Abstract
Coronary blood flow is tightly coupled to myocardial oxygen consumption to maintain a consistently high level of myocardial oxygen extraction over a wide range of physical acitivity. This tight coupling has been proposed to depend on periarteriolar oxygen tension, signals released from cardiomyocytes (adenosine acting on KATP channels) and the endothelium (prostanoids, nitric oxide, endothelin) as well as neurohumoral influences (catecholamines, endothelin), but the contribution of each of these regulatory pathways, and their interactions, to exercise hyperaemia in the human heart are still incompletely understood. Thus, in the human heart, nitric oxide, prostanoids, adenosine and KATP channels each contribute to resting tone, but evidence for a critical contribution to exercise hyperaemia is lacking. In dogs KATP channel activation together with adenosine and nitric oxide contribute to exercise hyperaemia in a non-linear redundant fashion. In contrast, in swine nitric oxide, adenosine and KATP channels contribute to resting coronary resistance vessel tone control in a linear additive manner, but are not mandatory for exercise hyperaemia in the heart. Rather, exercise hyperaemia in swine appears to involve KCa channel opening that is mediated, at least in part, by exercise-induced β-adrenergic activation, possibly in conjunction with exercise-induced blunting of an endothelin-mediated vasoconstrictor influence. In view of these remarkable species differences in coronary vasomotor control during exercise, future studies are required to determine whether exercise hyperaemia in humans follows a canine or porcine control design.
From birth to death, the heart continuously contracts at a rate of at least 60 beats per minute. The continuous work results in a high metabolic rate and, consequently, myocardial oxygen consumption is already very high under resting conditions. One way the heart has adapted to the high oxygen demands is by achieving a high level of oxygen extraction, reaching as much as 70–80% of the arterially delivered oxygen (Feigl, 1983; Laughlin et al. 1996). This high level of oxygen extraction is facilitated by a high capillary density of 3000–4000 mm−2 (Laughlin & Tomanek, 1987). Due to the high level of oxygen extraction under basal resting conditions and because the heart depends principally on aerobic metabolism, an increase in myocardial oxygen consumption such as occurs during exercise must be accompanied by an increase in oxygen supply, i.e. an increase in coronary blood flow. Thus, tight (metabolic) control of the coronary resistance vessels is critical for maintaining an adequate oxygen supply to the myocardium, as the inability of the coronary vasculature to dilate in response to increments in myocardial oxygen demand will almost immediately result in myocardial ischaemia and loss of cardiac function.
A sensitive way to study alterations in coronary vascular tone in relation to myocardial metabolism is the relation between coronary venous oxygen tension (PO2) and myocardial oxygen consumption (Tune et al. 2004). For example, an increase in coronary resistance vessel tone will limit coronary blood flow and hence oxygen supply, forcing the myocardium to increase oxygen extraction in order to maintain oxygen consumption, which results in a lower coronary venous PO2. Conversely, a decrease in resistance vessel tone will increase coronary flow and oxygen supply, allowing the myocardium to reduce oxygen extraction, so that coronary venous PO2 increases. The coronary venous PO2 thus represents an index of myocardial tissue oxygenation, that is the balance between oxygen supply and demand, which is determined by coronary resistance vessel tone (Tune et al. 2004).
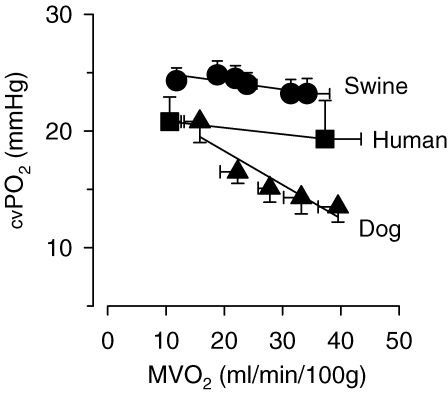
In dogs, the increase in coronary blood flow and myocardial oxygen supply that occurs during treadmill exercise does not fully match the increased oxygen demand of the myocardium, so that even during mild to moderate levels of exercise an increase in oxygen extraction and hence a decrease in coronary venous PO2 occurs (Fig. 1). The decrease in coronary venous PO2 that occurs in the dog during exercise is, at least in part, due to α-adrenergic vasoconstriction (Heyndrickx et al. 1982; Bache et al. 1987), indicating that an increase in α-adrenergic coronary vasoconstriction can compete with metabolic vasodilatation during exercise. In contrast, in swine, myocardial oxygen extraction and coronary venous PO2 are maintained even during heavy treadmill exercise, while in humans minimal changes in oxygen extraction occur at mild to moderate levels of exercise although an increase in oxygen extraction and decrease in coronary venous oxygen content have been reported in humans during heavy exercise (Laughlin et al. 1996). The relatively constant coronary venous PO2 over a wide range of myocardial work in swine, as compared with dogs, has been attributed to a lack of significant α-adrenergic control of vasomotor tone in porcine coronary resistance vessels during treadmill exercise (Duncker et al. 1998a). These findings indicate that considerable interspecies differences of coronary vasomotor control exist during exercise.
Figure 1.
Relation between myocardial oxygen consumption (MVO2) and coronary venous oxygen tension (cv PO2) at rest and during treadmill exercise in swine (Haitsma et al. 2001), humans (Heiss et al. 1976) and dogs (Bache & Dai, 1990) Note that exercise does not alter coronary venous oxygen tension in swine, whereas it is already reduced at low levels of exercise in dogs. Humans demonstrate an intermediate oxygen tension response. Data are mean ±s.e.m.
Mechanism of exercise hyperaemia: the usual suspects
The vasodilator mechanisms that mediate exercise hyperaemia in the heart have been the subject of intense research efforts over the past 40 years in dogs and, more recently, in swine. It is now recognized that regulation of coronary vascular resistance is the result of a balance between a myriad of vasodilator and vasoconstrictor influences, which are exerted by the myocardium, endothelium and neurohumoral systems (Laughlin et al. 1996; Duncker & Bache, 2000). Classically, candidate contributors to exercise-induced coronary resistance vessel dilatation should fulfil several criteria: first, it must have vasodilator properties and exogenous administration must mimic exercise hyperaemia. Second, its production must increase during exercise and correlate with exercise intensity. Third, and most importantly, blocking its action through inhibition of endogenous production or blocking its receptor(s) must abolish or reduce the increase in coronary blood flow during exercise (Feigl, 1983).
Adenosine
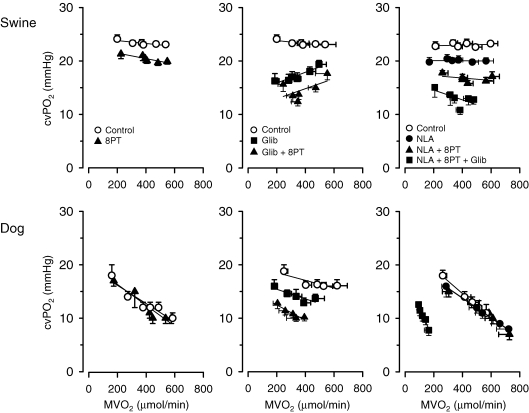
Due to its potent vasodilator action, and because it is the ultimate breakdown product of ATP, it was already proposed in the 1960s that adenosine may play a role in coupling coronary vasomotor tone to cardiac metabolism (Berne & Rubio, 1974). Adenosine formed from the action of nucleotide phosphorylase on AMP can be transported out of myocytes into the interstitial space (Sparks & Bardenheuer, 1986). Upon entering the interstitial space adenosine can interact with A2 receptors on coronary vascular smooth muscle to produce vasodilatation and an increase in coronary blood flow (Olsson et al. 1992). In the canine heart, adenosine release has been reported to be enhanced during exercise (Watkinson et al. 1979; Bacchus et al. 1982), although recent work suggests that interstitial adenosine concentrations may not increase significantly during exercise (Tune et al. 2000). However, studies in dogs have generally failed to demonstrate an effect of intracoronary adenosine deaminase or adenosine receptor blockade on basal coronary flow or coronary venous PO2 (Fig. 2) (Bache et al. 1988; Ishibashi et al. 1998), although one study reported a decrease in resting coronary venous PO2 (Tune et al. 2000). Overall, these findings indicate that endogenous adenosine production is neither mandatory for maintaining resting coronary blood flow, nor obligatory for the increase in coronary blood flow produced by exercise in the dog heart. In contrast, there is evidence that adenosine contributes to regulation of coronary vasomotor tone in the human heart. Thus, during exercise in normal young adult human subjects, adenosine receptor blockade resulted in coronary vasoconstriction both at rest and during exercise (Edlund et al. 1989). Similarly, adenosine receptor blockade produced vasoconstriction in the porcine coronary circulation that was similar at rest and during exercise (Fig. 2) (Duncker et al. 1998b; Merkus et al. 2003a). These findings suggest that, although endogenous adenosine exerts a vasodilator influence on the coronary circulation both at rest and during exercise, adenosine is (similar to observations in dogs) not mandatory for the exercise hyperaemia in swine and humans.
Figure 2.
Integrative control of coronary vasomotor tone in dogs (lower panels) and swine (upper panels) at rest and during treadmill exercise Shown are the effects of adenosine receptor blockade with 8-phenyltheophylline (8PT; left panels), the effects of KATP channel blockade with glibenclamide (Glib) and additional adenosine receptor blockade (middle panels), and the effects of NO synthase inhibition with N-nitro-l-arginine (NLA) and additional adenosine receptor blockade and KATP channel blockade (right panels) on the relation between myocardial oxygen consumption (MVO2) and coronary venous oxygen tension (CVPO2) in the left ventricles. Data in dogs are from references (Bache et al. 1988 (left panel); Duncker et al. 1995 (centre panel); Ishibashi et al. 1998 (right panel)). Swine data are from Merkus et al. (2003a). Data are mean ±s.e.m.
Nitric oxide
Due to its location inbetween the blood stream and the cardiac myocytes, the endothelium has been proposed to be the oxygen sensor of the heart. In response to changes in either vascular or interstitial oxygen, the endothelium was hypothesized to produce factors that could directly influence smooth muscle tone such as nitric oxide (NO), prostanoids and endothelin-1 (ET) (Pohl, 1990; Duncker & Bache, 2000). NO is formed by endothelial NO synthase through the conversion of l-arginine to l-citrulline, and causes vasodilatation via an elevation of cyclic GMP and subsequent opening of vascular smooth muscle K+ channels. Endothelial NO production can be stimulated by specific receptors, hypoxia or by mechanical deformation (Duncker & Bache, 2000).
The NO production in response to an increase in shear stress has been proposed to recruit vasodilatation in proximal coronary arteries in response to an increase in flow obtained by a metabolic stimulus in the distal microcirculation. However, studies in awake dogs (Altman et al. 1994; Ishibashi et al. 1998) have generally shown that inhibition of NO has no effect on coronary blood flow either at rest or during treadmill exercise (Fig. 2), although one study reported small decreases in coronary venous PO2 (Tune et al. 2000). In contrast, the contribution of NO to regulation of vascular tone in the coronary resistance vessels is more apparent in the human (Lefroy et al. 1993; Quyyumi et al. 1995; Nishikawa et al. 1997) and porcine heart (Duncker et al. 2000). However, similar to observations in dogs, NO inhibition did not blunt exercise hyperaemia in either human or swine. These studies suggest that although species differences may exist with respect to the role of NO in regulation of coronary vasomotor tone under basal conditions, NO is not mandatory for the exercise hyperaemia in dogs, humans or swine.
KATP channels
Potassium channels sensitive to intracellular ATP content (KATP channels) have been identified in vascular smooth muscle cells (Brayden, 1996). Opening probability of these channels depends on the relative levels of intracellular ATP and ADP and therefore reflects the metabolic status of the vascular smooth muscle cells, but can also be modified by prostacyclin, adenosine and noradrenaline. Opening of the KATP channel results in an outward flux of potassium that increases the membrane potential of the sarcolemma of the smooth muscle cells. This hyperpolarization closes voltage-dependent calcium channels, leading to a decreased influx of calcium, thereby causing vasodilatation.
In awake dogs and swine, KATP channel blockade caused coronary vasoconstriction at rest and during treadmill exercise, but did not blunt the exercise-induced coronary vasodilatation (Fig. 2) (Duncker et al. 1993, 2001). In humans, KATP channel blockade reduced resting blood flow (Farouque et al. 2002), but its effect on exercise hyperaemia has not been tested to date.
Integrated control by NO, adenosine and KATP channelsens
The observations that blockade of adenosine, nitric oxide or KATP channels alone did not interfere with exercise hyperaemia were initially interpreted to suggest that these pathways were not important in the metabolic control of coronary vasomotor tone. However, there was evidence that these pathways were not independent controllers of coronary vasomotor tone but that they could potentially influence each other. Hence, control of vasomotor tone could involve a redundancy design of multiple pathways, in which blockade of one pathway results in a compensatory increase in activity of other pathways (Feigl, 1983; Laughlin et al. 1996).
In awake dogs, inhibition of NO synthase alone or in combination with adenosine receptor blockade does not affect the relation between oxygen consumption and coronary venous PO2 (Ishibashi et al. 1998), suggesting that increased adenosine production does not compensate when NO production is lost. In contrast, a compensatory role for adenosine in the maintenance of coronary flow was demonstrated when KATP channels were blocked (Fig. 2). Following KATP channel blockade with intracoronary glibenclamide, which produced a significant decrease in coronary blood flow that was accompanied by a decrease in regional wall thickening, adenosine receptor blockade resulted in a significant further decrease in coronary flow and regional wall thickening, in particular during exercise (Duncker et al. 1995). Under these conditions, additional blockade of NO synthase resulted in a further reduction in coronary flow at rest, while the exercise hyperaemia was virtually abolished (Ishibashi et al. 1998). These studies suggest that exercise hyperaemia in the canine heart is regulated via a myriad of vasodilator systems that act in a non-linear redundant fashion, so that when one system fails, back-up systems take over to ensure adequate oxygen supply to the myocardium. The observation that only KATP channel blockade decreased coronary blood flow and caused myocardial ischaemia, suggests a hierarchy in these regulatory systems in that the principal coronary vasodilator pathway in the dog is the KATP channel, with adenosine and NO primarily acting as back-up systems (Ishibashi et al. 1998).
In contrast to the hierarchical organization of coronary blood flow regulation in dogs, in swine individual blockade of adenosine, NO and KATP channels produced an increase in coronary vasomotor tone, while combined blockade of these pathways had an additive vasoconstrictor effect (Merkus et al. 2003a). These findings indicate that loss of KATP channel activity was not compensated by increased adenosine or NO, suggesting that adenosine, NO and KATP channels act in a linear additive fashion in swine. Moreover, despite the intense coronary vasoconstriction induced by blockade of adenosine, KATP channels and nitric oxide (forcing oxygen extraction to increase over 90%) and despite signs of anaerobic metabolism and impaired left ventricular function under resting conditions, the responses of coronary blood flow and oxygen supply to subsequent exercise were essentially unperturbed (Fig. 2). Apparently, other vasodilator pathways that are not recruited under resting conditions can be recruited during exercise when NO, adenosine and KATP channels are blocked.
Mechanism of exercise hyperaemia: alternative candidates
Prostanoids
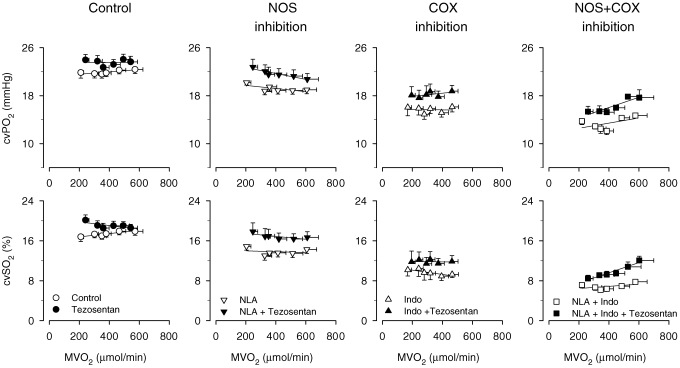
Several other vasodilator pathways could mediate the exercise hyperaemia in the porcine heart. For example, prostanoids have been proposed to contribute to metabolic dilatation of coronary resistance vessels in man (Friedman et al. 1981; Duffy et al. 1999), although this is not a ubiquitous finding (Edlund et al. 1989). In contrast, prostanoids do not appear to be important in the regulation of coronary vascular tone at rest or during exercise in the dog (Dai & Bache, 1984). A study in swine suggests that although endogenous prostanoids exert a vasodilator influence on porcine coronary resistance vessels at rest (Merkus et al. 2004), prostanoids are not mandatory for the exercise-induced vasodilatation. Furthermore, a contribution of prostanoids to exercise hyperaemia was also not observed after inhibition of NO synthesis (Merkus et al. 2004). In fact, prostanoids and NO appeared to contribute to coronary vasomotor tone in a linear additive fashion (Fig. 3).
Figure 3.
Integrative endothelial control of coronary vasomotor tone at rest and during exercise Effect of single and combined NO synthase (NLA, 20 mg kg−1i.v.) and COX blockade (Indo, 10 mg kg−1i.v.) on the responses to mixed ETA/ETB endothelin receptor blockade with tezosentan (3 mg kg−1i.v.) on the relation between myocardial oxygen consumption and coronary venous oxygen tension (CVPO2) and saturation (CVSO2) in the left ventricles of swine (Merkus et al. 2006b) during treadmill exercise. Data are mean ±s.e.m.
EDHF
Endothelium-derived hyperpolarizing factor (EDHF) has been shown to contribute to vasodilatation in response to pulsatile stretch as well as agonists such as substance P, acetylcholine and bradykinin (Fisslthaler et al. 2000). The presence of cytochrome P450 2C9, the enzyme responsible for the production of coronary EDHF, has been established in the porcine heart and has been shown to contribute to vasodilatation of isolated coronary arteries in reponse to agonists such as bradykinin. However, a role for this enzyme in exercise hyperaemia in the heart remains to be established.
Endothelin
Although exercise hyperaemia in the heart has generally been ascribed to an increase in vasodilators that counteract the high level of ‘intrinsic’ coronary vasomotor tone, it could also be evoked by reducing the influence of the vasoconstrictors responsible for this basal tone. In the absence of α-adrenergic constriction, angiotensin II and endothelin (ET) are the prime candidates for inducing basal tone at rest. Angiotensin II receptor blockade caused coronary vasodilatation at rest but did not modify exercise hyperaemia (Merkus et al. 2006a). In contrast, ET receptor antagonism induced coronary vasodilatation at rest, while the vasodilator effects of ET receptor antagonism progressively waned with increasing exercise intensity (Fig. 3) (Takamura et al. 2000; Merkus et al. 2003b). These data are consistent with the concept that withdrawal of ‘intrinsic’ coronary tone can contribute to exercise hyperaemia. The withdrawal of ET-mediated vasoconstriction is mediated, at least in part, by a factor released from the cardiac myocytes (Merkus et al. 2002). In addition, we recently observed that NO and prostanoids contributed to this withdrawal of ET-mediated vasoconstriction during exercise (Fig. 3) (Merkus et al. 2006b).
Autonomic nervous system
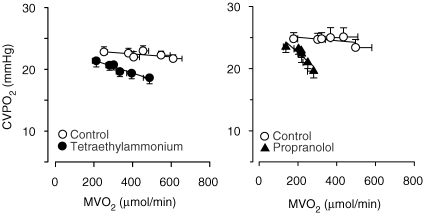
An important question in the concept of metabolic control in the porcine coronary circulation is how coronary flow reserve can be recruited during exercise in the presence of blockade of NO, adenosine and KATP channels while the heart fails to recruit such vasodilator reserve under resting conditions, despite signs of overt ischaemia. One possibility is that ‘extracardiac’ factors (i.e. independent of local intramyocardial factors) which are activated during exercise contribute to the regulation of coronary vasomotor tone. One such factor is sympathetic activity which is minimal under resting conditions but increases markedly during exercise. The coronary bed is richly innervated by sympathetic nerve fibres and sympathetic activity can directly modulate the increase in coronary blood flow through the vascular α- and β-adrenoceptors. In view of the virtual absence of α-adrenoceptors in the porcine coronary circulation (Dunker et al. 1998a), an increase in sympathetic activity causes vasodilation by stimulation of the β-adrenoceptors located on the coronary vasculature, which can contribute to the exercise-induced coronary vasodilatation in a feed-forward manner (Fig. 4).
Figure 4.
Role of KCa channels andβ-receptors in coronary vasomotor control Effect of KCa channel blockade with tetra-ethyl-ammonium (20 mg kg−1i.v.; left panel; Merkus et al. 2006c) and of β-blockade with propranolol (0.5 mg kg−1i.v.; right panel; Duncker et al. 1998a) on the relation beween myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of swine during treadmill exercise. Data are mean ±s.e.m.
Observations in humans (Ekstrom-Jodal et al. 1972; Jorgensen et al. 1973), dogs (Bassenge et al. 1972; Heyndrickx et al. 1980) and swine (Duncker et al. 1998a) indicate that the exercise-induced increase in sympathetic activity contributes to coronary vasodilatation in a feed-forward manner through β-adrenergic stimulation. However, in the dog the vasodilator effect of the increased β-adrenoceptor stimulation is counteracted by the vasoconstrictor effect of α-adrenoceptor stimulation, while it is unopposed in the porcine coronary vasculature. This difference in coronary vasomotor control may explain at least in part why coronary blood flow fails to increase during exercise in the canine coronary circulation upon blockade of NO, adenosine and KATP channels (Ishibashi et al. 1998), while exercise hyperaemia remains essentially unperturbed in the porcine coronary circulation (Merkus et al. 2003a).
Alternative K+ channels
Voltage-dependent K+ channels constitute a diverse family of outward rectifying K+ channels present in the vascular smooth muscle sarcolemma (Gutterman et al. 2005). These channels are sensitive to membrane potential so that depolarization will induce opening of KV channels, thereby opposing vasoconstriction. KV channels were recently reported to play a role in maintaining resting coronary blood flow (Rogers et al. 2006). However, although these channels are sensitive to β-adrenoceptor stimulation and other cAMP-mediated vasodilator responses (Gutterman et al. 2005), and may therefore contribute to exercise-induced coronary vasodilatation, the role for KV channels in mediating the coronary vasodilatation that occurs in response to exercise has not been investigated to date.
Large conductance KCa channels are abundantly expressed in coronary vascular smooth muscle cells (Gollasch et al. 1996). Small changes in their open probability have a significant effect on the sarcolemmal membrane potential and therefore on vasomotor tone. Membrane depolarization and increases in intracellular Ca2+ concentration are the main activators of KCa channels, thereby providing an important negative feedback mechanism to blunt vasoconstrictor responses. In addition many endogenous vasoactive substances exert their actions through modulation of KCa channel activity. In general, vasodilators act through stimulation of protein kinase A and G (PKA and PKG) resulting in increased opening of KCa channels, while vasoconstrictor substances such as endothelin and angiotensin II activate PKC and decrease the opening probability of KCa channels (Brayden, 1996).
In anaesthetized dogs, KCa channel blockade had no effect on coronary blood flow (Rogers et al. 2006), which is in accordance with the concept that in dogs KATP channels are the principal K+ channel involved in metabolic regulation of coronary vasomotor tone. In swine, KCa channel blockade produced slight coronary vasoconstriction that was progressively amplified with increasing levels of exercise, suggesting that KCa channels are an effector of exercise-induced coronary vasodilatation (Fig. 4) (Merkus et al. 2006c). The upstream vasodilator pathways that activate the KCa channels in swine remain to be determined. A potential candidate is β-adrenoceptor activation, which is known to act, at least in part, via KCa channels (Scornik et al. 1993). In addition the progressive withdrawal of the vasoconstrictor influence of ET, which is known to inhibit opening of KCa channels (Brayden, 1996), could contribute to activation of these channels during exercise.
Conclusions
Despite intense research efforts over the past 40 years, the mechanism of exercise hyperaemia remains incompletely understood. In dogs, KATP channel activation together with adenosine and nitric oxide contribute to exercise hyperaemia in a non-linear redundant fashion. In contrast, in swine, nitric oxide, prostanoids, adenosine and KATP channels contribute to resting coronary resistance vessel tone control in a linear additive manner, but are not mandatory for exercise hyperaemia in the porcine heart. Rather, exercise hyperaemia in swine appears to involve KCa channel opening that is mediated, at least in part, by exercise-induced β-adrenergic activation, possibly in conjunction with exercise-induced blunting of an endothelin-mediated vasoconstrictor influence. In the human heart, nitric oxide, prostanoids, adenosine and KATP channels each contribute to resting tone, but evidence for a critical contribution to exercise hyperaemia is currently lacking. Hence, future studies are required to determine whether exercise hyperaemia in humans follows the canine or porcine control design.
Acknowledgments
Dr Merkus is supported by a stipend of the Netherlands Heart Foundation (2000T042).
References
- Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res. 1994;28:119–124. doi: 10.1093/cvr/28.1.119. [DOI] [PubMed] [Google Scholar]
- Bacchus AN, Ely SW, Knabb RM, Rubio R, Berne RM. Adenosine and coronary blood flow in conscious dogs during normal physiological stimuli. Am J Physiol Heart Circ Physiol. 1982;243:H628–H633. doi: 10.1152/ajpheart.1982.243.4.H628. [DOI] [PubMed] [Google Scholar]
- Bache RJ, Dai XZ. Myocardial oxygen consumption during exercise in the presence of left ventricular hypertrophy secondary to supravalvular aortic stenosis. J Am Coll Cardiol. 1990;15:1157–1164. doi: 10.1016/0735-1097(90)90258-q. [DOI] [PubMed] [Google Scholar]
- Bache RJ, Dai XZ, Herzog CA, Schwartz JS. Effects of nonselective and selective α1-adrenergic blockade on coronary blood flow during exercise. Circ Res. 1987;61:II36–41. [PubMed] [Google Scholar]
- Bache RJ, Dai XZ, Schwartz JS, Homans DC. Role of adenosine in coronary vasodilation during exercise. Circ Res. 1988;62:846–853. doi: 10.1161/01.res.62.4.846. [DOI] [PubMed] [Google Scholar]
- Bassenge E, Kucharczyk M, Holtz J, Stoian D. Treadmill exercise in dogs under β-adrenergic blockade: adaptation of coronary and systemic hemodynamics. Pflugers Arch. 1972;332:40–55. [PubMed] [Google Scholar]
- Berne RM, Rubio R. Regulation of coronary blood flow. Adv Cardiol. 1974;12:303–317. doi: 10.1159/000395474. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Potassium channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 1996;23:1069–1076. doi: 10.1111/j.1440-1681.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Dai XZ, Bache RJ. Effect of indomethacin on coronary blood flow during graded treadmill exercise in the dog. Am J Physiol Heart Circ Physiol. 1984;247:H452–H458. doi: 10.1152/ajpheart.1984.247.3.H452. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation. 1999;100:1951–1957. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Bache RJ. Regulation of coronary vasomotor tone under normal conditions and during acute myocardial hypoperfusion. Pharmacol Ther. 2000;86:87–110. doi: 10.1016/s0163-7258(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of KATP channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol. 2001;280:H22–H33. doi: 10.1152/ajpheart.2001.280.1.H22. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Stubenitsky R, Tonino PA, Verdouw PD. Nitric oxide contributes to the regulation of vasomotor tone but does not modulate O2-consumption in exercising swine. Cardiovasc Res. 2000;47:738–748. doi: 10.1016/s0008-6363(00)00143-7. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward β-adrenergic control. Circ Res. 1998a;82:1312–1322. doi: 10.1161/01.res.82.12.1312. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Stubenitsky R, Verdouw PD. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am J Physiol Heart Circ Physiol. 1998b;275:H1663–H1672. doi: 10.1152/ajpheart.1998.275.5.H1663. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of KATP channels in coronary vasodilation during exercise. Circulation. 1993;88:1245–1253. doi: 10.1161/01.cir.88.3.1245. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, van Zon NS, Pavek TJ, Herrlinger SK, Bache RJ. Endogenous adenosine mediates coronary vasodilation during exercise after KATP channel blockade. J Clin Invest. 1995;95:285–295. doi: 10.1172/JCI117653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund A, Sollevi A, Wennmalm A. The role of adenosine and prostacyclin in coronary flow regulation in healthy man. Acta Physiol Scand. 1989;135:39–46. doi: 10.1111/j.1748-1716.1989.tb08548.x. [DOI] [PubMed] [Google Scholar]
- Ekstrom-Jodal B, Haggendal E, Malmberg R, Svedmyr N. The effect of adrenergic β-receptor blockade on coronary circulation in man during work. Acta Med Scand. 1972;191:245–248. [PubMed] [Google Scholar]
- Farouque HM, Worthley SG, Meredith IT, Skyrme-Jones RA, Zhang MJ. Effect of ATP-sensitive potassium channel inhibition on resting coronary vascular responses in humans. Circ Res. 2002;90:231–236. doi: 10.1161/hh0202.103713. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Fleming I, Busse R. EDHF: a cytochrome P450 metabolite in coronary arteries. Semin Perinatol. 2000;24:15–19. doi: 10.1016/s0146-0005(00)80048-8. [DOI] [PubMed] [Google Scholar]
- Friedman PL, Brown EJ, Jr, Gunther S, Alexander RW, Barry WH, Mudge GH, Jr, Grossman W. Coronary vasoconstrictor effect of indomethacin in patients with coronary-artery disease. N Engl J Med. 1981;305:1171–1175. doi: 10.1056/NEJM198111123052002. [DOI] [PubMed] [Google Scholar]
- Gollasch M, Ried C, Bychkov R, Luft FC, Haller H. K+ currents in human coronary artery vascular smooth muscle cells. Circ Res. 1996;78:676–688. doi: 10.1161/01.res.78.4.676. [DOI] [PubMed] [Google Scholar]
- Gutterman DD, Miura H, Liu Y. Redox modulation of vascular tone: focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol. 2005;25:671–678. doi: 10.1161/01.ATV.0000158497.09626.3b. [DOI] [PubMed] [Google Scholar]
- Haitsma DB, Bac D, Raja N, Boomsma F, Verdouw PD, Duncker DJ. Minimal impairment of myocardial blood flow responses to exercise in the remodeled left ventricle early after myocardial infarction, despite significant hemodynamic and neurohumoral alterations. Cardiovasc Res. 2001;52:417–428. doi: 10.1016/s0008-6363(01)00426-6. [DOI] [PubMed] [Google Scholar]
- Heiss HW, Barmeyer J, Wink K, Hell G, Cerny FJ, Keul J, Reindell H. Studies on the regulation of myocardial blood flow in man. I. Training effects on blood flow and metabolism of the healthy heart at rest and during standardized heavy exercise. Basic Res Cardiol. 1976;71:658–675. doi: 10.1007/BF01906411. [DOI] [PubMed] [Google Scholar]
- Heyndrickx GR, Muylaert P, Pannier JL. α-Adrenergic control of oxygen delivery to myocardium during exercise in conscious dogs. Am J Physiol Heart Circ Physiol. 1982;242:H805–H809. doi: 10.1152/ajpheart.1982.242.5.H805. [DOI] [PubMed] [Google Scholar]
- Heyndrickx GR, Pannier JL, Muylaert P, Mabilde C, Leusen I. Alteration in myocardial oxygen balance during exercise after β-adrenergic blockade in dogs. J Appl Physiol. 1980;49:28–33. doi: 10.1152/jappl.1980.49.1.28. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide mediated mechanisms account for coronary vasodilation during exercise. Circ Res. 1998;82:346–359. doi: 10.1161/01.res.82.3.346. [DOI] [PubMed] [Google Scholar]
- Jorgensen CR, Wang K, Wang Y, Gobel FL, Nelson RR, Taylor H. Effect of propranolol on myocardial oxygen consumption and its hemodynamic correlates during upright exercise. Circulation. 1973;48:1173–1182. doi: 10.1161/01.cir.48.6.1173. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Korthuis R, Duncker DJ, Bache RJ. Regulation of blood flow to cardiac and skeletal muscle during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Intergration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. pp. 705–769. [Google Scholar]
- Laughlin MH, Tomanek RJ. Myocardial capillarity and maximal capillary diffusion capacity in exercise-trained dogs. J Appl Physiol. 1987;63:1481–1486. doi: 10.1152/jappl.1987.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Lefroy DC, Crake T, Uren NG, Davies GJ, Maseri A. Effect of inhibition of nitric oxide synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation. 1993;88:43–54. doi: 10.1161/01.cir.88.1.43. [DOI] [PubMed] [Google Scholar]
- Merkus D, Duncker DJ, Chilian WM. Metabolic regulation of coronary vascular tone: role of endothelin-1. Am J Physiol Heart Circ Physiol. 2002;283:H1915–H1921. doi: 10.1152/ajpheart.00223.2002. [DOI] [PubMed] [Google Scholar]
- Merkus D, Haitsma DB, Fung TY, Assen YJ, Verdouw PD, Duncker DJ. Coronary blood flow regulation in exercising swine involves parallel rather than redundant vasodilator pathways. Am J Physiol Heart Circ Physiol. 2003a;285:H424–H433. doi: 10.1152/ajpheart.00916.2002. [DOI] [PubMed] [Google Scholar]
- Merkus D, Haitsma DB, Sorop O, Boomsma F, de Beer VJ, Lamers JM, Verdouw PD, Duncker DJ. Coronary vasoconstrictor influence of angiotensin II is reduced in remodeled myocardium after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006a;291:H2082–H2089. doi: 10.1152/ajpheart.00861.2005. [DOI] [PubMed] [Google Scholar]
- Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res. 2003b;59:745–754. doi: 10.1016/s0008-6363(03)00479-6. [DOI] [PubMed] [Google Scholar]
- Merkus D, Houweling B, Zarbanoui A, Duncker DJ. Interaction between prostanoids and nitric oxide in regulation of systemic, pulmonary, and coronary vascular tone in exercising swine. Am J Physiol Heart Circ Physiol. 2004;286:H1114–H1123. doi: 10.1152/ajpheart.00477.2003. [DOI] [PubMed] [Google Scholar]
- Merkus D, Sorop O, Houweling B, Boomsma F, van den Meiracker AH, Duncker DJ. NO and prostanoids blunt endothelin-mediated coronary vasoconstrictor influence in exercising swine. Am J Physiol Heart Circ Physiol. 2006b;291:H2075–H2081. doi: 10.1152/ajpheart.01109.2005. [DOI] [PubMed] [Google Scholar]
- Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006c;291:H2090–H2097. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Kanki H, Ogawa S. Role of nitric oxide in coronary vasomotion during handgrip exercise. Am Heart J. 1997;134:967–973. doi: 10.1016/s0002-8703(97)80022-1. [DOI] [PubMed] [Google Scholar]
- Olsson RA, Bunger R, Spaan JA. Coronary circulation. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. The Heart and Cardiovascular System. New York: Raven Press; 1992. pp. 1393–1426. [Google Scholar]
- Pohl U. Endothelial cells as part of a vascular oxygen-sensing system: hypoxia-induced release of autacoids. Experientia. 1990;46:1175–1179. doi: 10.1007/BF01936931. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO., 3rd Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle KCa channels by Gs alpha independent of phosphorylation by protein kinase A. Am J Physiol Heart Circ Physiol. 1993;265:H1460–H1465. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- Sparks HV, Jr, Bardenheuer H. Regulation of adenosine formation by the heart. Circ Res. 1986;58:193–201. doi: 10.1161/01.res.58.2.193. [DOI] [PubMed] [Google Scholar]
- Takamura M, Parent R, Cernacek P, Lavallee M. Influence of dual ETA/ETB-receptor blockade on coronary responses to treadmill exercise in dogs. J Appl Physiol. 2000;89:2041–2048. doi: 10.1152/jappl.2000.89.5.2041. [DOI] [PubMed] [Google Scholar]
- Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation. 2000;101:2942–2948. doi: 10.1161/01.cir.101.25.2942. [DOI] [PubMed] [Google Scholar]
- Watkinson WP, Foley DH, Rubio R, Berne RM. Myocardial adenosine formation with increased cardiac performance in the dog. Am J Physiol Heart Circ Physiol. 1979;236:H13–H21. doi: 10.1152/ajpheart.1979.236.1.H13. [DOI] [PubMed] [Google Scholar]