Abstract
Muscles and tendons are highly adaptive to changes in chronic loading, though little is known about the adaptative time course. We tested the hypothesis that, in response to unilateral lower limb suspension (ULLS), the magnitude of tendon mechanical adaptations would match or exceed those of skeletal muscle. Seventeen men (1.79 ± 0.05 m, 76.6 ± 10.3 kg, 22.3 ± 3.8 years) underwent ULLS for 23 days (n = 9) or acted as controls (n = 8). Knee extensor (KE) torque, voluntary activation (VA), cross-sectional area (CSA) (by magnetic resonance imaging), vastus lateralis fascicle length (Lf) and pennation angle (θ), patellar tendon stiffness and Young's modulus (by ultrasonography) were measured before, during and at the end of ULLS. After 14 and 23 days (i) KE torque decreased by 14.8 ± 5.5% (P < 0.001) and 21.0 ± 7.1% (P < 0.001), respectively; (ii) VA did not change; (iii) KE CSA decreased by 5.2 ± 0.7% (P < 0.001) and 10.0 ± 2.0% (P < 0.001), respectively; Lf decreased by 5.9% (n.s.) and 7.7% (P < 0.05), respectively, and θ by 3.2% (P < 0.05) and 7.6% (P < 0.01); (iv) tendon stiffness decreased by 9.8 ± 8.2% (P < 0.05) and 29.3 ± 11.5% (P < 0.005), respectively, and Young's modulus by 9.2 ± 8.2% (P < 0.05) and 30.1 ± 11.9% (P < 0.01), respectively, with no changes in the controls. Hence, ULLS induces rapid losses of KE muscle size, architecture and function, but not in neural drive. Significant deterioration in tendon mechanical properties also occurs within 2 weeks, exacerbating in the third week of ULLS. Rehabilitation to limit muscle and tendon deterioration should probably start within 2 weeks of unloading.
Muscle wasting and weakness are among the most common results of chronic disuse and unloading. The antigravity muscles, such as the knee extensors and the plantar flexors, appear to be the most affected (di Prampero & Narici, 2003). Losses of up 30% in muscle size after 90–120 days bed rest have been reported (Reeves et al. 2002; Alkner & Tesch, 2004; Shackelford et al. 2004). The onset of muscle atrophy seems to occur within a few days of disuse and after just 7 days of bed rest, a 3% decrease in thigh muscle volume is observable (Ferrando et al. 1995).
However, the losses of contractile strength and power with unloading seem to be consistently greater than the decreases in muscle size and volume (Berg et al. 1991; LeBlanc et al. 1992; Antonutto et al. 1999; Alkner & Tesch, 2004). Thus a loss of muscle mass can only partially explain the decrease in muscle function, with a reduction in muscle activation probably accounting for much of it. (Duchateau, 1995; Antonutto et al. 1999; Koryak, 2001; Ruegg et al. 2003); furthermore, a decrease in the intrinsic force of single muscle fibres has been shown to contribute to the reduced muscle function (Larsson et al. 1996; Trappe et al. 2004; Fitts et al. 2007).
Also, the mechanical properties of animal tendon tissues have been shown to adapt to disuse (Booth & Gould, 1975; Almeida-Silveira et al. 2000; Matsumoto et al. 2003). Although few data exist on the effects of disuse on human tendons, recent findings after long-term head down bed rest showed decreased stiffness and greater mechanical hysteresis in human tendons in vivo after 20 and 90 days (Kubo et al. 2000, 2004; Reeves et al. 2005). Similar results are observed in patients with spinal cord injury (Maganaris et al. 2006).
When comparing the relative decrease in tendon stiffness after 90 days of bed rest (−58%) with corresponding data on changes in muscle size, it appears that tendon properties deteriorate to a greater extent and at a greater relative rate. The adaptability of tendons to changes in loading is also illustrated by the response to chronic overloading since a 65% increase in tendon stiffness was found after 14 weeks of resistive training in elderly individuals (Reeves et al. 2003) whereas muscle volume and force increased only by 6% and 11%, respectively (Reeves et al. 2004). Muscle contraction is heavily influenced by the stiffness of the series-elastic component, the tendon and its aponeurosis. Therefore, from a functional point of view, it seems essential to consider both the muscular and tendinous adaptations together when investigating the causes of the loss of muscle strength with disuse. Hitherto, however, no studies have investigated the extent of muscular and tendinous adaptations to chronic disuse simultaneously and the impact thereof on muscle function.
Hence, the aim of the present study was to investigate the time courses of adaptations of key factors influencing the mechanical output of the quadriceps femoris muscle during 23 days of unilateral lower limb suspension. The hypothesis was put forward that, as for increased loading, the tendon adaptations to unloading would match or even exceed those of skeletal muscle and that these would be reflected by changes in function of the quadriceps–patellar tendon muscle–tendon complex as a whole.
Methods
Participants
Seventeen men gave written, informed consent to participate in this study. All procedures conformed to the Declaration of Helsinki and were approved by the Ethics Committee of the Institute for Biophysical and Clinical Research into Human Movement at the Manchester Metropolitan University. Nine participants (height: 179.3 ± 4.7 cm, mass: 72.4 ± 8.6 kg, age: 19.1 ± 0.6 years, BMI: 22.5 ± 2.5 kg m−2) were randomly assigned to an intervention group undergoing unilateral lower-limb suspension (ULLS), and eight (height: 179.6 ± 6.8 cm, mass: 81.3 ± 10.4 kg, age: 25.9 ± 2.1 years, BMI: 25.1 ± 2.0 kg m−2) to a non-intervention control group. Participants were screened by physicians and excluded for any of the following: chronic disease with regular clinical treatment, regular drug or alcohol intake, regular smoking, any metabolic or hormonal disorder, psychiatric conditions, any blood clotting disorder, any muscle or bone disease, metal implants, any inflammatory disease, participation in sports at a competitive level, fractures during the past 24 month, epilepsy and a known predisposition to develop a deep venous thrombosis (DVT) (factor 5 mutation, prothrombin mutation, plasma levels of protein S and protein C). It has been shown previously (Bleeker et al. 2004) that ULLS is associated with an increased risk of DVT. Participants were monitored every two days by measuring plasma D-dimer concentration and performing colour coded Doppler sonography in order to detect development of DVT in the early, sub clinical phase.
Experimental design
In the weeks preceding the limb suspension period, the participants were asked to visit the laboratory on several occasions to become familiar with all testing procedures. All baseline data were collected in the week before starting the suspension. Subsequent measurements were performed on days 14 and 23 of suspension. A typical testing session started with MRI scans being taken from the suspended leg, after which the participant was moved to an isokinetic dynamometer where the neurophysiological measurements were performed. Once this was completed, strength and ultrasonography measurements were performed.
For an investigation of alteration in tissue protein synthesis and associated changes in gene expression and cell signalling, biopsies of muscle (100 mg) and tendon (2 mg) were taken in five subjects undergoing ULLS at days 0 and 10 and in four subjects at days 10 and 21, except for one subject for whom the tendon sample was not taken. The results will be reported separately. None of the subjects reported any long lasting discomfort after these procedures and we have no evidence that they influenced the biomechanical and neurophysiological properties studied.
Unilateral unloading procedure
Unilateral lower limb suspension (Berg et al. 1991) was applied during a period of 23 days, to induce atrophy of the dominant leg. The non-dominant leg of the participants was fitted with a platform shoe and the dominant leg was kept in slightly flexed position by use of straps, suspending the foot of the dominant leg above the ground while walking with crutches. The knee joint was maintained at about 10 deg of knee flexion (full knee extension corresponds to 0 deg), and the ankle angle was kept at 0 deg (the sole of the foot at right angles to the tibial axis). Participants had to walk on crutches for the whole duration of the suspension period and were asked to refrain from loading the leg in any way, including driving vehicles. We had no evidence that the subjects altered the extent of their activity or diet as a result of the suspension but of course the pattern of their activity must have changed.
Maximal voluntary contraction
Maximal isometric knee extension and flexion torque were measured using an isokinetic dynamometer (Cybex NORM, Ronkonkoma, NY, USA), at 80 deg of knee flexion. The hip angle was set at 85 deg (full hip extension corresponds to 0 deg). All measurements were performed on the suspended leg except for those in the control subjects in which the dominant leg was repeatedly examined. The centre of rotation of the knee joint was aligned with the dynamometer axis of rotation, using a laser pointer attached to it. The participant was secured to the chair with straps placed around the hips and shoulders to avoid movement. All contractions were performed in randomized order with a 2 min resting period separating two contractions. Participants were instructed to perform a maximal isometric contraction and to hold the maximal torque level for ∼2–3 s, until a verbal signal was given to stop contracting. Furthermore, a knee flexion maximal voluntary contraction (MVC) was performed to allow for the calculation of BF coactivation during knee extension. The torque signal from the tested leg was corrected for gravitational force by weighing the leg on the isokinetic dynamometer before each testing session to account for changes in muscle cross-sectional area. In total, each participant performed six knee extensions, of which the first two were rate of torque development contractions (see Electromechanical delay and rate of torque development), the third, fourth and fifth were performed to determine maximum voluntary muscle activation (see Maximal voluntary activation), and the sixth contraction was a ramp contraction performed to assess tendon mechanical properties (see Ultrasonography).
Electromyography measurements
Surface electromyography (EMG) activity of the mm. vastus lateralis (VL) and biceps femoris (BF) was recorded while performing maximal isometric contractions using a custom-built 16 channel surface EMG acquisition system, and was normalized to the maximal muscle compound action potential amplitude (M-wave, see Maximal voluntary activation). Specially developed software (EMGACQ, LISiN, Bioengineering Center, Polytechnics of Turin, Italy) was used for data acquisition and data were stored on a portable computer. A single differential mode acquisition method was used, in which each EMG trace corresponds to the difference between the signals detected underneath two consecutive electrodes of the array. The reference electrodes were placed on the contralateral ankle. Before placing the electrodes, the skin was shaved, gently rubbed using sandpaper and cleansed using alcohol swabs to reduce interelectrode impedance below 5 kΩ. The location of all electrodes with respect to the muscle length and anatomical landmarks was recorded onto an acetate transparent sheet to ensure identical placement on subsequent testing sessions. The raw EMG signal was acquired with a sampling frequency of 2000 Hz, and processed with a multi-channel analog–digital converter (Biopac EMG 100B Systems, USA). The raw signal was filtered using analog high (10 Hz) and low pass (500 Hz) filters, and was amplified with a gain of 2000, before being corrected for offset. Root mean square (RMS) was calculated over 500 ms around the peak isometric torque, taking into account the electromechanical delay (EMD, see below). The extent of BF coactivation was estimated over 500 ms around the peak MVC torque by multiplying the ratio of BF RMS during antagonist and agonist MVCs by 100.
Electromechanical delay and rate of torque development
Both EMD and rate of torque development (RTD) were calculated during a trial in which the participants were instructed to reach their MVC torque as fast as possible. EMD was defined as the delay between the onset of EMG and torque development. The onset of EMG was determined as an increase of 15 μV above baseline VL EMG RMS, and torque onset was determined as an increase of 2 N m above baseline level (Reeves et al. 2003). Rate of torque development (RTD) was determined over the first 100 ms after the onset of torque. The rise in torque was divided by the time interval to yield RTD.
Maximal voluntary activation
To assess the maximal voluntary activation of the m. quadriceps femoris, the stimulation intensity administered to elicit the maximal M-wave was determined. The maximal M-wave of the VL muscle was obtained by administering twitches (400 V) to the femoral nerve, using an electrical stimulator (model DS7, Digitimer stimulator, Welwyn, Garden City, UK). A hand-held monopolar cathode (0.5 cm diameter) was placed in the femoral triangle, where the femoral nerve is relatively superficial and easily accessible. The exact placing of the cathode electrode was determined by applying 50 mA stimulations. The anode (76 by 127 mm, Versa-Stim, Conmed) was placed on the gluteal fold. Maximal M-wave peak-to-peak amplitude was measured by applying 50 mA increments until no further increase in M-wave amplitude and twitch torque was observed with a further 50 mA increase in stimulation intensity. The established stimulation intensity was kept the same throughout the testing session.
The central activation ratio (CAR) was used to give an index of maximal voluntary activation using doublets. The supramaximal doublet (pulse width of 200 μs at 100 Hz, 400 V) intensity was determined previously during the M-wave measurement. The doublet was applied upon the plateau phase of the MVC. CAR was calculated as follows (Behm et al. 2001; Bampouras et al. 2006):
Signals of torque, electrical stimuli application, and electromyographic (EMG) activity were displayed on the screen of a computer (Apple Macintosh, G4), interfaced with a data acquisition system (Acknowledge, Biopac Systems, Santa Barbara, CA, USA) used for analog-to-digital conversion.
Muscle cross sectional area
Anatomical cross sectional area (ACSA) of the m. quadriceps femoris was measured using a 0.2 tesla MRI scanner (E-Scan, Esaote Biomedica, Genova, Italy). Axial plane scans were acquired using a spin echo T1 half-Fourier sequence with the following scanning parameters: time of echo, 250 ms; time of repetition, 500 ms; field of view, 256 × 256; matrix, 256 × 192; 5 mm slice thickness; and 0.5 mm interslice gap. Oil filled capsules were secured to the skin using surgical tape as external markers to identify positions on the scan. Measurements were performed at the proximal 40% position of femur length, which was determined beforehand as the distance between the caudal part of the trochanter major and the distal boundary of the femoral condyles, using ultrasonography. Scans were performed with the participants lying in a supine position with the knee fully extended in a relaxed state. The investigators were trained in analysing the scan images until a coefficient of variation of less than 1% was obtained. Within-investigator coefficients of variation for the VL, rectus femoris (RF), vastus medialis (VM) and vastus intermedius (VI) muscles were 0.43, 0.35, 0.30 and 0.31%, respectively.
Ultrasonography
Patellar tendon mechanical properties (i.e. tendon elongation, tendon stiffness, stress and strain), and material properties (Young's modulus) were measured in vivo during ramp maximal voluntary contractions in which the participant was requested to build up to his maximal torque in 4–5 s and hold this level for about 3 s. Measurements were taken after the various MVC contractions so that the tendon was conditioned, to ensure reproducibility (Rigby, 1964; Wood et al. 1988). Measurements were performed at 80 deg of knee flexion, using real-time B-mode ultrasonography (ATL-HDI 3000, Bothell), with a 40 mm, 7.5 MHz linear-array probe. Furthermore, an echo-absorptive external marker was fixed on the skin, across the longitudinal axis of the patellar tendon, at about half-length between its two osteotendinous junctions. If any movement of the marker (with respect to the dermal surface and subcutaneous adipose tissue layer) was observed during the measurement, this trial would be removed from further analysis to avoid over- or underestimation of tendon elongation, depending on the movement direction of the marker. Due to limitations in the size of the ultrasound probe, we were not able to include both the apex of the patella and the tibia in the same image. Therefore, we could not account for any translational movement of the tibia during the contraction. Using a larger ultrasound probe, Hansen et al. (2006) showed that translation of the tibia during isometric contractions may contribute ∼45% of the total patella-tibia displacement, indicating that recording the displacement of the patellar bone only, as in our study, underestimates the true elongation of the tendon. Nonetheless, assuming that the relative translation of the tibia is similar between contractions of different intensity, the validity of our comparative results of tendon elongation between different time points in this experiment is not jeopardized.
VL muscle architecture was measured in vivo at rest. Scans were acquired at mid-belly, in the mid-sagittal plane. To ensure that all subsequent scanning measurements were taken in the same anatomical location, the ultrasound probe was positioned in the mid-sagittal plane, orthogonal to the mediolateral axis, and its positioning was marked on acetate paper using moles and small angiomas (which may be assumed to maintain a fixed position) as reference points. In each ultrasound image obtained at rest, the fascicular path was determined as the interspaces between echoes coming from the perimysial tissue surrounding the fascicle. Fascicle length (Lf) and pennation angle (θ) were measured using Matlab (Matlab, The MathWorks Inc., Natick, MA, USA). Where the fascicle extended beyond the image, the non-visible part was estimated by extrapolating the fascicle and aponeurosis in a proximal direction (Reeves et al. 2004). Pennation angle was calculated as the angle between the fascicle and the deep aponeurosis of the VL. In each scan, the average length and pennation angle of three fascicles was used for analysis.
Ultrasound scanning was synchronized with all signals captured on the acquisition system using a custom-built external voltage trigger, the action of which was visible on both ultrasound and acquisition systems. Ultrasound scanning was maintained above 25 Hz and was video recorded using SVHS (JVC HR-S6700) for further analysis. Images were digitized and acquired with a frame acquiring rate of 25 Hz using frame-capture software for further analysis (Adobe Premiere version 5.1, Adobe Systems).
Tendon dimensions
Resting tendon length was measured along the sagittal plane from the apex of the patella to the superior aspect of the tibial tuberosity. Tendon CSA (cm2) was subsequently assessed at 25, 50 and 75% of the resting length, along the axial plane. Three measurements were averaged at each site and the mean of the three sections was used for calculation of tendon stress. The intraclass correlation coefficients were 0.99 for patellar tendon CSA and patellar tendon length. Typical errors were ± 1.5 mm2 for tendon CSA and ± 0.6 mm for tendon length. In relative terms, this means that we would have detected any change greater than about 1% in either tendon CSA or slack length.
Estimation of tendon forces and stress
Patellar tendon moment arm length was measured for calculation of tendon forces, using a 0.2 tesla MRI scanner (E-Scan, Esaote Biomedica, Genova, Italy). Sagittal-plane scans were taken using a spin echo T1 half-Fourier sequence with the following scanning parameters: time of echo, 250 ms; time of repetition, 500 ms; field of view, 256 × 256; matrix, 256 × 192; 5 mm slice thickness; and 0.5 mm interslice gap. The patellar tendon moment arm length was defined as the perpendicular distance from the midpoint of the patellar tendon to the tibio-femoral contact point (Baltzopoulos, 1995; Kellis & Baltzopoulos, 1999; Tsaopoulos et al. 2006), which was determined as the mid-point on the line connecting the lateral and medial tibio-femoral contact points. Due to constraints in the size of the coil, it was only possible to image the knee joint in full extension. The measured moment arm length was multiplied by the ratio of the patella moment arm length between full extension and the particular joint angle previously reported (Baltzopoulos, 1995) to estimate the moment arm length for each participant (Reeves et al. 2003). True knee extensor torque, being the sum of the measured net knee extension torque and the estimated antagonistic knee flexor torque, was divided by the estimated patellar tendon moment arm length at 80 deg to yield patellar tendon force (Reeves et al. 2003). Antagonistic knee flexor torque was determined assuming a linear relationship between BF RMS and knee flexor torque (Reeves et al. 2003). Patellar tendon stress (MPa) was calculated by dividing tendon force by tendon CSA.
Estimation of tendon elongation and strain
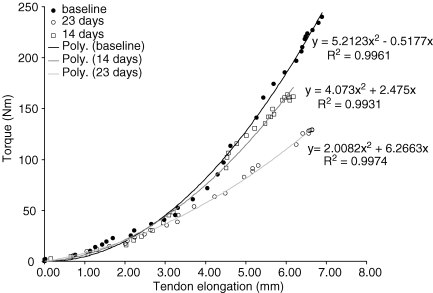
Tendon elongation was measured along the sagittal plane, by measuring the distance between its proximal insertion into the apex of the patella and an eco-absorptive external marker fixed on the skin with surgical tape. At least 25–30 frames were analysed per contraction using digitizing software (NIH Image version 1.61, National Institutes of Health, Bethesda, MD, USA), and were synchronized with the corresponding torque values. The average of three measurements for each frame was calculated. The torque–elongation relationship was fitted with a second-degree polynomial equation, which was used to determine the elongation at 10% intervals of the maximal isometric torque. Figure 1 shows typical torque–elongation relationships at the different time points. Tendon strain was calculated as the ratio (%) of tendon displacement to the initial resting tendon length.
Figure 1.
Typical knee extensor torque–patellar tendon elongation relationship for one individual at baseline (•), 14 days (□) and 23 days (○) of suspension
Estimation of tendon stiffness and Young's modulus
To calculate tendon stiffness, first, the highest 30% interval of maximal tendon force, of the weakest contraction from the weakest participant over all testing sessions was identified. The rationale here was that no extrapolation would be needed since this absolute tendon force interval would be present in all participants. This approach furthermore ensured that in the successive measurements the comparative stiffness values corresponded to the same absolute force intervals. Tendon stiffness (N mm−1) was then calculated as the gradient of the corresponding force–elongation interval in all participants. Young's modulus (GPa) over this interval was calculated by multiplying the stiffness value by the ratio of tendon resting length over tendon CSA.
Statistics
Student's t test for independent samples was applied to compare baseline values between the suspended and control groups. Data were normally distributed as indicated by the Shapiro–Wilks test. The number of participants (n) to be involved in this investigation has been estimated on the basis of a power analysis. It was calculated that the minimum sample size (n) required to detect a difference equal to the standard deviation and with a probability equal to 0.05 was eight participants for the structural (fascicle length and muscle cross-sectional area by ultrasound and MRI, respectively), functional (isometric contraction force, muscle power, tendon elongation) and neuromuscular (muscle activation capacity) measurements involved in the investigation. To determine the effect of the suspension period on the investigated variables, a one-way ANOVA for repeated measures was conducted with a Bonferroni post hoc adjustment for further analysis. Pre and post values for the controls were compared using paired samples t tests. Statistical significance was set as α= 0.05. Unless stated otherwise, results are means ± standard deviation.
Results
At baseline the comparison between the suspended group and the control group did not reveal any differences in any of the tested variables between the two groups.
Muscle strength and activation
MVC torque (Fig. 2, Table 1) decreased by 14.8% after 14 days (P < 0.001, with a rate of 1.06% day−1), and by 21.0% after 23 days (P < 0.001, with a rate of 0.91% day−1). A significant difference was also found between the torque values at 14 and 23 days (P < 0.05, Table 1). Muscle activation based on CAR did not change over the course of the suspension period (N.S., Fig. 2, Table 1). Maximal M-wave peak-to-peak amplitude did not differ at any of the time points. In line with this, the ratio of EMG RMS normalized to maximal M-wave did not change during the intervention (N.S., Table 1). No changes in the control group were found between pre and post MVC torque values or in activation (Table 1).
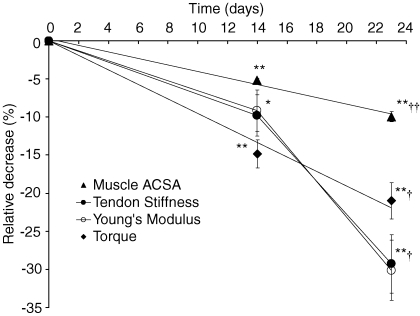
Figure 2.
Relative decrease (%) at 14 and 23 days of suspension for m. quadriceps femoris ACSA (s), patellar tendon stiffness (•), patellar tendon Young's modulus (○) and torque (♦) Data are displayed as means ±s.e.m. *Significantly different from baseline (P < 0.05); **significantly different from baseline (P < 0.01); †significantly different from 14 days (P < 0.05); ††significantly different from 14 days (P < 0.01). Lines connecting the tendon stiffness and Young's modulus data points are added for clarity.
Table 1.
Knee extensor torque and activation
| Suspended | Control | ||||
|---|---|---|---|---|---|
| Baseline | 14 days | 23 days | Pre | Post | |
| MVC torque (N m) | 289.4 ± 54.0 | 248.5 ± 58.58** | 230.6 ± 57.4**† | 326.7 ± 98.2 | 326.5 ± 97.3 |
| CAR (%) | 98.4 ± 0.7 | 97.8 ± 1.3 | 97.3 ± 1.7 | 98.6 ± 1.3 | 98.4 ± 1.0 |
| RMS/M-wave | 0.067 ± 0.027 | 0.064 ± 0.030 | 0.063 ± 0.023 | 0.053 ± 0.015 | 0.053 ± 0.016 |
| EMD (ms) | 32.8 ± 5.6 | 35.4 ± 6.9 | 38.3 ± 7.4* | 34.3 ± 8.3 | 32.4 ± 7.5 |
| RTD (Nm s−1) | 1206.1 ± 464.3 | 697.8 ± 266.5** | 749.6 ± 365.6** | 1429.0 ± 360.5 | 1361.9 ± 319.5 |
Significantly different from baseline, P < 0.05
significantly different from baseline, P < 0.01
significantly different from 14 days, P < 0.05.
Muscle cross sectional area
Suspension of the lower limb resulted in significant reductions in muscle anatomical CSA (ACSA, Table 2). The whole quadriceps muscle ACSA (determined as the sum of the individual muscles' ACSAs) decreased by 5.2% (P < 0.001, Fig. 2) after 14 days (0.37 ± 0.05% day−1), and by 10.0% (P < 0.001, Fig. 2) after 23 days (0.51 ± 0.21% day−1). Atrophy of the individual muscles of the quadriceps followed a similar pattern (Table 2). No changes in muscle ACSA were found in the control group, either for the whole quadriceps or for any of the individual muscles (Table 2).
Table 2.
Quadriceps muscle CSA
| Suspended | Control | ||||
|---|---|---|---|---|---|
| Baseline | 14 days | 23 days | Pre | Post | |
| Quadriceps (cm2) | 56.27 ± 9.48 | 53.35 ± 8.86** | 50.65 ± 7.79**†† | 66.89 ± 13.21 | 66.47 ± 13.40 |
| VM (cm2) | 20.39 ± 3.61 | 19.39 ± 3.29** | 18.53 ± 2.93**†† | 25.48 ± 3.58 | 25.43 ± 3.61 |
| RF (cm2) | 2.75 ± 1.04 | 2.64 ± 0.98* | 2.45 ± 0.96**†† | 2.83 ± 1.48 | 2.86 ± 1.45 |
| VL (cm2) | 16.50 ± 3.63 | 15.94 ± 3.48** | 14.88 ± 3.11**†† | 19.31 ± 5.57 | 19.04 ± 5.71 |
| VI (cm2) | 16.63 ± 2.70 | 15.37 ± 2.47** | 14.80 ± 2.30*** | 19.27 ± 5.57 | 19.14 ± 5.53 |
Significantly different from baseline, P < 0.05;
significantly different from baseline, P < 0.01;
significantly different from 14 days, P < 0.05
significantly different from 14 days, P < 0.01.
Muscle architecture
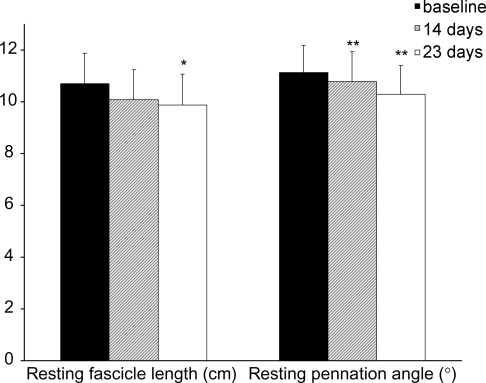
Resting fascicle length did not decrease significantly, but was reduced by 5.9% at day 14, and by 7.7% (P < 0.05) at day 23 (Fig. 3). Pennation angle at rest decreased by 3.2% (P < 0.005) at day 14 and by 7.6% (P < 0.01) at day 23 (Fig. 3). No differences in resting muscle fascicle length or pennation angle were observed in the control group.
Figure 3.
VL muscle fascicle length and pennation angle at baseline (filled bars), and 14 days (dashed bars) and 23 days (open bars) of suspension *Significantly different from baseline (P < 0.05); **significantly different from baseline (P < 0.01).
Electromechanical delay and rate of torque development
EMD was not significantly different between baseline and 14 days, but increased by 16.7% after 23 days (P < 0.05, Table 1). No differences in EMD were found in the controls (Table 1).
RTD decreased by 42.1% (P < 0.01) at 14 days and by 37.9% (P < 0.01) at 23 days (Table 1). No differences were found in the control group for either EMD or RTD (Table 1).
Tendon dimensions and properties
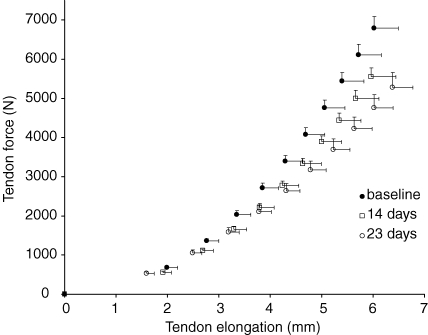
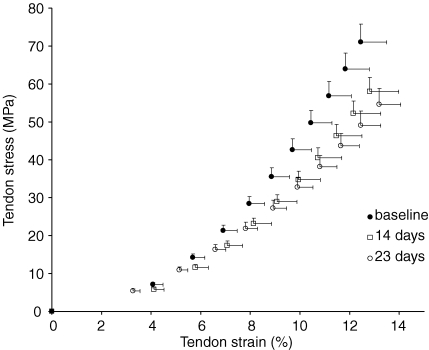
Both tendon CSA (100.7 ± 12.4 mm2versus 101.1 ± 11.6 mm2, N.S.) and resting length (48.6 ± 3.0 mm versus 48.5 ± 2.8 mm, N.S.) did not differ between baseline and post measurements (Table 3). Tendon elongation at the highest tendon force of the weakest contraction of the weakest subject increased significantly after both 14 (9.1%, P < 0.005) and 23 (16.9%, P < 0.05) days of suspension (Table 3). Since resting tendon length did not change throughout the suspension period, a similar pattern was observed for tendon strain at the corresponding tendon force (Table 3). Maximum tendon force decreased significantly (main effect P < 0.001) after suspension. A decrease in maximal tendon force of 18.3% (P < 0.001) was found after 14 days and decreased further by 22.3% (P < 0.001) after 23 days (Table 3). The average tendon force–elongation relationships at the three time points are shown in Fig. 4. Tendon stiffness decreased by 9.8% (P < 0.05) after 14 days and decreased further by 29.3% (P < 0.005) after 23 days (Fig. 2, Table 3). Following the trend shown by decreases in tendon stiffness, the tendon's Young's modulus was reduced by 9.2% (P < 0.05) after 14 days and by 30.1% (P < 0.01) after 23 days (Fig. 2, Table 3). The relative rate of decline in Young's modulus during the last 9 days of suspension (1.31%/day) was significantly higher (P < 0.05) than during the initial 14 days (0.65%/day). Furthermore, the relative rate of decline in Young's modulus during the last 9 days was greater than the rate of decline in muscle ACSA (P < 0.05), indicating that the impact of tendon deterioration on muscle function was greater during this period of suspension. Maximal tendon stress also decreased significantly, by 18.5% (P < 0.001) after 14 days and by 23.3% (P < 0.005) after 23 days (Table 3), which is similar to the changes observed in tendon force since tendon CSA did not change. The average tendon stress–strain relationships at the different time points are shown in Fig. 5. No changes were found in the control group for any of the variables mentioned above (Table 3).
Table 3.
Patellar tendon properties
| Suspended | Control | ||||
|---|---|---|---|---|---|
| Baseline | 14 days | 23 days | Pre | Post | |
| Max. tendon force (N) | 6796.9 ± 873.1 | 5554.5 ± 682.5** | 5283.5 ± 1136.8** | 7289.7 ± 1732.2 | 7062.6 ± 1986.5 |
| Resting length (mm) | 48.6 ± 3.0 | — | 48.5 ± 2.8 | 50.0 ± 5.2 | 49.9 ± 5.1 |
| CSA (mm2) | 100.7 ± 12.4 | — | 101.1 ± 11.6 | 125.5 ± 17.3 | 125.3 ± 17.3 |
| Elongation (mm) | 4.3 ± 1.1 | 4.7 ± 1.1** | 5.0 ± 1.0* | 3.5 ± 0.4 | 3.4 ± 0.4 |
| Stiffness (N mm−1) | 1605.3 ± 512.5 | 1447.6 ± 492.3* | 1135.4 ± 284.7**† | 1898.5 ± 292.2 | 1924.5 ± 355.8 |
| Young's modulus (GPa) | 0.83 ± 0.32 | 0.75 ± 0.32* | 0.58 ± 0.18**† | 0.77 ± 0.19 | 0.78 ± 0.21 |
| Stress (MPa) | 71.1 ± 14.1 | 58.0 ± 11.0** | 54.5 ± 12.7** | 59.4 ± 17.6 | 57.8 ± 18.7 |
| Strain (%) | 8.9 ± 2.3 | 9.7 ± 2.5 | 10.4 ± 2.1 | 7.1 ± 1.3 | 7.0 ± 1.2 |
Significantly different from baseline, P < 0.05
significantly different from baseline, P < 0.01
significantly different from 14 days, P < 0.05.
Figure 4.
Patellar tendon force–elongation relationships at baseline (•), and after 14 days (□) and 23 days (○) of suspension Data are displayed as mean and s.e.m.
Figure 5.
Patellar tendon stress–strain relationships at baseline (•) and after 14 days (□) and 23 days (○) of suspension Data are displayed as means and s.e.m.
Discussion
The present study was designed to investigate the time course of muscular and tendinous adaptations to 23 days unilateral lower limb suspension (ULLS) in healthy young men. For the first time, to our knowledge, we present simultaneous data on the time course of deterioration of the main determinants of skeletal muscle mechanical output with prolonged unloading. The data show significant losses in knee extensor torque, muscle CSA and patellar tendon stiffness, after just 14 days of ULLS. Voluntary muscle activation did not change throughout the entire ULLS period, suggesting that the reduced muscle function was mainly due to muscle atrophy, alterations in muscle architecture, tendon deconditioning, and possibly to decreased muscle fibre specific tension.
Effect of unloading on muscle strength
In the present study, knee extensor torque decreased by 14.8% after 14 days, and by 21.0% after 23 days. The average rate of strength loss was 1.06% day−1 over the first 14 days and 0.68% day−1 over the following 9 days. Although the rate of strength loss was not significantly greater during the first 2 weeks of unloading, these data suggest that the most crucial period for the loss of muscle strength is in the early phases of the disuse period and that the rate of strength loss already begins to slow down after this period. The reductions in muscle strength with unloading can be mainly attributed to adaptations in the key variables investigated in this study. The first and most apparent contributing factor is a decrease in muscle size (LeBlanc et al. 1995; Akima et al. 2000; LeBlanc et al. 2000; Alkner & Tesch, 2004; Tesch et al. 2004, 2005). Also, a decrease in muscle fascicle length and pennation angle as observed in this study may have caused a shift in the torque–angle relationship which could have contributed to the decreased torque. Secondly, since decreases in muscle strength after unloading cannot be exclusively ascribed to a reduction in muscle size (Berg et al. 1991; Dudley et al. 1992; Adams et al. 1994; Ploutz-Snyder et al. 1995; Schulze et al. 2002; Tesch et al. 2004), a reduction in muscle activation capacity, often shown to be compromised by chronic disuse (Dudley et al. 1992; Duchateau, 1995; Koryak, 1995; Berg et al. 1997; Koryak, 2001; Ruegg et al. 2003) is also a potential factor. Nevertheless, we did not find any change in muscle activation as indicated by the CAR results. This contradicts results from other ULLS studies (Dudley et al. 1992; Schulze et al. 2002) in which a reduced muscle activation, based on maximal integrated EMG values, was found. However, measurements of EMG root mean square normalized to maximal M-wave amplitude we performed in this study confirmed the results we obtained with the CAR method. The apparent inconsistency might arise from the different method of EMG analysis, as previous investigators did not normalize EMG activity to the maximal M-wave. Such a procedure is important since changes in absolute values of EMG have little meaning unless normalized to maximum EMG activity ascertained by electrical stimulation (Crone et al. 1999). Also, the observation that EMG activity did not change is probably due to the fact that ULLS, contrary to immobilization by leg casting or, to some extent, bed rest, tends to preserve afferent activity, which is known to be fundamental for maintaining activation capacity (Gandevia et al. 1990).
The third factor potentially contributing to the disproportionate loss of muscle strength compared to muscle size is a reduction in single fibre specific tension. Although not assessed in the present study, this has been found after bed rest by several authors (Larsson et al. 1996; Widrick et al. 1999; Trappe et al. 2004) and is thought to be due to a decrease in the number of active cross-bridges, as suggested by a decrease in myofibrillar density, rather than in the force per cross-bridge (Widrick et al. 1999; D'Antona et al. 2003). A decrease in thin filament density observed in hindlimb suspended rats (Riley et al. 2005) and also in astronauts after spaceflight (Widrick et al. 1999) has also been proposed as a cause of this phenomenon. Indeed, Riley et al. (2005) found a significant reduction in thin filament density (−25%) associated with a 17% decrease in fibre specific tension in the soleus muscle of rats suspended for 14 days. Last but not least are changes in tendon tensile properties since tendons influence the length at which muscle fibres operate (Narici & Maganaris, 2006) and thus, ultimately, influence force production. The role of muscle ACSA and tendon tensile properties and their time courses are described in more detail in the following paragraphs.
Effect of unloading on muscle anatomical cross sectional area and muscle architecture
In the present study, quadriceps muscle ACSA decreased by 5.2% and 10.0% after 14 days and 23 days, respectively. The rate of atrophy was ∼0.4% day−1 over the first 14 days, and about 0.5% over the following nine days, with no significant difference between the two periods implying that atrophy is almost a linear phenomenon during the first 23 days of limb suspension. The magnitude of this decrease is consonant with reported reductions in human quadriceps muscle size after lower limb unloading. For example, a decline in knee extensor muscle CSA of 7% after 21 days (Schulze et al. 2002), and of 16% after 35 days (Dudley et al. 1992; Hather et al. 1992) of lower limb suspension have been previously reported. Similar rates of muscle atrophy as in ULLS are observed during bed rest (Berry et al. 1993; Kubo et al. 2000; Akima et al. 2001; Alkner & Tesch, 2004).
Suspension also resulted in decreases in fascicle length and pennation angle, in line with the reduction in muscle ACSA. Changes in muscle architecture have been reported earlier after limb immobilization (Narici & Ceretelli, 1998) and bed rest (Kawakami et al. 2001; Reeves et al. 2002). Our findings show changes in muscle architecture after only 14 days of unloading suggesting fast remodelling of sarcomeres in series and in parallel. Although the exact mechanisms regulating this process have yet to be confirmed, they are likely to involve costameric proteins sensitive to mechanotransduction, among which focal adhesion kinase has been pinpointed as a major candidate (Fluck et al. 2002). The functional implications of these changes will be discussed together with tendon adaptations below.
The mechanisms of this fall in human muscle CSA over periods of less than 28 days are not known although it seems likely that in periods between 28 days and 49 days the major driver is a fall in muscle protein synthesis (Gibson et al. 1987; Ferrando et al. 2002), without any increase in muscle protein breakdown. Resolution of the question of whether there is an early (within 1 week) increase of bulk muscle protein breakdown and its time course must await direct measurement of this arm of protein turnover in early disuse. In a separate paper we will address some of these questions using data on the time course of muscle and tendon protein synthesis and associated gene and cell signalling changes.
Disuse leads not only to a decrease in contractile material in the muscle, but also to an increase in intramuscular connective tissue as shown in immobilized rat muscles (Jozsa et al. 1990; Jarvinen et al. 2002), which is also the case in elderly human muscle as compared to that in young individuals (Kent-Braun et al. 2000). A fall in muscle fibre size with no change in connective tissue would increase the relative contribution of perifibrillar connective tissue. Furthermore, it has been shown that there is an increased intramuscular fat content in hemiparetic muscle atrophy in stroke patients (Ryan et al. 2002). Taken together, these data imply that the actual loss of contractile material within the muscle may be even greater than reported in the present study, and may have contributed to the disproportionate loss of muscle strength compared to that of muscle size. The relative contribution of these factors in our studies is unknown and must be a subject for further study, but any contribution will likely be small given the time frame of the present study.
Effect of unloading on patellar tendon properties
Alongside the changes in muscle size, the patellar tendon underwent significant alterations. Tendon elongation increased by 9.1% and 16.9% after 14 and 23 days of ULLS as a result of a decrease in tendon stiffness (9.8% and 29.3%, respectively, after 14 and 23 days of ULLS). Hence, we show that in addition to muscle atrophy, ULLS also induces marked changes in the mechanical behaviour of the patellar tendon in humans, as seen with bed rest (Kubo et al. 2000). The present results indicate a rapid deterioration of tendon tissue with unloading which could affect the mechanical behaviour of the muscle–tendon unit as a whole. Our observations are in line with those obtained after 20 days of bed rest in which reductions in tensile stiffness of the VL deep aponeurosis of 32% (Kubo et al. 2000) and 28% (Kubo et al. 2004) were found. In the present study, tendon stiffness decreased with an average rate of 0.65% day−1 during the initial 14 days, accelerating to 1.98% day−1 during the next 9 days. These data are evidence of a variable time course in tendon adaptations to unloading. Data from a recent study (Reeves et al. 2005) showed a reduction in Achilles tendon stiffness and Young's modulus of 58% and 57%, respectively, after 90 days of bed rest. Seen over a greater time window, these data, combined with data from spinal cord injury patients (Maganaris et al. 2006), suggest that the rate of tendon deterioration has a differential time course. Within the first 23 days of unloading, it seems that tendon deteriorates at a slower pace initially after which it accelerates, before proceeding at a slower pace again and becoming asymptotic after an extended time duration.
Tendon dimensions did not change with unloading, which is in agreement with previous studies (Kubo et al. 2000, 2004; Reeves et al. 2005). However, we cannot exclude the possibility that a reduction in the area occupied by the tendon's collagen fibres occurred, but went undetected because of masking by increases in the area of extracellular matrix (Tsuchida et al. 1997; Kotani et al. 1998). The lack of changes in tendon dimensions explains why the changes in Young's modulus (i.e. stiffness normalized for tendon dimensions) mirror those in tendon stiffness, which implies alterations in the material properties of the tendon.
The alterations in tendon material properties (including a decrease in tendon thermal shrinkage following disuse; Peacock, 1963) may be influenced by alterations in the structure and packing of the collagen fibres (Danielsen & Andreassen, 1988), as a result of differences either in the cross-link pattern of the collagen (possibly due to reduced acyivities of lysyl-oxidase (Prockop & Kivirikko, 1995) or galactosylhydroxylysyl glucosyltransferase (Savolainen et al. 1988).
The decrease in tendon stiffness resulted in an increase in tendon deformation at a given level of force exerted by the muscle. For any given muscle–tendon length during contraction a greater stretch of the tendon would imply that the muscle fibres shorten more at a given force level and place the fibres in a non-optimal region of the length–tension relationship. Previous in vivo experimental measurements have shown that at the joint angle of 80 deg tested in the present study the m. vastus lateralis operates close to the highest part of the ascending limb and the plateau region of the length–tension relationship (Ichinose et al. 1997; Reeves et al. 2004). Therefore, an increased shortening of the contractile muscle fibres through increased tendon deformation would cause the sarcomeres to operate at shorter lengths, and further away from their optimum. However, the observed reduction in fascicle length should act to oppose this shift: shorter fascicles means fewer sarcomeres in series, implying that, at any given muscle–tendon length, the remaining sarcomeres would be working at a longer length than before suspension. Because of this opposing effect, the above two alterations may, to some extent, mitigate each other. The observed decrease in pennation angle suggests a loss of sarcomeres in parallel, which is reflected in the reduced muscle ACSA. The possible reduction in muscle fibre specific tension discussed above should further contribute to the loss in contractile force-generating capacity. Furthermore, decreased tendon stiffness results in larger strain values at a given force, which may increase the possibility of tendon strain injuries, as suggested by Reeves et al. (2003).
The changes in patellar tendon stiffness were expected to affect the speed of contractile force transmission to the skeleton. In the present study, two variables relating to the speed of force transmission from muscle to bone were assessed: the RTD and EMD. The RTD is dependent on both stiffness of the tendon and on the force–velocity characteristics of the contractile component, whereas EMD is dependent on propagation of the action potential over the muscle fibre membrane and excitation–contraction coupling of the contractile component, and is also influenced by the properties of the series elastic component (Wilkie, 1949). After 23 days, the EMD increased (+17%) alongside the fall in RTD (−38%), meaning that the time over which excitation–contraction coupling of the contractile component and stretching of the series elastic component occur was longer. It is noteworthy that RTD fell by 42% after the initial 14 days of unloading, and did not change thereafter. Both decreases in RTD (Duchateau, 1995; Bamman et al. 1998; Kubo et al. 2000) and an increase in EMD (Kubo et al. 2000) have been reported after bed rest. In agreement with our findings, Bamman et al. (1998) observed a 54% reduction in RTD after 14 days of bed rest. Furthermore Kubo et al. (2000) found RTD to be decreased by 47% after 20 days of bed rest. Comparing these results to our data, it seems that RTD appears to be affected by an initial drop in tendon stiffness beyond a certain threshold, showing little change thereafter. EMD has been found to increase by 21% after 20 days of bed rest (Kubo et al. 2000), which is similar to our observation. Unlike RTD, EMD only increased significantly after a much greater fall in tendon stiffness. Data from Muraoka et al. (2004) show that the EMD is not affected when tendon slack is taken up by stretching the myotendinous complex. In the present study, because of large flexion joint angle (80 deg) used for testing RTD and EMD, most of the tendon slack would have been taken up. Therefore, any changes in excitation–contraction coupling could have been initially masked by the amount of stretch of the tendon. Only after the large drop post-suspension in tendon stiffness would the increase in EMD become apparent. Unloading is known to increase human single muscle fibre maximum shortening velocity (Larsson et al. 1996; Widrick et al. 1997; 1999; Yamashita-Goto et al. 2001; Trappe et al. 2004). This suggests that the decrease in EMD and RTD observed in the present study can be mainly attributed to a decrease in patellar tendon stiffness, the effect of which exceeded that of any possible increase in maximum shortening velocity.
Concluding remarks
The present findings show rapid deterioration of muscle structural and functional properties and of tendon mechanical characteristics with 23 days of ULLS, while maximal voluntary activation remained unaltered. Furthermore, ULLS seems to affect tendon tensile properties at a faster rate than that observed for muscle atrophy during the second period of suspension. After 14 days of suspension the divergence between the two time courses increases further, suggesting that the impact of tendon mechanical behaviour on muscle strength increases beyond that observed during the first 2 weeks of suspension. These findings have important clinical implications for musculoskeletal recovery after unloading on Earth and in space. They strongly suggest that tendon, in addition to muscle, should be given more attention during rehabilitation programmes, which should preferably commence within the first 2 weeks of the disuse period.
Acknowledgments
The authors are particularly grateful to the participants whom volunteered to the present study, for their commitment and endurance. Furthermore, the authors would like to thank Dr Joern Rittweger for the medical screening and monitoring of the volunteers. Funding by the European Space Agency (ESA MESM grant no. 15097/01/NL/SH, Prof M. Narici) and by BBSRC (Prof M.J. Rennie) is acknowledged.
References
- Adams GR, Hather BM, Dudley GA. Effect of short-term unweighting on human skeletal muscle strength and size. Aviat Space Environ Med. 1994;65:1116–1121. [PubMed] [Google Scholar]
- Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand. 2001;172:269–278. doi: 10.1046/j.1365-201x.2001.00869.x. [DOI] [PubMed] [Google Scholar]
- Akima H, Kubo K, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Leg-press resistance training during 20 days of 6 degrees head-down-tilt bed rest prevents muscle deconditioning. Eur J Appl Physiol. 2000;82:30–38. doi: 10.1007/s004210050648. [DOI] [PubMed] [Google Scholar]
- Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol. 2004;93:294–305. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- Almeida-Silveira MI, Lambertz D, Perot C, Goubel F. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur J Appl Physiol. 2000;81:252–257. doi: 10.1007/s004210050039. [DOI] [PubMed] [Google Scholar]
- Antonutto G, Capelli C, Girardis M, Zamparo P, di Prampero PE. Effects of microgravity on maximal power of lower limbs during very short efforts in humans. J Appl Physiol. 1999;86:85–92. doi: 10.1152/jappl.1999.86.1.85. [DOI] [PubMed] [Google Scholar]
- Baltzopoulos V. A videofluoroscopy method for optical distortion correction and measurement of knee-joint kinematics. Clin Biomech (Bristol, Avon) 1995;10:85–92. doi: 10.1016/0268-0033(95)92044-m. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol. 1998;84:157–163. doi: 10.1152/jappl.1998.84.1.157. [DOI] [PubMed] [Google Scholar]
- Bampouras TM, Reeves ND, Baltzopoulos V, Maganaris CN. Muscle activation assessment: effects of method, stimulus number, and joint angle. Muscle Nerve. 2006;34:740–746. doi: 10.1002/mus.20610. [DOI] [PubMed] [Google Scholar]
- Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24:925–934. doi: 10.1002/mus.1090. [DOI] [PubMed] [Google Scholar]
- Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991;70:1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol. 1997;82:182–188. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- Berry P, Berry I, Manelfe C. Magnetic resonance imaging evaluation of lower limb muscles during bed rest – a microgravity simulation model. Aviat Space Environ Med. 1993;64:212–218. [PubMed] [Google Scholar]
- Bleeker MW, Hopman MT, Rongen GA, Smits P. Unilateral lower limb suspension can cause deep venous thrombosis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1176–R1177. doi: 10.1152/ajpregu.00718.2003. [DOI] [PubMed] [Google Scholar]
- Booth FW, Gould EW. Effects of training and disuse on connective tissue. Exerc Sport Sci Rev. 1975;3:83–112. [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Hultborn H, Orsnes GB. Amplitude of the maximum motor response (Mmax) in human muscles typically decreases during the course of an experiment. Exp Brain Res. 1999;124:265–270. doi: 10.1007/s002210050621. [DOI] [PubMed] [Google Scholar]
- D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen CC, Andreassen TT. Mechanical properties of rat tail tendon in relation to proximal-distal sampling position and age. J Biomech. 1988;21:207–212. doi: 10.1016/0021-9290(88)90171-6. [DOI] [PubMed] [Google Scholar]
- di Prampero PE, Narici MV. Muscles in microgravity: from fibres to human motion. J Biomech. 2003;36:403–412. doi: 10.1016/s0021-9290(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Duchateau J. Bed rest induces neural and contractile adaptations in triceps surae. Med Sci Sports Exerc. 1995;27:1581–1589. [PubMed] [Google Scholar]
- Dudley GA, Duvoisin MR, Adams GR, Meyer RA, Belew AH, Buchanan P. Adaptations to unilateral lower limb suspension in humans. Aviat Space Environ Med. 1992;63:678–683. [PubMed] [Google Scholar]
- Ferrando AA, Paddon-Jones D, Wolfe RR. Alterations in protein metabolism during space flight and inactivity. Nutrition. 2002;18:837–841. doi: 10.1016/s0899-9007(02)00930-9. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Stuart CA, Brunder DG, Hillman GR. Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviat Space Environ Med. 1995;66:976–981. [PubMed] [Google Scholar]
- Fitts RH, Romatowski JG, Peters JR, Paddon-Jones D, Wolfe RR, Ferrando AA. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am J Physiol Cell Physiol. 2007;293:C313–320. doi: 10.1152/ajpcell.00573.2006. [DOI] [PubMed] [Google Scholar]
- Fluck M, Ziemiecki A, Billeter R, Muntener M. Fibre-type specific concentration of focal adhesion kinase at the sarcolemma: influence of fibre innervation and regeneration. J Exp Biol. 2002;205:2337–2348. doi: 10.1242/jeb.205.16.2337. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990;113:1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72:503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon) 2006;21:54–58. doi: 10.1016/j.clinbiomech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Hather BM, Adams GR, Tesch PA, Dudley GA. Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol. 1992;72:1493–1498. doi: 10.1152/jappl.1992.72.4.1493. [DOI] [PubMed] [Google Scholar]
- Ichinose Y, Kawakami Y, Ito M, Fukunaga T. Estimation of active force-length characteristics of human vastus lateralis muscle. Acta Anat (Basel) 1997;159:78–83. doi: 10.1159/000147969. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Jarvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil. 2002;23:245–254. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Thoring J, Reffy A, Jarvinen M, Kvist M. The effect of tenotomy and immobilisation on intramuscular connective tissue. A morphometric and microscopic study in rat calf muscles. J Bone Joint Surg Br. 1990;72:293–297. doi: 10.1302/0301-620X.72B2.2312572. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84:7–12. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- Kellis E, Baltzopoulos V. In vivo determination of the patella tendon and hamstrings moment arms in adult males using videofluoroscopy during submaximal knee extension and flexion. Clin Biomech (Bristol, Avon) 1999;14:118–124. doi: 10.1016/s0268-0033(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol. 2000;88:662–668. doi: 10.1152/jappl.2000.88.2.662. [DOI] [PubMed] [Google Scholar]
- Koryak Y. Contractile properties of the human triceps surae muscle during simulated weightlessness. Eur J Appl Physiol Occup Physiol. 1995;70:344–350. doi: 10.1007/BF00865032. [DOI] [PubMed] [Google Scholar]
- Koryak YU. Electrically evoked and voluntary properties of the human triceps surae muscle: effects of long-term spaceflights. Acta Physiol Pharmacol Bulg. 2001;26:21–27. [PubMed] [Google Scholar]
- Kotani Y, Cunningham BW, Cappuccino A, Kaneda K, McAfee PC. The effects of spinal fixation and destabilization on the biomechanical and histologic properties of spinal ligaments. An in vivo study. Spine. 1998;23:672–682. doi: 10.1097/00007632-199803150-00006. discussion 682–673. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur J Appl Physiol. 2000;83:463–468. doi: 10.1007/s004210000309. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T. Effects of resistance training during bed rest on the viscoelastic properties of tendon structures in the lower limb. Scand J Med Sci Sports. 2004;14:296–302. doi: 10.1046/j.1600-0838.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch. 1996;432:320–328. doi: 10.1007/s004240050139. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol. 2000;89:2158–2164. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviat Space Environ Med. 1995;66:1151–1154. [PubMed] [Google Scholar]
- LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol. 1992;73:2172–2178. doi: 10.1152/jappl.1992.73.5.2172. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33:85–92. doi: 10.1002/mus.20441. [DOI] [PubMed] [Google Scholar]
- Matsumoto F, Trudel G, Uhthoff HK, Backman DS. Mechanical effects of immobilization on the Achilles' tendon. Arch Phys Med Rehabil. 2003;84:662–667. doi: 10.1016/s0003-9993(02)04834-7. [DOI] [PubMed] [Google Scholar]
- Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H. Influence of tendon slack on electromechanical delay in the human medial gastrocnemius in vivo. J Appl Physiol. 2004;96:540–544. doi: 10.1152/japplphysiol.01015.2002. [DOI] [PubMed] [Google Scholar]
- Narici M, Cerretelli P. Changes in human muscle architecture in disuseatrophy evaluated by ultrasound imaging. J Gravit Physiol. 1998;5:73–74. [PubMed] [Google Scholar]
- Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208:433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock EE., Jr Comparison of collagenous tissue surrounding normal and immobilized joints. Surg Forum. 1963;14:440–441. [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Tesch PA, Crittenden DJ, Dudley GA. Effect of unweighting on skeletal muscle use during exercise. J Appl Physiol. 1995;79:168–175. doi: 10.1152/jappl.1995.79.1.168. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Reeves NJ, Maganaris CN, Ferretti G, Narici MV. Influence of simulated microgravity on human skeletal muscle architecture and function. J Gravit Physiol. 2002;9:P153–P154. [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol. 2005;98:2278–2286. doi: 10.1152/japplphysiol.01266.2004. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. Muscle Nerve. 2003;28:74–81. doi: 10.1002/mus.10392. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol. 2004;89:675–689. doi: 10.1113/expphysiol.2004.027797. [DOI] [PubMed] [Google Scholar]
- Rigby BJ. Effect of cyclic extension on the physical properties of tendon collagen and its possible relation to biological ageing of collagen. Nature. 1964;202:1072–1074. doi: 10.1038/2021072a0. [DOI] [PubMed] [Google Scholar]
- Riley DA, Bain JL, Romatowski JG, Fitts RH. Skeletal muscle fiber atrophy: altered thin filament density changes slow fiber force and shortening velocity. Am J Physiol Cell Physiol. 2005;288:C360–C365. doi: 10.1152/ajpcell.00386.2004. [DOI] [PubMed] [Google Scholar]
- Ruegg DG, Kakebeeke TH, Gabriel JP, Bennefeld M. Conduction velocity of nerve and muscle fiber action potentials after a space mission or a bed rest. Clin Neurophysiol. 2003;114:86–93. doi: 10.1016/s1388-2457(02)00329-2. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- Savolainen J, Myllyla V, Myllyla R, Vihko V, Vaananen K, Takala TE. Effects of denervation and immobilization on collagen synthesis in rat skeletal muscle and tendon. Am J Physiol Regul Integr Comp Physiol. 1988;254:R897–R902. doi: 10.1152/ajpregu.1988.254.6.R897. [DOI] [PubMed] [Google Scholar]
- Schulze K, Gallagher P, Trappe S. Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc. 2002;34:303–313. doi: 10.1097/00005768-200202000-00019. [DOI] [PubMed] [Google Scholar]
- Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–129. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol. 2005;93:463–468. doi: 10.1007/s00421-004-1236-9. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol. 2004;96:1451–1458. doi: 10.1152/japplphysiol.01051.2003. [DOI] [PubMed] [Google Scholar]
- Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol. 2004;557:501–513. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaopoulos DE, Baltzopoulos V, Maganaris CN. Human patellar tendon moment arm length: measurement considerations and clinical implications for joint loading assessment. Clin Biomech (Bristol, Avon) 2006;21:657–667. doi: 10.1016/j.clinbiomech.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Yasuda K, Kaneda K, Hayashi K, Yamamoto N, Miyakawa K, Tanaka K. Effects of in situ freezing and stress-shielding on the ultrastructure of rabbit patellar tendons. J Orthop Res. 1997;15:904–910. doi: 10.1002/jor.1100150617. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915–930. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Romatowski JG, Bain JL, Trappe SW, Trappe TA, Thompson JL, Costill DL, Riley DA, Fitts RH. Effect of 17 days of bed rest on peak isometric force and unloaded shortening velocity of human soleus fibers. Am J Physiol Cell Physiol. 1997;273:C1690–C1699. doi: 10.1152/ajpcell.1997.273.5.c1690. [DOI] [PubMed] [Google Scholar]
- Wilkie DR. The relation between force and velocity in human muscle. J Physiol. 1949;110:249–280. doi: 10.1113/jphysiol.1949.sp004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TO, Cooke PH, Goodship AE. The effect of exercise and anabolic steroids on the mechanical properties and crimp morphology of the rat tendon. Am J Sports Med. 1988;16:153–158. doi: 10.1177/036354658801600211. [DOI] [PubMed] [Google Scholar]
- Yamashita-Goto K, Okuyama R, Honda M, Kawasaki K, Fujita K, Yamada T, Nonaka I, Ohira Y, Yoshioka T. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol. 2001;91:417–424. doi: 10.1152/jappl.2001.91.1.417. [DOI] [PubMed] [Google Scholar]