Abstract
Rationale: Recent studies have suggested that both embryonic stem cells and adult bone marrow stem cells can participate in the regeneration and repair of diseased adult organs, including the lungs. However, the extent of airway epithelial remodeling with adult marrow stem cells is low, and there are no available in vivo data with embryonic stem cells. Human umbilical cord blood contains both hematopoietic and nonhematopoietic stem cells, which have been used clinically as an alternative to bone marrow transplantation for hematologic malignancies and other diseases.
Objectives: We hypothesized that human umbilical cord blood stem cells might be an effective alternative to adult bone marrow and embryonic stem cells for regeneration and repair of injured airway epithelium.
Methods: Human cord blood was obtained from normal deliveries at the University of Vermont. Cultured plastic adherent cells were characterized as mesenchymal stem cells (MSCs) by flow cytometry and differentiation assays. Cord blood–derived MSCs (CB-MSCs) were cultured in specialized airway growth media or with specific growth factors, including keratinocyte growth factor and retinoic acid. mRNA and protein expression were analyzed with PCR and immunofluorescent staining. CB-MSCs were systematically administered to immunotolerant, nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice. Lungs were analyzed for presence of human cells.
Measurements and Main Results: When cultured in specialized airway growth media or with specific growth factors, CB-MSCs differentially expressed Clara cell secretory protein (CCSP), cystic fibrosis transmembrane conductance regulator (CFTR), surfactant protein C, and thyroid transcription factor-1 mRNA, and CCSP and CFTR protein. Furthermore, CB-MSCs were easily transduced with recombinant lentiviral vectors to express human CFTR. After systemic administration to immunotolerant, NOD-SCID, mice, rare cells were found in the airway epithelium that had acquired cytokeratin and human CFTR expression.
Conclusions: CB-MSCs appear to be comparable to MSCs obtained from adult bone marrow in ability to express phenotypic markers of airway epithelium and to participate in airway remodeling in vivo.
Keywords: cord blood stem cells, lung epithelium, lung remodeling, mesenchymal stem cells
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Cord blood–derived mesenchymal stem cells may be a feasible alternative to embryonic or adult stem cells for lung remodeling. However, their use for lung epithelial remodeling has not been well studied.
What This Study Adds to the Field
Human cord blood–derived mesenchymal stem cells can be induced in vitro to express phenotypic markers of airway epithelium and can localize to airway epithelium after systemic administration to immunotolerant mice.
Recent studies have suggested that both embryonic stem cells and adult bone marrow stem cells can participate in the regeneration and repair of diseased adult organs, including the lungs (1–3). These findings suggest a possible therapeutic role for stem cells in the treatment of cystic fibrosis (CF) and other chronic lung diseases. For example, if stem cells expressing normal cystic fibrosis transmembrane conductance regulator (CFTR) can be induced to repopulate the airway epithelium, then much of the morbidity associated with CF could potentially be reduced (4–6). However, investigations using human embryonic stem cells are currently limited by scientific, ethical, and political considerations (7, 8). Furthermore, although adult marrow–derived stem cells have been demonstrated to engraft as airway epithelium, the extent of epithelial incorporation appears to be low (2, 9, 10). It is therefore unclear whether adult marrow–derived stem cells will be clinically useful for the treatment of lung diseases.
We hypothesized that stem cells obtained from umbilical cord blood (CB) could be an effective alternative to both embryonic and adult marrow–derived stem cells for regeneration of injured lung tissue. Blood from umbilical cord and placenta is safely and easily obtained immediately after birth and is a rich source of fetal origin hematopoietic stem cells (HSCs) and non-HSCs, including mesenchymal stem cells (MSCs) (11–13). Human CB-HSCs have been successfully and safely used in clinical transplantation for hematologic malignancies and other hematologic diseases for many years (11). In addition, studies using either CB mononuclear cells (MNCs) (14, 15) or plastic adherent CB stem cells (16–20) have demonstrated capacity to differentiate in vitro into nonhematopoeitic tissues of all germ layers (ectoderm, mesoderm, and endoderm) under specific culture conditions. It has recently been reported that a population of multilineage progenitor cells isolated from human CB can be induced to express phenotypic markers of type 2 alveolar epithelial cells in vitro (19). However, there is no other available information as to whether other types of lung cells, notably airway epithelial cells, can be derived from human CB stem cells.
In the current study, we demonstrate that human umbilical cord–derived MSCs (CB-MSCs), obtained from deliveries of normal infants, can be induced in vitro to express markers of airway epithelial phenotype, including Clara cell secretory protein (CCSP) and CFTR. Furthermore, we demonstrate that CB-MSCs are easily and effectively transduced with recombinant lentiviral vectors, including a CFTR-expressing vector, and thus may conceivably be used in autologous transplantation for CF lung disease if sufficient lung incorporation could be achieved. Finally, a small number of CB-MSCs appear to engraft in the airway epithelium after systemic (i.e., tail vein) administration to immunotolerant (NOD-SCID) mice.
METHODS
Additional details on all methods and results are included in the online supplement.
Animals
Adult NOD-SCID mice (Jackson Laboratories, Bar Harbor, ME) were used. All studies were subject to Institutional Animal Care and Use Committee review at the University of Vermont (UVM; Burlington, VT) and conformed to institutional and Association for Assessment and Accreditation of Laboratory Animal Care standards for humane treatment of laboratory animals.
Isolation and Characterization of CB-MSCs
Thirty-one human CB samples were obtained from term, normal deliveries at UVM. All studies were subjected to institutional review board review at UVM and informed consent was obtained from all donors. CB-MNCs were isolated by Ficoll gradient centrifugation (Fisher BioReagents, Pittsburgh, PA), resuspended in 1:1 mixture of CB basal medium (20) and human bone marrow MSC conditioned medium, plated in standard culture dishes (Corning, Pittsburgh, PA), and maintained until colonies were established. Passage 2–4 cells were assessed by flow cytometry for expression of MSC cell surface markers (21) and for differentiation into adipocytes, osteoblasts, and chondroblasts (22–24).
Induction of Lung Epithelial Phenotypic Differentiation
Passage 2–4 CB-MSCs were cultured in CB basal medium, mouse tracheal epithelial cell (MTEC) medium (25), small airway growth medium (SAGM) (26), 50 ng/ml keratinocyte growth factor (KGF) (Sigma, St. Louis, MO), or 10 μg/ml retinoic acid (RA) (Sigma) for 1, 2, or 4 weeks.
Total RNA was extracted from CB-MSCs using TRIzol and purified using an RNeasy kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized with random primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Glyceraldehyde phosphate dehydrogenase (GADPH), CCSP, CFTR, aquaporin (AQP)-5, thyroid transcription factor (TTF)-1, surfactant protein C (SPC), α-smooth muscle actin, and collagen, type I, α1 (Col1A1) were amplified by polymerase chain reaction (PCR) (40 cycles). Quantitative PCR was performed on cDNA with Assays on Demand predesigned CCSP, CFTR, and hypoxanthineguanine phosphoribyl transferase (HPRT) primer/probe sets (Applied Biosystems, Foster City, CA), using TaqMan Universal PCR Master Mix and Applied Biosystems 7900HT Sequence Detection System.
In parallel, cells were fixed with 4% paraformaldehyde (PFA) (5 min, room temperature), stained with goat anti-rat CCSP (courtesy of Barry Stripp, Ph.D., University of Pittsburgh), mouse anti-human CFTR (Chemicon, Billerica, MA), and appropriate secondary antibodies (Invitrogen), and analyzed with Zeiss confocal microscopy (Zeiss, Thornwood, NY).
Transduction of CB-MSCs
CB-MSCs were incubated with recombinant lentivirus encoding either yellow fluorescent protein (YFP) or CFTR at multiplicity of infection (MOI) 1 to 100 (courtesy of Donald Anson, Ph.D., and David Parsons, M.D., University of Adelaide, Australia) for 24 hours at 37°C. YFP direct fluorescence and CFTR fluorescent immunostaining were assessed with an Olympus IX70 inverted microscope (Olympus, Center Valley, PA).
Engraftment of CB-MSCs in NOD-SCID Mouse Lung
Six hours after sublethal irradiation (1.4 Gy, RS 2000 Biological Irradiator; Rad Source Technologies, Boca Raton, FL), each experimental mouse received 2 × 106 CB-MSCs in 200 μl phosphate-buffered saline (PBS) intravenously. Control animals received equal volume of PBS. Mice were subsequently killed and lungs assessed at 1 day, 2 weeks, and 1 or 3 months after transplantation.
Genomic DNA was extracted from each lung (15 mg) using a DNA extraction kit (Stratagene, La Jolla, CA). A total of 300 ng of DNA was amplified for human Alu sequences (27). Standard curves were generated using serial dilution of CB-MSC genomic DNA into control mouse lung genomic DNA (27).
Immunofluorescent staining was performed on nonadjacent 5-μm paraffin-embedded lung sections using rabbit anti-human β2-microglobulin (Dako, Carpinteria, CA) and mouse anti-human pancytokeratin (CK) (Sigma) or mouse anti-human CFTR and appropriate secondary antibodies (Invitrogen). Five random fields (×40) from each of eight sections per animal were analyzed with Zeiss confocal microscopy.
Statistical Analyses
Cell counts on histologic sections were compared using Student t test (28).
RESULTS
Culture of CB-MSCs
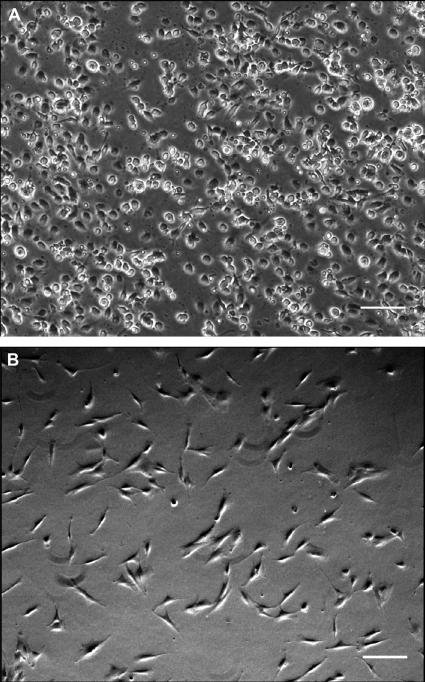
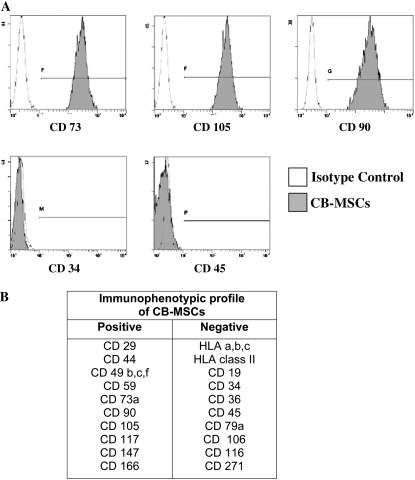
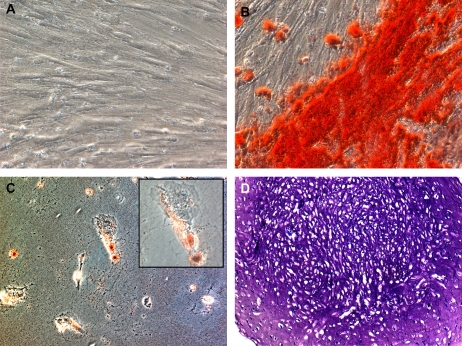
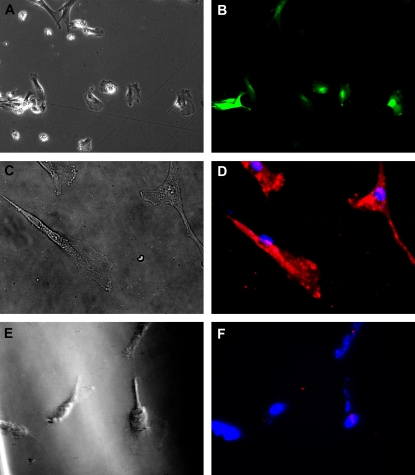
We were able to isolate and expand plastic adherent MNCs (CB-MNCs) from 12 of 31 human CB samples. The other 19 samples were complicated by either bacterial contamination or failure to grow in culture after 4 weeks. After several days in culture, the cells had a fibroblast-like morphology similar to adult bone marrow–derived MSCs (Figure 1). CB-MNCs from four separate CB isolations were assessed by flow cytometry and all expressed CD73, CD90, and CD105 but not CD34, CD45, or HLA antigens, and were identified as MSCs according to the most recent criteria from the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) (21) (Figure 2). In parallel, CB-MNCs from three of these samples also appropriately differentiated into chondroblasts, osteoblasts, and adipocytes as per 2006 ISCT criteria (21) (Figure 3). Given the consistency with different samples, each CB sample successfully cultured and expanded was believed to represent CB-MSCs.
Figure 1.
Morphologies of cord blood mononuclear cells (CB-MNCs) and cord blood mesenchymal stem cells (CB-MSCs). (A) Passage 0 cultures demonstrate an initial mixed cell population; (B) CB-MSCs after one passage showed uniform fibroblastic-like morphology. Standard phase contrast microscopy. Original magnification, ×10 and filter Ph1.
Figure 2.
(A) Cord blood (CB) plastic adherent cells express CD73, CD105, and CD90, but not CD34 and CD45. (B) CB plastic adherent cells between passage 2 and 4 express cell surface markers that meet the International Society for Cellular Therapy criteria for mesenchymal stem cells (21).
Figure 3.
Cord blood mesenchymal stem cells (CB-MSCs) can differentiate into osteoblast, adipocytes, and chondroblasts in vitro. CB-MSCs were incubated to confluency in CB basal medium and then cultured in CB basal medium or in different induction media for 21 days. (A–C) Phase contrast micrograph of CB-MSCs cultured in (A) CB basal medium and stained with Alizarin Red S and Oil Red O, (B) osteogenic medium for 21 days and stained with Alizarin Red S for calcium deposits (shown in red), or (C) adipogenic medium and stained with Oil Red O for lipid droplets (shown in red). Chondrogenesis (D) was produced by incubating 200,000 CB-MSCs as a micromass pellet in chrondrogenic media for 21 days and 5-μm paraffin-embedded sections were stained with Toluidine blue for histologic assessment. Photomicrographs demonstrate representative findings from passage 4 CB-MSCs. Original magnification, ×20, except inset, ×40. Specific constituents of each media formulation are included in the online supplement Methods.
CB-MSCs Acquire Lung Epithelial Markers In Vitro under Specific Culture Conditions
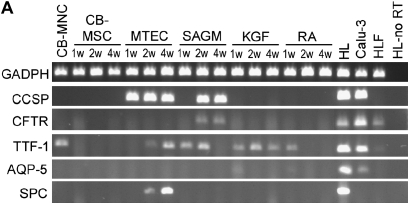
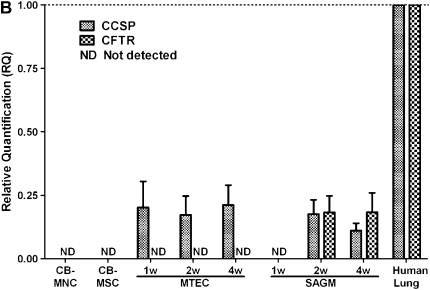
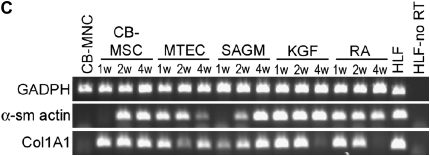
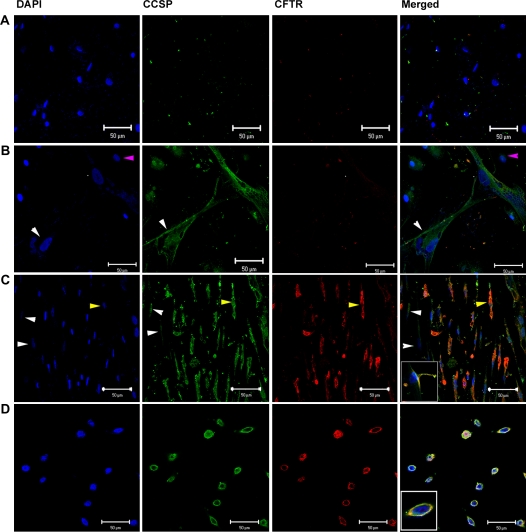
To determine if phenotypic markers of airway or alveolar cells could be induced in vitro, passage 2–4 CB-MSCs from 8 of the 12 successful CB-MSC cultures were cultured for 1, 2, or 4 weeks in media known to promote growth and differentiation of airway or alveolar epithelial cells (MTEC medium or SAGM) (25, 29) or in CB basal medium supplemented with growth factors known to promote development of lung epithelium (KGF or RA) (30, 31). Representative results from one of the eight CB-MSC samples is depicted in Figures 4 and 5. In comparison to CB-MSCs cultured in CB basal medium, cells grown in MTEC medium acquired CCSP mRNA expression by 1 week and maintained this up to 4 weeks (Figure 4A). Furthermore, after 2 weeks in MTEC medium, these cells also expressed SPC and TTF-1 mRNA. Cells cultured in SAGM demonstrated both CCSP and CFTR mRNA expression after 2 weeks' culture. These cells also expressed TTF-1 mRNA for 2 weeks in SAGM but TTF-1 expression was lost at 4 weeks. Neither KGF nor RA induced expression of specific differentiated airway or alveolar epithelial markers after up to 4 weeks in culture. However, cells cultured in KGF expressed TTF-1 mRNA throughout the study period and cells cultured in RA expressed TTF-1 mRNA at 1 week but not at subsequent time points. AQP-5 mRNA was not expressed under any culture condition. Quantitative PCR confirmed expression of CCSP and CFTR after culture in either MTEC medium or SAGM in five of the eight CB-MSC samples (Figure 4B). To further characterize these cells, we performed reverse transcriptase–PCR for α-smooth muscle actin and Col1A1 mRNA. These genes were chosen to evaluate for possibility of fibroblast or myofibroblast differentiation. We found that CB-MSCs also expressed α-smooth muscle actin and Col1A1 under several culture conditions (Figure 4C). Immunofluorescent staining confirmed protein expression of CCSP and both CCSP and CFTR in cells cultured in MTEC medium or in SAGM, respectively, for 2 weeks (Figure 5). (Positive and negative immunohistochemistry controls are shown in Figure E1 of the online supplement.)
Figure 4.
Cord blood mesenchymal stem cells (CB-MSCs) express Clara cell secretory protein (CCSP) and cystic fibrosis transmembrane conductance regulator (CFTR) mRNA when cultured in specialized airway growth medium. (A) Representative reverse transcriptase–polymerase chain reaction (RT-PCR) gel demonstrating expression of lung epithelial markers under different culture conditions (40 cycles RT-PCR). (B) Quantitative PCR confirming CCSP and CFTR mRNA expression in CB-MSCs cultured in MTEC medium and SAGM compared with cells maintained in CB basal medium. Bars represent means ± SE of five samples cultured in either MTEC medium or in SAGM. (C) RT-PCR gels demonstrating expression of fibroblast and myofibroblast markers under different culture conditions (40 cycles RT-PCR). Data represent representative findings from one of eight similar experiments using different CB preparations. CB-MNC = cord blood mononuclear cells; HL = human lung; HLF = human lung fibroblast; HLF-no RT = human lung fibroblast and no reverse transcriptase; KGF = keratinocyte growth factor; MTEC = mouse tracheal epithelial cells; RA = retinoic acid; SAGM = small airway growth medium.
Figure 5.
Cord blood mesenchymal stem cells (CB-MSCs) express Clara cell secretory protein (CCSP) and cystic fibrosis transmembrane conductance regulator (CFTR) protein after culture in specialized airway growth medium (MTEC medium and SAGM). Representative immunofluorescent staining photomicrography of (A) CB-MSCs cultured in CB basal medium, (B) CB-MSCs cultured in MTEC medium, (C) CB-MSCs cultured in SAGM, inset shows a higher magnification of a cell and (D) human bronchial epithelial cells (positive control inset shows a higher magnification of a cell). Pink arrows indicate cells that do not express either CCSP or CFTR, white arrows indicate cells that express only CCSP but not CFTR, and yellow arrows indicate cells that express both CCSP and CFTR. Blue = DAPI (4′-6-diamidino-2-phenylindole) nuclear stain, green = CCSP, red = CFTR, orange = costaining of CCSP and CFTR. Original magnification, ×40. Images were adjusted for brightness and contrast with Photoshop version 6.0 (Adobe Systems, San Jose, CA). Data represent representative findings from one of eight similar experiments using different CB preparations.
CB-MSCs Can Be Transduced to Express CFTR Using Recombinant Lentiviral Vectors
We hypothesized that CB-MSCs may be useful to correct gene defects such as absent or defective CFTR in CF airway epithelium. To determine if CB-MSCs could be induced to express CFTR, for example for use in autologous transplantation in patients with CF, CB-MSCs (passage 2–4) were transduced in vitro with recombinant lentiviral vectors encoding with either YFP or CFTR. We initially used lentivirus YFP to identify an appropriate MOI for CB-MSCs. At 48 hours after transduction with recombinant lentiviral vectors, approximately 90% of CB-MSCs expressed YFP at an optimal MOI of 80. Lentivirus CFTR was subsequently used at an approximate appropriate MOI of 100 and successfully transduced more than 90% of CB-MSCs as demonstrated by immunofluorescent staining for CFTR protein (Figure 6).
Figure 6.
Cord blood mesenchymal stem cells (CB-MSCs) can be transduced with recombinant lentivirus yellow fluorescent protein (YFP) and cystic fibrosis transmembrane conductance regulator (CFTR). Phase contrast (A, C, E) and fluorescent (B, D, F) microscopy of CB-MSCs expressing either YFP (A, B) or CFTR (C, D) after lentiviral transduction. YFP direct fluorescence was pseudocolored to green. E and F represent nontransduced control CB-MSCs. Original magnification, ×40. Images were adjusted for brightness and contrast with Photoshop version 6.0. Data represent representative findings from one of two similar experiments using different CB preparations.
Systemic Administration of Human CB-MSCs to NOD-SCID Mice Results in Localization of Human Cells to Airway Epithelium and Expression of Cytokeratin
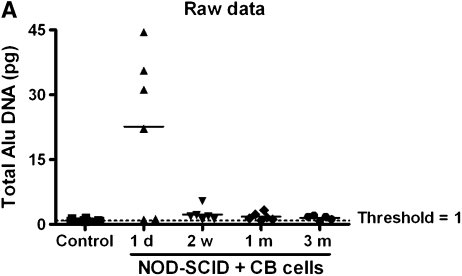
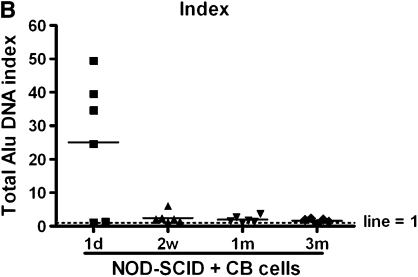
CB-MSCs were administered intravenously to NOD-SCID mice (1 × 106 cells/mouse by tail vein injection) after low-dose irradiation (1.4 Gy). Abundant human genomic DNA (human Alu sequences) was detected in lung homogenates at the 1-day time point (Figures 7A and B). However, at subsequent time points, 2 weeks, and 1 and 3 months, only low levels of human Alu sequences DNA were detected with an index between 1 and 2 (Figure 7B).
Figure 7.
Quantitative polymerase chain reaction of human Alu genomic DNA in recipient lungs. (A) Quantity of DNA (ng) detected in recipient lung. (B) Index of DNA quantity compared with control (nontransplanted) lungs. The horizontal bars represent the mean value for each experimental condition. CB = cord blood; NOD-SCID = nonobese diabetic/severe combined immunodeficiency mice.
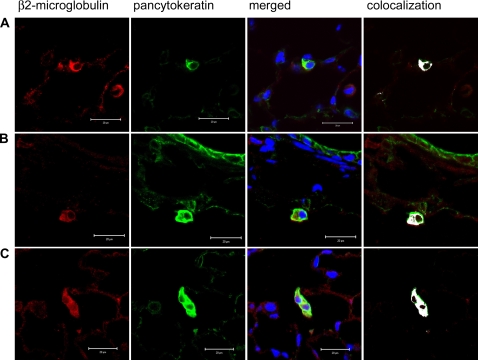
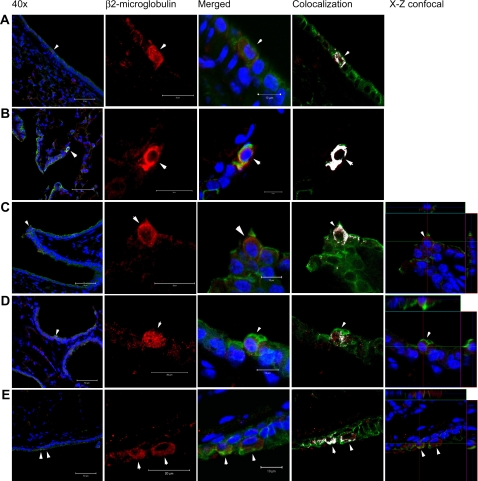
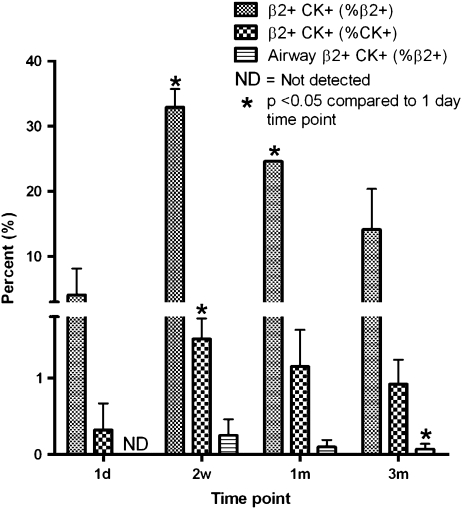
In parallel, cells of human origin were identified in recipient NOD-SCID mouse lungs by immunofluorescent staining for human β2-microglobulin. Human origin cells were predominantly found in the parenchyma (Figure 8), but a small number were detected in the airway epithelium (Figure 9, with enlarged versions of individual panels in Figure E2). We found that up to 3.36% of the total airway epithelial cells counted 1 day after CB-MSC administration were β2-microglobulin positive. This number was reduced to 0.72%, 0.86%, and 0.5% at subsequent time points (Table 1). After costaining for cytokeratin using a mouse anti-human/mouse pancytokeratin (CK) antibody directed against CK 1, 4–6, 8, 10, 13, 18, and 19, we found approximately 4.07% of the total β2-microglobulin–positive cells also stained positive for cytokeratin in lungs of mice assessed 1 day after MSC administration (Table 1 and Figure 10). This increased to a maximum average of 32.89% at 2 weeks but fell to 14.13% at 3 months. In parallel, 0.32% of total CK+ cells were also β2-microglobulin positive at 1 day. This also increased to a maximum at 2 weeks (1.51%) before decreasing by 3 months (0.92%). Most of the dual-labeled cells were located in alveolar regions. However, based on anatomic location and morphology, up to 0.25% of CK+ airway epithelial cells were dual labeled (β2+ CK+). Again, the maximum number of dual-labeled cells was observed at 2 weeks (Table 2 and Figure 10). Importantly, cultured CB-MSCs did not stain positively for cytokeratin (Figure E3). Positive and negative immunohistochemistry controls are shown in Figure E1.
Figure 8.
Human β2-microglobulin– and pancytokeratin-positive cells are mostly detected in NOD-SCID mice alveolar walls after systemic administration of cord blood mesenchymal stem cells (CB-MSCs). (A–C) NOD-SCID lung sections 2 weeks, 1 month, and 3 months after CB-MSC administration, respectively. Blue = DAPI (4′-6-diamidino-2-phenylindole) nuclear stain, green = pancytokeratin, red = β2-microglobulin, white = colocalization of pancytokeratin and β2-microglobulin. Original magnification, ×200. Representative photomicrographs from one of four mice per experimental condition.
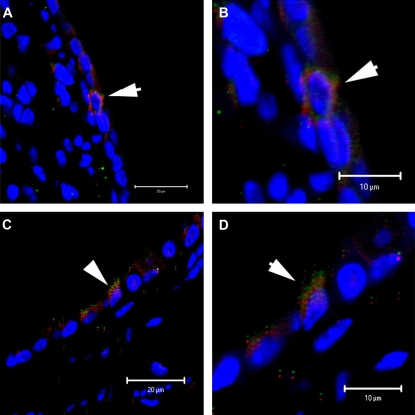
Figure 9.
Human β2-microglobulin positive cells can be detected in NOD-SCID mice airways after systemic administration of cord blood mesenchymal stem cells (CB-MSCs). (A–C) NOD-SCID lung sections 2 weeks after CB-MSC administration, (D) NOD-SCID lung section 1 month after CB-MSC administration, and (E) NOD-SCID lung section 3 months after CB-MSC administration. Blue = DAPI (4′-6-diamidino-2-phenylindole) nuclear stain, green = pancytokeratin, red = β2-microglobulin, white = colocalization of pancytokeratin and β2-microglobulin. Columns 2–5, original magnification, ×200. (See original size in Figure E2). Representative photomicrographs from one of four mice per experimental condition.
TABLE 1.
DISTRIBUTION OF DONOR-DERIVED PANCYTOKERATIN-POSITIVE CELLS IN NOD-SCID MOUSE LUNGS AT VARIOUS TIME POINTS
| Time Point | Mouse No. | β2+ (% total cells) | β2+ CK+ (% total β2+) | β2+ CK+ (% total CK+) |
|---|---|---|---|---|
| 1 d | 1 | 2.45 | 4.08 | 0.26 |
| 2 | 2.59 | 0.00 | 0.00 | |
| 3 | 5.96 | 8.13 | 0.69 | |
| Mean ± SD | 3.36 ± 1.99 | 4.07 ± 4.07 | 0.32 ± 0.35 | |
| 2 wk | 1 | 0.60 | 36.14 | 1.65 |
| 2 | 0.79 | 31.51 | 1.20 | |
| 3 | 0.75 | 31.03 | 1.68 | |
| Mean ± SD | 0.72 ± 0.1 | 32.89 ± 2.82 | 1.51 ± 0.27 | |
| 1 mo | 1 | 0.76 | 28.87 | 1.35 |
| 2 | 1.41 | 13.21 | 0.61 | |
| 3 | 0.53 | 31.71 | 1.50 | |
| Mean ± SD | 0.86 ± 0.46 | 24.6 ± 0.1 | 1.15 ± 0.48 | |
| 3 mo | 1 | 0.79 | 19.33 | 1.29 |
| 2 | 0.43 | 15.84 | 0.76 | |
| 3 | 0.38 | 7.22 | 0.70 | |
| Mean ± SD | 0.5 ± 0.22 | 14.13 ± 6.23 | 0.92 ± 0.32 |
Definition of abbreviations: β2 = β2-microglobulin; CK = pancytokeratin; NOD-SCID = nonobese diabetic/severe combined immunodeficiency mice.
Pancytokeratin-positive cells (no. = 4,472–5,919) were examined for dual staining with human β2-microglobulin and pancytokeratin (β2+ CK+) in five random high-power field (×40) on eight nonadjacent 5-μm sections for each lung (n = 3 for each experimental condition at each time point). β2+ (% total cells) = percentage of β2+ cells to total cells counted. β2+ CK+ (% total β2+) = percentage of cells dual stained for β2-microglobulin and pancytokeratin to total β2+ cells. β2+ CK+ (% total CK+) = percentage of cells dual stained for β2-microglobulin and pancytokeratin to total CK+ cells.
Figure 10.
Quantitative determination of donor-derived cells in recipient lungs. Bars represent means ± SD from quantitative determinations on lung sections from three mice per experimental condition. β2+ CK+ = β2-microglobulin– and pancytokeratin-positive cells.
TABLE 2.
DONOR-DERIVED CELLS LOCALIZED IN NOD-SCID MOUSE AIRWAY AFTER SYSTEMIC ADMINISTRATION OF CORD BLOOD-DERIVED MESENCHYMAL CELLS
| Time Point | Mouse No. | Airway β2+ CK+ (% total CK+) |
|---|---|---|
| 1 d | 1 | 0.00 |
| 2 | 0.00 | |
| 3 | 0.00 | |
| Mean ± SD | 0.00 | |
| 2 wk | 1 | 0.11 |
| 2 | 0.16 | |
| 3 | 0.50 | |
| Mean ± SD | 0.25 ± 0.21 | |
| 1 mo | 1 | 0.00 |
| 2 | 0.13 | |
| 3 | 0.17 | |
| Mean ± SD | 0.1 ± 0.09 | |
| 3 mo | 1 | 0.11 |
| 2 | 0.14 | |
| 3 | 0.25 | |
| Mean ± SD | 0.17 ± 0.07 |
For definition of abbreviations, see Table 1.
β2-Microglobulin and pancytokeratin airway epithelial cells, as determined by morphology and anatomic location, were counted in five random high-power field (×40) on eight nonadjacent 5-μm sections for each lung (n = 3 for each experimental condition at each time point).
To further confirm human cells engrafted as mouse airway epithelial cells, we used human-specific anti-CFTR antibody costaining with the anti-human β2-microglobulin antibody. We were able to demonstrate rare positive dual-stained cells (β2+ CFTR+) (Figure 11). Positive and negative immunohistochemistry controls are shown in Figure E1.
Figure 11.
Human β2-microglobulin– and cystic fibrosis transmembrane conductance regulator (CFTR)–positive cells can be detected in NOD-SCID mice airways after systemic administration of cord blood mesenchymal stem cells (CB-MSCs). (A and B) NOD-SCID lung sections 2 weeks and 1 month after CB-MSC administration, respectively. (C and D) Corresponding higher power images of (A) and (B). Representative photomicrographs from one of four mice per experimental condition.
DISCUSSION
We have demonstrated that human CB-MSCs are easily isolated from normal human CB samples and that these cells can be induced in vitro to express markers of airway epithelial phenotype, including CCSP and CFTR. Furthermore, systemically administrated CB-MSCs can localize to the airway and alveolar epithelium of immunotolerant (NOD-SCID) mice and acquire cytokeratin expression in both airway and alveolar regions and human CFTR expression in those cells that appear to have engrafted as airway epithelium.
Umbilical CB is safely and easily obtained immediately after birth and is a rich source of fetal origin HSCs and non-HSCs (11–13). Human CB HSCs have been used in clinical transplantation for hematologic malignancies for many years (11, 32). Non-HSCs derived from CB, including CB-MSCs, appear to have the capacity to develop into nonhematopoeitic cell types, and in vitro studies have demonstrated differentiation of both CB-MSCs and CB-HSCs into nonhematopoietic tissues of all germ layers under specific culture conditions (15–19, 33). In vivo, both CB-MSCs and CB-HSCs have been investigated for treatment of neurologic, liver, and cardiac diseases (14, 15, 17, 34, 35). Although the mechanisms by which CB-MSCs are induced to migrate to specific tissues and acquire phenotypic and functional characteristics of those tissues are unknown, the studies to date demonstrate not only the multipotent differentiation potential of CB-MSCs but also the possibility for therapeutic use of CB-MSCs.
Few studies to date have evaluated differentiation of CB-MSCs into lung epithelial or interstitial cells either in vitro or in vivo. In lung samples obtained from three female recipients of sex-mismatched CB-HSC transplantation, up to 8% chimerism of lung epithelium and 34% chimerism of lung vascular endothelium with donor-derived cells were observed (36). No obvious pulmonary chimerism was observed in a parallel patient who received adult bone marrow–derived HSCs. Although this study has come under question with respect to the methods used to demonstrate chimerism (2, 9), a more recent study has demonstrated a population of multilineage progenitor cells isolated from normal human CB that can be induced in vitro to express SPC and also develop lamellar bodies, characteristic of type 2 alveolar epithelial cells (19). However, it is unclear whether airway epithelial cells could also be similarly derived in vitro or what would happen if the cells were administered in vivo. It has also been reported in abstract form that human CB-MSCs can abrogate hyperoxia-induced lung damage when administered to neonatal rats (37). However, these findings have not yet been reported in peer-reviewed form, and the fate of these cells after systemic administration of CB-MSCs in vivo is unknown.
In our current study, we found that standard approaches for obtaining and culturing plastic-adherent MNCs resulted in homogenous populations of cells that exhibited appropriate MSC cell surface markers and differentiated along appropriate lineages (21). However, despite ready availability of CB samples, CB-MSCs could not be reliably isolated from all term CB samples, as only 38% of all samples obtained yielded CB-MSCs. This yield was comparable to that reported for obtaining multilineage progenitor cells (19). We used only CB samples obtained from term normal deliveries in which the mother was healthy and prenatal testing was negative for infectious diseases such as HIV or hepatitis B or C. We did not otherwise evaluate any other maternal factors that might conceivably affect MSC yield from the CB samples. For example, gestational age when CB samples are obtained plays an important role in the yield of CD34+ stem cell isolation, but factors affecting MSC yields are less well understood (38).
Different culture conditions significantly affected expression of lung epithelial phenotypic markers. In comparison to CB-MSCs maintained in CB basal medium, CB-MSCs cultured in vitro in MTEC medium expressed CCSP, pro-SPC, and TTF-1, but not any other airway or alveolar epithelial markers assessed, including AQP-5. Surprisingly, neither KGF nor RA induced expression of differentiated lung epithelium–specific markers. However, both KGF and RA induced an expression of TTF-1 mRNA. Furthermore, culture of CB-MSCs in SAGM resulted in mRNA and protein expression of CCSP, CFTR, and TTF-1, but not pro-SPC or AQP-5. This contrasts with recent findings using human CB origin multilineage progenitor cells in which culture in SAGM resulted in SPC mRNA expression (19). The multilineage progenitor cells described in that study were in many ways similar to the MSCs used in our current study, including fibroblastic morphology, expression of CD73, CD90, and CD105, but no expression of CD34 and CD45, and appropriate differentiation capacity along mesenchymal lineages. Differences in the initial isolation and handling of these cells might play an important role in subsequent responses to specific culture conditions. Further characterization and comparisons between the two cell populations are needed.
We also found that CB-MSCs could be induced to express α-smooth muscle actin and col1A1 mRNA. We had assessed these to determine the specificity of CB-MSC differentiation and were surprised to find that the different culture conditions used, including the basal CB media, induced expression of both. This may reflect either some degree of fibroblast or myfibroblast contamination in the CB-MSC preparations or that the CB-MSCs differentiated in vitro to mixed populations of cells under the conditions investigated. It is also possible that a nonspecific increase in expression of multiple genes in culture may have occurred and that the cells did not fully differentiate into airway or alveolar epithelial cells. We are further attempting to clarify these results.
Systemic administration of CB-MSCs to immunotolerant (NOD-SCID) mice resulted in only a small number of human cells remaining in the lung for longer than 1 day after administration. Presumably, as with administration of other cell types, including adult bone marrow MSCs, most of the cells only temporarily lodged in the capillary beds of the pulmonary vasculature before either being cleared or transiting on to other sites (39, 40). Interestingly, we found approximately 4.07% of the β2-microglobulin–positive cells stained positively for cytokeratin at 1 day and increased to a maximum of 33% at 2 weeks (Table 1). Because the CB-MSCs initially administered did not express CK, this suggests that even brief residence in the microenvironment of the lung plays an important role in acquisition of CK expression. Furthermore, most of the dual-labeled (β2+ CK+) cells were located in alveolar regions with only up to 0.25% of CK+ airway epithelial cells also staining positive for β2-microglobulin (Figure 8 and Table 2). To further clarify that human cells can engrafted as mouse airway epithelial cells, we used a human-specific anti-CFTR antibody costaining with β2-microglobulin. We were able to find rare cells that dual-stained for β2-microglobulin and CFTR. This further supports our hypothesis that human CB-MSCs can engraft as mouse airway epithelial cells. The rare events were found in our low-dose irradiation NOD-SCID mouse model; the engraftment might increase with a more pronounce injury models as was demonstrated in previous study that the rate of engraftment increase with amount of irradiation (41).
The apparent rate of engraftment of CB-MSCs is similar to the low levels of engraftment we and others have observed with systemically administered adult mouse marrow–derived MSCs (2, 5, 9, 42). There are, however, a number of variables that might have affected engraftment in these studies. One important variable is rigorous understanding of what cells were actually used. Nomenclatures concerning MSCs are currently controversial. Cells obtained from human CB were divided in HSCs and non-HSCs. Several terms were used in reference to “nonhematopoietic” stem cells from human CB, including mesenchymal stem cells or multipotent progenitor/mesenchymal stem cells. Therefore, further characterization beyond surface markers and differentiation potential of each cell, both from bone marrow and cord blood, will need to be done.
Furthermore, we used a low level of nonmyeloablative radiation to cause mild lung injury and increase recruitment of the CB-MSCs to lung. However, the dose may have been too low, because higher doses of irradiation have been described as necessary for lung epithelial engraftment by adult marrow–derived stem cells (41). We had initially attempted to use naphthalene to induce lung injury, as we have done in previous studies (5). However, the NOD-SCID mouse strain proved particularly susceptible to systemic toxic effects of the naphthalene. Other injury models may result in more extensive engraftment or physiologic effect as has been suggested with use of hyperoxia in neonatal rats (37). Furthermore, the NOD-SCID mice retain natural killer T cells, which may have played a role in clearing the human CB-MSCs (43). Studies in other immunotolerant mouse strains (i.e., NOD-SCID-γc null) (44) without any residual innate or adaptive immunity may demonstrate enhanced engraftment.
Nonetheless, our current study supports the hypothesis that human CB-MSCs can acquire airway epithelial cell phenotype in vitro under specific culture conditions. Further elucidation of specific factors and growth conditions—for example, culture at air–liquid interface as has been described for development of airway epithelium from mouse embryonic stem cells (45)—will allow more robust methods of deriving airway epithelial cells from the CB-MSCs. Furthermore, the easy ability to transduce the CB-MSCs suggests a role for ex vivo manipulation of the cells to correct genetic defects such as defective CFTR. Although we observed only rare airway epithelial cells that appeared to be derived from human CB-MSCs in this model, other lung injury models may demonstrate more robust engraftment. Future studies will include understanding the cell populations, mechanisms of recruitment and phenotypic conversion, and function of these cells in order for this approach to be clinically feasible.
Supplementary Material
Acknowledgments
The authors thank Don Anson and David Parsons for providing the lentivirus YFP and lentivirus CFTR, Darwin Prockop and the Center for Gene Therapy at Tulane University for assistance with CB-MSCs FACS analysis, and the UVM Image Analyses core facilities for imaging analyses.
Supported by a postdoctoral fellowship grant from the Cystic Fibrosis Foundation (V.S.), Vermont Lung Center T32 HL76122 (Charles Irvin, principal investigator) NIH/NHBLI Multidisciplinary Training in Lung Biology (V.S.), HL081289 from the NHLBI (D.J.W.), research grants from the Cystic Fibrosis Foundation (D.J.W.), and the American Lung Association (D.J.W.), National Center for Research Resources COBRE P20 RR-155557 (Vermont Lung Center; Charles Irvin, principal investigator).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200706-859OC on December 6, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Aliotta JM, Passero M, Meharg J, Klinger J, Dooner MS, Pimentel J, Quesenberry PJ. Stem cells and pulmonary metamorphosis: new concepts in repair and regeneration. J Cell Physiol 2005;204:725–741. [DOI] [PubMed] [Google Scholar]
- 2.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc 2006;3:193–207. [DOI] [PubMed] [Google Scholar]
- 3.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA 2005;102:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow–derived cells. Am J Respir Crit Care Med 2006;173:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC, Egan ME, Krause DS. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci USA 2006;103:2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JK, Hogan BL, Randell SH, Stripp BR, Weiss DJ. Human embryonic stem cell research. Am J Respir Crit Care Med 2006;173:1043–1045. [DOI] [PubMed] [Google Scholar]
- 8.Baschetti R. The dawn of science-based moral reasoning. Med Hypotheses 2006;68:4–8. [DOI] [PubMed] [Google Scholar]
- 9.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stripp BR, Shapiro SD. Stem cells in lung disease, repair, and the potential for therapeutic interventions: state-of-the-art and future challenges. Am J Respir Cell Mol Biol 2006;34:517–518. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein P. Why cord blood? Hum Immunol 2006;67:398–404. [DOI] [PubMed] [Google Scholar]
- 12.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 2004;200:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogler G, Sensken S, Wernet P. Comparative generation and characterization of pluripotent unrestricted somatic stem cells with mesenchymal stem cells from human cord blood. Exp Hematol 2006;34:1589–1595. [DOI] [PubMed] [Google Scholar]
- 14.Hirata Y, Sata M, Motomura N, Takanashi M, Suematsu Y, Ono M, Takamoto S. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem Biophys Res Commun 2005;327:609–614. [DOI] [PubMed] [Google Scholar]
- 15.Sharma AD, Cantz T, Richter R, Eckert K, Henschler R, Wilkens L, Jochheim-Richter A, Arseniev L, Ott M. Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. Am J Pathol 2005;167:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koponen JK, Kekarainen T, Heinonen SE, Laitinen A, Nystedt J, Laine J, Ylä-Herttuala S. Umbilical cord blood–derived progenitor cells enhance muscle regeneration in mouse hindlimb ischemia model. Mol Ther 2007;15:2172–2177. [DOI] [PubMed] [Google Scholar]
- 17.Wang TT, Tio M, Lee W, Beerheide W, Udolph G. Neural differentiation of mesenchymal-like stem cells from cord blood is mediated by PKA. Biochem Biophys Res Commun 2007;357:1021–1027. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JA, Gang EJ, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport 2004;15:1731–1734. [DOI] [PubMed] [Google Scholar]
- 19.Berger M, Adams S, Tigges B, Sprague S, Wang XJ, Collins D, McKenna D. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy 2006;8:480–487. [DOI] [PubMed] [Google Scholar]
- 20.Sueblinvong V, Loi R, Poynter ME, Bernstein IM, Weiss DJ. Development of airway epithelium from cord blood stem cells [abstract]. Proc Am Thorac Soc 2006;3:A25. [Google Scholar]
- 21.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 22.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 2004;103:1662–1668. [DOI] [PubMed] [Google Scholar]
- 23.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem 1999;72:570–585. [PubMed] [Google Scholar]
- 24.Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 2004;104:2728–2735. [DOI] [PubMed] [Google Scholar]
- 25.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- 26.Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, Bishop AE. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng 2006;12:867–875. [DOI] [PubMed] [Google Scholar]
- 27.McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy 2003;5:7–18. [DOI] [PubMed] [Google Scholar]
- 28.Zar J. Biostatistic analysis. Englewood Cliffs, NJ: Prentice-Hall; 1974.
- 29.Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng 2002;8:541–550. [DOI] [PubMed] [Google Scholar]
- 30.Gomperts BN, Belperio JA, Fishbein MC, Keane MP, Burdick MD, Strieter RM. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol 2007;37:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci 2004;359:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J. Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant 2006;38:83–93. [DOI] [PubMed] [Google Scholar]
- 33.Buzanska L, Machaj EK, Zablocka B, Pojda Z, Domanska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci 2002;115:2131–2138. [DOI] [PubMed] [Google Scholar]
- 34.Di Campli C, Piscaglia AC, Rutella S, Bonanno G, Vecchio FM, Zocco MA, Monego G, Michetti F, Mancuso S, Pola P, et al. Improvement of mortality rate and decrease in histologic hepatic injury after human cord blood stem cell infusion in a murine model of hepatotoxicity. Transplant Proc 2005;37:2707–2710. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa F, Yasukawa M, Yoshida S, Nakamura K, Nagatoshi Y, Kanemaru T, Shimoda K, Shimoda S, Miyamoto T, Okamura J, et al. Human cord blood- and bone marrow-derived CD34+ cells regenerate gastrointestinal epithelial cells. FASEB J 2004;18:1958–1960. [DOI] [PubMed] [Google Scholar]
- 36.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:318–322. [DOI] [PubMed] [Google Scholar]
- 37.Canadian Cord Blood Registry. Cell Therapy Technologies announces successful use of cord blood stem cells to prevent oxygen induced lung damage in a neonatal rat model [press release]. 2006. May 31. Available from: www.ccbr.ca/Official_Documents/Press_Release.pdf
- 38.Dimitriou H, Perdikogianni C, Stiakaki E, Vorgia P, Hatzidaki E, Kalmanti M. The impact of mode of delivery and gestational age on cord blood hematopoietic stem/progenitor cells. Ann Hematol 2006;85:381–385. [DOI] [PubMed] [Google Scholar]
- 39.Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol 1997;97:97–104. [DOI] [PubMed] [Google Scholar]
- 40.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation 2005;112:1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells 2006;24:1986–1992. [DOI] [PubMed] [Google Scholar]
- 42.Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT, Weiss DJ. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther 2005;12:680–686. [DOI] [PubMed] [Google Scholar]
- 43.Thomsen M, Yacoub-Youssef H, Marcheix B. Reconstitution of a human immune system in immunodeficient mice: models of human alloreaction in vivo. Tissue Antigens 2005;66:73–82. [DOI] [PubMed] [Google Scholar]
- 44.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174:6477–6489. [DOI] [PubMed] [Google Scholar]
- 45.Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol 2005;32:87–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.