Abstract
We recently reported lower glycolytic flux (ATPGLY) and increased reliance on oxidative ATP synthesis (ATPOX) in contracting muscle of older compared to young humans. To further investigate this age-related difference in the pathways of ATP synthesis, we used magnetic resonance spectroscopy to determine the rates of ATPOX, ATPGLY and net phosphocreatine hydrolysis in vivo during maximal muscle contractions under free-flow (FF) and ischaemic (ISC) conditions in the ankle dorsiflexors of 20 young (27 ± 3 years; 10 male, 10 female) and 18 older (70 ± 5 years; 10 male, 8 female) adults. We hypothesized that ATPGLY would be higher in young compared to old during FF contractions, but that old would be unable to increase ATPGLY during ISC to match that of the young, which would suggest impaired glycolytic ATP synthesis with old age. Peak glycolytic flux during FF was lower in older (0.8 ± 0.1 mm ATP s−1) compared to young (1.4 ± 0.1 mm ATP s−1, P < 0.001) subjects. During ISC, peak ATPGLY increased in old to a level similar to that of young (1.4 ± 0.2 mm ATP s−1, 1.3 ± 0.2 mm ATP s−1, respectively; P = 0.86), suggesting that glycolytic function remains intact in aged muscle in vivo. Notably, older adults fatigued less than young during both FF and ISC (P ≤ 0.004). These results provide novel evidence of unimpaired in vivo glycolytic function in the skeletal muscle of older adults during maximal isometric dorsiflexion, and suggest a potential role for differences in metabolic economy and as a result, metabolite accumulation, in the fatigue resistance of the old.
Although advanced age is commonly accompanied by losses of skeletal muscle mass and strength (Lexell, 1995), the effect of old age on muscle fatigability (i.e. the temporary decline in muscle force production during repeated muscle activity) is less clear. Numerous investigators have demonstrated greater muscle fatigue with advanced age (Davies & White, 1983; Cupido et al. 1992). In contrast, others have reported enhanced fatigue resistance with old age in many muscle groups (Narici et al. 1991; Bemben et al. 1996; Ditor & Hicks, 2000; Kent-Braun et al. 2002; Lanza et al. 2004). This apparent paradox may be explained in part by morphological changes to ageing muscle. For example, lower mass and strength may reduce intramuscular pressure and blood flow occlusion during contractions in older subjects (Wigmore et al. 2004), resulting in relatively greater perfusion during intense contractions in older compared to young muscle. The progressive shift to a greater proportion of oxidative type I muscle (Jakobsson et al. 1990) and lower motor unit discharge rates with old age (Kamen et al. 1995), may also contribute to an age-related fatigue resistance.
Several research groups have demonstrated age-related alterations in muscle bioenergetics that are consistent with increased resistance to fatigue with ageing. For example, during high intensity muscle contractions, young subjects show greater intramuscular acidification, phosphocreatine (PCr) depletion, and inorganic phosphate (Pi) accumulation than older adults (Coggan et al. 1993; Chilibeck et al. 1998; Kent-Braun & Ng, 2000). Furthermore, a recent study demonstrated a significant association between fatigue and diprotonated inorganic phosphate (H2PO4−) during incremental isometric contractions in which older adults fatigued less than young (Kent-Braun et al. 2002). Collectively, these data suggest that age-related fatigue resistance may be related to the manner in which adenosine triphosphate (ATP) is synthesized to meet the energetic demands of muscular activity.
We recently used phosphorous magnetic resonance spectroscopy (31P-MRS) to demonstrate significantly blunted glycolytic flux in older compared to young men during maximal ankle dorsiflexion (Lanza et al. 2005). While there are currently no clear explanations for the decreased glycolytic flux with old age, we propose two plausible mechanisms. First, the capacity for glycolytic ATP synthesis may be impaired with old age, as suggested by reduced glycolytic enzyme activities observed in older muscle (Larsson et al. 1978; Pastoris et al. 2000) and reduced lactate production in stimulated hindlimbs of older compared to young rats (Campbell et al. 1991; Hepple et al. 2004). Alternatively, older muscle may preferentially rely on oxidative phosphorylation due to its ability to adequately meet the energetic demands of contraction without increasing glycolytic flux to the same extent as young muscle, thus minimizing the accumulation of inhibitory metabolites.
The purpose of the present study was to determine whether glycolytic ATP production in vivo is impaired with old age. We used 31P-MRS to determine the ATP flux through oxidative phosphorylation, anaerobic glycolysis, and net PCr breakdown during high intensity contractions performed with intact blood flow and during ischaemia where blood flow to the working muscle was restricted, thus limiting oxidative ATP synthesis. We hypothesized that (1) glycolytic flux would be higher in young compared to old during free-flow contractions (FF), (2) old would fatigue less than young during FF, consequent to their greater reliance on oxidative ATP synthesis and blunted metabolite accumulation compared to young, (3) old would be unable to increase glycolytic flux in response to ischaemia (ISC) to the same level of the young, consistent with the notion that glycolytic function is impaired with old age, and (4) old would fatigue more than young during ISC due to their inability to adequately meet the energetic demands of the contractions.
Methods
Subjects
Thirty-eight healthy, non-smoking young (10 male, 10 female, 27 ± 1 years, mean ±s.e.m.) and older (10 male, 8 female, 70 ± 1 years) subjects were studied. A subset of these data from the young age group (n = 6 male, 6 female) has been published previously (Lanza et al. 2006). Potential subjects were screened and excluded if they had a history of diabetes, cardiovascular disease, peripheral vascular disease (ankle : brachial systolic blood pressure < 1 (McDermott et al. 2001), stroke, or neurological disorders. Subjects taking anti-hypertensive, cardiac, or lipid-lowering medications were excluded due to the potential effects of these drugs on muscle function and blood flow. Each subject provided written informed consent in accordance with the procedures approved by the Human Subjects Review Boards at the University of Massachusetts and Yale University School of Medicine, and conforming to the standards set by the Declaration of Helsinki. Subjects were excluded from the study if they engaged in structured exercise more than 20 min per day, twice per week. To quantify physical activity level, each subject wore a uniaxial accelerometer (Manufacturing Technology, Fort Walton Beach, FL, USA) for seven consecutive days during waking hours, while maintaining their usual daily level of physical activity. Activity counts were averaged over a 5 day period and used to verify that activity levels were similar across age groups.
Experimental design
We used 31P-MRS to measure intracellular concentrations of PCr, Pi, ATP, and phosphomonoesters (PME) in the dorsiflexors during three contraction protocols. First, oxidative capacity was determined from the kinetics of PCr recovery following a 16 s isometric maximal voluntary contraction (MVC) (Conley et al. 2000; Lanza et al. 2005). Next, the rates of ATP synthesis from oxidative phosphorylation, anaerobic glycolysis, and net PCr breakdown in the creatine kinase reaction were determined during an intermittent MVC protocol; once with intact blood flow and once with circulatory occlusion (Kemp et al. 1994; Lanza et al. 2006).
Subjects were tested on two occasions, separated by at least 48 h. The first visit consisted of a habituation session at the University of Massachusetts, where subjects were familiarized with the contraction protocols, and their ability to fully activate the dorsiflexor muscles was confirmed during an MVC. The second visit took place at the Magnetic Resonance Research Center at Yale University, where subjects performed the contraction protocols with metabolic measures using 31P-MRS.
Habituation session
Subjects were positioned supine with either their left or their right foot (randomized) secured to a plate (ankle angle ∼120 deg) interfaced with a strain gauge for the measurement of dorsiflexion force (Kent-Braun et al. 2002). Electrodes for muscle stimulation and surface electromyography were attached to the lower leg to assess central activation, as previously described (Kent-Braun & Le Blanc, 1996). Subjects performed three baseline MVCs (3–4 s duration, 2 min recovery between each), the highest of which was taken as their isometric strength. Subjects were verbally encouraged and received visual force feedback from a series of light-emitting diodes.
Completeness of voluntary activation of the muscle was assessed during the final baseline MVC by superimposing a train (50 Hz, 500 μs pulse, 500 ms train duration) of supramaximal electrical stimuli. A central activation ratio (CAR) was calculated as MVC divided by the sum of MVC and any additional force produced by the superimposed train. A CAR less than 1.0 indicates incomplete voluntary activation. Two minutes following baseline MVC and CAR measures, subjects performed a contraction protocol consisting of six MVCs, each of 12 s duration, with 12 s rest intervals between contractions. Verbal encouragement and visual feedback were provided.
The contralateral leg was tested in the same manner, but with circulatory occlusion to the lower leg induced 30 s prior to the initiation of the intermittent MVC protocol. Lower leg ischaemia was maintained by inflating a cuff around the proximal thigh to a pressure of 220 Torr using a rapid cuff inflator (Hokanson, Inc. Belleview, WA, USA). During the habituation and metabolic testing sessions, the free-flow protocol (FF) was always performed before the ischaemic protocol (ISC) to maximize subject compliance and minimize any effects of ischaemia on subsequent contractions. The protocols were randomized as to which protocol was performed by each leg.
The analog signal from dorsiflexion force was acquired and digitized at 500 Hz (DAQ pad 6020E, National Instruments, Austin, TX, USA). Custom data-processing programs (Matlab, Mathworks, Inc, Novi, MI, USA) were used to determine baseline strength and the force–time integrals (FTI) for each contraction during FF and ISC. Fatigue was calculated as the relative decline in FTI:
| (1) |
Metabolic testing
Subjects were instructed to avoid strenuous physical activity and alcohol during the 24 h preceding the lab visit, and to abstain from caffeine for 6 h prior to testing. Following a minimum of 4 h of fasting and ∼30 min prior to testing, each subject consumed a supplement bar (22 g carbohydrate, 6 g fat, 15 g protein) to standardize caloric intake. Subjects were positioned supine on the bed of a 4.0 tesla superconducting magnet (Bruker Biospin, Rheinstetten, Germany) with one leg affixed to an exercise apparatus, as described for the habituation session. A probe assembly consisting of a 6 cm diameter 1H surface coil and a coplanar 3 × 5 cm elliptical 31P surface coil was secured over the tibialis anterior muscle, which is the main dorsiflexor muscle. The subject was then positioned in the isocentre of the magnet, as confirmed using gradient-echo scout images. Magnetic field homogeneity was optimized by localized shimming on the proton signal of tissue water (width at half-maximal height of PCr = 11.1 ± 0.7 Hz, n = 38). Prior to acquisition of MRS data, subjects performed two MVCs (3–4 s duration) separated by 2 min of rest to establish MVC force for that day.
Muscle oxidative capacity
Two minutes following the baseline MVCs, subjects performed a sustained MVC for 16 s. Phosphorous data were acquired (125 μs hard pulse, nominal 60 deg flip angle, 2 s repetition time, 2048 data points, 8000 Hz spectral width) during a 1 min rest interval prior to the contraction, during the contraction, and throughout a 10 min recovery period. Individual FIDs were averaged to yield 4 s resolution during the contraction, 8 s resolution during the first 5 min of recovery, and 30 s resolution during the last 5 min of recovery. Spectral analysis and peak integration were performed using NUTS software (Acorn NMR, Livermore, CA, USA) as described elsewhere (Lanza et al. 2005; Lanza et al. 2006). Phosphocreatine recovery following the contraction was fitted by a mono-exponential curve from which the rate constant, kPCr, was used to calculate muscle oxidative capacity (Qmax, mm ATP s−1) (Meyer, 1989; Conley et al. 2000):
| (2) |
Free-flow ATP flux
Immediately following the oxidative capacity measure, subjects performed the FF contraction protocol (as described in habituation session) with simultaneous measures of muscle force and phosphorous metabolites. The 31P-MRS measures were acquired 1 min before, during, and 10 min following the contraction protocol, with identical parameters to those described for muscle oxidative capacity. The rate of oxidative ATP synthesis (ATPOX, mm s−1) was determined from the rate of PCr recovery during the rest intervals between contractions (Boska, 1991; Kemp & Radda, 1994):
| (3) |
Glycolytic flux (ATPGLY, mm s−1) was determined from the changes in pH and phosphorous metabolites during each 12 s contraction as previously described (Lanza et al. 2006). Intramuscular pH was calculated based on the chemical shift (σ) of Pi relative to PCr (Hoult et al. 1974). When distinct Pi splitting was evident, the pH corresponding to each Pi pool was calculated separately as previously described (Lanza et al. 2006). The pH change, after correcting for proton efflux (υeff), buffering capacity (β), the consumption of protons by the creatine kinase reaction (θ), and the small contribution from oxidative metabolism (m), reflects glycolytic flux (Walter et al. 1999; Kemp & Radda, 1994; Lanza et al. 2006):
| (4) |
The rate of ATP synthesis from the net breakdown of PCr via the CK reaction (ATPCK, mm s−1) was determined from the change in PCr during each contraction. Since the synthesis of ATP is stoichiometric with the hydrolysis of PCr in the creatine kinase reaction, the calculation of ATPCK takes the simple form (Kemp & Radda, 1994):
| (5) |
The overall rate of ATP turnover (ATPTOT mm s−1) was determined as the sum of the flux through the three pathways:
| (6) |
Ischaemic ATP flux
Subjects were removed from the magnet after the FF protocol and allowed to rest for ∼1 h before performing the baseline MVCs, 16 s MVC, and ISC protocol on the opposite leg, as described above. The cuff was released ∼5 s following the completion of the final contraction. During ISC, ATPOX was assumed to be negligible based on studies showing that PCr does not recover following ischaemic contractions until the cuff is released, indicating that complete vascular occlusion is accomplished using cuff pressures similar to those used in the present study (Quistorff et al. 1993; Nakagawa et al. 2005). Furthermore, oxygen trapped within the muscle during cuff occlusion would be sufficient to generate only ∼4 mm ATP (Harris et al. 1975; Kemp et al. 1994), an amount that would be exhausted within ∼10 s during an MVC.
Glycolytic flux was calculated in a similar manner to that described for FF contractions, except that proton efflux and oxidative proton production were considered to be negligible:
| (7) |
The rate of ATP synthesis from PCr hydrolysis was calculated as described for the FF protocol, as was total ATP flux.
Spectral analyses
Free-induction decays were Fourier-transformed following 10 Hz line broadening. The resulting spectra were phased manually, and the underlying broad peak due to phosphorous in bone was removed by fitting a 5th order polynomial to the baseline region and subtracting this baseline from each spectrum. Peaks corresponding to PCr, PME, Pi, and γ-, α- and β-ATP were then fitted with Lorentzian-shaped curves (NUTS software) to quantify the area of each peak. When two distinct Pi peaks were observed, two Lorentzian-shaped curves were used, as previously described (Lanza et al. 2005, 2006). Corrections for partial saturation of each metabolite were applied (Lanza et al. 2005). Millimolar concentrations of phosphorous metabolites were calculated assuming that [PCr]+[Cr]= 42.5 mm and resting [ATP]= 8.2 mm (Harris et al. 1974), as previously described (Lanza et al. 2005, 2006).
Statistical analyses
The data were first examined for possible effects of sex and age-by-sex interactions on strength, fatigue, QMAX, ATPOX, ATPGLY, ATPCK and ATPTOT during FF and ISC using linear models, with sex treated as a categorical variable and age treated as a continuous variable. There were no significant effects of sex nor any age × sex interactions (P > 0.05), so all data were collapsed by sex and compared across age groups.
Our primary hypotheses were tested using a three factor (age, condition, time) repeated measures ANOVA to compare ATPGLY across age groups during FF and ISC, and a two factor (age, condition) repeated measures ANOVA to examine fatigue across age groups during FF and ISC. In addition, Student's t test for unpaired data was used to compare baseline [PCr], [Pi], [ADP], [AMP], [PME], [ATP], [H2PO4−], pH, MVC, Qmax and peak ATPGLY across age groups. The non-normal distribution of CAR values necessitated non-parametric analyses using the Mann–Whitney U test.
To fully evaluate the metabolic response to the protocols, two-factor (age, condition) repeated measures ANOVA was used to compare end-exercise [PCr], [Pi], [ADP], [AMP], [PME], [ATP], [H2PO4−], pH and fatigue in young and older subjects during FF and ISC. Two factor (age, time) repeated measures ANOVA was used to examine ATPOX during the FF condition. Three factor (age, condition, time) repeated measures ANOVA was used to compare ATPCK and ATPTOT across age groups during FF and ISC. The appropriate covariance structures were defined for each analysis. Post hoc pairwise comparisons were conducted using Tukey's procedure where significant age-by-condition interactions were evident.
The relationship between FTI (% initial) and H2PO4− was determined for each subject using linear regression analysis. All analyses were performed using SAS software (SAS Institute, Inc., Cary, NC, USA). Data are presented as means ±s.e.m. with significance established at P < 0.05.
Results
Subject characteristics
The young and older groups were similar in height (172 ± 2, 171 ± 3 cm, respectively), mass (69 ± 3, 77 ± 4 kg, respectively), and physical activity level (265 ± 19, 295 ± 31 counts day−1 1000−1, respectively).
Muscle oxidative capacity
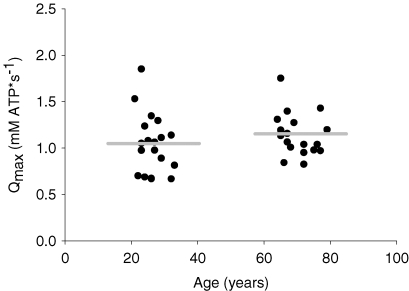
Resting [PCr] was similar across age groups (Table 1). Young and older subjects depleted PCr to similar levels during the 16 s MVC prior to FF (young = 22.5 ± 0.6 mm, older = 23.5 ± 0.6) and ISC (young = 23.5 ± 0.5 mm, older = 23.6 ± 0.5). The rate constants of PCr recovery following the 16 s MVC were similar across the two legs (data not shown). Therefore, age-group comparisons of Qmax were performed using each subject's average value for the two trials. The average rate constant of PCr recovery was similar in young (0.027 ± 0.002) and older subjects (0.031 ± 0.001), as was oxidative capacity (Fig. 1).
Table 1.
Intracellular pH and metabolite concentrations measured prior to FF and ISC protocols
| Free-flow | Ischaemia | |||||
|---|---|---|---|---|---|---|
| Metabolites at rest | Young | Older | P | Young | Older | P |
| [PCr] (mm) | 38.1 ± 0.2 | 38.2 ± 0.2 | 0.73 | 38.2 ± 0.3 | 37.8 ± 0.3 | 0.38 |
| [Pi] (mm) | 4.4 ± 0.2 | 4.3 ± 0.2 | 0.73 | 4.3 ± 0.3 | 4.7 ± 0.3 | 0.38 |
| pH | 7.00 ± 0.01 | 7.00 ± 0.01 | 0.95 | 7.02 ± 0.01 | 7.02 ± 0.00 | 0.60 |
| H2PO4− (mm) | 2.1 ± 0.6 | 1.5 ± 0.1 | 0.39 | 1.5 ± 0.1 | 1.6 ± 0.1 | 0.42 |
| PME (mm) | 2.9 ± 0.4 | 2.9 ± 0.3 | 0.97 | 2.7 ± 0.3 | 2.7 ± 0.2 | 0.99 |
| ADP (mm) | 0.008 ± 0.000 | 0.007 ± 0.007 | 0.46 | 0.008 ± 0.001 | 0.009 ± 0.001 | 0.36 |
| AMP (μm) | 0.007 ± 0.001 | 0.006 ± 0.001 | 0.60 | 0.007 ± 0.001 | 0.009 ± 0.002 | 0.38 |
Values are means ±s.e.m.P-values reflect comparisons across age groups using unpaired t tests.
Figure 1.
Muscle oxidative capacity (Qmax), determined from PCr recovery kinetics, was similar in older compared to young subjects Data points represent individual subjects. Horizontal grey bars represent the mean values for each group.
Force and voluntary activation
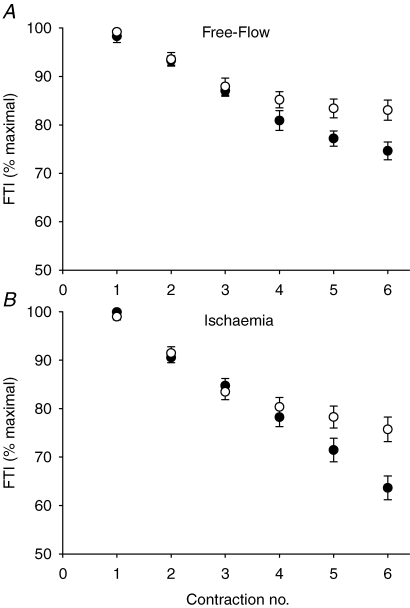
Isometric strength (MVC) was similar in both age groups for the right (young, Y, 303 ± 22; older, O, 285 ± 14 N) and left legs (Y, 301 ± 20; O, 301 ± 17 N). All subjects, regardless of age, were able to achieve full voluntary activation of the dorsiflexors during baseline MVCs, as indicated by the CAR measure in the right (Y, 0.99 ± 0.01; O, 0.99 ± 0.00) and left legs (Y, 1.00 ± 0.00; O, 0.99 ± 0.01). Central activation was measured during the habituation sessions, but not during the metabolic testing sessions. For all habituation and testing protocols, subjects were well-motivated and received consistent instruction, verbal encouragement, and visual feedback during each contraction. Thus, we have no reason to expect that central activation was different across the two testing sessions. Young subjects fatigued more than older subjects during FF contractions (Fig. 2). All subjects fatigued more during ISC compared to FF contractions, with the older group once again fatiguing less than the young during this protocol (Fig. 2, Page < 0.001, Pcondition < 0.001, Page-by-condition= 0.20).
Figure 2.
Changes in FTI during FF and ISC The relative decline of the force–time integral during free-flow (A) and ischaemic (B) contractions in young (•) and older (○) subjects. Young fatigued more than older subjects during FF and ISC contractions. Data are presented as means ±s.e.m.
Metabolite changes during FF and ISC
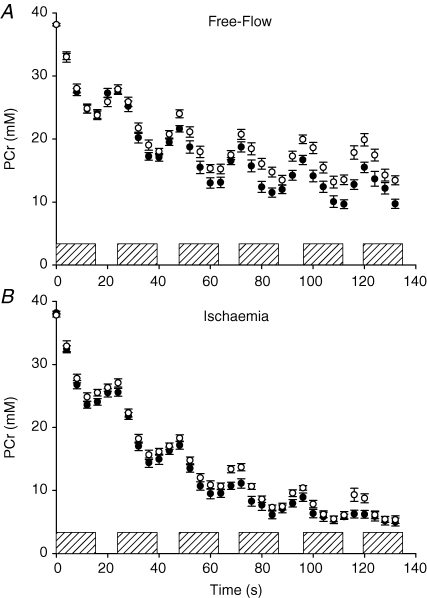
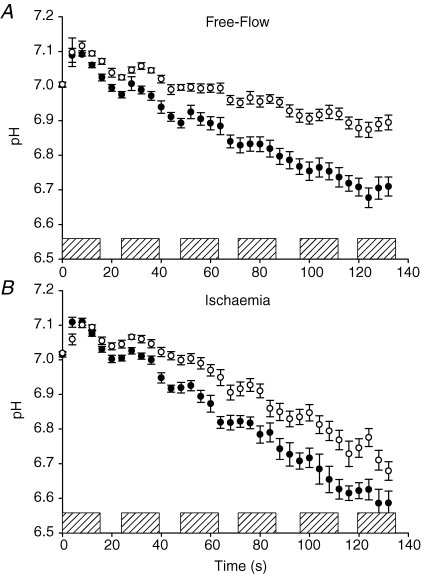
Resting pH and concentrations of all phosphorus metabolites were similar across age groups prior to FF and ISC (Table 1). Phosphocreatine decreased and Pi increased more in young compared to older subjects during FF but not during ISC (Fig. 3, Table 2). Intracellular pH declined more in young compare to old during FF and ISC, although there was a trend toward a significant age-by-condition interaction (Table 2), suggesting that the difference in pH between young and old was less pronounced during ISC compared to FF (Fig. 4, Table 2). Similarly, diprotonated inorganic phosphate increased more in young than older subjects during both protocols, with a trend toward a significant age-by-condition interaction (Table 2), suggesting a greater age-related difference in [H2PO4−] in FF than ISC (Table 2). A trend toward greater phosphomonoester accumulation in young compared to older subjects was observed during FF and ISC (Table 2). Both ADP and AMP increased to a greater extent during ISC compared to FF, but these changes were similar in young and older subjects (Table 2). ATP did not change in either group during FF or ISC (data not shown).
Figure 3.
Phosphocreatine during FF and ISC protocols A, phosphocreatine declined more in young (•) compared to older (○) subjects during free-flow contractions. B, similar PCr breakdown was observed across age groups during ischaemic contractions. Hatched bars represent each 12 s MVC. Data are presented as means ±s.e.m.
Table 2.
Intracellular pH and metabolite concentrations measured at the end of FF and ISC
| Free-flow | Ischaemia | ||||||
|---|---|---|---|---|---|---|---|
| Metabolites at end-exercise | Young | Older | Young | Older | P (age) | P (cond) | P (age-by-cond) |
| [PCr] (mm) | 9.7 ± 0.7† | 13.4 ± 0.7*† | 5.0 ± 0.6 | 5.4 ± 0.7 | — | — | <0.01 |
| [Pi] (mm) | 32.8 ± 0.7† | 29.1 ± 0.8*† | 37.5 ± 0.6 | 37.1 ± 0.6 | — | — | <0.01 |
| pH | 6.71 ± 0.03 | 6.89 ± 0.03 | 6.59 ± 0.03 | 6.68 ± 0.03 | <0.01 | <0.01 | 0.09 |
| H2PO4− (mm) | 16.5 ± 0.9 | 11.9 ± 0.8 | 21.8 ± 0.8 | 19.6 ± 0.8 | <0.01 | <0.01 | 0.09 |
| PME (mm) | 9.1 ± 0.7 | 7.1 ± 0.7 | 10.5 ± 0.9 | 9.3 ± 0.8 | 0.06 | <0.01 | 0.57 |
| ADP (mm) | 0.15 ± 0.02 | 0.12 ± 0.02 | 0.38 ± 0.08 | 0.33 ± 0.07 | 0.87 | <0.01 | 0.41 |
| AMP (μm) | 2.6 ± 0.7 | 1.7 ± 0.6 | 17.0 ± 6.1 | 14.1 ± 560 | 0.64 | <0.01 | 0.81 |
Values are means ±s.e.m.P-values reflect main effects of age, condition, and age-by-condition interactions from 2-way (age, condition) repeated measures ANOVA.
Significant (P <0.05) effects of age within a given protocol.
Significant effects of protocol within each age group.
Figure 4.
Intracellular pH during FF and ISC protocols pH decreased to a greater extent in young (•) than older (○) subjects during free-flow contractions (A), and ischaemic contractions (B) Hatched bars represent each 12 s MVC. Data are presented as means ±s.e.m.
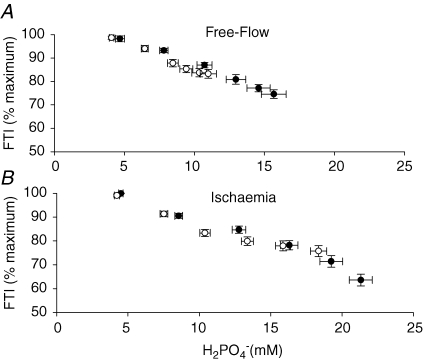
To examine the role of intracellular metabolic changes in fatigue, the relationship between FTI (% initial) and H2PO4− (mm) was determined for each subject (Fig. 5). These regression analyses revealed strong linear associations between FTI and intracellular H2PO4− during FF in young (r = 0.88 ± 0.05) and older (r = 0.82 ± 0.07) subjects. Linear relationships were found also during ISC in young (r = 0.90 ± 0.05) and older (r = 0.82 ± 0.06) individuals.
Figure 5.
Relationships between FTI and diprotonated inorganic phosphate A, the fall of FTI during free-flow contractions was strongly associated with the accumulation of H2PO4− in young (•) and older (○) subjects. B, although significant linear associations were also observed in both age groups during ischaemia, the older subjects appeared to reach a plateau in fatigue while H2PO4− continued to decline (see text for discussion).
ATP synthesis during FF and ISC
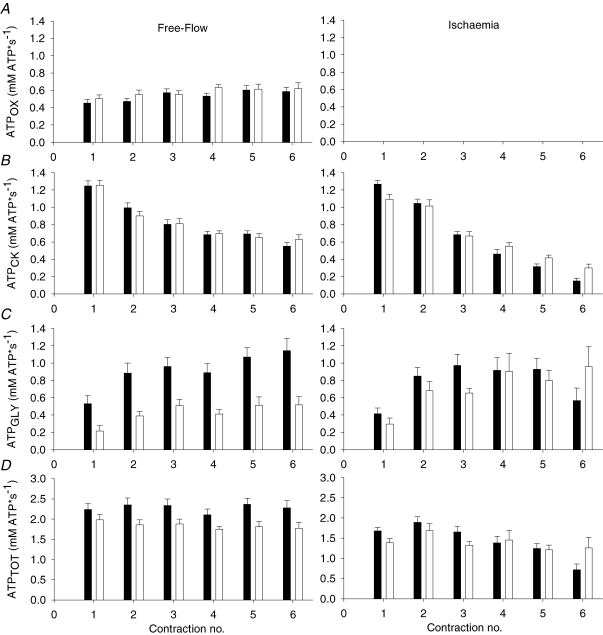
ATPOX increased with time during FF contractions (Ptime= 0.004) to a similar extent in young and older subjects (Fig. 6A). ATPCK decreased similarly in young and older subjects during FF and ISC (Fig. 6B), and to a greater extent in ISC than FF (Pcondition-by-time < 0.001). ATPGLY increased with time during FF and ISC (Ptime < 0.001, Fig. 6C). A significant age-by-condition interaction (Page-by-condition= 0.013) indicated that ATPGLY was lower in older compared to young subjects during FF (P < 0.001), but similar across age groups during ISC. Peak ATPGLY was higher (P < 0.001) in young (1.4 ± 0.1 mm ATP s−1) than older subjects (0.8 ± 0.1 mm ATP s−1) during FF, but similar in both age groups during ISC (1.3 ± 0.2 mm ATP s−1, 1.4 ± 0.2 mm ATP s−1, respectively; P = 0.86). The overall ATP synthesis rate (ATPTOT) was higher in young than older subjects during FF but not during ISC, as revealed by a significant age-by-condition interaction (Page-by-condition= 0.037) and post hoc comparisons across age groups within both conditions (Fig. 6D). A significant condition-by-time interaction was observed for this variable (Pcondition-by-time < 0.001), indicating that ATPTOT decreased more in both age groups as the contractions progressed during ISC compared to FF.
Figure 6.
ATP synthesis rates A, oxidative ATP synthesis (ATPOX) was similar in young (▪) and older (□) subjects during free-flow contractions (left panel) and assumed to be negligible during ischaemia (right panel). B, ATP derived from net PCr breakdown (ATPCK) was similar across age groups during FF and ISC contractions. C, glycolytic flux (ATPGLY) was higher in young than older subjects during FF, but similar in both age groups during ISC. D, total ATP synthesis rates (ATPTOT) were lower in old during FF, but not during ISC. Data are presented as means ±s.e.m.
Discussion
Despite blunted glycolytic flux in older compared to young adults during FF, older adults increased glycolytic flux to a level similar to that of young during ISC, when oxidative phosphorylation was no longer a viable source of ATP. This metabolic adaptation suggests that glycolytic function remains intact in the ankle dorsiflexor muscles of heathly older adults. As expected, the greater fatigability of young compared to old during FF was related to inhibitory metabolite accumulation during contractions. The mechanisms of the age-related fatigue resistance during ISC are less clear. While our results provide further evidence for the role of muscular energetics in the age-related differences in muscle fatigue, they also suggest that increased metabolic economy may be a potential mechanism allowing older muscle to engage in maximal work with lower glycolytic flux compared to young muscle.
Muscle oxidative capacity
The rate constant of PCr recovery was similar in young and older subjects, as was the calculated oxidative capacity, Qmax. Although others have reported that oxidative capacity is impaired with old age in vivo (McCully et al. 1993; Taylor et al. 1997; Conley et al. 2000), the results of the present study are consistent with previous reports that oxidative capacity is preserved in the tibialis anterior (Kent-Braun & Ng, 2000; Lanza et al. 2005), forearm (Kutsuzawa et al. 2001), and plantarflexor muscles of older adults (Chilibeck et al. 1998). Studies in vitro reveal similar discrepancies with some reports of reduced oxidative enzyme activities (Coggan et al. 1992; Pastoris et al. 2000), increased mitochondrial DNA alterations (Short & Nair, 2001), and reduced mitochondrial protein synthesis (Rooyackers et al. 1996), while others found no effects of age on mitochondrial function by a variety of methods (Grimby et al. 1982; Aniansson et al. 1986; Barrientos et al. 1996). The diversity of muscle groups under investigation may be a primary explanation for these discrepant results. Indeed, numerous studies demonstrate that neural (Galea, 1996), structural (Grimby et al. 1982), mechanical (Lanza et al. 2003), and enzymatic (Houmard et al. 1998) changes with age exhibit muscle-group specificity. This consideration, as well as the impact of varying health characteristics and physical activity patterns on age-related changes in oxidative capacity, is reviewed elsewhere (Russ & Kent-Braun, 2004).
Pathways of ATP synthesis
The oxidative ATP synthesis rates were similar in young and older subjects during FF, as observed previously (Lanza et al. 2005), and were comparable to the rates reported in previous studies using similar methods (Boska, 1991; Jubrias et al. 2001; Lanza et al. 2005). The similarity in ATPOX across age groups in the present study is consistent with our observations of preserved oxidative capacity with ageing. Phosphocreatine recovery is typically attributed exclusively to oxidative ATP synthesis, based on demonstrations that PCr does not recover in the absence of oxygen (Quistorff et al. 1993; Nakagawa et al. 2005). This dogmatic view has been challenged recently by reports of a transient portion of PCr recovery that can be attributed to anaerobic glycolysis (Crowther et al. 2002b; Lanza et al. 2006). As a result, attempts have been made to adjust the calculation of oxidative flux to account for this transient glycolysis. In two studies, glycolytic PCr resynthesis was quantified during ischaemic contractions and used to derive correction factors that were then applied during equivalent free-flow contractions (Jubrias et al. 2001; Lanza et al. 2006). However, these corrections assumed equal glycolytic flux during the postcontraction interval during FF and ISC, which may be inappropriate in the present study, where glycolytic flux was higher during ISC than FF in older subjects. Therefore, we did not correct for the glycolytic portion of PCr recovery in this study, and as a result have likely overestimated ATPOX. This issue remains unresolved until more is known about the contribution of glycolysis to the postcontraction recovery of PCr.
The rates of ATP synthesis from net PCr hydrolysis in the creatine kinase reaction were similar in young and older subjects during FF and ISC. We have previously shown that ATPCK is unaffected by old age during FF (Lanza et al. 2005). Using a steady-state saturation transfer MRS method, Horska et al. (2000) also found that flux through the creatine kinase reaction does not differ by age. In contrast to these studies in vivo, experiments using biopsy tissue have revealed significant age-related reductions in creatine kinase activity in vitro (Kaczor et al. 2006; Gelfi et al. 2006). Although the effects of ageing on the temporal ATP-buffering capacity of the creatine kinase reaction have not been studied extensively, the literature to date suggests that this pathway is unaffected by the ageing process in vivo.
Glycolytic flux was lower in older compared to young subjects during FF, as reported recently (Lanza et al. 2005) and inferred from blunted intracellular acidosis during muscle contractions (Coggan et al. 1993; Taylor et al. 1997; Chilibeck et al. 1998; Kent-Braun et al. 2002). Contrary to our hypothesis, ATPGLY increased in older subjects when oxidative ATP synthesis was eliminated during ischaemia, suggesting that the functionality of glycolytic ATP synthesis in vivo remains robust with old age. Therefore, the blunted glycolytic flux observed during FF is likely to reflect the ability of older muscle to adequately meet energetic needs without increasing glycolytic flux to the same extent as young. It is important to note that we did not measure the capacity for anaerobic glycolysis, but rather the functionality of this pathway for ATP production under different conditions in vivo. It is certainly possible that the glycolytic flux we measured during maximal isometric contractions, even under ischaemic conditions, is below the upper limit that is possible in vivo. For example, dynamic contractions, which are more metabolically demanding than isometric contractions (Newham et al. 1995), may result in higher glycolytic flux than observed in the present study.
Studies of age-related alterations in glycolytic function in humans have been limited primarily to enzymatic analyses, which showed similar (Grimby et al. 1982; Coggan et al. 1992) or lower (Larsson et al. 1978; Pastoris et al. 2000) activity of glycolytic enzymes in old compared to young muscle. The ability to generate ATP glycolytically could decline with age as a result of reduced type II fibre area. By multiplying the proportional area of type I (Y, 64.5%; O, 81.1%) and type II fibres (Y, 35.5%; O, 18.9%; Jakobsson et al. 1990) by their respective PFK activities (type I, 25.8, type II, 49.4 mmol min−1 (kg dry weight)−1; Essen et al. 1975), we estimate that the reduction in overall muscle PFK activity due to reduced type II fibre proportions is not likely to exceed ∼11% in old muscle. This prediction is higher than our earlier estimations based on α-glycerol phosphate dehydrogenase (GPDH) activity; however, PFK is likely to be a better proxy for the potential for glycolytic flux than GPDH (Lanza et al. 2005). Regardless, both estimates suggest that the age-related fibre type shift cannot fully explain the observation that glycolytic flux is blunted in older compared to young under FF conditions.
Our results in vivo are in contrast to stimulated rat hind-limb experiments, which show reduced lactate production in older compared to young rats (Campbell et al. 1991; Hepple et al. 2004). However, this result was exclusive to white gastrocnemius muscle, whereas soleus, plantaris, and red gastrocnemius demonstrated no age-related impairment in glycolytic function. Thus, it seems that, at least in rat skeletal muscle, glycolytic function may be impaired with age in fast glycolytic fibres but preserved in more oxidative fibres. Nevertheless, despite the mixed composition of human tibialis anterior muscle (Jakobsson et al. 1990) we observed that glycolytic function is preserved with age in this muscle group. Further studies are warranted to determine if this result is consistent across other muscle groups with more glycolytic fibre composition.
Although our results oppose the notion of impaired glycolytic capacity as a mechanism to explain blunted glycolytic flux in old muscle, there remain numerous potential mechanisms that warrant discussion. We have previously proposed that glycolytic flux may be higher in younger subjects due to constricted blood flow during muscle contractions (Kent-Braun et al. 2002; Lanza et al. 2005). The larger, stronger muscles of younger individuals would be expected to generate greater intramuscular pressure and thus occlude blood flow to the working muscle to a greater extent than the smaller, weaker muscles of older subjects. However, this mechanism is unlikely to explain the greater glycolytic flux in young subjects in the present study since all subjects, regardless of age, generated > 60% MVC during the free-flow protocol, which is a level force beyond which skeletal muscle perfusion is fully occluded during voluntary dorsiflexion (Wigmore et al. 2004).
Activation of glycolysis during muscle contraction is believed to be regulated by a ‘dual control’ model whereby intracellular calcium (Ca2+) and metabolic by-products of muscle contraction (Pi, AMP, ADP) synergistically regulate glycolytic flux (Quistorff et al. 1993; Connett & Sahlin, 1996; Crowther et al. 2002a). Although [AMP] and [ADP] were similar in young and old during both protocols here, [Pi] was higher in the young during FF, but similar across age groups during ISC. During FF, [Pi] was at (older) or above (young) the reported Michaelis constant (Km) of glycogen phosphorylase for Pi of ∼27 mm (Chasiotis et al. 1982), consistent with the notion of Pi as a regulator of age-related differences in glycolytic flux in FF. During ISC, [Pi] increased to a similar, high (∼37 mm) level in young and old, and ATPGLY was similar in both groups.
When examining the change in [Pi] in both groups from FF to ISC (Table 2), it is somewhat surprising to note the lack of increase in ATPGLY in the young, given their ∼5 mm increase in [Pi] from FF to ISC. It is possible that [Pi] in young was sufficiently above the km during FF, such that a further increase in [Pi] during ISC had little effect on ATPGLY. In contrast, the change in [Pi] from FF to ISC in the old (∼8 mm) apparently occurred at a steeper portion of the saturation curve between Pi and ATPGLY, and thus was able to further activate glycolysis in the old. Additionally, the inhibitory effects of intracellular acidosis on glycolytic flux (Hill, 1955; Chase & Kushmerick, 1988) may have been different in young and old across conditions. That is, the young may have reached an intracellular pH during both FF and ISC at which glycolysis became inhibited, while the older group may have attained this level of acidosis only during ISC.
While the differences in Pi accumulation and intracellular pH provide an attractive mechanism to explain the current results, the importance of intracellular Ca2+ in regulation of ATPGLY should not be overlooked. It is possible that the age-related decline in motor unit discharge rates (MUDR) (Kamen et al. 1995; Connelly et al. 1999) and the leftward shift in the force–frequency relationship with old age (Ng & Kent-Braun, 1999; Allman & Rice, 2004) may combine to generate high force with relatively fewer activation pulses in the old. This effect would translate to less Ca2+ release from the sarcoplasmic reticulum, lower intracellular Ca2+, and lower glycolytic flux during high-intensity contractions in older compared to young muscle. This possibility needs to be explored.
Total ATP flux
Because there was no change in [ATP] during these protocols, ATPTOT can serve as a proxy for ATP demand. We observed that ATPTOT was lower in old compared to young during FF. Although one might expect differences in absolute force production to contribute to the observed differences in the overall ATP demand, the similar strength of the two age groups, and the use of volumetric units (mm s−1), render our flux measures independent of muscle size. Thus, our data suggest that the ATP requirements of force production are lower in older muscle, possibly as a result of more economical force production due to the combination of contractile slowing and lower MUDR mentioned above.
Previous reports of age-related changes in metabolic economy are scarce and thus far limited to animal studies, which have shown increased contractile economy with age (de Haan et al. 1993; Hepple et al. 2004). The age-related shift of muscle toward a slower, more oxidative and economical fibre-type composition (Jakobsson et al. 1990; Lexell, 1995; Hepple et al. 2004) is a potential source of increased metabolic economy with age. We are unaware of any studies that have investigated age-related changes in metabolic economy in humans to date.
Force and voluntary activation
As expected, young subjects fatigued more than older subjects during FF, in agreement with some previous studies (Bemben et al. 1996; Ditor & Hicks, 2000), but in contrast to others (Davies & White, 1983; Lennmarken et al. 1985; Cupido et al. 1992). In the present study, the strong association between fatigue and [H2PO4−] during FF supports the contention of a metabolic basis for the observed fatigue resistance with old age. We hypothesized that older subjects would fatigue more than young during ISC because of an inability to increase glycolytic flux in compensation for suppressed oxidative ATP synthesis. Although all subjects fatigued more during ISC compared to FF, the fatigue resistance of older subjects persisted despite occlusion of blood flow. As discussed above, older adults were capable of increasing their reliance on substrate-level phosphorylation during ISC as necessary to meet the energetic demands of the contraction protocol. Similar to FF, we again observed a strong association between fatigue and [H2PO4−] during ISC, suggesting that metabolite accumulation may still be a potent mechanism to explain greater fatigue in young than older muscle, even in the absence of blood flow. However, there was a trend for the age-related difference in H2PO4− accumulation to be less pronounced during ISC than FF, which suggests that something other than the direct effects of accumulating metabolites on muscle force development may have been contributing to the fatigue resistance of the older subjects. Recent observations from our laboratory (Chung et al. unpublished) reveal that greater central and peripheral activation failure in the young may explain a portion of the age-related fatigue resistance during ischaemic MVCs. However, we did not measure activation during fatigue in the present study, and thus cannot ascertain the neural contribution to fatigue.
In summary, we have shown that, during maximal voluntary ankle dorsiflexion, glycolytic flux was lower in older compared to young subjects during FF, but similar across age groups during ISC. These data suggest that glycolytic function remains intact with old age in this muscle group, and that the ankle dorsiflexors of older individuals retain the ability to meet the energetic demands of contractile activity under a variety of conditions. In addition, the results point to an age-related increase in metabolic economy as a potential mechanism that may allow older muscle to engage in maximal work with less fatigue compared to young. Further study is needed to determine whether the present findings are consistent across other morphologically and functionally distinct muscle groups.
Acknowledgments
Many thanks to Danielle Wigmore, PhD, Linda Chung, MS, Damien Callahan, and Stephen Foulis for assistance in data collection and analysis. We also wish to thank John Buonaccorsi, PhD for expert statistical advice, Mike Tevald, PT, PhD, Douglas Befroy, DPhil, Douglas Rothman, PhD, David Russ, PhD, Barry Braun, PhD for thoughtful discussion, and Peter Brown and Mark Abildgaard for technical assistance. This study was funded by NIH R01AG21094 and American College of Sports Medicine Doctoral Student Grant.
References
- Allman BL, Rice CL. An age-related shift in the force-frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol. 2004;96:1026–1032. doi: 10.1152/japplphysiol.00991.2003. [DOI] [PubMed] [Google Scholar]
- Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–591. doi: 10.1002/mus.880090702. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Casademont J, Rotig A, Miro O, Urbano-Marquez A, Rustin P, Cardellach F. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun. 1996;229:536–539. doi: 10.1006/bbrc.1996.1839. [DOI] [PubMed] [Google Scholar]
- Bemben MG, Massey BH, Bemben DA, Misner JE, Boileau RA. Isometric intermittent endurance of four muscle groups in men aged 20–74 yr. Med Sci Sports Exerc. 1996;28:145–154. doi: 10.1097/00005768-199601000-00026. [DOI] [PubMed] [Google Scholar]
- Boska M. Estimating the ATP cost of force production in the human gastrocnemius/soleus muscle group using 31P MRS and 1H MRI. NMR Biomed. 1991;4:173–181. doi: 10.1002/nbm.1940040404. [DOI] [PubMed] [Google Scholar]
- Campbell CB, Marsh DR, Spriet LL. Anaerobic energy provision in aged skeletal muscle during tetanic stimulation. J Appl Physiol. 1991;70:1787–1795. doi: 10.1152/jappl.1991.70.4.1787. [DOI] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988;53:935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis D, Sahlin K, Hultman E. Regulation of glycogenolysis in human muscle at rest and during exercise. J Appl Physiol. 1982;53:708–715. doi: 10.1152/jappl.1982.53.3.708. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, McCreary CR, Marsh GD, Paterson DH, Noble EG, Taylor AW, Thompson RT. Evaluation of muscle oxidative potential by 31P-MRS during incremental exercise in old and young humans. Eur J Appl Physiol Occup Physiol. 1998;78:460–465. doi: 10.1007/s004210050446. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Abduljalil AM, Swanson SC, Earle MS, Farris JW, Mendenhall LA, Robitaille PM. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol. 1993;75:2125–2133. doi: 10.1152/jappl.1993.75.5.2125. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- Connett RJ, Sahlin K. Control of glycolysis and glycogen metabolism. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 870–911. [Google Scholar]
- Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting skeletal muscle. I. Turning it on. Am J Physiol Endocrinol Metab. 2002a;282:E67–E73. doi: 10.1152/ajpendo.2002.282.1.E67. [DOI] [PubMed] [Google Scholar]
- Crowther GJ, Kemper WF, Carey MF, Conley KE. Control of glycolysis in contracting skeletal muscle. II. Turning it off. Am J Physiol Endocrinol Metab. 2002b;282:E74–E79. doi: 10.1152/ajpendo.2002.282.1.E74. [DOI] [PubMed] [Google Scholar]
- Cupido CM, Hicks AL, Martin J. Neuromuscular fatigue during repetitive stimulation in elderly and young adults. Eur J Appl Physiol Occup Physiol. 1992;65:567–572. doi: 10.1007/BF00602367. [DOI] [PubMed] [Google Scholar]
- Davies CT, White MJ. Contractile properties of elderly human triceps surae. Gerontology. 1983;29:19–25. doi: 10.1159/000213090. [DOI] [PubMed] [Google Scholar]
- de Haan A, de Ruiter CJ, Lind A, Sargeant AJ. Age-related changes in force and efficiency in rat skeletal muscle. Acta Physiol Scand. 1993;147:347–355. doi: 10.1111/j.1748-1716.1993.tb09511.x. [DOI] [PubMed] [Google Scholar]
- Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78:781–790. [PubMed] [Google Scholar]
- Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Galea V. Changes in motor unit estimates with aging. J Clin Neurophysiol. 1996;13:253–260. doi: 10.1097/00004691-199605000-00010. [DOI] [PubMed] [Google Scholar]
- Gelfi C, Vigano A, Ripamonti M, Pontoglio A, Begum S, Pellegrino MA, Grassi B, Bottinelli R, Wait R, Cerretelli P. The human muscle proteome in aging. J Proteome Res. 2006;5:1344–1353. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]
- Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand. 1982;115:125–134. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Kaijser L, Nordesjo LO. The effect of circulatory occlusion on isometric exercise capacity and energy metabolism of the quadriceps muscle in man. Scand J Clin Laboratory Invest. 1975;35:87–95. [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Laboratory Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Hepple RT, Hagen JL, Krause DJ, Baker DJ. Skeletal muscle aging in F344BN F1-hybrid rats. II. Improved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol A Biol Sci Med Sci. 2004;59:1111–1119. doi: 10.1093/gerona/59.11.1111. [DOI] [PubMed] [Google Scholar]
- Hill AV. The influence of the external medium on the internal pH of muscle. Proc R Soc Lond B Biol Sci. 1955;144:1–22. doi: 10.1098/rspb.1955.0030. [DOI] [PubMed] [Google Scholar]
- Horska A, Fishbein KW, Fleg JL, Spencer RG. The relationship between creatine kinase kinetics and exercise intensity in human forearm is unchanged by age. Am J Physiol Endocrinol Metab. 2000;279:E333–E339. doi: 10.1152/ajpendo.2000.279.2.E333. [DOI] [PubMed] [Google Scholar]
- Hoult DI, Busby SJ, Gadian DG, Radda GK, Richards RE, Seeley PJ. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974;252:285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Jakobsson A, Borg K, Edstrom L. Fiber-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Acta Neuropath. 1990;80:459–468. doi: 10.1007/BF00294604. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90:1663–1670. doi: 10.1152/jappl.2001.90.5.1663. [DOI] [PubMed] [Google Scholar]
- Kaczor JJ, Ziolkowski W, Antosiewicz J, Hac S, Tarnopolsky MA, Popinigis J. The effect of aging on anaerobic and aerobic enzyme activities in human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:339–344. doi: 10.1093/gerona/61.4.339. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q. 1994;10:43–63. [PubMed] [Google Scholar]
- Kemp GJ, Thompson CH, Barnes PR, Radda GK. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med. 1994;31:248–258. doi: 10.1002/mrm.1910310303. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93:1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Kutsuzawa T, Shioya S, Kurita D, Haida M, Yamabayashi H. Effects of age on muscle energy metabolism and oxygenation in the forearm muscles. Med Sci Sports Exerc. 2001;33:901–906. doi: 10.1097/00005768-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97:967–975. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE, Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol. 2006;577:353–367. doi: 10.1113/jphysiol.2006.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lennmarken C, Bergman T, Larsson J, Larsson LE. Skeletal muscle function in man: force, relaxation rate, endurance and contraction time-dependence on sex and age. Clin Physiol. 1985;5:243–255. [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Medical Sci. 1995;50 doi: 10.1093/gerona/50a.special_issue.11. Spec No: 11–16. [DOI] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75:813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol. 1989;257:C1149–C1157. doi: 10.1152/ajpcell.1989.257.6.C1149. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Ratkevicius A, Mizuno M, Quistorff B. ATP economy of force maintenance in human tibialis anterior muscle. Med Sci Sports Exerc. 2005;37:937–943. [PubMed] [Google Scholar]
- Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol. 1991;71:1277–1281. doi: 10.1152/jappl.1991.71.4.1277. [DOI] [PubMed] [Google Scholar]
- Newham DJ, Jones DA, Turner DL, McIntyre D. The metabolic costs of different types of contractile activity of the human adductor pollicis muscle. J Physiol. 1995;488:815–819. doi: 10.1113/jphysiol.1995.sp021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AV, Kent-Braun JA. Slowed muscle contractile properties are not associated with a decreased EMG/force relationship in older humans. J Gerontol A Biol Sci Med Sci. 1999;54:B452–B458. doi: 10.1093/gerona/54.10.b452. [DOI] [PubMed] [Google Scholar]
- Pastoris O, Boschi F, Verri M, Baiardi P, Felzani G, Vecchiet J, Dossena M, Catapano M. The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp Gerontol. 2000;35:95–104. doi: 10.1016/s0531-5565(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Johansen L, Sahlin K. Absence of phosphocreatine resynthesis in human calf muscle during ischaemic recovery. Biochem J. 1993;291:681–686. doi: 10.1042/bj2910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. Is skeletal muscle oxidative capacity decreased in old age? Sports Med. 2004;34:221–229. doi: 10.2165/00007256-200434040-00002. [DOI] [PubMed] [Google Scholar]
- Short KR, Nair KS. Does aging adversely affect muscle mitochondrial function? Exerc Sport Sci Rev. 2001;29:118–123. doi: 10.1097/00003677-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem. 1997;174:321–324. [PubMed] [Google Scholar]
- Walter G, Vandenborne K, Elliott M, Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol. 1999;519:901–910. doi: 10.1111/j.1469-7793.1999.0901n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore DM, Damon BM, Pober DM, Kent-Braun JA. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol. 2004;97:2385–2394. doi: 10.1152/japplphysiol.01390.2003. [DOI] [PubMed] [Google Scholar]