Abstract
Following a suprathreshold transcranial magnetic stimulation (TMS) to the primary motor cortex (M1) during voluntary muscle contraction, a motor evoked potential (MEP) occurs in the target muscle followed by a silent period (SP) in the electromyographic (EMG) activities. The present study investigated how short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) change during the SP. The time course of MEP and motor threshold during the SP were examined in the right first dorsal interosseous muscle. Using a triple-pulse protocol, SICI and ICF were tested at different times during the SP. The effects of different intensities of the conditioning stimulation (CS) for SICI and ICF were also investigated during the SP and at rest. During the SP, MEP was inhibited and motor threshold was increased, whereas MEP latency and background EMG level were same as those at rest. SICI decreased during the SP over a wide range of CS intensities. ICF increased at higher CS intensity. We conclude that SICI is suppressed and ICF is facilitated during the SP and the effects are separate from the interruption of voluntary drive.
Transcranial magnetic stimulation (TMS) has become a widely used method in neuroscience research (Hallett, 2000) and can be used to test several intracortical inhibitory and facilitatory circuits. A suprathreshold TMS pulse applied to the primary motor cortex (M1) during voluntary muscle contraction causes a motor evoked potential (MEP) in the target muscle followed by a period of suppression in the ongoing electromyographic (EMG) activity, known as the silent period (SP). The SP was found to be prolonged in some patients with stroke (Classen et al. 1997) and shortened in Parkinson's disease (Priori et al. 1994).
Previous study investigating the effects of different levels of muscle contraction suggested that proprioceptive input induced by muscle twitch plays no major role in generating the SP (Inghilleri et al. 1993). Using the H-reflex to test the spinal motoneuron pools during the SP, it was proposed that the early part of the SP is at least in part related to spinal inhibition, whereas the later part depends on the interruption of voluntary drive at cortical level (Fuhr et al. 1991). However, a loss of cortical voluntary drive onto the spinal motoneuron may not be the only cause of the SP. When a suprathreshold conditioning stimulation is applied 50–200 ms prior to the test stimulation at rest, the test MEP is inhibited, a phenomenon referred to as long interval intracortical inhibition (LICI) (Valls-Soléet al. 1992; Wassermann et al. 1996; Sanger et al. 2001). Furthermore, it is likely that the SP is mediated by γ-aminobutyric acid (GABA), a widely distributing cortical inhibitory transmitter. Intrathecal administration of baclofen, a GABAB receptor agonist, produced a dose-dependent increase in the SP (Siebner et al. 1998). Tiagabine, a GABA uptake blocker, prolonged the SP induced by TMS whereas the SP induced by peripheral nerve stimulation was unchanged (Werhahn et al. 1999). Therefore, it is likely that the SP is related to cortical inhibitory mechanisms.
Paired-pulse TMS protocols can be used to study intracortical neural circuits in M1. Short interval intracortical inhibition (SICI) can be elicited by subthreshold conditioning stimulation (CS) followed by suprathreshold test stimulation (TS) at interstimulus intervals of 1–5 ms, whereas intracortical facilitation (ICF) can be elicited at the intervals of 6–15 ms (Kujirai et al. 1993; Ziemann et al. 1996b). LICI can be elicited at interstimulus intervals of 50–200 ms with suprathreshold CS and TS (Valls-Soléet al. 1992; Wassermann et al. 1996). Using a triple-pulse protocol, it was suggested that in the resting state SICI and LICI were mediated by different neural populations and the LICI inhibited SICI (Sanger et al. 2001). In addition, ICF showed a trend to increase at the presence of LICI. However, the effects of voluntary contraction on the interactions between LICI, SICI and ICF have not been investigated. Voluntary contraction increases the excitability of the motor pathway and was found to decrease both SICI and ICF (Ridding et al. 1995; Hanajima et al. 2002). Additionally, voluntary contraction can cancel the suppressive effects of low-frequency rTMS on the M1 (Touge et al. 2001; Todd et al. 2006). Therefore, examining the interaction between LICI and SICI/ICF in the presence of voluntary contraction will lead to further understanding of intracortical inhibitory and facilitatory circuits in the M1 and may help in the interpretation of future studies in disease states. The purpose of the present study is to examine how SICI and ICF change during the SP. We hypothesize that SICI will be decreased and ICF increased during the SP compared to rest.
Methods
Subjects
We studied 11 right-handed healthy subjects (six women and five men, aged 18–37 years). Handedness (laterality quotient, 96.6 ± 8.1) was confirmed using the Oldfield Handedness Inventory (Oldfield, 1971). All subjects provided written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the University Health Network (Toronto) Research Ethics Board.
EMG recording
Surface EMG was recorded from the right first dorsal interosseous (FDI) muscle with 9 mm diameter Ag–AgCl surface electrodes. The active electrode was placed over the muscle belly, and the reference electrode over the metacarpophalangeal joint of the index finger. The signal was amplified (1000×), band-pass filtered (2 Hz to 2.5 kHz, Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada), digitized at 5 kHz by an analog-to-digital interface (Micro1401, Cambridge Electronics Design, Cambridge, UK) and stored in a computer for off-line analysis. The EMG signal passed through a leaky integrator and the EMG level was displayed to the subject on an oscilloscope. During the SP recording, subjects abducted their index finger to produce 20% of maximum EMG (calculated during maximum voluntary contraction, MVC) with the aid of visual and auditory feedback.
Transcranial magnetic stimulation
Three Magstim 200 stimulators (Magstim, Whitland, Dyfed, UK), two Bistim modules and a figure-of-eight shaped coil (outside diameter of each loop was 9.5 cm) were used to apply TMS to the left M1. Two stimulators were connected via a Bistim module. This Bistim module and the third Magstim 200 stimulator were connected to a second Bistim module. The TMS coil was connected to the second Bistim module. This setup allowed us to deliver three pulses of different stimulus intensities through the same coil at very short interstimulus intervals. This arrangement is associated with about 15% power attenuation (Sanger et al. 2001). The trigger pulses for TMS were delivered from a Micro1401 interface (Cambridge Electronics Design) controlled by Signal Software (3.07).
The handle of the coil pointed backward at 30–45 deg from the mid-sagittal line. The induced current was anterior-medially directed, approximately perpendicular to the central sulcus. With this orientation, corticospinal tract neurons are activated trans-synaptically and produce early I waves (Kaneko et al. 1996; Di Lazzaro et al. 2001). The optimal position for activation of the right FDI muscle was marked with a pen as the motor hot-spot. Special attention was paid to maintaining the position and orientation of the coil.
Rest motor threshold (RMT) and active motor threshold (AMT) were determined. RMT was defined as the minimum stimulator output that evoked MEPs of more than 50 μV in at least 5 out of 10 trials when FDI muscle was completely relaxed. AMT was the minimum stimulator output that induced MEPs of more than 200 μV in at least 5 out of 10 consecutive trials during voluntary contractions of 20% maximum.
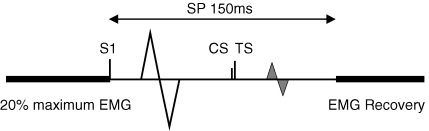
Up to three stimuli were used in each trial and we named them stimulus 1 (S1), CS and TS (Fig. 1). S1 was applied first and used to elicit the SP, while CS and TS were used to elicit SICI or ICF during the SP. In all experiments the intensity of S1 was adjusted to evoke an SP of about 150 ms (calculated from the time of TMS delivery to the first return of EMG activity). The time between trials was at least 6 s. Breaks were allowed between experiments to avoid fatigue.
Figure 1.
Experimental design Subject performed 20% of maximum EMG. Up to three stimulations were included in the experiment. S1 alone can evoke a large MEP followed by a SP of 150 ms. CS and TS refer to the conditioning and test stimulations for eliciting SICI and ICF. They were delivered at different times during the SP. The fluctuation of the second MEP (grey) was measured to evaluate the SICI and ICF during the SP.
Experiment 1: time course of MEP inhibition during the SP
TMS was applied while the subjects (n = 11) maintained a constant muscle contraction of 20% maximum. Two stimuli, S1 and TS, were used. TS intensity was set at 140% RMT and was delivered after S1 at one of 12 intervals from 70 to 180 ms in increments of 10 ms. We did not select the S1–TS intervals less than 70 ms because they might be included in the MEP evoked by S1, and the early part of the SP was at least partly due to the decreased spinal excitability (Fuhr et al. 1991; Ziemann et al. 1993; Hallett, 1995). Ten trials of each S1–TS interval and of TS alone (total of 130 trials) were delivered in random order for each subject.
Experiment 2: MEP threshold during the SP
Three S1–TS intervals of 80, 110 and 140 ms were tested in all 11 subjects. The motor thresholds (MT) in the presence of S1 are termed MT80, MT110 and MT140. The S1–TS interval of 80 ms was selected because it was just after the MEP evoked by S1. The interval of 110 ms was about the mid point of the SP and the interval of 140 ms was just before EMG recovery. The MT at 170 ms after S1 (after EMG recovery) was also determined and was termed MT170.
Experiment 3: SICI and ICF during the SP
SICI and ICF during the SP were investigated using a triple-pulse protocol in eight subjects. The level of muscle contraction (20% maximum) and S1 intensity (to generate SP of 150 ms) was identical to experiments 1 and 2. TS was delivered at intervals of 80, 110 and 140 ms after the S1. Since SICI and ICF changed with the different test MEP amplitudes (Sanger et al. 2001; Roshan et al. 2003), the TS intensity was adjusted at each interval to evoke a MEP of 1 mV (in at least 5 out of 10 trials) in the presence of S1. CS was delivered before the TS, at CS–TS intervals of 1 and 2.5 ms for SICI, and 10 ms for ICF. The CS–TS intervals of 1 and 2.5 ms were selected because previous studies reported maximum SICI at these intervals, and they are likely to be mediated by different mechanisms (Fisher et al. 2002; Roshan et al. 2003). The CS intensity was set at 95% of AMT. Ten trials for each condition (three CS–TS intervals of 1, 2.5, 10 ms and TS alone, total of 40 trials) were delivered in random order. Different S1–TS intervals (80, 110 and 140 ms) were tested in separate sessions. In another session, we recorded SICI and ICF at rest (without S1) with the same interstimulus intervals and CS (95% AMT) and TS (evoke ∼1 mV MEP) intensities used in the main experiment.
We performed three additional control experiments. The first control experiment examined whether SICI and ICF returned back to baseline after the EMG recovery. SICI and ICF at an S1–TS interval of 170 ms were investigated in eight subjects. The CS intensity was 95% AMT and the TS intensity was adjusted to evoke 1 mV MEPs. The results were compared to active SICI and ICF during constant muscle contraction of 20% maximum without the preceding S1 pulse.
The second control experiment was conducted in six subjects and was designed to address the possibility that the effects of CS might also be inhibited during the SP. Since MEP latencies and background EMG levels during the SP were similar to the rest condition (see Results), we adjusted the CS to 80% MT determined during the SP in the presence of S1. SICI (1 and 2.5 ms) and ICF (10 ms) were investigated at S1–TS intervals of 80, 110 and 140 ms and CS intensities used were 80% of MT80, MT110 and MT140, respectively. TS intensity was adjusted to evoke a MEP of 1 mV in the presence of S1 at different S1–TS intervals. The results were compared to SICI and ICF in the rest (CS 80% RMT) or active (during 20% maximum, CS 95% AMT) conditions.
The third control experiment investigated the influence of higher TS intensities used during the SP on the SICI and ICF in six subjects. We matched the TS intensities instead of MEP size during the SP and at rest. The TS intensity that evoked 1 mV MEPs during the SP was used both during the SP and at rest (without S1), resulting in higher MEP amplitude at rest. CS intensity was set at 95% of AMT and the S1–TS interval of 110 ms was investigated.
Experiment 4: effect of different CS intensities
SICI and ICF change with different CS intensities. In six subjects we examined how the SP modifies SICI and ICF by testing different CS intensities from 0.3 to 0.9 MT110 in increments of 0.1 MT110 at the S1–TS interval of 110 ms. The results are compared to the same protocol performed at rest with CS intensities from 0.3 to 0.9 RMT. During both the SP and at rest, the TS intensity was adjusted to evoke 1 mV MEPs. The configuration of stimulation pulses was same as in experiment 3. Different CS intensities were tested in separate runs.
Data analysis
SP durations were calculated from the time of TMS delivery to the first recovery of sustained EMG activity. MEP amplitudes were measured peak-to-peak. In experiment 1, the MEP amplitudes evoked by paired pulses (S1–TS) were expressed as a percentage of the mean MEP amplitude of TS alone. In experiment 3 and 4, SICI and ICF during the SP were calculated by expressing the amplitude of the MEPs evoked by the triple TMS pulses (S1–CS–TS) as a percentage of the mean amplitude of MEP evoked by S1–TS (without CS). For the MEP recorded without the S1, the amplitude of each CS–TS evoked MEP was expressed as a percentage of the mean MEP amplitude evoked by TS alone. Values above 100% indicate facilitation and values below 100% indicate inhibition.
The background EMG area for 10 ms preceding the TMS artifact was measured and was normalized to the maximum EMG (background EMG area during MVC). The background EMG levels at different S1–TS intervals were compared to those at rest or active. MEP latency was measured at every S1–TS interval.
Statistical analysis
For experiments 1 and 2, one-way repeated measures analysis of variance (ANOVA) was used to examine the effects of interstimulus interval (within-subject factor) between S1 and TS on MEP amplitude and MEP threshold. Student's paired t test with Bonferroni's correction for multiple comparisons was used to determine which interstimulus intervals showed significant change from baseline. A two-way repeated measures ANOVA (S1–TS intervals and CS–TS intervals were the within-subject factors) was conducted for experiment 3. Additionally, one-way repeated measures ANOVA (S1–TS intervals as the within-subject factor) was applied at each CS–TS interval (1, 2.5 and 10 ms) to examine whether SICI and ICF varied at different times during the SP (80, 110 and 140 ms). Post hoc paired t tests with Bonferroni's correction were used for multiple comparisons. In experiment 4, three-way repeated measures ANOVA (CS intensities, CS–TS intervals and states (during the SP versus rest) were the within-subject factors) and same post hoc tests were performed to determine whether SICI and ICF were different during the SP and at rest at various CS intensities. In addition, two-way repeated measures ANOVA (CS intensities and CS–TS intervals of 1 and 2.5 ms were the within-subject factors) were conducted to examine whether SICI varied at different CS–TS intervals at rest and during the SP. SPSS (15.0) software was used for statistical analysis. The significance level was set at P < 0.05. Unless otherwise stated, values are reported as means ± standard deviation.
Results
Experiment 1: Time course of MEP inhibition during the SP
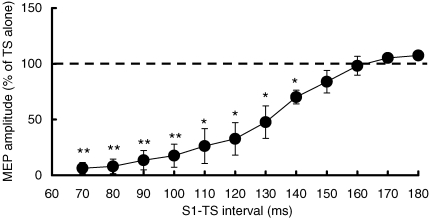
S1 intensity used was 79.6 ± 9.9% (n = 11) of stimulator output, producing MEPs of 4.01 ± 1.29 mV in amplitude and SP durations of 151.2 ± 4.3 ms. Figure 2 shows that MEPs were inhibited during the SP. ANOVA showed a significant effect of S1–TS interval on MEP amplitude (F12120= 23.21, P < 0.001). Post hoc testing showed significant MEP inhibition at the S1–TS intervals from 70 to 140 ms (70–100 ms, P < 0.01; 110–140 ms, P < 0.05) compared to TS alone (MEP amplitude 1.62 ± 0.64 mV). At S1–TS intervals longer than 150 ms, the conditioned MEP returned to the control level (TS alone).
Figure 2.
Time course of MEP during the SP Mean values and standard deviations (n = 11) of MEP amplitudes at S1–TS intervals from 70 to 180 ms. Each MEP amplitude was a percentage value of that evoked by TS alone (dash line). **P < 0.01, *P < 0.05, comparing to MEP evoked by TS alone.
Experiment 2: MEP threshold during the SP
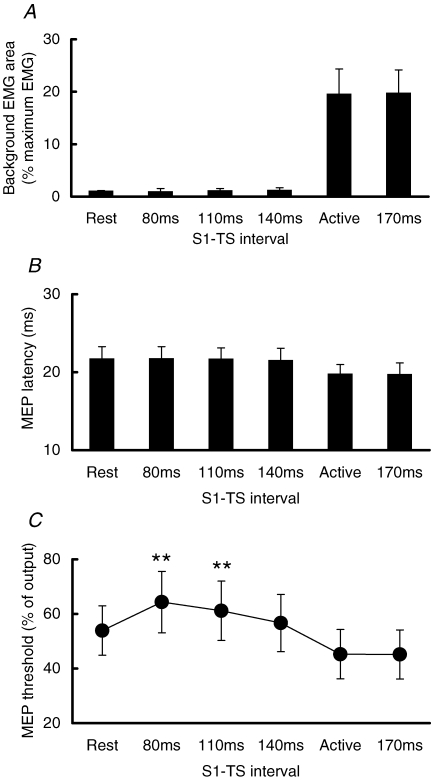
Figure 3A shows the background EMG area 10 ms before the TMS artifact at different times. Background EMG during the SP (80, 110, 140 ms after S1) was the same as the rest condition and probably represented the noise level of the system. After EMG recovery (170 ms after S1), the background EMG was similar to that of constant 20% maximum contraction. MEP latencies are shown in Fig. 3B. The MEP latencies during the SP (S1–TS intervals of 80, 110, 140 ms) were similar to that at rest and were about 2 ms longer than active MEPs during constant muscle contraction or after the SP (S1–TS interval of 170 ms). Figure 3C shows the MEP thresholds at different S1–TS intervals for all subjects. ANOVA showed a significant effect of S1–TS interval on MEP threshold (F3,30= 22.10, P < 0.001). Post hoc testing indicated that MT80 and MT110 were significantly higher than RMT (both P < 0.01), and MT140 was not significantly different from RMT (P > 0.05). A paired t test showed that MT170 was not different from AMT (P = 0.68).
Figure 3.
Background EMG area, MEP latency and threshold at different times during the SP Mean values and standard deviations (n = 11) of background EMG areas (A), MEP latencies (B) and MEP thresholds (C) at S1–TS intervals of 80, 110, 140 and 170 ms. Note that the intervals of 80, 110 and 140 ms were during the SP, and 170 ms was after EMG recovery. **P < 0.01, comparing to MEP threshold at rest (RMT).
Experiment 3: SICI and ICF during the SP
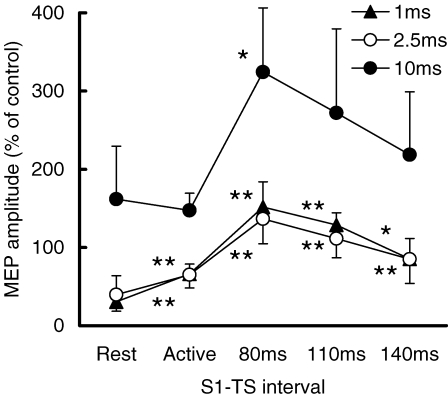
The TS intensities to evoke 1 mV MEPs were 78.1 ± 13.7% of stimulator output at S1–TS intervals of 80 ms, 74.3 ± 10.1% at 110 ms and 68.9 ± 12.3% at 140 ms. They were higher than that used at rest (66.4 ± 13.1%). The MEP amplitude induced by S1–TS was 0.99 ± 0.45 mV at S1–TS intervals of 80 ms, 1.14 ± 0.34 mV at 110 ms and 1.14 ± 0.49 mV at 140 ms. They were matched to the MEP amplitude obtained at rest (1.10 ± 0.29 mV).
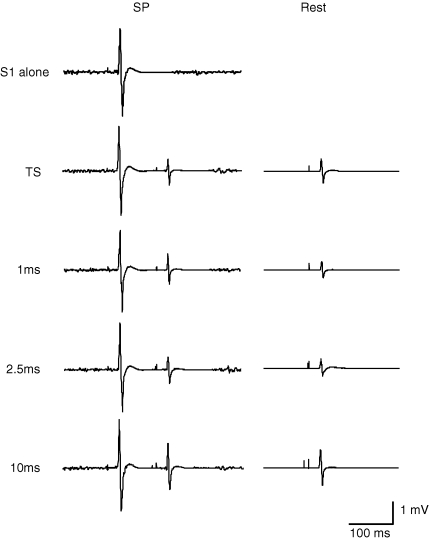
SICI was suppressed and ICF was increased during the SP. Figure 4 shows the recordings from a representative subject. The left panel shows the recordings during the SP at S1–TS interval of 110 ms, and the right panel showed the recordings at rest. The results from all subjects are shown in Fig. 5A. ANOVA showed significant effects of both S1–TS interval (F3,42= 23.15, P < 0.001) and CS–TS interval (F2,42= 41.38, P < 0.001) on MEP amplitude. The interaction between S1–TS and CS–TS intervals was also significant (F6,42= 8.83, P < 0.001). We conducted further analysis with separate one-way repeated measures ANOVA for each CS–TS interval. At all three CS–TS intervals, the effects of S1–TS intervals were significant (1 ms, F3,21= 8.12, P < 0.001; 2.5 ms, F3,21= 11.43, P < 0.001; 10 ms, F3,21= 10.31, P < 0.001). Post hoc testing showed that at CS–TS intervals of 1 and 2.5 ms, SICI was weaker during the SP at S1–TS intervals of 80, 110, 140 ms compared to rest (P < 0.01 for all comparisons). For a CS–TS interval of 10 ms, ICF during the SP was significantly increased compared to rest at S1–TS interval of 80 ms (P < 0.01) and 110 ms (P < 0.05), but the difference at 140 ms was not significant.
Figure 4.
Typical recordings of SICI and ICF during the SP (S1–TS interval of 110 ms) and at rest Every trace represents the average of 10 trials. Traces on the left side are recordings during the SP (with S1 applied 110 ms preceding the TS); those on the right side are recordings at rest. The top trace showed S1 alone was delivered. The second row shows the recordings without CS delivery (S1–TS during the SP, TS alone at rest). The traces from the third to the fifth row showed the CS–TS intervals of 1, 2.5 and 10 ms (triple-pulse during the SP, CS–TS at rest).
Figure 5.
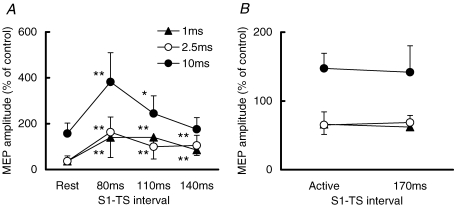
SICI and ICF during the SP when CS was 0.95 AMT Mean values and standard deviations (n = 8) of MEP amplitudes at different S1–TS intervals. Triangles show the MEPs at a CS–TS interval of 1 ms, open circles show those of 2.5 ms and filled circles show those of 10 ms. A, SICI and ICF during the SP (S1–TS intervals of 80, 110 and 140 ms) were compared to those at rest. B, SICI and ICF after EMG recovery (S1–TS interval of 170 ms) were compared to those at the active state. **P < 0.01, *P < 0.05, comparing the MEP during the SP to that at rest.
For the first control experiment, at S1–TS interval of 170 ms (Fig. 5B), both SICI and ICF returned to a similar level to during constant contraction at 20% maximum (Active). Two-way repeated measures ANOVA showed a significant effect of CS–TS interval (F2,14= 45.83, P < 0.001) but no significant effect of test condition (Active versus S1–TS 170 ms; F1,14= 0.45, P > 0.05).
In the second control experiment, CS was adjusted to 80% MT during the SP. There was also strong suppression of SICI at CS–TS intervals of 1 ms and 2.5 ms and increase in ICF during the SP (Fig. 6). Two-way repeated measures ANOVA showed significant effects of S1–TS interval (F4,40= 38.80, P < 0.001) and CS–TS interval (F2,40= 22.05, P < 0.001) on MEP amplitude. The interaction between S1–TS and CS–TS intervals was not significant (F8,40= 1.46, P > 0.05). Post hoc testing showed that at a CS–TS interval of 1 ms, active SICI and SICI at S1–TS intervals of 80, 110, 140 ms were reduced compared to rest (P < 0.01 for all comparisons). At a CS–TS interval of 2.5 ms, active SICI (P < 0.01) and SICI at S1–TS intervals of 80 (P < 0.01), 110 (P < 0.01), 140 ms (P < 0.05) were also reduced compared to rest. For ICF (CS–TS interval of 10 ms), stronger facilitation was found at S1–TS interval of 80 ms (P < 0.05), compared to rest.
Figure 6.
SICI and ICF during the SP when CS was adjusted to 0.8 MT Mean values and standard deviations (n = 6) of MEP amplitudes at different S1–TS intervals. Triangles show the MEPs at a CS–TS interval of 1 ms, open circles show those of 2.5 ms and filled circles show those of 10 ms. Note that CS intensities were adjusted to MT80, MT110 and MT140 for different S1–TS intervals, respectively. **P < 0.01, *P < 0.05, comparing the MEP during the SP to that at rest.
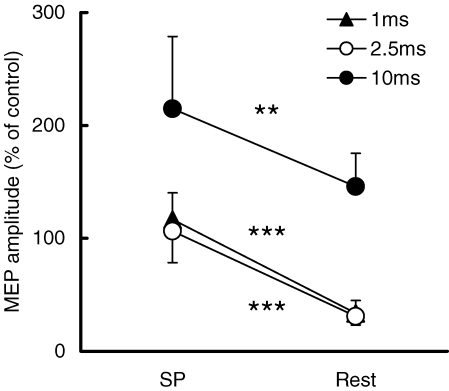
In the third control experiment, SICI and ICF at rest were investigated using the TS intensity that evoked 1 mV MEP at S1–TS interval of 110 ms (74.3 ± 10.1% stimulator output). With this TS intensity, MEP amplitude during the SP at S1–TS interval of 110 ms was 1.10 ± 0.39 mV and at rest (TS alone) was 1.42 ± 0.25 mV. Figure 7 shows that even with matched TS intensity, there was strong SICI at rest but not during the SP, and ICF increased during the SP. Paired t tests confirmed SICI was decreased (CS–TS interval 1 ms, P < 0.001; 2.5 ms, P < 0.001) and ICF was increased (P < 0.01) during the SP compared to rest.
Figure 7.
Different SICI and ICF during the SP and at rest when same TS intensity was used Mean values and standard deviations (n = 6) of MEP amplitudes at S1–TS interval of 110 ms and at rest. Triangles show the MEPs from a CS–TS interval of 1 ms, open circles show those of 2.5 ms and filled circles show those of 10 ms. Note that same intensity of TS was used during the SP and at rest. ***P < 0.001, **P < 0.01, comparing the MEP during the SP to that at rest.
Experiment 4: Effect of different CS intensities
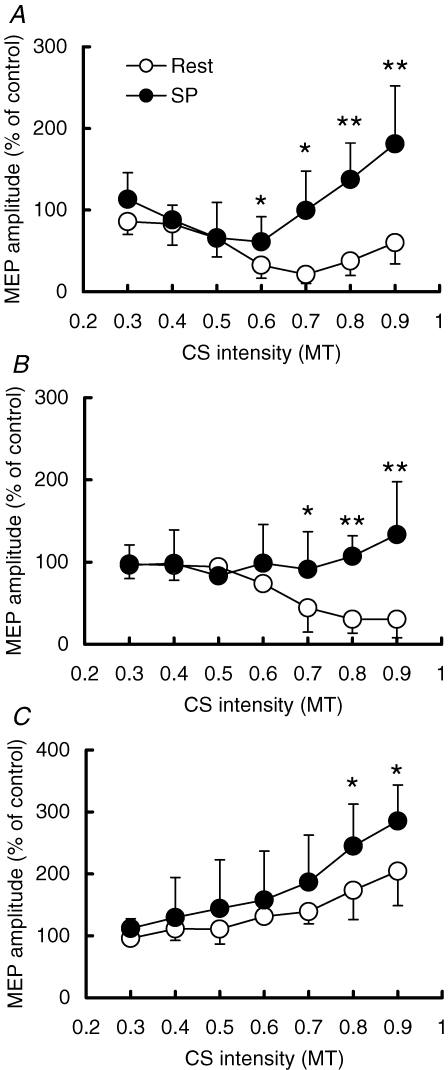
The results of different CS intensities during the SP (S1–TS interval of 110 ms) and at rest are shown in Fig. 8. Three-way repeated measures ANOVA showed significant effects of CS intensity (F6,60= 8.12, P < 0.001), CS–TS interval (F2,60= 24.33, P < 0.001) and state (Rest versus SP, F1,60= 34.21, P < 0.01) on MEP amplitude. There was a strong interaction between the CS intensity and state (F6,60= 9.22, P < 0.001), indicating that the effects of CS intensities differ depending on the activation state. At rest, post hoc testing showed that MEPs were inhibited at CS intensities from 0.5 to 0.9 MT (P < 0.05) at a CS–TS interval of 1 ms. There was no MEP inhibition at any CS intensities during the SP. SICI was significantly reduced during the SP compared to rest from 0.6 to 0.9 MT (0.6, 0.7 MT, P < 0.05; 0.8, 0.9 MT, P < 0.01). For a CS–TS interval of 2.5 ms, MEPs at rest were significantly inhibited at CS intensities from 0.6 to 0.9 MT (P < 0.05). There was significantly less SICI during the SP than at rest from 0.7 to 0.9 MT (0.7 MT, P < 0.05; 0.8, 0.9 MT, P < 0.01). For a CS–TS interval of 10 ms, MEPs at rest were facilitated at CS intensities from 0.6 to 0.9 MT (P < 0.05). ICF during the SP was significantly increased compared to rest at CS intensities of 0.8 and 0.9 MT (P < 0.05). In addition, two-way repeated measures ANOVA showed that SICI at CS–TS intervals of 1 and 2.5 ms were different at rest (F1,30= 8.46, P < 0.05), but not during the SP (F1,30= 0.36, P = 0.57). The effects of different CS intensities on MEP were significant both at rest (F6,30= 15.17, P < 0.001) and during the SP (F6,30= 4.95, P < 0.01). Post hoc tests showed that resting SICI was stronger at 1 ms than at 2.5 ms at CS intensities from 0.5 to 0.7 MT (P < 0.05) at rest. At a CS intensity of 0.9 MT, SICI at 2.5 ms became stronger (P < 0.05).
Figure 8.
Effect of variations in CS intensities on SICI and ICF Open circles show mean MEP amplitudes (n = 6) at rest, and filled circles show those during the SP. Horizontal axis indicates the CS intensity. Note that the intensities used during the SP were adjusted to the ratios of MT110, and those used at rest were the ratios of RMT. Vertical axis indicates the MEP amplitude. A, SICI at a CS–TS interval of 1 ms. B, SICI at a CS–TS interval of 2.5 ms. C, ICF at a CS–TS interval of 10 ms **P < 0.01, *P < 0.05, comparing the MEP during the SP to that at rest.
Discussion
We examined how SICI and ICF are modulated during the SP. The main findings are the following. (1) MEP is inhibited and threshold increases during the SP although the MEP latency and background EMG level are the same as those at rest. (2) Shortly after the recovery of ongoing EMG, MEP amplitude, threshold, latency, SICI and ICF return to the values during constant voluntary muscle contraction. (3) SICI is suppressed and ICF is increased during the SP. (4) Reduction of SICI occurs over a wide range of CS intensities, whereas increase in ICF only occurs at higher CS intensity.
Interruption of voluntary drive during the SP
Previous studies concluded that the SP induced by TMS is of different origin from the MEP and is due to several different mechanisms (Hallett, 1995). In general, MEP indicates the excitability of the motor pathway and threshold indicates the most sensitive part in the pathway (Rothwell, 1997). Pharmacological studies suggested that MT reflects membrane excitability but it is also influenced by non-NMDA glutamatergic agents (Ziemann et al. 1996a; Di Lazzaro et al. 2003; Ziemann, 2004). The present study showed that MEP was reduced and MT was increased during the SP, suggesting that the excitability of the motor pathway is inhibited by S1 (during the SP). This is consistent with the previous studies of resting (Valls-Soléet al. 1992) and active (Wassermann et al. 1996) LICI. The increased MT (MT80 and MT110) during the SP was similar to that reported by Tergau et al. (1999). Based on the idea that the difference between RMT and AMT indicates the effect of voluntary drive, they reported that voluntary drive is completely suppressed during the early to middle part of the SP although the results may also be interpreted as an interruption of the access of voluntary drive to pyramidal tract neurons. Our result that background EMG during the SP is the same as at rest is consistent with this finding. Moreover, we measured the MEP latencies at different times during the SP. If TMS is applied at rest, pyramidal tract neurons will fire when summation of multiple I-waves leads its membrane potential to threshold level. When the voluntary drive is projected to the M1 during the voluntary muscle contraction, it can raise the excitability of pyramidal tract neurons. Therefore, with only the I1 wave pyramidal tract neurons may achieve their firing threshold, leading to the shortened latency during voluntary muscle contraction. The present results showed that MEP latencies during the SP are the same as that at rest and about 2 ms longer than that at the active state. This evidence also supports that voluntary drive is interrupted during the SP.
Changes in SICI and ICF during the SP
The interruption of voluntary drive may not be the only cause of the SP. We investigated SICI and ICF during the SP. It was found that SICI was suppressed and ICF was increased at all three S1–TS intervals tested, at the beginning, middle and end of the SP. We performed three control experiments. The first showed that shortly after EMG recovery at the S1–TS interval of 170 ms, the background EMG, SICI and ICF all returned to the same levels as constant muscle contraction. Therefore, normalization of SICI and ICF follows a similar time course as the termination of the SP. This finding supports the hypothesis that mechanisms mediating the changes in SICI and ICF during the SP and mediating the EMG suppression itself are similar. In the second control experiment we used CS intensities adjusted to reflect higher MT during the SP and showed that the suppression of SICI and increase in ICF during the SP cannot be explained by suppression of the effects of the CS. In the main experiment, we adjusted the TS intensity to generate a MEP of ∼1 mV both during the SP and at rest in order to recruit a similar amount of pyramidal neurons, resulting in higher TS intensity used during the SP than at rest. The third control experiment showed that the results cannot be explained by different TS intensities used during the SP and at rest.
Since both SICI (Nakamura et al. 1997; Di Lazzaro et al. 1998) and LICI (Chen et al. 1999; Di Lazzaro et al. 2002) predominately inhibit late I-waves, it could be argued that epidural volleys evoked by the second suprathreshold stimulus consisted of mainly early I-waves and were less susceptible to SICI. While this is a possible mechanism, it is unlikely to account for our results for several reasons. First, the third control experiment showed that when the higher TS intensities used during the SP were applied at rest leading to higher test MEP amplitudes, the degree of SICI was comparable to that obtained with a target MEP of 1 mV used in the main experiment (Figs 5A and 7, values for ‘Rest’ columns). These findings suggest that the extra I-waves recruited by the stronger, adjusted TS applied during the SP have similar susceptibility to SICI as the I-waves produced by the TS that evoked 1 mV MEPs at rest. Moreover, over a range of test MEP amplitudes from 0.2 mV to 4 mV, LICI decreases but SICI increases with higher test MEP amplitude (Sanger et al. 2001; Daskalakis et al. 2004), suggesting that LICI predominately affects neurons activated at low intensities while neurons activated at high intensities are more susceptible to SICI. Thus, SICI and LICI are likely to have a predilection for different circuits involved in producing MEPs. In addition, while changes in the composition of I-waves may lead to reduced inhibition, this mechanism cannot explain the finding that application of SICI during the SP resulted in no MEP inhibition or even MEP facilitation (Figs 5–7).
Mechanism of decreased SICI during the SP
Since SICI is likely to be due to the combined effects of inhibition and facilitation (Roshan et al. 2003; MacKinnon et al. 2005), we varied the CS intensity in experiment 4 to investigate whether the suppressed SICI during the SP is caused by a loss of inhibition or by increased facilitation. No SICI or ICF was found at low CS intensities (0.3 and 0.4 RMT). SICI at CS–TS intervals of 1 and 2.5 ms increased with higher CS intensity up to 0.9 RMT. These results are consistent with previous reports (Chen et al. 1998; Butefisch et al. 2003; MacKinnon et al. 2005) and support the view that the threshold of inhibitory interneurons was lower than that of facilitatory neurons. During the SP (in the presence of S1), there was no SICI at any CS intensity at a CS–TS interval of either 1 ms or 2.5 ms. Therefore, decreased SICI during the SP was not due to a shift in the stimulus (CS intensity)–response curve for SICI. Since the difference between SICI at rest and during the SP was present at low CS intensities when inhibition just began to emerge, suppression of SICI during the SP is likely to be due to decreased inhibition rather than increased facilitation. This is different from observations in the unaffected hemisphere of stroke patients (Butefisch et al. 2003) or in patients with Parkinson's disease (MacKinnon et al. 2005) where differences between patients and control only emerge at higher CS intensities and changes in SICI are attributed to increased facilitation rather than reduced inhibition. Increased ICF during the SP was observed at higher CS intensities than decreased SICI, also supporting the notion that reduction in SICI during the SP is due to decreased inhibition rather than increased facilitation.
Possible roles of GABAA and GABAB receptors
GABA is the most important inhibitory neurotransmitter in the brain and is distributed throughout all layers of the cortex. GABAA receptors mediate short-lasting Cl−-dependent inhibition, whereas the GABAB receptors mediate long-lasting K+-dependent inhibition (McCormick, 1992; Deisz, 1999). In vitro studies showed that GABAB receptors cause presynaptic inhibition of GABA release in neocortical and hippocampal neurons in rats and humans (Davies et al. 1990; Deisz, 1999). Previous studies have suggested that SICI is mediated by the GABAA receptor (Ziemann et al. 1996a, b). Because of the long duration of LICI and the SP, they may be mediated by GABAB receptors (Chen et al. 1998; Siebner et al. 1998). Sanger et al. (2001) showed that LICI inhibits SICI at rest and suggested that this may be due to GABAB mediated presynaptic inhibition of GABA release. GABAB mediated inhibition of GABAA effects also explain the results of pharmacological studies. The GABA uptake inhibitor tiagabine was found to prolong the SP and increase LICI but decrease SICI (Werhahn et al. 1999). Administration of the GABAB receptor agonist baclofen decreases SICI and increases LICI in the resting state in normal subjects (McDonnell et al. 2006). The current findings suggest that inhibition of SICI by LICI also occurs in the active state during the SP. The most likely mechanism is GABAB mediated inhibition of GABA release, probably at the presynaptic level.
Differences between SICI at 1 and 2.5 ms
Our results showed that the CS intensity recruitment curve for CS–TS intervals of 1 and 2.5 ms had different shapes at rest (Fig. 8A and B). At CS intensities of 0.5–0.7 RMT, SICI at 1 ms was stronger than that at 2.5 ms. This finding is consistent with previous studies which suggested that SICI at 1 and 2.5 ms is due to different mechanisms (Fisher et al. 2002; Roshan et al. 2003).
With high CS intensities of 0.9 RMT there was less inhibition at a CS–TS interval of 1 ms than at 2.5 ms. This difference may be due to short-interval intracortical facilitation or I-wave facilitation (Chen & Garg, 2000; Ziemann & Rothwell, 2000; Ilic et al. 2002). The CS may raise the excitability of pyramidal neurons and the TS may fire these neurons because of temporal summation. A CS–TS interval of 1 ms is likely to involve the first wave (I1 wave) and a CS–TS interval of 2.5 ms is likely to involve the second wave (I2 wave) of short-interval intracortical facilitation. Greater facilitation at a CS–TS interval of 1 ms compared to 2.5 ms can be explained by the lower threshold and greater extent of facilitation for the I1 wave compared to the I2 wave of short-interval intracortical facilitation.
It has been suggested that SICI at 1 ms may be due to the neural refractoriness, while that SICI at 2.5 ms may be due to the synaptic inhibition (Fisher et al. 2002). Our results showed that SICI at 1 ms and 2.5 ms were suppressed to a similar extent during the SP. Since the SP is unlikely to affect neural refractoriness, the results may support the view that synaptic inhibition is responsible for the SICI at 1 ms (Roshan et al. 2003) although contributions from neuronal refractoriness cannot be completely ruled out.
Increase in ICF during the SP
We found that resting ICF began at higher CS intensities than SICI and increased with higher CS intensities (Fig. 8C), similar to previous reports (Chen et al. 1998). Sanger et al. (2001) reported that LICI caused a non-significant increase in ICF in the resting state. In the present study, we found that S1 significantly increased ICF during voluntary muscle contraction. One possible explanation is that the SP can facilitate the ICF directly, and this facilitatory effect only occurs on interneurons with high threshold (CS intensities of 0.8 and 0.9 MT). However, other mechanisms are likely to be involved and the mechanisms underlying ICF remain unclear. The interruption of voluntary drive during the SP (Tergau et al. 1999) may also cause the increase in ICF during the SP, since voluntary drive inhibits the ICF (Ridding et al. 1995; Hanajima et al. 2002). That is, during the voluntary muscle contraction the baseline of the ICF is inhibited by the voluntary drive. When the S1 was delivered it interrupted the voluntary drive, leading to increased ICF indirectly. Moreover, it was reported that ICF was not associated with any increase in descending corticospinal volleys (Di Lazzaro et al. 2006). Therefore, ICF may be mediated by multiple mechanisms and include facilitatory effects at both the cortical and spinal levels.
Possible interactions between SP, voluntary drive, SICI and ICF
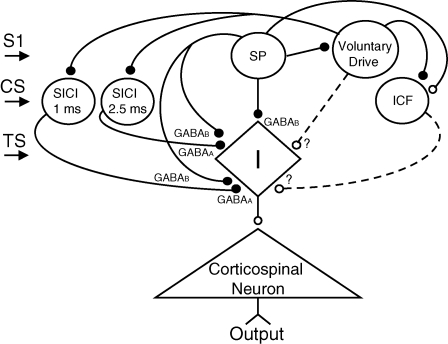
Figure 9 shows a possible model to explain the present findings. We suppose that different neural populations mediate the SP, SICI at 1 ms, SICI at 2.5 ms and ICF. These different groups of interneurons modify a group of common interneurons (labelled I, leading to I waves) that give rise to facilitatory input to corticospinal neurons. However, it should be noted that the mechanisms of generation of I waves remain uncertain (Ziemann & Rothwell, 2000). The CS activates the SICI and ICF neurons, S1 activates the SP neurons and the TS activates the common interneurons. While SICI is shown as due to somatic firing of inhibitory neurons, GABAA inhibition may also occur through glutamate receptor mediated axo-axonic excitation of the nerve terminal of inhibitory neurons (Ren et al. 2007). The voluntary drive is projected to the M1 from other brain areas and is shown to raise the excitability of the common interneurons to produce EMG activities, although it may also have a direct effect on the corticospinal neuron. Without the S1, the common interneurons receive inhibitory inputs from SICI (mediated by GABAA receptors) and facilitatory inputs from ICF neurons. This may enable the common interneurons to keep the balance between inhibition and facilitation, by which it has a high sensitivity to the voluntary drive. When S1 activates the SP neurons, they inhibit the common interneurons via the GABAB receptor leading to reduced output from corticospinal neurons (Inghilleri et al. 1993; Wassermann et al. 1996; Chen et al. 1998; Siebner et al. 1998; Tergau et al. 1999) (Figs 2 and 3C). We propose that the SP neurons also inhibit SICI neurons presynaptically, causing reduction of SICI during the SP. The SP neurons also interrupt voluntary drive (Tergau et al. 1999) and may facilitate the ICF neurons, causing the increase of ICF during the SP. However, other mechanisms may contribute to the increase of ICF during the SP since the neural origin of ICF has not been fully elucidated (Di Lazzaro et al. 2006).
Figure 9.
Proposed model The diamond (labelled I) at the centre indicates a group of common interneurons. They project to the pyramidal neurons that produce the output to the spinal motoneurons. The common interneurons receive inputs from the SICI, ICF and SP neurons. Voluntary drive is projected onto the common interneurons from other brain areas. The small filled circles show inhibitory connections, and small open circles show facilitatory connections. S1 activates the SP neuron. CS activates the SICI (1 ms and 2.5 ms) and ICF neurons. TS activates the common interneuron. SICI (1 ms and 2.5 ms) is shown as mediated by GABAA receptor, whereas the SP neuron is mediated by GABAB receptor. The SP neurons are shown to inhibit SICI through the presynaptic inhibition and facilitate ICF. The question marks and dash lines indicate the interactions are not proven.
Conclusions
The SP is not due to the interruption of voluntary drive alone as there are changes in intracortical inhibitory and facilitatory circuits during the SP. Suppression of SICI during the SP may be related to GABAB mediated presynaptic inhibition of GABAA activity. Since SP or active LICI have been reported to be abnormal in several movement disorders such as stroke (Classen et al. 1997), Parkinson's disease (Priori et al. 1994) and dystonia (Chen et al. 1997), investigations of how intracortical circuits are modulated during voluntary movement in these conditions may help to further understand their pathophysiology.
Acknowledgments
The authors are grateful to Dr Aimee Nielson for her comments on the manuscript. This study was supported by the Canadian Institutes of Health Research (MOP 62917).
References
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Caños M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic stroke. Brain. 1997;120:605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA. GABAB receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999;38:1755–1766. doi: 10.1016/s0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002;113:1673–1679. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Fisher J, Nakamura Y, Bestmann S, Rothwell C, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation. Negative effects. Adv Neurol. 1995;67:107–113. [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired- pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human motor cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral-cortex. J Clin Neurophysiol. 1992;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson's disease. Ann Neurol. 2005;58:516–524. doi: 10.1002/ana.20599. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edingburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial magnetic brain stimulation in normal subjects, patients with Parkinson's disease and drug-induced parkinsonism. Brain. 1994;117:317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- Ren M, Yoshimura Y, Takada N, Horibe S, Komatsu Y. Specialized inhibitory synaptic actions between nearby neocortical pyramidal neurons. Science. 2007;316:758–761. doi: 10.1126/science.1135468. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Meth. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124:447–454. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Todd G, Flavel SC, Ridding MC. Low-intensity repetitive transcranial magnetic stimulation decreases motor cortical excitability in humans. J Appl Physiol. 2006;101:500–505. doi: 10.1152/japplphysiol.01399.2005. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscle. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelényi A, Hömberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]