Abstract
Rationale: Idiopathic pulmonary fibrosis is a progressive disease with high mortality. Although most patients have a slow, progressive course, some patients will have an acute deterioration in function or acute exacerbation, which carries a poor prognosis. In some cases, acute deterioration is associated with infection. Herpesviruses have been associated with this disease. Fibrocytes have also been shown to be important in the pathogenesis of pulmonary fibrosis.
Objectives: To develop a murine model for infectious exacerbation of preexisting fibrosis, and provide mechanistic insight into the role of herpesviruses in fibrotic disease.
Methods: We used a model of fluorescein isothiocyanate–induced pulmonary fibrosis in mice. Infection with a murine gammaherpesvirus was given at time of established lung fibrosis. Measurements were made at the time of peak lytic viral replication.
Measurements and Main Results: We demonstrate that infection with gammaherpesvirus can exacerbate established fluorescein isothiocyanate–induced fibrosis evidenced by increased total lung collagen, histologic changes of acute lung injury, and diminished lung function. Gammaherpesvirus can exacerbate preexisting fibrosis in a Th1 cytokine environment and in the absence of Th2 cytokines. Gammaherpesvirus increases fibrocyte recruitment to the lung in wild-type, but not CCR2−/− mice, in part because viral infection up-regulates production of CCL2 and CCL12, chemokines important for fibrocyte recruitment. In contrast, mouse adenovirus infection did not exacerbate collagen deposition.
Conclusions: These data provide a new model for gammaherpesvirus exacerbation of established pulmonary fibrosis. The up-regulation of chemokines during viral infection and subsequent recruitment of fibrocytes to the lung likely contribute to augmentation of pulmonary fibrosis.
Keywords: chemokine, fibrocyte, Th1/Th2 cytokines, interstitial lung disease
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Herpesviruses have been associated with idiopathic pulmonary fibrosis and exacerbations of fibrosis are associated with high morbidity and mortality.
What This Study Adds to the Field
This study describes a new animal model for herpesvirus-induced exacerbation of established lung fibrosis and provides a mechanistic description for pathogenesis that includes fibrocyte recruitment.
Idiopathic pulmonary fibrosis (IPF) is a progressive, fibrotic lung disease with 3- and 5-year median survival rates of less than 40% (1). Causes of IPF may relate to epithelial cell injury, abnormal fibroproliferation, inflammation, and deposition of extracellular matrix components (2, 3). Standard therapies have shown little benefit and most patients progress to respiratory failure.
Many patients with IPF have a slow progressive disease course over months to years postdiagnosis (4). However, some patients experience acute deterioration in pulmonary function (4–8) without clear cause. This is referred to as acute exacerbation of IPF. Histology shows diffuse alveolar damage or organizing pneumonia plus usual interstitial pneumonitis (5–8). Nine different studies have reported mortality rates of 78% or greater (reviewed in Reference 8). High-dose corticosteroids and immunosuppression have shown no treatment efficacy. Infections also cause rapid deterioration in patients with IPF associated with high mortality (9–11). In the placebo arm of a recent study, 32 patients succumbed to IPF-related death; 47% were defined as acute deterioration and 27% of acute deaths were related to infection (4). Mechanisms whereby infections promote fibrogenesis are unknown. No animal models exist for acute or infectious exacerbation of established pulmonary fibrosis.
Recent studies have highlighted the importance of extrapulmonary derived fibroblasts in the development of pulmonary fibrosis (12–14). Fibrocytes are circulating leukocytes that express collagen 1, CD45, CD34, CD11b, and major histocompatibility class II (15). In culture, fibrocytes have spindle morphology and transforming growth factor-β1 can induce α-smooth muscle actin (14, 16, 17). Fibrocytes are important in dermal wound healing and asthma remodeling (16, 17). Bone marrow–derived collagen-producing cells have been demonstrated in lungs post-bleomycin (18), and adoptive transfer of fibrocytes augments fluorescein isothiocyanate (FITC)–induced pulmonary fibrosis (13). CCL12, a murine chemokine with homology to human CCL2, is the ligand primarily responsible for CCR2-mediated recruitment of fibrocytes and enhancement of FITC fibrosis in mice (13).
Herpesvirus infections have been associated with IPF. Increased incidence of Epstein-Barr virus (EBV), cytomegalovirus, and human herpesviruses (HHV)-7 and -8 are found in lungs of patients with IPF (19–22). It is possible that some IPF acute exacerbations may in reality be attributable to occult or subclinical viral infection. Murine gammaherpesvirus-68 (γHV68) is a murine homolog of human EBV and HHV-8 (23). It produces mononucleosis-like syndrome in mice and establishes latency in B lymphocytes (24). Lung epithelial cells can harbor latent γHV68 (25). Infection with γHV68 1 week before bleomycin administration increases fibrosis scores and inflammation compared with virus or bleomycin alone (26). Th2-biased mice develop lung, spleen, and lymph node fibrosis post-γHV68 (27, 28). These data support the hypothesis that viral infections can augment fibrotic responses. Thus, we sought to establish an animal model to further investigate the pathogenesis of virus-induced exacerbations of established fibrosis. Some of these results have previously been reported in abstract form (29).
METHODS
Mice
C57Bl/6 and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). Male and female mice were used between the ages of 6 and 8 weeks. IL-4/13−/− (30) and IL-13−/− mice (31) on BALB/c background were the gift of Andrew McKenzie (Medical Research Council, Cambridge, UK). These mice and CCR2−/− mice (12) were bred at the University of Michigan. Animal protocols were approved by the University Committee on the Use and Care of Animals.
FITC Model of Pulmonary Fibrosis
Intratracheal FITC inoculation (50 μl of a 28-mg/ml solution in saline) was performed as described (32).
Viral Infection
Mice were anesthetized with ketamine and xylazine. γHV68 (5 × 104 plaque forming units [pfu]; American Type Culture Collection, Manassas, VA) or 1 × 105 pfu mouse adenovirus (MAV-1; a kind gift from Dr. Kathy Spindler, University of Michigan) (33) was suspended in 20 μl saline and delivered intranasally to each mouse. Mock infections were 20 μl of saline. γHV68 was inactivated by exposure to ultraviolet (UV) light using a UV cross-linker set on 120,000 microjoules for four consecutive cycles as previously described (34).
Lung Collagen Measurements
Total lung collagen measurements were made as previously described (13) using the Sircol collagen dye binding assay (Accurate Chemical & Scientific Corp., Westbury, NY) or via hydroxyproline assay (32).
Lung Function Measurements
Lung function was measured as previously described (35) and as detailed in the online supplement.
Histology
Frozen or paraffin-embedded sections of lung were stained with either hematoxylin and eosin or Masson's trichrome stain. In some cases, γHV68 infection was detected using a rabbit polyclonal anti-γHV68 serum (36) kindly donated by Dr. Skip Virgin (Washington University School of Medicine, St. Louis, MO) and CCL2 was detected using a goat anti-murine CCL2 IgG (no. AB-479-na; R&D Systems, Minneapolis, MN). Primary antibodies were detected using appropriate secondary reagents and were visualized by peroxidase, alkaline phosphatase, phycoerythrin, or Cy3.
Virus Plaque Assay
Lytic viral plaques in lung homogenates were determined as described in the online supplement.
Real-Time Reverse Transcriptase–Polymerase Chain Reaction
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed on an ABI Prism 7000 thermocycler (Applied Biosystems, Foster City, CA) using a previously described protocol (37). Gene-specific primers and probes (Table 1) were designed using Primer Express software (Applied Biosystems).
TABLE 1.
PRIMERS AND PROBES FOR REAL-TIME POLYMERASE CHAIN REACTION ANALYSIS
| β-Actin forward primer | CTGCCTGACGGCCAAGTC | IFN-γ forward primer | CTGCGGCCTAGCTCTGAGA |
| β-Actin reverse primer | CAAGAAGGAAGGCTGGAAAAGAG | IFN-γ reverse primer | CAGCCAGAAACAGCCATGAG |
| β-Actin probe | AACGAGCGGTTCCGATGCCCTG | IFN-γ probe | CACACTGCATCTTGGCTTTGCAGCTC |
| gB (ORF8) forward primer | CGCTCATTACGGCCCAAA | TNF-α forward primer | CAGCCGATGGGTTGTACCTT |
| gB (ORF8) reverse primer | ACCACGCCCTGGACAACTC | TNF-α reverse primer | TGTGGGTGAGGAGCACGTAGT |
| gB (ORF8) probe | TTGCCTATGACAAGCTGACCACCA | TNF-α probe | TCCCAGGTTCTCTTCAAGGGACAAGGC |
| DNA polymerase (ORF9) forward primer | ACAGCAGCTGGCCATAAAGG | IL-13 forward primer | AGCAACATCACACAAGACCAGACT |
| DNA polymerase (ORF9) reverse primer | TCCTGCCCTGGAAAGTGATG | IL-13 reverse primer | GGCCAGGTCCACACTCCAT |
| DNA polymerase (ORF9) probe | CCTCTGGAATGTTGCCTTGCCTCCA | IL-13 probe | CCCTGTGCAACGGCAGCATGG |
| CCL12 forward primer | TGGCTGGACCAGATGCG | ||
| CCL12 reverse primer | GACGTGAATCTTCTGCTTAACAACA | ||
| CCL12 probe | TGAGCACCCCAGTCACGTGCTGTTA |
ELISA
Cytokine levels were measured in lung homogenates using the Duoset ELISA Development System kits for mouse IFN-γ, tumor necrosis factor (TNF)-α, and IL-13 from R&D Systems.
Flow Cytometry
Cells obtained by collagenase digestion (38) were stained for CD45 and intracellular collagen 1 as previously described (13). Cells were analyzed on a flow cytometer (FACScan; BD Biosciences, Mountain View, CA).
Statistical Analyses
All calculations were performed using Prism 3.0 software (GraphPad Software, San Diego, CA). Values expressed are means ± SEM. Student t tests or Mann-Whitney U tests were used for comparisons of two groups; analysis of variance was used for comparisons of three or more groups. A P value of less than 0.05 was considered significant.
RESULTS
γHV68 Infection Exacerbates FITC-induced Fibrosis
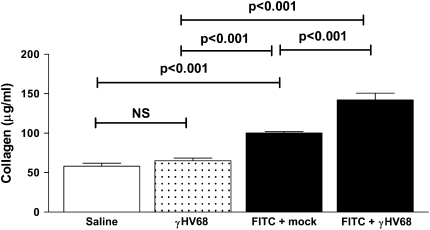
Mice were given intratracheal saline, γHV68, or FITC at Day 0. At Day 14, a time of established FITC-induced fibrosis (32), the FITC-treated mice were then given 5 × 104 pfu γHV68 intranasally or were mock infected. Lungs were harvested on Day 21 and lung collagen content was measured using the Sircol assay. Subsequent γHV68 infection resulted in significantly more collagen deposition in the lungs than did FITC treatment followed by mock infection (100.2 ± 1.6 vs. 142.1 ± 8.7 μg/ml, P < 0.001; Figure 1). It should be noted that γHV68 infection alone did not increase lung collagen content above saline control levels. Thus, acute γHV68 infection alone does not induce fibrosis in wild-type mice, but γHV68 infection exacerbates the FITC-induced fibrotic response in the lungs.
Figure 1.
Murine gammaherpesvirus-68 (γHV68) infection augments fluorescein isothiocyanate (FITC)–induced pulmonary fibrosis. Wild-type mice were given FITC or saline or γHV68 intratracheally on Day 0. On Day 14, FITC-treated mice received γHV68 or mock infection intranasally. Lungs were harvested for collagen determination on Day 21. Data represent n = 10–12 mice per group collected in two independent experiments.
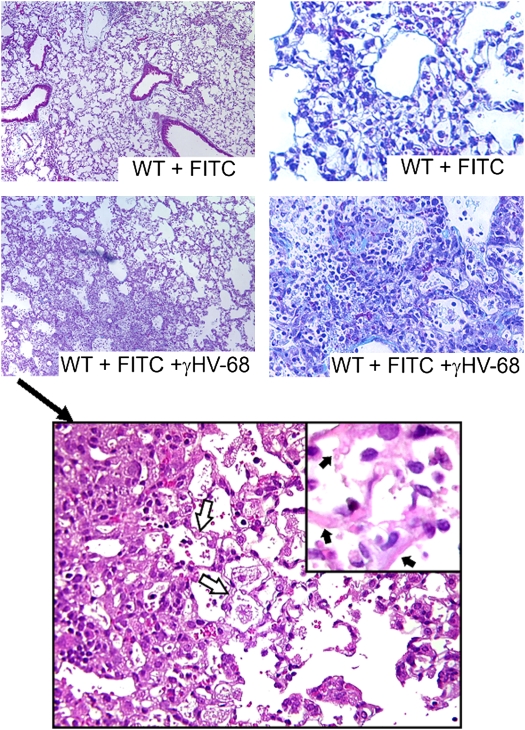
We looked at lung histology to confirm the results of our biochemical measurements of collagen content. Using the same protocol as above, lungs were harvested on Day 21, and inflated, fixed, and embedded in paraffin. Sections were then stained with hematoxylin and eosin or Masson's trichrome. Representative sections shown in Figure 2 (top panels) demonstrate that there is increased inflammation and collagen deposition in mice given γHV68 infection after FITC as opposed to mock-infected controls. Higher power views of the histology seen with FITC + γHV68 are shown in the bottom panel of Figure 2. There is evidence of interstitial edema, intraalveolar hemorrhage, alveolar epithelial denudation, and sloughing off of injured/dead epithelial cells. At the higher power, the mononuclear infiltrate is also evident. These histologic findings are consistent with evidence of diffuse alveolar damage and are similar to findings noted in cases of acute exacerbation in human IPF. However, FITC alone is also capable of producing similar pathologic patterns. As such, whereas these changes are consistent with diffuse alveolar damage and acute lung injury, they likely represent quantitative changes in response to the viral infection rather than qualitative changes.
Figure 2.
Histologic analysis confirms that murine gammaherpesvirus-68 (γHV68) infection leads to increased fibrotic response to fluorescein isothiocyanate (FITC). Top panels: Wild-type (WT) mice were given FITC intratracheally on Day 0. On Day 14, FITC-treated mice received γHV68 (lower panels) or mock infection (upper panels) intranasally. On Day 21, lungs were harvested for processing. Paraffin-embedded sections of lung were stained with hemotoxylin and eosin (left panels, ×100 original magnification) and Masson's trichrome (right panels, ×200 original magnification). Bottom panel: Higher power magnifications of FITC + γHV68–treated lungs (×400 original magnification; inset at ×1,000 original magnification). The open arrows point to examples of interstitial edema. Chronic inflammatory cells and intraalveolar hemorrhage are also evident. The inset highlights the evidence of alveolar epithelial denudation as indicated by the solid arrows, due to sloughing off of injured/dead epithelial cells. Results shown are representative of n = 3 mice per group.
Exacerbation of FITC-induced Fibrosis by γHV68 Is Associated with Impaired Lung Function
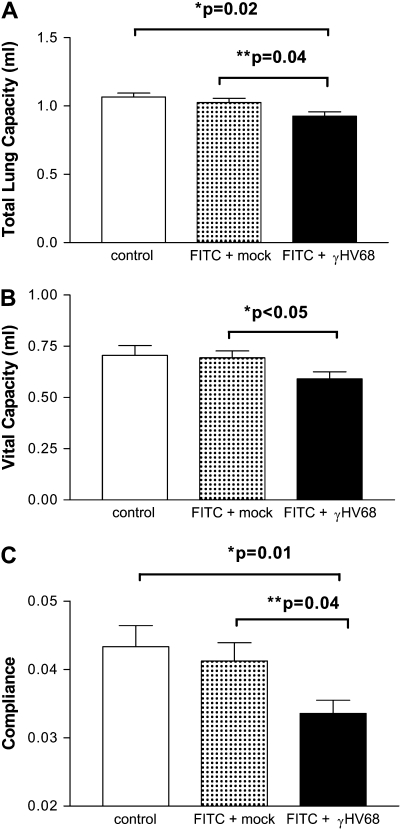
We next wanted to determine whether the increased deposition of collagen noted in FITC + γHV68–treated mice would translate to impaired lung function. Mice were treated with saline or FITC on Day 0. On Day 14, FITC-treated mice received 5 × 104 pfu γHV68 intranasally or were mock infected. On Day 21, total lung capacity, vital capacity, and lung compliance were measured. Figure 3 demonstrates that γHV68 infection results in significant impairments in total lung capacity (Figure 3A), vital capacity (Figure 3B), and lung compliance (Figure 3C) when compared with FITC-treated mice given mock infection. Thus, γHV68 exacerbation of FITC-induced fibrosis is associated with impaired lung function. Despite these changes, however, survival of the mice was not affected through Day 28.
Figure 3.
Infection with murine gammaherpesvirus-68 (γHV68) after fluorescein isothiocyanate (FITC) impairs lung function. Mice were given an injection of FITC on Day 0 or were given saline (control). On Day 14, FITC-treated mice were given a γHV68 infection or were mock infected. Total lung capacity (A), vital capacity (B), and compliance (C) were all measured on Day 21 as described in the online supplement; n = 5 for control group and n = 10 for FITC + mock or FITC + γHV68. γHV68 infection resulted in significant impairment of lung function compared with FITC + mock in each measurement.
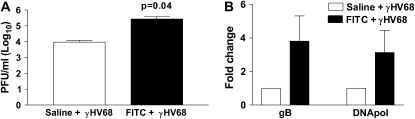
Increased Fibrosis Is Associated with Increased Lytic Viral Replication and Increased Viral Load
Our time point for harvest on Day 7 post–γHV68 infection was chosen because our initial experiments revealed that peak viral replication in unmanipulated mice occurs on Day 7 postinfection (data not shown). To determine whether virus replication was enhanced in FITC-treated mice, animals were given intratracheal FITC or saline at Day 0 and were then infected intranasally with γHV68 (5 × 104 pfu) at Day 14. Lungs were harvested at Day 21 (7 d after γHV68 infection) and plaque assays were performed (Figure 4A). There was lytic virus present at this time point, and mice that had been pretreated with FITC had significantly greater viral loads compared with those pretreated with saline (9,434 ± 2,644 vs. 273,600 ± 119,200 pfu, P = 0.04). Virus gene expression was measured using real-time RT-PCR in both groups of mice. We analyzed expression of the glycoprotein B (gB) gene, which encodes part of the virus capsid, and the viral DNA polymerase gene, both of which are expressed during lytic infection (Figure 4B). There is expression of both of these genes on Day 21 post-FITC (7 d after viral infection), and there is an approximately 3.5-fold increase in expression of both genes in mice that were given FITC and γHV68 versus those given saline followed by γHV68 infection.
Figure 4.
There is active lytic viral replication at Day 7 after murine gammaherpesvirus-68 (γHV68) infection, and previous fibrotic insult leads to increased viral load. Wild-type mice were given either saline or fluorescein isothiocyanate (FITC) intratracheally on Day 0. On Day 14, they were given γHV68 (5 × 104 pfu) intranasally. Lungs were harvested on Day 21. (A) Viral plaque assay demonstrates that there is active viral replication at Day 7 postinfection, and mice given FITC are more susceptible to the γHV68 infection (n = 5, P = 0.04). (B) Real-time polymerase chain reaction demonstrates increased lytic viral gene expression in FITC-treated lungs at Day 7 postinfection. Viral gene expression of mice given virus post-saline (open bars) was set at 1 and gene expression of mice given FITC and virus (solid bars) is expressed in comparison. Viral gene expression of both genes is increased approximately 3.5-fold in mice that were given FITC before infection (n = 3). DNApol = DNA polymerase; gB = glycoprotein B.
Lytic Viral Replication Is Required for Exacerbation of FITC-induced Fibrosis
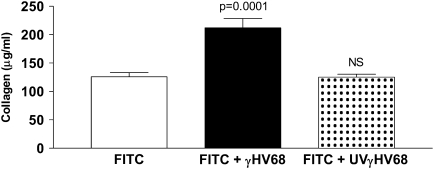
We next wanted to know whether or not lytic viral replication was needed to exacerbate the fibrotic response or whether inactive viral antigen would have the same result. To accomplish this, we used a protocol to produce UV light–inactivated γHV68 (UVγHV68), which has previously been shown to limit viral replication (34). Mice were again given FITC intratracheally on Day 0. They were then infected intranasally with either 5 × 104 pfu of γHV68 or UVγHV68 or were mock infected on Day 14. Lungs were harvested on Day 21 and collagen content was determined via Sircol assay (Figure 5). Wild-type γHV68 was able to exacerbate FITC-induced fibrosis as seen previously (FITC + mock = 125.7 ± 7.5 vs. FITC + γHV68 = 212.0 ± 16.4 μg/ml collagen, P < 0.001). However, the UVγHV68 was unable to augment fibrosis (FITC + mock = 125.7 ± 7.5 vs. FITC + UVγHV68 = 125.1 ± 5.2 μg/ml collagen). Therefore, lytic viral replication, not just viral antigen, is necessary for viral exacerbation of FITC-induced fibrosis.
Figure 5.
Lytic viral replication is necessary to augment the fluorescein isothiocyanate (FITC)–induced fibrotic response. Wild-type mice were given FITC intratracheally on Day 0. On Day 14, FITC-treated mice received 5 × 104 pfu murine gammaherpesvirus-68 (γHV68) (solid bar) or ultraviolet (UV)-inactivated γHV68 (stippled bar) intranasally. Lungs were harvested for collagen determination on Day 21. Infection with the UV-inactivated virus does not exacerbate FITC-induced fibrosis. Data represent n = 5–7 mice per group.
MAV-1 Infection Does Not Significantly Augment the Fibrotic Response to FITC
To determine whether the augmentation of fibrosis seen with γHV68 infection was virus specific, we treated mice on Day 0 with saline or FITC and on Day 14 gave FITC-treated mice 1 × 105 pfu MAV-1 intranasally or mock infected the mice. Collagen content was measured on Day 21 by hydroxyproline assay. Collagen in the lungs of saline-treated mice was measured at 51 ± 2.3 mg/ml (n = 3). FITC + mock infection resulted in 83.5 ± 4.6 mg/ml collagen content (n = 5). Mice treated with FITC + MAV-1 had collagen contents that were slightly elevated (95.5 mg/ml, n = 5), but this increase did not reach statistical significance compared with FITC + mock infection. Thus, it appears that there is specificity in the ability of γHV68 to exacerbate fibrosis in this model.
γHV68 Exacerbation of Fibrosis Occurs in the Presence of a Th1 Immune Response
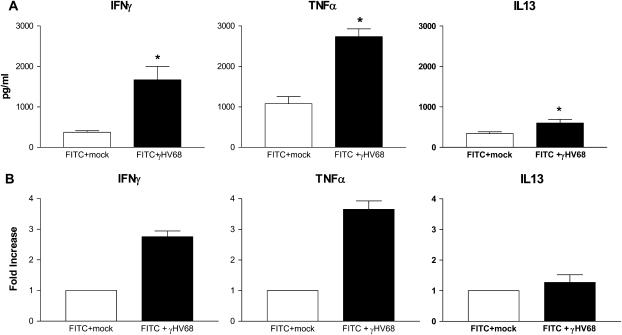
Because previous models have shown that γHV68 infection can lead to fibrosis in a Th2-biased environment (27, 28) and FITC fibrosis is associated with Th2 cytokine production (39), we wanted to know if the cytokine milieu in our model was Th2 skewed at the time of viral exacerbation of fibrosis. Mice were again given FITC on Day 0. On Day 14, they were infected intranasally with γHV68 (5 × 104 pfu) or were mock infected. Lungs were harvested on Day 21. Whole lung cytokine levels were measured by ELISA (Figure 6A) and cytokine gene expression was measured by real-time RT-PCR (Figure 6B). After γHV68 infection, there was an increase in both cytokine levels and gene expression when compared with mock-infected mice for all three cytokines analyzed. Notably, the exacerbation of fibrosis by γHV68 was associated with a strong Th1 response. Compared with mock-infected mice, IFN-γ levels were strongly induced in the γHV68-infected mice post-FITC (1,669 ± 339 vs. 373 ± 38 pg/ml, P < 0.05), and mRNA expression was elevated 2.75-fold. TNF-α was produced at even higher levels in γHV68 versus mock-treated mice (2,736 ± 192 vs. 1,078 ± 176 pg/ml). IL-13 was also elevated, but the overall amounts of IL-13 produced were much lower than the levels of IFN-γ.
Figure 6.
Murine gammaherpesvirus-68 (γHV68) augments the fibrotic response to fluorescein isothiocyanate (FITC) in the presence of a significant Th1 immune response. Wild-type mice were given FITC intratracheally on Day 0 and then given either mock (open bars) or γHV68 (5 × 104 pfu; solid bars) intransally on Day 14. (A) Lungs were homogenized in antiprotease buffer for ELISA or (B) in Trizol for RNA extraction on Day 21 (n = 4–5 per group), *P < 0.05 compared with FITC + mock infection. TNFα = tumor necrosis factor-α.
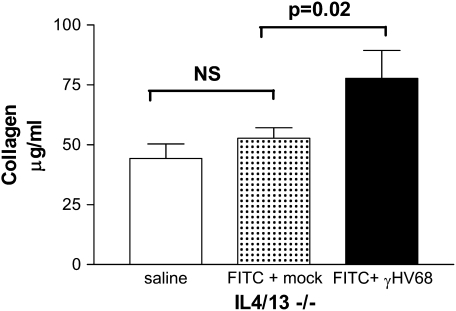
To determine whether Th2 cytokines were critical for the exacerbation of fibrosis, we tested the ability of γHV68 to exacerbate FITC-induced fibrosis in the Th2-deficient IL-4/13−/− mice. BALB/c and IL-4/13−/− mice were injected with FITC on Day 0. The mice were then given 5 × 104 pfu γHV68 or mock infection on Day 14, and lungs were harvested on Day 21 for collagen measurement via Sircol assay. Figure 7 demonstrates that γHV68 can exacerbate FITC-induced fibrosis in IL-4/13−/− mice (52.68 ± 4.43 vs. 77.73 ± 11.67 μg/ml, P = 0.01). We have seen similar results in IL-13−/− mice with a 40% increase in collagen after γHV68 infection (data not shown). Therefore, the Th2 cytokines IL-4 and IL-13 are not required for acute γHV68 infection to exacerbate already established fibrosis, and γHV68 infection can exacerbate FITC-induced fibrosis despite the presence of significant increases in IFN-γ levels.
Figure 7.
Murine gammaherpesvirus-68 (γHV68) infection exacerbates fluorescein isothiocyanate (FITC)–induced fibrosis in IL-4/13−/− mice. Wild-type and IL-4/13−/− mice were given FITC intratracheally on Day 0. On Day 14, FITC-treated mice received γHV68 (5 × 104 pfu; solid bar) or mock infection (stipled bar) intranasally. Lungs were harvested for collagen determination on Day 21. Data represent n = 6 mice per group. NS = not significant.
γHV68 Infection Induces Recruitment of Fibrocytes to the Lung
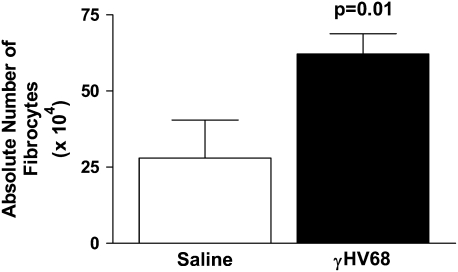
Because fibrocytes are known to be important mediators of fibrosis post-FITC, we next determined whether γHV68 infection could induce fibrocyte recruitment. Mice were infected intranasally with 5 × 104 pfu γHV68 or were mock infected on Day 0. On Day 5, lungs were harvested for collagenase digestion to collect lung leukocytes. The leukocytes were then stained for CD45+ col 1+ cells, and analyzed by flow cytometry to identify fibrocytes. Absolute numbers of fibrocytes were then calculated. Figure 8 demonstrates that fibrocytes are recruited to the lung in significantly increased numbers 5 days post–γHV68 infection (P = 0.01).
Figure 8.
Murine gammaherpesvirus-68 (γHV68) infection induces fibrocyte recruitment to the lungs. Mice were infected intransally with 5 × 104 pfu γHV68 or saline on Day 0. On Day 5, lungs were digested with collagenase and DNase, cells were enumerated, and then stained for CD45 and col 1. Percentage of fibrocytes in each lung was determined by flow cytometry, n = 6. Absolute numbers were calculated, P = 0.01.
Increased CCL2 and CCL12 Production Post–γHV68 Infection
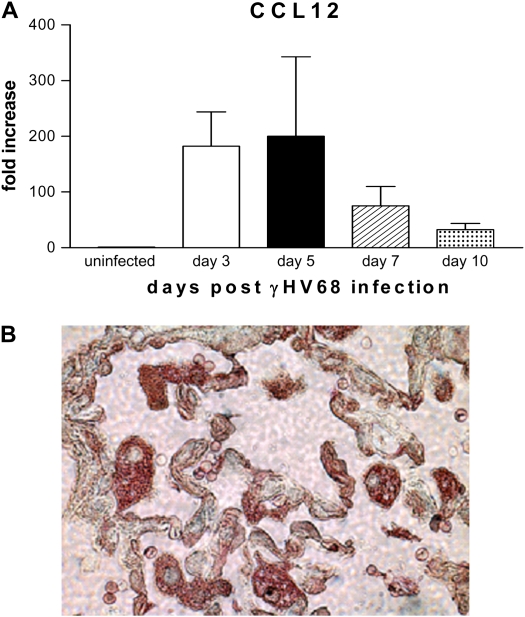
Having demonstrated that fibrocytes were recruited by γHV68 infection, we next wanted to assess the production of chemokines in response to γHV68 infection. Our previous results demonstrated that γHV68 infection results in CCL2 (monocyte chemoattractant protein-1) elaboration (40). Although CCL2 is a chemotactic ligand for CCR2-mediated fibrocyte recruitment and an inducer of collagen synthesis by fibrocytes (12), we have previously demonstrated that CCL12 is the preferred ligand for CCR2-mediated fibrocyte recruitment to the lung in response to FITC alone (13). Thus, we examined the ability of γHV68 infection to stimulate CCL12 expression. Mice were infected intranasally with γHV68 (5 × 104 pfu) and lungs were harvested at Days 0, 3, 5, 7, and 10 postinfection. Whole lung RNA was extracted and real-time RT-PCR was performed to quantify CCL12 gene expression (Figure 9A). Gene expression in control, uninfected mice (Day 0) was set to 1, and other time points are expressed relative to this condition. There is a greater than 150-fold increase in CCL12 mRNA expression between Days 3 and 5 postinfection. CCL12 levels then begin to decrease at Days 7 and 10 postinfection but remain elevated compared with baseline. Thus, γHV68 infection induces expression of both CCL2 and CCL12, chemokines known to be critical for fibrocyte recruitment to the lungs, and the timing of CCL12 production precedes fibrocyte recruitment.
Figure 9.
Murine gammaherpesvirus-68 (γHV68) infection induces increased CCR2 ligand production. (A) Wild-type mice were infected with 5 × 104 pfu γHV68 intransally on Day 0. Lungs were harvested on Days 0, 3, 5, 7, and 10. Whole lung RNA was extracted and real-time polymerase chain reaction was performed for CCL12 expression. Uninfected controls (Day 0) were set at 1 and all other values are expressed relative to control (n = 4). (B) Frozen tissue sections taken from mice on Day 21 post–FITC + γHV68 infection were stained for expression of γHV68 using a virus-specific antiserum (brown) and expression of CCL2 (red). γHV68 infection could be detected in epithelial cells and CCL2 production was noted both in infected epithelial cells as well as in alveolar macrophages.
To confirm that γHV68 infection resulted in CCR2-binding chemokine release, paraffin sections of FITC + γHV68–infected lungs were stained with an antiserum to detect γHV68 infection (brown staining) as well as with an antibody to detect CCL2 expression (red staining). Figure 9B demonstrates evidence of γHV68 infection in alveolar epithelial cells. CCL2 expression is noted both in infected epithelial cells as well as in alveolar macrophages located in the region of infected lung.
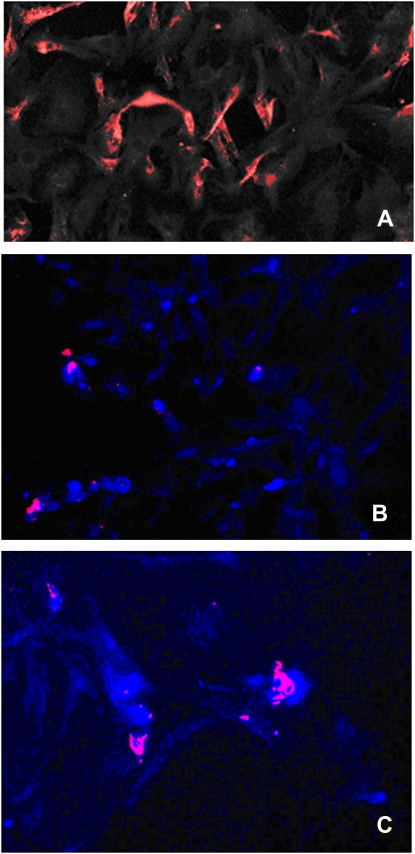
To confirm that alveolar epithelial cells could be both reservoirs of γHV68 replication and CCL2 release, alveolar epithelial cells were purified on Day 21 from mice that had been treated with FITC on Day 0 and γHV68 on Day 14. Epithelial cells were plated on fibronectin-coated eight-chambered glass slide (BD Biosciences, Bedford, MA) and immunofluorescent staining was performed using γHV68-specific and anti-CCL2 antisera. Figure 10A demonstrates that γHV68 infection (red staining) is readily detected in these purified alveolar epithelial cells. Figures 10B and 10C demonstrate colocalization of γHV68 infection (red) and CCL2 production (blue).
Figure 10.
Lung epithelial cells are reservoirs for murine gammaherpesvirus-68 (γHV68) replication and CCL2 production. Epithelial cells were purified from mice on Day 21 post–FITC + γHV68 treatment. Isolated cells were plated on fibronectin-coated eight-chambered glass slides and fixed, and immunofluorescent staining was performed. (A) Staining with anti-γHV68 polyclonal antisera detected with a phycoerythrin (PE)-conjugated secondary antibody. (B) Colocalization of γHV68 staining (red, PE) with anti-CCL2 (Cy5, blue) at ×200 original magnification and (C) a different section of this slide at ×400 original magnification.
γHV68 Can Infect Multiple Cell Types
Figures 9 and 10 demonstrate that γHV68 infection is detected in epithelial cells in vivo and ex vivo. To determine whether other cell types were potential reservoirs for γHV68 replication, we isolated fibroblasts, fibrocytes, alveolar macrophages, and interstitial macrophages from untreated mice and infected these cell types in vitro with 0.01 or 0.1 pfu/cell γHV68 and measured viral gene expression 72 hours later. In all cases, we could detect a dose-dependent increase in viral gene expression by real-time PCR analysis (data not shown), suggesting that each of these cell types is a potential reservoir for γHV68 replication in vivo.
Fibrocyte Recruitment in Response to FITC + γHV68 Is Impaired in CCR2−/− Mice
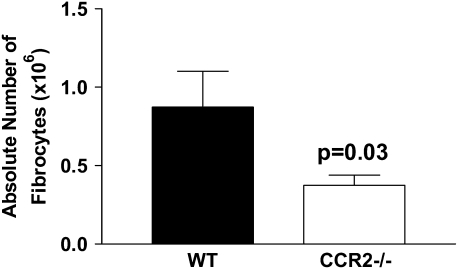
CCL12 is the preferred ligand for the CCR2 chemokine receptor on fibrocytes (13). To confirm that fibrocytes were being recruited to the lungs of FITC + γHV68–treated lungs in a CCR2-dependent manner, we treated wild-type (C57Bl/6) or CCR2−/− mice with FITC on Day 0. On Day 14, mice were infected with 5 × 104 pfu γHV68 and lungs were removed for collagenase digestion and flow cytometry analysis for CD45+ col 1+ cells on Day 21. Figure 11 demonstrates that recruitment of fibrocytes in response to FITC + γHV68 is significantly inhibited in CCR2−/− mice compared with wild-type mice.
Figure 11.
CCR2−/− mice have decreased recruitment of fibrocytes in response to fluorescein isothiocyanate (FITC) and murine gammaherpesvirus-68 (γHV68) infection. Wild-type and CCR2−/− mice were given FITC intratracheally on Day 0. On Day 14, all mice received γHV68 (5 × 104 pfu) intranasally. On Day 21, lungs were digested with collagenase and DNase. The cells were enumerated and stained for CD45 and col 1. Percentage of fibrocytes were determined by flow cytometry, n = 5. Actual cell numbers were then calculated, P = 0.03.
DISCUSSION
Our results demonstrate that infection with γHV68 can cause rapid exacerbation of established FITC-induced lung fibrosis. This experimental model demonstrates the ability of a gammaherpesvirus to exacerbate disease rapidly despite the presence of acute Th1 antiviral responses. In addition, our results provide new mechanistic insight into the pathogenesis of virus-induced exacerbations by demonstrating that the active viral infection results in the release of CCL2 and CCL12 and the recruitment of CCR2-expressing fibrocytes to the lung. Our studies extend previous observations in several important ways. One previous report demonstrated the ability of γHV68 infection 7 days before the administration of bleomycin to augment collagen deposition within the lungs of wild-type mice (26); however, no mechanism was reported for these findings. To our knowledge, our results are the first to demonstrate that fibrocytes are recruited to any infectious agent and to γHV68 in particular. It is likely that the mechanism of increased fibrocyte recruitment that we have identified could also explain the augmentation of fibrosis when the infection occurs before the fibrotic stimulus. Adoptive transfer of fibrocytes has previously been shown by us to augment FITC-induced fibrotic responses (13). The only other descriptions of γHV68 involvement in lung fibrosis come from studies of chronic infection (45–180 d) in IFN-γ receptor–deficient mice (27, 28, 41). These studies suggest that the virus has the potential to be fibrogenic in a Th2-skewed environment over long periods of time. In contrast, our results demonstrate that acute γHV68 infection can increase fibrosis in wild-type mice and that this rapid exacerbation is not dependent on the presence of IL-4 or IL-13. In fact, the rapid exacerbation of fibrosis in our model occurs in the face of a strong antiviral Th1 response. Finally, we suggest that this model system shares many features of a newly described aspect of IPF disease pathogenesis in humans, namely acute exacerbations of IPF. As such, we suggest that viral infections should be given strong consideration as mediators of rapid deterioration noted in some patients with IPF.
Our results demonstrate that, when γHV68 is administered intranasally on Day 14 post-FITC, a time point of established fibrosis (32), the deposition of collagen is increased when measured on Day 21 in two different genetic backgrounds (C57Bl/6 and BALB/c). This time point, 7 days postinfection, corresponds to the period of peak lytic viral replication. Furthermore, because UV-inactivated γHV68 infection did not exacerbate disease, viral replication is required for augmentation of fibrosis, and fibrotic mice experience enhanced γHV68 gene expression and higher viral titers than nonfibrotic mice. Our results further suggest that MAV-1 infection is unable to exacerbate FITC fibrosis. Although these results may suggest some specificity for the ability of gammaherpesviruses to exacerbate fibrosis, caution should be exercised in this interpretation. It is not yet clear whether there are overlapping cell tropisms between MAV-1 and γHV68; thus, this disparity may reflect differences in sites of viral replication during acute respiratory infection. Further experiments will be needed with additional viruses to fully address this point.
Our results demonstrate that γHV68 infection results in an approximately 200-fold induction of CCL12 within the first 3–5 days postinfection. We have previously shown that CCL2 gene expression is also increased after γHV68 infection and peaks between Days 6 and 9 postinfection (40). CCL2 and CCL12 can be produced by a variety of cells in the lung, including alveolar epithelial cells, mesenchymal cells, and leukocytes. Alveolar and interstitial macrophages likely play a role in the recognition of γHV68 and the subsequent release of chemokines. Histologic analysis in Figures 9 and 10 suggests that both alveolar macrophages and alveolar epithelial cells are sources of chemokine release in response to γHV68 infection. In vitro infection of the alveolar macrophage cell line, MH-S, or primary alveolar macrophages results in the production of both CCL2 and CCL12 (J.B.W. and B.B.M., unpublished observations). Production of both of these chemokines is likely important to the pathogenesis of the exacerbation. CCR2 appears to be expressed on all murine fibrocytes (12). We have previously shown that CCL12 is the preferred CCR2 ligand for fibrocyte recruitment to FITC alone (13), and our current studies suggest that CCR2 mediates the recruitment of fibrocytes in response to FITC + γHV68 infection as well. CCL2, another CCR2 ligand, is known to up-regulate the production of collagen by fibrocytes (12). Thus, infection with γHV68 likely results in both increased recruitment and activation of fibrocytes. These data suggest that one mechanism by which γHV68 may exacerbate lung fibrosis is through up-regulation of chemokines (CCL2 and CCL12), leading to enhanced recruitment of fibrocytes and increased extracellular matrix deposition by fibrocytes.
Fibrotic responses are often associated with imbalances in Th1/Th2 cytokines (42). A Th2 pattern of cytokines predominates in the pulmonary interstitium of patients with IPF (43). In addition, we have previously shown IL-13 to promote fibrosis post-FITC via direct activation of collagen synthesis in fibroblasts (39). Given the requirement of a Th2 bias for chronic γHV68 infection to induce fibrosis (27, 28, 41), we anticipated that IL-13 would be required for γHV68 to acutely augment fibrosis between Days 14 and 21 post-FITC, a time period in which fibroblast proliferation and accumulation is occurring. Thus, we were surprised to discover that γHV68 was able to augment collagen production in FITC-treated IL-13−/− and IL-4/13−/− mice to a similar extent as in wild-type mice. Thus, in this model of acute viral exacerbation of fibrosis, these Th2 cytokines are likely not required for the augmented production of extracellular matrix. In fact, the exacerbation occurs in the presence of a strong antiviral Th1 response associated with abundant IFN-γ production. It should be noted that our results are consistent with recent findings in experimental silicosis, which also indicate that Th2 cytokines are not required for the development of that inflammatory and fibrotic disease (44). Th1 cytokines may play some role in the pathogenesis of fibrotic responses in both humans and animals. Patients with sarcoidosis develop fibrotic responses and these responses are associated with IFN-γ and IL-12 p40 up-regulation (45). Neutralization of IL-12 was demonstrated to ameliorate apoptosis, inflammation, and fibrosis in bleomycin-treated mice (46) and IFN-γ−/− mice have also been reported to be protected from bleomycin-induced inflammation, weight loss, mortality, and fibrosis (47). Thus, the presence of Th1 cytokines induced by the virus in our model may in fact augment the fibrotic response.
Other potential mechanisms exist by which γHV68 could contribute to the fibrotic response seen in the lung. Alveolar epithelial cell injury is a consistent finding in IPF. There is evidence for impaired reepithelialization in the pathogenesis of pulmonary fibrosis (3). It is possible that herpesviruses may infect alveolar epithelial cells and that viral infection can induce cell lysis and/or apoptosis. Figures 9 and 10 clearly demonstrate the ability of γHV68 to infect epithelial cells. Furthermore, the pathology noted in Figure 2 is consistent with findings of alveolar epithelial cell injury and diffuse alveolar damage. Virus-induced alterations in inflammatory cell accumulation or activation may also influence lung fibrogenesis. The initial host response to virus infection leads to an influx of activated macrophages, lymphocytes, and neutrophils (48), which could also contribute to the fibrotic response. Interestingly, neutrophils from patients with IPF display a more activated phenotype than those from normal control subjects (3), and increased neutrophil activation could increase oxidants and epithelial cell injury (3). These possibilities will be the subject of future investigations.
In summary, we have described an animal model for acute viral exacerbation of established fibrosis. γHV68 can exacerbate established pulmonary fibrosis and this is due in part to up-regulation of chemokines (CCL2 and CCL12), leading to a profibrotic environment with increased fibrocyte recruitment and collagen deposition. As we are now beginning to recognize the morbidity and mortality associated with acute exacerbations of pulmonary fibrosis in our hospitals and intensive care units, it becomes imperative that we have a better understanding of the cause and mechanisms involved in these acute deteriorations so that better treatment strategies can be developed. Our model represents an attractive experimental method in consideration of previous data that suggest a possible role for herpesviruses, including EBV and HHV-8, in the pathogenesis of IPF (19–22). The current definition of an acute exacerbation of pulmonary fibrosis excludes infection (6, 7). However, most studies have only used conventional culture and serologic methods for ruling out infection. Evidence of herpesvirus infection, particularly latent infection, can be very difficult to demonstrate, and it is possible that subclinical infection (latent infection, reactivating virus, or occult lytic infection) could contribute to the pathogenesis of at least some cases of acute exacerbation or deterioration in IPF. In this regard, it is interesting to note that the standard therapy for patients with IPF involves immunosuppressive therapies and corticosteroids (1). Potentially, these therapies may predispose patients with IPF to a greater risk of latent virus reactivation or primary infection. Our new model system should facilitate studies to unravel the pathogenesis of acute infectious fibrotic deteriorations.
As with any animal model, however, caution must be exercised when extrapolating these results to human disease. The virus used in our studies is γHV68, a rodent pathogen. Although there are extensive similarities between γHV68 pathogenesis in mice and EBV or HHV-8 pathogenesis in humans, more work is needed to evaluate whether the cell tropisms and the consequences of infection in each host are similar. In addition, our model system does not result in enhanced mortality during the first 14 days postinfection, whereas human acute exacerbations are associated with significant morbidity. There are likely other predisposing conditions, such as genetics and coinfections, that vary between our animal model and the human situation. Thus, whereas this model system likely does not replicate all of the cardinal manifestations associated with acute exacerbations in patients with chronic IPF, it should be instructive for studying pathologic mechanisms associated with infectious exacerbation of fibrosis.
Supplementary Material
Acknowledgments
The authors thank Carol Wilke for technical assistance with the FITC injections of mice and Dr. Sem Phan (University of Michigan) for the pathologic evaluation of our histology specimens.
Supported by NIH grants HL071586 and AI065543 (B.B.M.), HL080282 (J.B.W.), CA103632 (L.F.v.D.); a Research Enhancement Award Program grant from the Department of Veterans Affairs (P.J.C.); a Career Investigator Award from the American Lung Association of Michigan (B.B.M.); and a research gift from the Martin Edward Galvin fund for pulmonary fibrosis research. T.R.M. is supported by the Multidisciplinary Training Grant for Pulmonary Research T32HL07749.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200708-1184OC on January 10, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society; European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963–967. [DOI] [PubMed] [Google Scholar]
- 5.Churg A, Muller NL, Silva CI, Wright JL. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007;31:277–284. [DOI] [PubMed] [Google Scholar]
- 6.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143–150. [DOI] [PubMed] [Google Scholar]
- 7.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808–1812. [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Lloyd JE, Noth I, Olman MA, et al. Acute exacerbations of idiopathic pulmonary fibrosis [pulmonary perspective]. Am J Respir Crit Care Med 2007;176:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blivet S, Philit F, Sab JM, Langevin B, Paret M, Guerin C, Robert D. Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest 2001;120:209–212. [DOI] [PubMed] [Google Scholar]
- 10.Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med 2002;166:839–842. [DOI] [PubMed] [Google Scholar]
- 11.Stern JB, Mal H, Groussard O, Brugiere O, Marceau A, Jebrak G, Fournier M. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest 2001;120:213–219. [DOI] [PubMed] [Google Scholar]
- 12.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 16.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556–7562. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lok SS, Stewart JP, Kelly BG, Hasleton PS, Egan JJ. Epstein-Barr virus and wild p53 in idiopathic pulmonary fibrosis. Respir Med 2001;95:787–791. [DOI] [PubMed] [Google Scholar]
- 20.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003;41:2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:510–513. [DOI] [PubMed] [Google Scholar]
- 22.Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159:1336–1341. [DOI] [PubMed] [Google Scholar]
- 23.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol 1990;71:1365–1372. [DOI] [PubMed] [Google Scholar]
- 24.Flano E, Woodland DL, Blackman MA. A mouse model for infectious mononucleosis. Immunol Res 2002;25:201–217. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med 1998;187:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lok SS, Haider Y, Howell D, Stewart JP, Hasleton PS, Egan JJ. Murine gammaherpes virus as a cofactor in the development of pulmonary fibrosis in bleomycin resistant mice. Eur Respir J 2002;20:1228–1232. [DOI] [PubMed] [Google Scholar]
- 27.Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham KL, Stecenko AA. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L711–L721. [DOI] [PubMed] [Google Scholar]
- 28.Ebrahimi B, Dutia BM, Brownstein DG, Nash AA. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am J Pathol 2001;158:2117–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillan T, Toews G, Standiford T, Moore B. Murine gammaherpesvirus-68 enhances fibrotic response to fluorescein isothiocyanate (FITC)–induced pulmonary fibrosis [abstract]. Proc Am Thorac Soc 2006;3:A38. [Google Scholar]
- 30.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med 1999;189:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol 1998;8:339–342. [DOI] [PubMed] [Google Scholar]
- 32.Moore BB, Paine R III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001;167:4368–4377. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg JB, Stempfle GS, Wilkinson JE, Younger JG, Spindler KR. Acute respiratory infection with mouse adenovirus type 1. Virology 2005;340:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Symensma TL, Martinez-Guzman D, Jia Q, Bortz E, Wu TT, Rudra-Ganguly N, Cole S, Herschman H, Sun R. Cox-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J Virol 2003;77:12753–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sisson T, Hansen J, Shah M, Hanson K, Du M, Ling T, Simon R, Christensen P. Expression of the reverse tetracycline-transactivator gene causes emphysema-like changes in mice. Am J Respir Cell Mol Biol 2006;34:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weck KE, Dal Canto AJ, Gould JD, O'Guin AK, Roth KA, Saffitz JE, Speck SH, Virgin HW. Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat Med 1997;3:1346–1353. [DOI] [PubMed] [Google Scholar]
- 37.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Olkiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. J Immunol 2006;177:5499–5508. [DOI] [PubMed] [Google Scholar]
- 38.Huffnagle GB, Strieter RM, Standiford TJ, McDonald RA, Burdick MD, Kunkel SL, Toews GB. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary cryptococcus neoformans infection. J Immunol 1995;155:4790–4797. [PubMed] [Google Scholar]
- 39.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 2004;172:4068–4076. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg JB, Lutzke ML, Efstathiou S, Kunkel SL, Rochford R. Elevated chemokine responses are maintained in lungs after clearance of viral infection. J Virol 2002;76:10518–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora AL, Torres-Gonzalez E, Rojas M, Xu J, Ritzenthaler J, Speck SH, Roman J, Brigham K, Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-γ receptor–deficient mice. Am J Respir Crit Care Med 2007;175:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukacs NW, Hogaboam C, Chensue SW, Blease K, Kunkel SL. Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest 2001;120:5S–8S. [DOI] [PubMed] [Google Scholar]
- 43.Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA). Clin Exp Immunol 1995;101:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misson P, Brombacher F, Delos M, Lison D, Huaux F. Type 2 immune response associated with silicosis is not instrumental in the development of the disease. Am J Physiol Lung Cell Mol Physiol 2007;292:L107–L113. [DOI] [PubMed] [Google Scholar]
- 45.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, et al. Enhanced expression of IL-12 associated with th1 cytokine profiles in active pulmonary sarcoidosis type 2 immune response associated with silicosis is not instrumental in the development of the disease. J Immunol 1996;156:4952–4960. [PubMed] [Google Scholar]
- 46.Maeyama T, Kuwano K, Kawasaki M, Kunitake R, Hagimoto N, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by monoclonal antibody to interleukin-12. Am J Physiol Lung Cell Mol Physiol 2001;280:L1128–L1137. [DOI] [PubMed] [Google Scholar]
- 47.Chen ES, Greenlee BM, Wills-Karp M, Moller DR. Attenuation of lung inflammation and fibrosis in interferon-γ-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol 2001;24:545–555. [DOI] [PubMed] [Google Scholar]
- 48.Sarawar SR, Lee BJ, Anderson M, Teng YC, Zuberi R, Von Gesjen S. Chemokine induction and leukocyte trafficking to the lungs during murine gammaherpesvirus 68 (MHV-68) infection. Virology 2002;293:54–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.