Abstract
Exercise can increase skeletal muscle blood flow by 100-fold over values observed at rest. As this value was 3 to 4 times higher than so-called ‘textbook’ values at the time it raised a number of issues about cardiovascular control. However, there is a continuing inability to identify the factor or combination of factors that explain this substantial increase in muscle blood flow. Moreover, these governing mechanism(s) must also explain the precise matching of muscle blood flow to metabolic demand and oxygen use or need. The difficulties identifying the mechanisms for exercise hyperaemia are especially disappointing due to the essentially concurrent discovery in the 1980s that the vascular endothelium was a key site of vasomotor control and that nitric oxide (NO) potentially released from nerves, endothelial cells, directly from tissues such as skeletal muscle, or perhaps released from red blood cells, might participate in vascular control in a way that would permit blood flow and metabolism to be closely matched.
The exercise hyperaemia laundry list
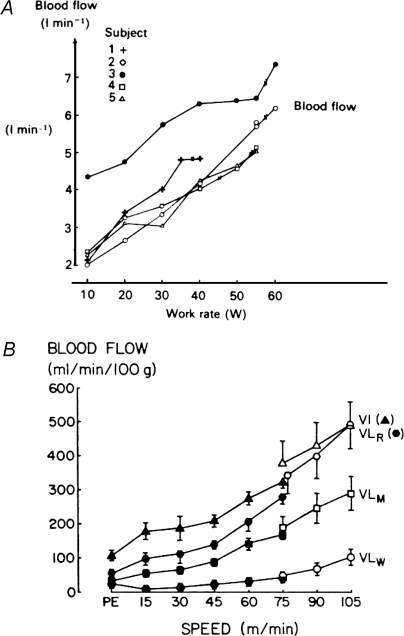
The general concept of exercise hyperaemia was clearly recognized in the second half of the 19th century with a number of key observations including those made by Gaskell in the 1870′s. Additionally, there were a number of precursor ideas suggesting that there may be a functional hyperaemia linking blood flow and muscle metabolism (Rowell, 2004). Presented in Fig. 1 are examples of the very high blood flows which can be obtained during exercise in isolated human quadriceps muscle (Andersen & Saltin, 1985) and in rat locomotor muscles during treadmill running at various rates (Armstrong & Laughlin, 1985). It was quickly appreciated that substances released by nerves, potential mechanical interactions between blood vessels and contracting muscles, substances released by or near active muscles, and/or substances carried in the blood, might contribute to exercise hyperaemia. Over the last 100+ years the above ideas have been repeatedly evaluated as new techniques were developed or new putative vasodilating substances discovered. The published studies and concepts established from about 1980 are comprehensively and brilliantly summarized in the Handbook of Physiology chapter authored by John T. Shepherd (Shepherd, 1983). Table 1 provides a list of criteria for candidate vasodilator substances from Dr Shepherd's chapter. Other than the observed high values for skeletal muscle blood flow during exercise, what new developments have emerged since Dr Shepherd's review?
Figure 1.
Examples of the very high blood flow values observed in exercising (A) human and (B) rat muscles in the 1980s The human data, when expressed per 100 g of active muscle, showed that muscle blood flow values between 200 and 300 ml min−1 (100 g)−1 of tissue were possible. (Figure adapted from Andersen & Saltin, 1985 (A) and Armstrong & Laughlin, 1985 (B), both used with permission.)
Table 1.
Criteria for candidate vasodilator substance(s)
| 1 | The substance(s) or its precursor(s) should be present in skeletal muscle (or perhaps blood or nerves) |
| 2 | The substance(s) should have access to the muscle resistance vessels. |
| 3 | The concentration in the interstitial fluid (or at the endothelium) must be sufficient to cause dilation and the concentration should be proportional to the contractile activity. |
| 4 | Exogenous administration of the substance(s) should be capable of causing prolonged dilation without sensations in humans. |
| 5 | Pharmacologic agents or physiological maneuvers which modify the blood flow responses to exercise should also modify the dilator responses to any putative substance given exogenously. |
Concepts are adapted from Shepherd, 1983.
Nerves
The three main ideas about the role of nerves in exercise hyperaemia include: (1) sympathetic withdrawal, (2) active vasodilatation via sympathetic vasodilator fibres, and (3) vasodilatation elicited by acetylcholine spillover from active motor nerves. Abrogation of sympathetic control to most resting skeletal muscles increases blood flow only about 3-fold and the observed peak values are much less than those obtained during even mild muscle exercise (Shepherd, 1983; Reed et al. 2000). This finding argues against a role for sympathetic withdrawal.
Clear evidence exists for active sympathetic cholinergic vasodilatation in the skeletal muscle of a variety of species. This vasodilatation is thought to be due to acetylcholine-stimulated NO release from the vascular endothelium (Matsukawa et al. 1993). In a number of animal preparations such vasodilatation can be evoked during stimulation of selected brainstem areas which may also participate in haemodynamic and cardiovascular responses to exercise. By contrast, selective local infusions of atropine and/or NO synthase inhibitors alone or in combination have little or no impact on blood flow to contracting muscles in whole animal models, including humans (Shoemaker et al. 1997; Frandsen et al. 2001). Another factor to consider here is evidence that humans lack sympathetic cholinergic vasodilator nerves identified in other species (Joyner & Halliwill, 2000; Reed et al. 2000). The combination of these observations argues against sympathetic active vasodilatation as a major contributor to exercise hyperaemia.
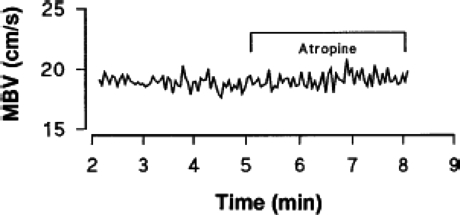
The failure of intra-arterial atropine to affect exercise hyperaemia, as shown in Fig. 2, and the minimal effects of NO synthase inhibition (Dyke et al. 1995; Shoemaker et al. 1997) suggest that acetylcholine spillover from active motor nerves is not essential for the normal exercise hyperaemic response. These observations are frustrating because of the solid evidence in some microcirculatory preparations, spatially and temporally linking (via acetylcholine spillover) the pattern of motor unit and muscle fibre recruitment to the pattern of resistance vessel dilatation during muscle contraction (VanTeeffelen & Segal, 2003).
Figure 2.
Steady state Doppler ultrasound recording of brachial artery blood velocity during rhythmic handgrip exercise Administration of the muscarinic antagonist atropine during forearm exercise did not affect the flow. This demonstrates that ongoing acetylcholine-mediated vasodilatation does not play a major role in exercise hyperaemia. (Figure adapted from Shoemaker et al. 1997, used with permission.)
Mechanical factors
Over the past 20 years the idea has emerged that the so-called ‘muscle pump’ and/or other mechanical interactions between the contracting skeletal muscles and the vasculature initiate the rise in flow with contractions. This idea is especially attractive because it could promote a rapid increase in blood flow by coupling local mechanical and haemodynamic events (Laughlin, 1987). While there is clear evidence that such interactions can promote a rapid increase in skeletal muscle blood flow, the magnitude of the increase appears to be modest. In human studies, when the exercising muscle is above heart level and the limb veins are empty there is little evidence (as shown in Fig. 3) that the muscle pump plays an obligatory role in exercise hyperaemia (Tschakovsky et al. 1996).
Figure 3.
Effects of external muscle compression on forearm blood flow The rise in flow with rhythmic cuff inflations (○) was small compared with that seen during muscle contractions and only occurred when the limb was in a dependent position (•). These data suggest that the possible contribution of the muscle pump to exercise hyperaemia is modest at best. (Figure from Tschakovsky et al. 1996, used with permission.)
Another important consideration with the muscle pump idea is the magnitude of the exercise hyperaemia. The question remains, can this mechanism provide either the necessary energy or change in blood vessel diameter in order to augment muscle blood flow enough to explain more than a small fraction of the total exercise hyperaemia response? It should be emphasized here that the muscle pump is probably essential for a normal systemic haemodynamic response to exercise because it facilitates venous return from the periphery and the associated increases in stroke volume and cardiac output that are hallmarks of whole body exercise in humans (Wang et al. 1960).
Substances released by muscle
Over the years, a number of substances thought to be released from active skeletal muscle have been implicated in exercise hyperaemia or hypothesized to be ‘the factor’ that links muscle blood flow to metabolism. In this context, ATP, ADP, adenosine and related compounds (i.e. phosphorus) have a long history as putative ‘factors’ controlling exercise hyperaemia. However, to the extent that these substances can be manipulated (especially blocked) pharmacologically, their importance as obligatory factors for the occurrence of a ‘normal’ exercise hyperaemia response is unclear. It does appear as if these factors play an important role during ischaemic exercise, where evidence suggests adenosine contributes importantly in compensatory vasodilator response (Koch et al. 1991). Potentially confounding the issue, recent evidence in humans suggests there may be adenosine responders and non-responders based on issues related to adenosine metabolism and re-uptake by skeletal muscle (Martin et al. 2007). Finally, an attractive property of ATP (mentioned again below) is that unlike adenosine, acetylcholine or nitroprusside, exogenous administration can evoke the massive dilatation observed during heavy exercise (Rosenmeier et al. 2004). By contrast, exogenous administration of the other substances typically only generates 50–75% of the total hyperaemic response.
Two other ‘newer’ compounds possibly playing a role in exercise hyperaemia are NO and vasodilating prostanoids. Nitric oxide could, theoretically, be released from vasodilator nerves, the vascular endothelium or locally by the contracting skeletal muscle (Furchgott, 1996). As noted above, local administration of NO synthase inhibition, at doses known to inhibit vasodilatation via acetylcholine and other physiological stimuli (Dietz et al. 1994; Dyke et al. 1995), or the systemic administration of NO synthase inhibitors has only minimal effect on skeletal muscle blood flow. This effect is difficult to study during systemic drug administration due to the impact of NO synthase inhibition on systemic blood pressure and engagement of counter-regulatory baroreflex-mediated changes in sympathetic tone. Along these lines, the largest contribution of nitric oxide to exercise hyperaemia has been observed in chronically instrumented exercising dogs with concurrent ganglionic blockade (Sheriff et al. 2000). Systemic NO synthase inhibition in these animals reduces vasodilatation in active skeletal muscles by about 30%. In most human studies, the effect on regional NO synthase inhibition is 20% or less during forearm exercise (Schrage et al. 2004) and there is no effect during leg exercise (Radegran & Saltin, 1999; Frandsen et al. 2001). However, in almost every model, NO synthase inhibition typically speeds the return of blood flow to baseline values in the immediate post-exercise period (Shoemaker et al. 1997; Frandsen et al. 2001).
There is some evidence that the release of vasodilator prostaglandins is enhanced during exercise, but depending on the experimental protocol and model, their overall contribution to exercise hyperaemia is modest at best. An interesting point here is that during both forearm exercise (Schrage et al. 2004) and leg exercise (Mortensen et al. 2007), combined inhibition of NO synthase inhibition and vasodilating prostaglandins seems to reduce blood flow to a greater degree than blockade of these substances independently.
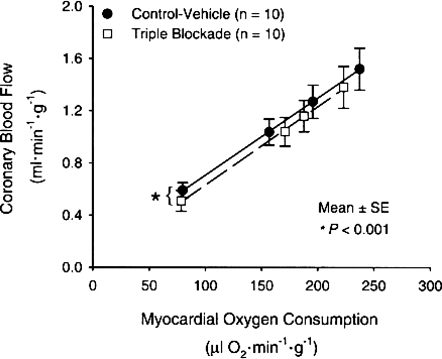
Some evidence suggests that KATP channels may contribute to exercise hyperaemia in a way that could link the energetic state in the active muscle to vasodilatation. However, the nature of how the signal may be spatially linked between the energetic state of active muscles and to what is happening in the nearby vasculature is unclear. In addition, relatively normal exercise hyperaemia responses have been observed during KATP channel blockade (Duncker et al. 2001). Finally, in a number of human and animal models including the coronary circulation, when so-called ‘double and triple blockade studies’ are performed and adenosine, NO synthase, prostanoids and/or KATP channels are all blocked (see Fig. 4), there is a remarkably normal exercise hyperaemia response (Tune et al. 2001).
Figure 4.
Effects of pharmacological blockade of adenosine receptors, KATP channels and NO synthase on the coronary blood flow responses to graded exercise in the dog These data demonstrate the robust nature of the cardiac muscle blood flow responses to exercise and highlight the inability to find a substance or combination of substances that explain this phenomenon. (Figure from Tune et al. 2001, used with permission.)
Substances carried in the blood
As is the case with substances released from contracting muscle, the idea that substances carried in the blood play a key role in exercise hyperaemia is well known. The early candidates for this were typically oxygen and/or carbon dioxide partial pressures in the blood. In this case, both oxygen and carbon dioxide can elicit vasodilatation but the magnitude of the total effect is once again too low to explain much of the overall exercise hyperaemia response (Shepherd, 1983). More recently, ideas have emerged that NO bound to haemoglobin may be released as oxygen is removed from the red blood cells, thus evoking vasodilatation in the active muscles. This is an attractive hypothesis because blood flow to areas where oxygen demand is high, would be elevated (Stamler et al. 1997). However, total body NO synthase inhibition, sufficient to block > 70% of NO production, has limited impact on exercise hyperaemia (Frandsen et al. 2001). Therefore, unless there is another source of NO for the red blood cells or some other mechanism for NO production, it seems unlikely that this mechanism plays a major and/or obligatory role in exercise hyperaemia.
In addition to NO, ATP may also be released from the red blood cell as oxygen is off-loaded from haemoglobin, spatially and temporally linking vasodilatation in the areas of the contracting muscle that are consuming oxygen (Ellsworth, 2004). There is evidence in a number of reduced preparations that ATP released from red blood cells as haemoglobin desaturates can participate in vasodilator responses (Ellsworth, 2004). This observation is absent in blood from patients with cystic fibrosis (CF) because the genetic mutation associated with this disease limits the release of ATP. However (as shown in Fig. 5), exercise hyperaemia in relatively healthy young CF patients is normal, making it seem unlikely that this mechanism for ATP release plays a major and/or obligatory role in exercise hyperaemia (Schrage et al. 2005). As noted above, ATP is the substance that when given exogenously evokes a vasodilator response that closely resembles the in vivo condition. Thus, a role for ATP in exercise hyperaemia remains an attractive hypothesis under normal conditions but the extent to which it is an obligatory contributor or the dominant factor is unclear.
Figure 5.
Blood flow responses to handgripping in patients with cystic fibrosis (CF) in comparison with controls The mutation responsible for CF also limits the ability of the red blood cells to release ATP as oxygen tension falls. These data suggest that ATP release from red blood cells is not a major contributor to exercise hyperaemia. (Figure from Schrage et al. 2005, used with permission.)
Summary
How best to summarize such a ‘negative’ collection of examples? The standard explanation(s) for the failure of any one factor or factors to explain exercise hyperaemia typically return to the ‘redundancy’ argument, that a combination of multiple factors contribute in a synergistic manner. However, attempts to block everything that can be blocked pharmacologically might at least show some consistent pattern of blunted responses. This, however, is not the case. The other common explanation is that there is some unknown factor or collection of factors that might explain this unexplainable response. This is certainly attractive coming from the typical ‘reductionist’ mind-set that continues to permeate much biological research. In this context, the discovery of NO ‘late’ in the history of putative vasodilator substances gives some optimism that there will ultimately be a simple explanation for, what would appear to be, the simple matching of muscle blood flow and metabolism under most circumstances. For now the most reasoned approach seems to be a combination cautious nihilism and cautious optimism.
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol. 1985;59:1322–1328. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of KATP+ channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol. 2001;280:H22–H33. doi: 10.1152/ajpheart.2001.280.1.H22. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276:1186–1188. [PubMed] [Google Scholar]
- Joyner MJ, Halliwill JR. Neurogenic vasodilation in human skeletal muscle: possible role in contraction-induced hyperaemia. Acta Physiol Scand. 2000;168:481–488. doi: 10.1046/j.1365-201x.2000.00700.x. [DOI] [PubMed] [Google Scholar]
- Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol. 1991;260:H436–H444. doi: 10.1152/ajpheart.1991.260.2.H436. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol. 1987;253:H993–H1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Curry TB, Eisenach JH, Charkoudian N, Joyner MJ. Adenosine transporter antagonism in humans augments vasodilator responsiveness to adenosine, but not exercise, in both adenosine responders and non-responders. J Physiol. 2007;579:237–245. doi: 10.1113/jphysiol.2006.123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa K, Shindo T, Shirai M, Ninomiya I. Nitric oxide mediates cat hindlimb cholinergic vasodilation induced by stimulation of posterior hypothalamus. Jpn J Physiol. 1993;43:473–483. doi: 10.2170/jjphysiol.43.473. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Reed AF, Tschakovsky ME, Minson CT, Halliwill JR, Torp KD, Nauss LA, Joyner MJ. Skeletal muscle vasodilatation during sympathoexcitation is not neurally mediated in humans. J Physiol. 2000;525:253–262. doi: 10.1111/j.1469-7793.2000.t01-1-00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol. 2005;99:1866–1871. doi: 10.1152/japplphysiol.00616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, Peripheral Circulation and Organ Blood Flow. Bethesda, MA, USA: American Physiological Society; 1983. pp. 319–370. [Google Scholar]
- Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation? Am J Physiol Heart Circ Physiol. 2000;279:H726–H732. doi: 10.1152/ajpheart.2000.279.2.H726. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Tune JD, Richmond KN, Gorman MW, Feigl EO. KATP+ channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2001;280:H868–H875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Marshall RJ, Shepherd JT. The effect of changes in posture and of graded exercise on stroke volume in man. J Clin Invest. 1960;39:1051–1061. doi: 10.1172/JCI104120. [DOI] [PMC free article] [PubMed] [Google Scholar]