow evoked by exercise (see Radegren & Hellsten, 2000; Clifford & Hellsten, 2004). In our recent studies, when vascular tone was restored after NOS inhibition, by infusion of NO donor, the dilatation evoked in rat hindlimb muscle by contractions at 4 Hz was fully restored and then reduced by ∼30%, by ZM24138 (Ray & Marshall, 2007 unpublished findings). This is fully consistent with exercise hyperaemia being evoked by adenosine acting on A2A receptors on vascular smooth muscle and not on endothelial A1 receptors that are stimulated during hypoxia and act via NO (see above).
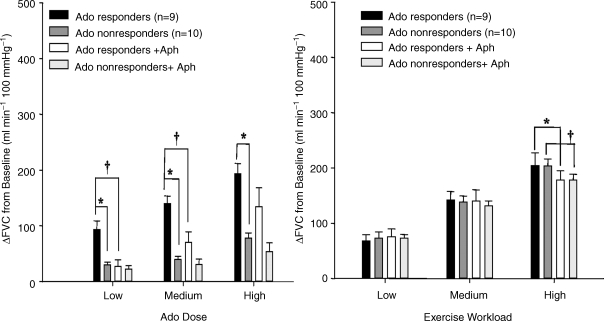
(iii) Martin et al. (2006a,b, 2007) recently reported that healthy male and female subjects can be designated ‘responders’ and ‘non-responders’ on the basis of their responsiveness to intra-arterially infused adenosine, although the two groups showed fully comparable exercise hyperaemia responses to three different workloads. Inhibition of NOS reduced muscle vasodilatation evoked by intra-arterial adenosine in ‘responders’, but not in ‘non-responders’, nor that evoked by exercise in either group (Martin et al. 2006a). Further, dipyridamole reduced vasodilatation evoked by intra-arterial adenosine in ‘responders’, so that it equalled that evoked in ‘non-responders’, but had no effect on exercise hyperaemia in either group (Martin et al. 2007). Moreover, the relatively weak adenosine receptor antagonist aminophylline, significantly reduced vasodilatation evoked by intra-arterial adenosine in ‘responders’ and had no effect in ‘non-responders’, but achieved a 15% attenuation of exercise hyperaemia in both groups (Martin et al. 2006b; Fig. 7). These results could be explained if the disparities between the groups mainly reflect greater endothelial uptake of intra-arterial adenosine in ‘non-responders’, so decreasing the concentration available to stimulate endothelial adenosine receptors that act via NO, and if intraluminal adenosine makes little contribution to exercise hyperaemia.
Figure 7.
Change in femoral vascular conductance above baseline Changes shown are for low, medium and high doses of adenosine (Ado) for Ado-responders and non-responders (A) and for low, medium and high exercise workloads for Ado-responders and non-responders (B), before and after infusion of the adenosine receptor antagonist aminophylline (Aph). Values are mean ±s.e.m.*P < 0.05 responders versus non-responders, †P < 0.05 before versus after Aph for responders or non-responders. Reproduced from Martin et al. (2006b) with permission from The American Physiological Society.
(iv) Finally, the finding that prolonged intrarterial infusion of adenosine leads to decrement of its vasodilator response, but does not affect exercise hyperaemia, has been used as evidence that adenosine does not contribute to exercise hyperaemia (Hester et al. 1982). However, A1 receptors that mediate a large part of the response to intraluminal adenosine (see above) rapidly desensitize in the continued presence of adenosine, whereas this is not a feature of A2A receptors (Ralevic & Burnstock, 1998) that mediate the adenosine component of exercise hyperaemia.
Thus, exercise hyperaemia differs in several important respects from vasodilatation induced by intraluminal adenosine and indeed, by intraluminal ATP, given ATP's effects are NO-dependent and partly mediated by adenosine.
Conclusion
Adenosine is responsible for 20–40% of the maintained phase of exercise hyperaemia at submaximal and maximal workloads. Adenosine is largely formed extracellularly from AMP released by skeletal muscle fibres, by the action of ecto 5′N associated with the sarcolemma. This adenosine causes dilatation by acting on A2A receptors on the extraluminal surface of the arterial smooth muscle. By contrast, adenosine released from endothelial cells, or generated from ATP released from endothelial cells or RBCs, produces vasodilatation via endothelial A1 receptors, and makes little contribution to exercise hyperaemia. ATP itself may make a small contribution to exercise hyperaemia by acting on P2Y receptors. However, this possibility is unattractive since the dilator effect of ATP would be expected to show a strong dependency on NO, which is not a characteristic of exercise hyperaemia.
Acknowledgments
The work of the author and colleagues that is described in this review was generously supported by The Wellcome Trust and British Heart Foundation.
References
- Achike FI, Ballard HJ. Influence of stimulation parameters on the release of adenosine, lactate and CO2 from contracting dog gracilis muscle. J Physiol. 1993;463:107–121. doi: 10.1113/jphysiol.1993.sp019586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard HJ, Cotterrell D, Karim F. Appearance of adenosine in venous blood from contracting gracilis muscle and its role in vasodilatation in the dog. J Physiol. 1987;387:401–413. doi: 10.1113/jphysiol.1987.sp016580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni FL, Phair RD, Sparks HV. The role of adenosine in prolonged vasodilatation following flow restriction exercise of canine skeletal muscle. Circ Res. 1979;44:759–766. doi: 10.1161/01.res.44.6.759. [DOI] [PubMed] [Google Scholar]
- Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Berne RM, Rubio R, Dobson JG, Curnish RR. Adenosine and adenine nucleotides as possible mediators of cardiac and skeletal muscle blood flow regulation. Circ Res. 1971;28–29(Suppl. 1):115–119. [PubMed] [Google Scholar]
- Bockman EL, Berne RM, Rubio R. Release of adenosine and lack of release of ATP from contracting skeletal muscle. Pflugers Arch. 1975;355:229–241. doi: 10.1007/BF00583686. [DOI] [PubMed] [Google Scholar]
- Bockman EL, Berne RM, Rubio R. Adenosine and active hyperemia in dog skeletal muscle. Am J Physiol. 1976;230:1531–1537. doi: 10.1152/ajplegacy.1976.230.6.1531. [DOI] [PubMed] [Google Scholar]
- Bockman EL, McKenzie JE. Tissue adenosine content in active soleus and gracilis muscle of cats. Am J Physiol Heart Circ Physiol. 1983;244:H552–H559. doi: 10.1152/ajpheart.1983.244.4.H552. [DOI] [PubMed] [Google Scholar]
- Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol. 1999;514:151–162. doi: 10.1111/j.1469-7793.1999.151af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signalling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Cheng B, Essackjee HC, Ballard HJ. Evidence for control of adenosine metabolism in rat oxidative skeletal skeletal muscle by changes in pH. J Physiol. 2000;522:467–477. doi: 10.1111/j.1469-7793.2000.t01-1-00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56:43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM. Adenosine and adenine nucleotides are independently released from both nerve terminals and the muscle fibres upon electrical stimulation of the innervated skeletal muscle of the frog. Pflugers Arch. 1993;424:503–510. doi: 10.1007/BF00374914. [DOI] [PubMed] [Google Scholar]
- Deussen A, Moser G, Schrader J. Contribution of endothelial cells to cardiac adenosine production. Pflugers Arch. 1986;406:608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Dobson JG, Rubio R, Berne RM. Role of adenosine nucleotides, adenosine, and inorganic phosphate in the regulation of skeletal muscle blood flow. Circ Res. 1971;29:375–384. doi: 10.1161/01.res.29.4.375. [DOI] [PubMed] [Google Scholar]
- Edmunds NJ, Moncada S, Marshall JM. Does nitric oxide allow endothelial cells to sense hypoxia and mediate hypoxic vasodilatation?In vivo and in vitro studies. J Physiol. 2003;546:521–527. doi: 10.1113/jphysiol.2002.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969;204:347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BD, Gorman MW, Sparks HV. Adenosine release into venous plasma during free flow exercise. Proc Soc Exp Biol Medical. 1986;181:364–370. doi: 10.3181/00379727-181-42266. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Hellsten Y. The effect of muscle contraction on the regulation of adenosine formation in rat skeletal muscle. J Physiol. 1999;518:761–768. doi: 10.1111/j.1469-7793.1999.0761p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. J Physiol. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, MacLean DA, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Hester RL, Guyton AC, Barber BJ. (Reactive and exercise hyperemia during high levels of adenosine infusion. Am J Physiol Heart Circ Physiol. 1982;243:H181–H186. doi: 10.1152/ajpheart.1982.243.2.H181. [DOI] [PubMed] [Google Scholar]
- Honig CR, Frierson JL. Role of adenosine in exercise vasodilation in dog gracilis muscle. Am J Physiol Heart Circ Physiol. 1980;238:H703–H715. doi: 10.1152/ajpheart.1980.238.5.H703. [DOI] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Coney AM, Marshall JM. Roles of norepinephrine and ATP in sympathetically evoked vasoconstriction in rat tail and hindlimb in vivo. Am J Physiol Heart Circ Physiol. 2001;281:H2432–H2440. doi: 10.1152/ajpheart.2001.281.6.H2432. [DOI] [PubMed] [Google Scholar]
- Karim F, Ballard HJ, Cotterrell D. Changes in adenosine release and blood flow in the contracting dog gracilis muscle. Pflugers Arch. 1988;412:1061–1112. doi: 10.1007/BF00583738. [DOI] [PubMed] [Google Scholar]
- Karim F, Goonewardene I. The effect of adenosine deaminase on freeflow exercise hyperemia. J Vasc Med Biol. 1989;1:180. [Google Scholar]
- Kille JM, Klabunde RE. Adenosine as a mediator of postcontraction hyperemia in dog gracilis muscle. Am J Physiol Heart Circ Physiol. 1984;246:H274–H282. doi: 10.1152/ajpheart.1984.246.2.H274. [DOI] [PubMed] [Google Scholar]
- Klabunde RE. Conditions for dipyridamole potentiation of skeletal muscle active hyperemia. Am J Physiol Heart Circ Physiol. 1986;250:H62–H67. doi: 10.1152/ajpheart.1986.250.1.H62. [DOI] [PubMed] [Google Scholar]
- Klabunde RE, Laughlin MH, Armstrong RB. Systemic adenosine deaminase administration does not reduce active hyperemia in running rats. J Appl Physiol. 1988;64:108–114. doi: 10.1152/jappl.1988.64.1.108. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL, Metting PJ. Adenosine is not essential for exercise hyperemia in the hindlimb in conscious dogs. J Physiol. 1990;429:63–75. doi: 10.1113/jphysiol.1990.sp018244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol. 1989;257:H1507–H1515. doi: 10.1152/ajpheart.1989.257.5.H1507. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol. 1999;87:2218–2224. doi: 10.1152/jappl.1999.87.6.2218. [DOI] [PubMed] [Google Scholar]
- Lo SM, Mo FM, Ballard HJ. Interstitial adenosine concentration in rat red or white skeletal muscle during systemic hypoxia or contractions. Exp Physiol. 2001;86:593–598. doi: 10.1113/eph8602226. [DOI] [PubMed] [Google Scholar]
- Lynge J, Hellsten Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol Scand. 2000;169:283–290. doi: 10.1046/j.1365-201x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Lynge J, Juel C, Hellsten Y. Extracellular formation and uptake of adenosine during skeletal muscle contraction in the rat: role of adenosine transporters. J Physiol. 2001;537:597–605. doi: 10.1111/j.1469-7793.2001.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Adenosine and muscle vasodilatation in acute systemic hypoxia. Acta Physiol Scand. 2000;168:561–573. doi: 10.1046/j.1365-201x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Curry TB, Eisenach JH, Charkoudian N, Joyner MJ. Adenosine transporter antagonism in humans augments vasodilator responsiveness to adenosine, but not exercise, in both adenosine responders and non-responders. J Physiol. 2007;579:237–245. doi: 10.1113/jphysiol.2006.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol. 2006a;101:492–499. doi: 10.1152/japplphysiol.00684.2005. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in responders and nonresponders. J Appl Physiol. 2006b;101:1678–1684. doi: 10.1152/japplphysiol.00546.2006. [DOI] [PubMed] [Google Scholar]
- McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol. 1997;272:H1886–H1891. doi: 10.1152/ajpheart.1997.272.4.H1886. [DOI] [PubMed] [Google Scholar]
- Metting PJ, Weldy DL, Ronau TF, Britton SL. Effect of aminophylline on hindlimb blood flow autoregulation during increased metabolism in dogs. J Appl Physiol. 1986;60:1857–1864. doi: 10.1152/jappl.1986.60.6.1857. [DOI] [PubMed] [Google Scholar]
- Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol. 2001;536:593–603. doi: 10.1111/j.1469-7793.2001.0593c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Regul Integr Comp Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- Pittman RN. Oxygen supply to contracting skeletal muscle at the microcirculatory level: diffusion vs. convection. Acta Physiol Scand. 2000;168:593–602. doi: 10.1046/j.1365-201x.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- Poucher SM. The role of the A2A adenosine receptor subtype in functional hyperaemia in the hindlimb of anaesthetized cats. J Physiol. 1996;492:495–503. doi: 10.1113/jphysiol.1996.sp021324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucher SM, Nowell CG, Collis MG. The role of adenosine in exercise hyperaemia of the gracilis muscle in anaesthetized cats. J Physiol. 1990;427:19–29. doi: 10.1113/jphysiol.1990.sp018158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor KG. Reduction of contraction-induced arteriolar vasodilation by adenosine deaminase or theophylline. Am J Physiol Heart Circ Physiol. 1984;247:H195–H205. doi: 10.1152/ajpheart.1984.247.2.H195. [DOI] [PubMed] [Google Scholar]
- Radegran G, Calbet JAL. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Radegren G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2000;168:575–592. doi: 10.1046/j.1365-201x.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Phamacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray CJ, Marshall JM. Measurement of nitric oxide release evoked by systemic hypoxia and adenosine from rat skeletal muscle in vivo. J Physiol. 2005;568:967–978. doi: 10.1113/jphysiol.2005.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio R, Berne RM, Dobson JG., Jr Sites of adenosine production in cardiac and skeletal muscle. J Physiol. 1973;225:938–953. doi: 10.1152/ajplegacy.1973.225.4.938. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, McKenzie JE. Adenosine and active hyperemia in soleus and gracilis muscle of cats. Am J Physiol Heart Circ Physiol. 1990;259:H1295–H1304. doi: 10.1152/ajpheart.1990.259.4.H1295. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol. 1996;495:553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135–2141. doi: 10.1161/01.cir.92.8.2135. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- Tabaie HM, Scott JB, Haddy FJ. Reduction of exercise dilation by theophylline. Proc Soc Exp Biol Medical. 1977;154:93–97. doi: 10.3181/00379727-154-39611. [DOI] [PubMed] [Google Scholar]
- Tullson P, Bangsbo J, Hellsten Y, Richter EA. IMP metabolism in human skeletal muscle following exhaustive exercise. J Appl Physiol. 1995;78:146–152. doi: 10.1152/jappl.1995.78.1.146. [DOI] [PubMed] [Google Scholar]