Abstract
Leishmania amazonensis was found to release nucleoside diphosphate kinase (NdK) - a stable enzyme capable of decreasing extracellular ATP. The release of this enzyme from Leishmania results in its progressive accumulation extracellularly as they replicate, peaking at the stationary phase in vitro. The released NdK is immunoprecipitable and constitutes ~40% of its total activities and proteins. The retention of a known cytosolic protein by wild type cells and a fluorescent protein by DsRed transfectants at stationary phase, which release NdK, indicates that this is a spontaneous event, independent of inadvertent cytolysis. Recombinant products of Leishmania NdK prepared were enzymatically and immunologically active. Both recombinant and native Leishmania NdK utilized ATP to produce expected nucleoside triphosphates in the presence of nucleoside diphosphates in excess. Both native and recombinant Leishmania NdK were also found to prevent ATP-induced cytolysis of J774 macrophages in vitro, as determined by assays for lactate dehydrogenase release from these cells and for their mitochondrial membrane potential changes. The results obtained thus suggest that Leishmania NdK not only serves its normal house-keeping and other important functions true to all cells, but also prevents ATP-mediated lysis of macrophages, thereby preserving the integrity of the host cells to the benefit of the parasite.
Keywords: Nucleoside diphosphate kinase, Secreted enzymes, Leishmania, Extracellular ATP, Macrophages, Necrosis/apoptosis
1. Introduction
Nucleoside diphosphate kinase (NdK) is a ubiquitous enzyme, which catalyzes the transfer of phosphate from NTP (nucleoside triphosphate) to NDP (nucleoside diphosphate) for maintaining appropriate NTP levels in cells. The enzyme is phosphorylated at the histidyl residue of its active site when ATP is supplied, for example, as the phosphate donor [1]. Additional functions have been ascribed to NdK in different organisms, e. g. regulation of gene expression in mammalian cells [2], participation in the purine salvage pathways of trypanosomatid protozoa [3] and bacterial pathogenesis [4].
Biochemical properties and functional significance of NdK in pathogenic trypanosomatids are of interest, but have not been extensively studied, e. g. Trypanosoma spp. [5, 6] and Leishmania spp. [7]. The latter are of particular interest not only as the causative agents of human leishmaniasis but also as a model for intracellular parasitism. These parasites are transmitted by sand fly vectors as promastigotes. In mammalian hosts, they infect macrophages and differentiate into amastigotes in the phagolysosomes of these mononuclear phagocytes - the exclusive and true host cells of these intracellular parasites. It is thus of interest to examine their NdK as a multifunctional enzyme in Leishmania infection.
Chakrabarty and others [4, 8–11] have demonstrated that many pathogenic microorganisms release enzymes that modulate levels of NTPs. Of particular interest is extracellular ATP (eATP), which is expected to accumulate at the site of inflammation [12–14], important in immunity against microbial infection. The released enzymes include adenylate kinase, 5′-nucleotidase (and/or ATPase/phosphatase), ecto-ATPase [15], ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) [16] and NdK. The last enzyme utilizes eATP as its principal substrate [9, 10, 17–20]. eATP is known to trigger the death of immune cells, including macrophages, by its binding to purinergic receptor of the P2X family, i. e. P2X7 [12, 13, 21, 22]. ATP-binding to P2X7 receptors of macrophages results in their cytolysis in vitro via necrotic [23, 24] or apoptotic pathways [25, 26], dependent presumably on the duration of exposure to eATP [27, 28]. Extracellular pathogenic bacteria, such as Pseudomonas aeruginosa [10, 18], have evolved strategies to avoid phagocytic activity of macrophages by releasing enzymes that increases the level of eATP, thereby triggering apoptosis of these and other professional phagocytes. In contrast, intracellular parasites, like Leishmania spp., are expected to develop strategies in the opposite direction by releasing eATP-utilizing enzymes, thereby prolonging the survival of their host cells.
Except for the above mentioned ATP-utilizing enzymes, like NdK, little is known about the microbial molecules of relevance from intracellular pathogens that mediate apoptosis/necrosis of host cells. Inhibition of this event has been found after infection of host cells in vitro by a number of different intracellular parasites. Examples include the infection of macrophages by Leishmania spp. [29–31] and Toxoplasma gondii [32, 33]. A 65 kDa heat-shock protein (HSP65) was found to be up-regulated in macrophages after infection by these organisms [34, 35] and Trypanosoma cruzi [36], but its relevance to the infection remains unknown. Many viral infections, such as hepatitis C are known to confer fas ligand-specific signals on the host cells for apoptosis [37], whereas T. cruzi infection inhibits this [38]. Identification of parasite molecules relevant to the life and death of their host cells is of potential interest for developing specific therapeutic strategies against the infectious diseases in question.
We report here the findings of NdK release by L. amazonensis into the culture supernatants. The release of NdK appears to be a spontaneous event, although the mechanism is unknown, since it is not accompanied by co-release of a known cytosolic protein. Nor is an episomally over-expressed fluorescent marker released under the same culture conditions. Additionally, we produced enzymatically active recombinant products. Evidence is presented, indicating that ATP-mediated cytolysis of J774 macrophages is prevented by the activities of this enzyme. We propose from these findings that Leishmania release NdK at the time of infection and beyond to prevent ATP-mediated cytolysis of their host cells by decreasing eATP, which may accumulate in such infection at the site of inflammation for modulation of immune cells.
2. Materials and Methods
2.1. Cell culture
Leishmania amazonensis (LV78, clone 12-1) were cultured continuously as promastigotes in vitro at 25°C in HEPES-buffered Medium 199 (pH 7.4) containing 10% heat-inactivated fetal bovine serum [39]. Culture starting with an inoculum of 106 cells/ml reached a peak cell density of ~108/ml in 4–5 days that was maintained throughout the stationary phase in the next several days. Cells were also grown in chemically defined medium [40] or incubated in serum-free Medium 199 or Hank’s Balanced Salt Solution (HBSS). These efforts were discontinued after repeated attempts due to the low release of NdK in the absence of serum proteins or BSA (Not shown).
2.2. Assays for NdK activities
Materials used for these assays included both cell lysates and culture supernatants. Portions of the cultures were withdrawn daily and centrifuged at 3,500 g for 5 min at 4°C to sediment cells. Cell pellets were washed and stored frozen. After centrifugation at 45,000 g for 1 h followed by filtration (Pore size=0.2 uM), the culture supernatants were concentrated 10-fold in a Centricon 10 (10 kDa cutoff) (Amicon Co).
The soluble fractions of the cell lysates and culture filtrates from serum-containing media were assayed for the activities of cytosolic and secreted NdK, respectively. Reaction mixtures in a total volume of 5 ul each consisted of the following components: an enzyme source in 1 ul aliquots (10X concentrated in the case of culture supernatants) and the substrates, i. e. 1.5 uCi of gamma[32P]-ATP (specific activity = ~3000 Ci/mmol) and NDPs (1 mM each of GDP, CDP, UDP) in TM buffer (50 mM Tris, pH 7.2 and 10 mM MgCl2) [17, 41]. Reaction was allowed to proceed at 25°C for 10 min and terminated by chilling on ice followed by the addition of 1% SDS (final concentration). Reaction products were resolved by polyethyleneimine thin-layer chromatography (PEI-TLC) and visualized by autoradiography [41]. Under the experimental conditions described, one unit of NdK activity is defined as 1 nmol of UTP formed per min, as estimated from its radioactivity with reference to the specific radioactivity of the ATP used by a STORM860 phosphor screen [17].
2.3. Western blot analyses
Various samples obtained were examined for NdK proteins using anti-Leishmania NdK antiserum at 10−3 dilutions and HRP-conjugated secondary antibodies at a ~10−4 dilution. Blots were developed with Pierce West Femto chemiluminescent substrates and exposed to X-ray films for visualization.
A cytosolic soluble protein of Leishmania [40] was included in this study as a marker for evaluation of cytolysis in culture. Samples used included 10X concentrates of daily collected culture supernatants, cell pellet (resuspended to 109/ml), water-soluble and –insoluble fractions of cell lysates (~109 cells/ml lysed in Tris-HCl buffer, pH 7.5 by 3 freeze-thaw cycles). Samples were each loaded at 10 ul equivalent to a total cell protein from 0.1 ml of the culture collected daily. Samples from day 1 cultures contained too few cells (~106/ml) and thus were concentrated 10X.
Sensitivity of Western blot analysis to detect P36 from minimal cell number was determined by using cytosolic fractions after 2-fold serial dilutions, giving proteins equivalent to the amounts from 1.625 × 105 to 107 cells. Blots were reacted first with rabbit anti-P36 antiserum at 5 × 10−3 [40] and then with goat anti-rabbit IgG conjugated to Alexafluor 680 (Molecular Probes) at 2 × 10−4 dilution. The blots were scanned for fluorescence intensities in a 700 channel reader (Odyssey, Li-Cor). The background signals were subtracted from the values obtained for plotting the linear regression of the relative fluorescence intensity versus the cell number.
2.4. Gamma[32P]ATP-phosphorylation of spent media for immunoprecipitation of released NdK
Culture supernatants were collected daily under standard conditions described in section 2.1 at 1.2 ml/day for 8 days. Proteins in each sample were phosphorylated at 37°C for 30 min with 10 uCi gamma [32P]-ATP in HEPES buffer (50 mM, pH 7.5) containing 1 mM DTT and 10 mM MgCl2 plus phosphatase inhibitors, i. e. 5 mM beta-glycerophosphate, 2.5 mM EGTA, 1 mM NaF and 0.1 mM sodium orthovanadate. Autophosphorylated NdK was immunoprecipitated from these samples with rabbit anti-rNdK at ~10−2 dilution for 30 min at 4°C. The subsequent collection of the immunoprecipitates with Protein A Sepharose, washing conditions and SDS-PAGE/autoradiography followed the procedures reported previously [42].
2.5. Biosynthetic pulse-label and chase of cells in culture for immunoprecipitation of NdK
Promastigotes grown to stationary phase were washed, resuspended in HBSS and biosynthetically labeled at 25°C for 1 h with 100 uCi/108 cells/ml of [35S] methionine/Cysteine (Specific Activity=1,175 Ci/mmole, Express Protein labeling Mix, Perkin-Elmer). Biosynthetically labeled cells were diluted with complete culture medium to 107/ml and chased for up to 60 h. Variable volumes of the samples were withdrawn at 12 hour intervals, giving ~108 cells for each time point. Each sample collected was centrifuged to separate cells and the culture supernatants. Sedimented cells were lysed at 4°C with 1% Nonidet-P 40 in 1 ml PBS and the lysate was cleared by centrifugation at 50,000 g for 30 min at 4 C. The culture supernatant was concentrated by Centricon (10 kDa cutoff) to ~1 ml containing 1% Nonidet-P 40. Detergent soluble proteins in both fractions from each time point were immunoprecipiated at 4°C with anti-Leishmania NdK antiserum at 10−2 dilution. The subsequent collection of the immunoprecipitates with Protein A Sepharose and SDS-PAGE/autoradiography were as described [42]. The levels of cytosolic and released NdK were estimated from band intensities (background subtracted) in the autoradiograms in Alphaimager (Kodak) for all samples collected at different time points after chase. The % of released NdK (Fig. S2B) was calculated for each time point as follows: Released NdK intensity/Released+Cytosolic NdK intensities × 100.
2.6. Enrichment of Leishmania cytosolic and secreted NdK
Promastigotes were grown in 700 ml batch cultures, which gave an optimal cell density of ~7 × 107/ml in stationary phase. Cultures were centrifuged as described to harvest spent media and cells. Cell pellets were resuspended to a density of 5 × 109 cells/ml in TM buffer and lysed by four freeze-thawing cycles. Cell lysates were cleared by centrifugation. Sedimented fractions were found to have negligible NdK activity and thus were not processed further (not shown).
Proteins in the water soluble cytosolic fractions and the culture filtrates were fractionated by precipitation with ammonium sulfate between 45% and 60% of saturation at 4°C. The precipitated proteins were centrifuged at 10,000 g for 20 min at 4°C and solubilized in a minimal volume of TM buffer, i. e. 20 ml and 70 ml for cytosolic and secreted protein fractions, respectively. Following dialysis against the same buffer, samples were subjected to FPLC on a Blue Sepharose column (2.6 cm × 25 cm). The Blue Sepharose-bound proteins were batch-eluted with KCl step gradients at 1 M and 2 M KCl. These fractions were dialyzed before use for enzyme assays and for SDS-PAGE in 15% gel followed by silver staining. Protein concentrations of all fractions were assayed by BCA reagent (Pierce) using BSA as standard.
2.7. Leishmania ndk cloning for recombinant NdK and anti-NdK antiserum production
The NdK gene (ndk) was PCR-amplified from 2 kb XhoI fragments of L. amazonensis genomic DNA. Forward and reverse primers used were designed on the basis of L. major ndk sequence (Leishmania Genome Project: LmjF32.2950) with BamH1 sites (bold-faced), i. e. 5′-TAGGATCCATGTCCTCCGAGCGCACC-3’ and 5’-GCGGATCCTTACTCATAGATCTGGGAC AC-3’. PCR products obtained were of the expected size (474 bp) and their identity verified as ndk by sequencing both strands to completion. The PCR products were cloned into pGEM-T vector (Promega, USA) (=pGEM-T-ndk) and expanded in E. coli DH5-alpha for subcloning into pET28a expression vector (Novagen, USA). The recombinant NdK thus produced consists of the 6X His sequence with the thrombin cleavage site in the N-terminal end. The pET28-ndk was transfected into a protease negative Escherichia coli (strain BL21-CodonPlus DE3, Stratagene). Transformants were induced with 1 mM IPTG to express His-NdK fusion protein and lysed in B-PER reagent (Pierce, USA). NdK fusion proteins expressed as inclusion bodies were solubilized in 50 mM CAPS buffer (pH 11) containing 0.3% N-lauryl sarcosine. After dialysis, solubilized proteins were subjected to batch purification of the fusion proteins with Probond Resin (Invitrogen) per manufacturer’s protocol. Purified His-NdK was digested with thrombin (1 U/100 ug recombinant proteins) under various conditions for up to 8 h at 37°C.
Anti-rNdK antisera were produced by immunizing rabbits with purified recombinant NdK via a commercial service facility (ProteinTech Group Co, Chicago). After collection of pre-immune sera to serve as controls, rabbits were each immunized intradermally with a total of ~900 ug rNdK in a period of ~3 months, i. e. a primary immunization with ~500 ug purified recombinant proteins plus a non-ulcerative adjuvant and four subsequent boosts of 100 ug antigens each. Immunodot blot analyses of the antisera in serial dilutions against rNdK at one ug per dot gave a titer of ~10−3 over the control with the preimmune sera. The antiserum produced is specific to Leishmania NdK, as it did not react with macrophage proteins (not shown).
2.8. Fluorescence assay for DsRed release from transfectants for evaluation of the extent of inadvertent cytolysis during in vitro cultivation
DsRed was PCR amplified from pDsRed-monomer-C1 (Clontech) using a set of forward (5’-GGATCCATGGTGCGCTCCAAG-3’) and reverse (5’-GGATCCTTATCTAGATCCGGTCG-3’) primers with BamH1 sites (bold-faced) to facilitate its cloning into p6.5 [39]. The reverse primer sequence was designed such that original BamH1 site in the 3’ end of the gene was obliterated without affecting the amino acid sequence of the product. The resulting 753 bp DsRed PCR product cloned into pGEM-T was obtained by BamHI digestion for ligation into this site of the p6.5 vector for transfecting L. amazonensis. This and control transfectants obtained were selected for tunicamycin-resistance and grown continuously in presence of this drug at 20 ug/ml.
Stock cultures of DsRed and control transfectants were inoculated into drug-free medium at 106cells/ml. Both cultures were sampled daily for six days at three ml aliquot each for separation of cells and culture supernatants. Sedimented cells were resuspended in three ml of fresh medium. Nonidet-P 40 was added to all samples at a final concentration of 1% before reading fluorescence intensity at excitation of 558 nm (slit width 10 nm) and emission of 583 nm (slit width 15 nm) in a spectrofluorimeter (LS50B, Perkin-Elmer). A standard curve of fluorescence intensity versus cell density was generated under the same setting using DsRed transfectants, which were serially diluted from 5 × 106 to 104 cells/ml. Fluorescent intensities of all collected samples were extrapolated to cell number against this standard curve.
2.9. Macrophage cytotoxicity assays
2.9.1. Lactate dehydrogenase (LDH) release assay
This assay was carried out by using Cytotox 96 Assay Kit (Promega, USA) [18]. Briefly, murine macrophages (J774) were plated in a 96 well tissue culture plate at 105 cells/200 ul DMEM/well and primed for 12 h with E. coli LPS at 100 ng/ml to activate P2X7 expression at 37°C in 5% CO2 [43]. The cells were then exposed to 1 or 3 mM eATP, ~1 U native NdK eluted from Blue Sepharose column (see above) and NDPs (0.1 mM each) in all possible combinations. LDH activities in the supernatants were colorimetrically determined after incubation for 3 h and percent release was calculated against the total LDH released from 0.2% Triton-X 100-lysed cells set as 100%.
2.9.2. Assay for macrophage mitochondrial membrane permeability changes
An Apoalert Mitochondrial Membrane Sensor kit (Invitrogen) was used as described [10]. In brief, J774 macrophages were seeded at 2.5 × 105 cells/9 mm2 cover slip in 30 ul RPMI containing 10% fetal bovine serum. Macrophages were primed with 100 ng/ml E. coli LPS in the same medium overnight at 37°C in 5% CO2. The primed cells were washed in HBSS HEPES-buffered to pH 7.4 and then treated for three h with 2 mM ATP in presence of Leishmania NdK partially purified or as recombinant NdK (~2 U) in complete RPMI 1640 medium (LPS+ATP+NdK or rNdK). Controls included J774 cells treated under the following conditions: LPS-ATP± native or recombinant NdK and LPS+ATP+ boiled native or recombinant NdK (brNdK). Treated cells were all washed in HBSS before exposure to Mitosensor reagent for 15 min at 37°C in 5% CO2. All preparations were finally rinsed once with HBSS and ~200 cells were read for each sample by epifluorescence microscopy (Carl Zeiss, Germany) using a filter set at band pass of 485 nm, fluorescence transmission of 510 nm and long pass of 520 nm. The fluorescent images were taken with Nikon Coolpix 4500 digital Camera.
2.10. Statistics
All experiments were carried out at least three times, except for NdK enrichment and molecular cloning of ndk, which were done twice and once, respectively. Data presented are representative of the results obtained and values presented are means of triplicate samples with standard errors.
3. Results
3.1. Leishmania releases nucleoside diphosphate kinase
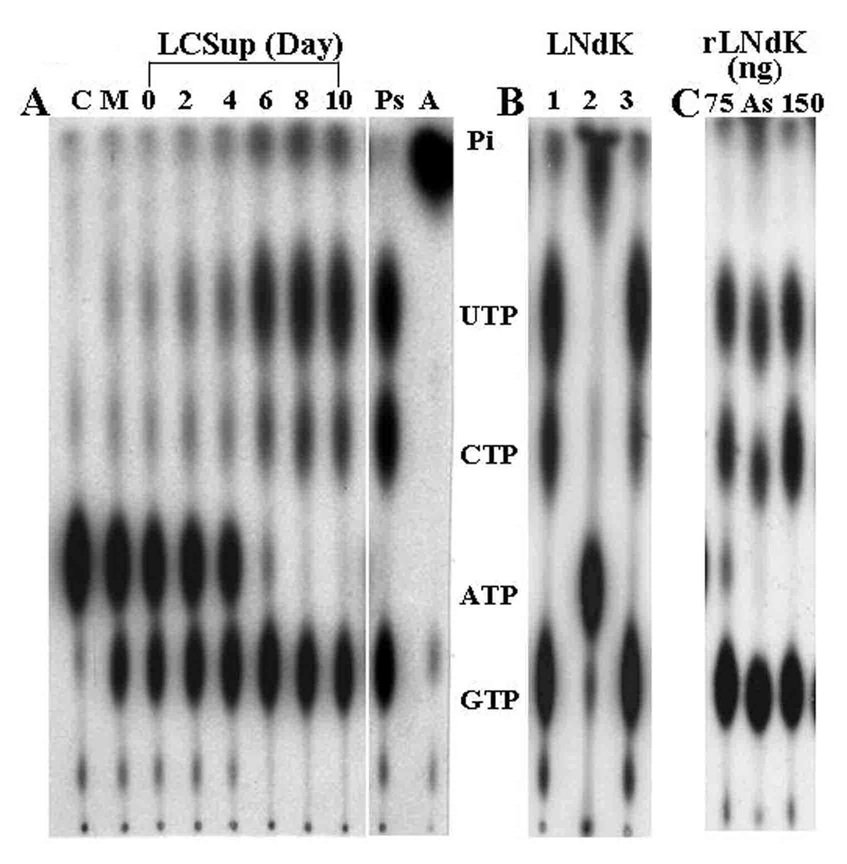
The release of NdK by L. amazonensis was indicated initially by finding the enzyme activities in the spent media, i. e. utilization of gamma[32P]-ATP and NDPs for the formation of expected products resolvable by TLC for visualization by autoradiography (Fig. 1A). In the presence of equimolar NDPs (minus ADP) in excess, additional NTPs emerged with time in the order of ~GTP>UTP>CTP until cells reached the stationary phase (Fig. 1A, Lanes 2–6). Thereafter, the relative intensity of NTPs became stabilized as ~GTP=UTP>CTP for the remaining period of the stationary phase (Lanes 8–10). The appearance of these NTPs is indicative of enzyme activities, since no products emerged without addition of the spent media as an enzyme source or after sample boiling before the assays (Fig. 1A, lane C). Authenticity of the reaction products as NTPs is indicated by their co-migration with those produced by purified Pseudomonas aeruginosa NdK under the same assay conditions (Fig. 1, Lane Ps). The ATP level diminished gradually and became depleted completely with the accumulation of the released NdK (Fig. 1A, lanes 6–10). This is expected, since CDP, UDP and GDP (NDPs) are present in overwhelming excess over gamma[32P]-ATP (molar ratio = 104:1), thereby favoring the formation of CTP, UTP and GTP instead of ATP. The final relative intensity of the NTPs seen with released NdK (Fig. 1A, Lanes 8–10) is comparable to those produced by extracellular NdK (Fig. 1B, LNdK) enriched by ammonium sulfate precipitation (Lane 1) and Blue Sepharose affinity after elution of NdK with 2 M KCl (Lane 3), but not with 1 M KCl (Lane 2). The kinetics of [32P]-labeled NTP products seen with NdK daily released from cells (Fig. 1A, Lanes 2–10) also approximates that observed with 75 and 150 ng of recombinant NdK (Fig. 1C, rLNdK, Lanes 75 and 150; Lane As = Ammonium sulfate precipitate fraction). Thus, all Leishmania NdK obtained in different ways appear to behave similarly, including their utilization of GDP for the formation of GTP, although the activity of unidentified enzyme(s) in the fetal bovine serum of the culture medium apparently contributed to the early signals observed for this NTP in the released samples collected (Fig. 1A, Lanes 2–6). This is indicated by the finding of GTP in day 0 (Fig. 1A, Lane 0) and in unused fresh medium (Fig. 1A, Lane M). The GTP in the culture filtrates at the very early time points may be attributable largely or even completely to the activity of the serum enzyme(s). The contribution of Leishmania released NdK to this may begin from day 2, judging from the emergence of CTP and UTP at this time point (Fig. 1A, lanes 2–4). These two products became prominent only at the stationary phase (Lanes 6–10).
Fig. 1. Leishmania nucleoside diphosphate kinase activities of culture supernatants after concentration [A] and enrichment [B], and recombinant products [C].
[A] Leishmania NdK released into culture media (LCSup). The culture supernatants daily collected and 10X concentrated were assayed for NdK activities (see Materials and Methods). The reaction products of [32P]-labeled NTPs were resolved by TLC and visualized by autoradiography. Lane C, Control without enzyme; Lane M, Control with 10X concentrates of uninoculated culture medium; Lanes 0, 2, 4, 6, 8, 10, Leishmania culture supernatants collected on day 0, 2, 4, 6, 8, 10, respectively; Lane Ps, Pseudomonas aeruginosa NdK; Lane A, Control with ATPase.
[B] Partially purified Leishmania NdK released into culture media (LNdK) (see Table 1). Lane 1, 45–60% ammonium sulfate precipitated samples (3.1 ug protein); Lanes 2–3, ineffective (1 M KCl) and effective (2 M KCl) stepwise elution of NdK bound to Blue Sepharose.
[C] Leishmania recombinant NdK (rLNdK). Lanes 75, 150, Reaction products using 75 and 150 ng of rLNdK as the enzyme source (see Fig. 7, Lane 4 for purity of the recombinant NdK); Lane As; Ammonium sulfate fraction of the culture supernatant used in Panel B included for comparison.
Immunoprecipitation of the culture supernatants with Leishmania NdK-specific antiserum resulted in the loss of activities therein to utilize ATP for the formation of CTP and UTP (Fig. 2, Lane C vs Lanes A and B). Released Leishmania NdK is thus solely responsible for these activities. This interpretation was ascertained by excluding GDP from the assay, since a serum enzyme appeared to co-exist and compete with Leishmania NdK in the culture supernatants for conversion of GDP into GTP (Fig. 1A, Lane M). This activity of the Leishmania NdK was clearly demonstrated with the recombinant NdK (Fig. 1C), which were free from the contamination of fetal bovine serum.
Fig. 2. Loss of nucleoside diphosphate kinase activities from culture supernatants after immunoprecipitation.
10X concentrated culture supernatants (A) of L. amazonensis promastigotes at stationary phase were reacted with pre-immune serum (B) and anti-Leishmania NdK immune serum (C) (see Materials and Methods). A separate aliquot of fresh culture medium was simultaneously prepared under the same conditions without antiserum addition as another negative control (D). Immunoprecipitates are removed by incubation with Protein G Sepharose. NdK activity of the post-reaction supernatants was assayed as described in the Materials and Methods for Fig. 1, except for the omission of GDP from the NDP mixture as the substrates.
Lane A–C, Reaction products of 10X concentrates reacted without antiserum, with pre-immune serum and anti-Ndk antiserum, respectively; Lane D, Negative control of unused medium.
Leishmania NdK released into the culture media was specifically recognized as such immunologically. We demonstrated this in two independent experiments by using both biosynthetically labeled and unlabeled cells (Fig. 3A and Fig. 4). To increase the sensitivity of detecting NdK released from unlabeled cells, we exploited its activity of autophosphorylation in the presence of gamma[32P]-ATP and phosphatase inhibitors without the addition of NDPs as phosphate acceptors. Proteins in the culture supernatants collected daily were phosphorylated under these conditions and immunoprecipitated. SDS-PAGE-autoradiography of these samples revealed a single band of ~17 kDa, the expected size of NdK (Fig. 3[A]). The phosphorylated NdK increased in intensity progressively with culture periods and peaked on days 4–5 when cells reached the stationary phase. In samples collected thereafter until day 8, there was no further increase in the intensity of autophosphorylated NdK, indicating that the enzyme release is growth phase-dependent. This is generally consistent with the kinetics of NdK activities observed in these daily collected samples (Fig. 1A, days 2–8). The NdK released by Leishmania was similarly detected immunologically by examining culture supernatants of cells, which were pulse-labeled biosynthetically for 1 h and chased for up to 60 h (Fig. 4). Immunoprecipitable NdKs from ~108 labeled cells and the corresponding culture supernatants were examined every 12 h by autoradiography. The results showed clearly that the intensities of NdK increased in the culture supernatants (Fig. 4 Right Panel) and decreased in the cells progressively with culture periods (Fig 4 Left Panel). In addition to the ~17 kDa NdK, 5–6 high molecular weight bands of unknown identity were observed in the cell samples collected at 12, 24 and 36 h (Fig. 4, Cytosolic). These proteins decreased in intensity as NdK with time. They are however not polymeric NdK, since they are absent in cytosolic fractions after immunoprecipitation of autophosphorylated NdK (Fig. S1). These proteins appear to be too discrete and persistent to represent non-specific protein contamination of the immunoprecipitates. It awaits further study to determine if they might be NdK-associated proteins and thus co-precipitated. Densitomerty of band intensities for both cellular and released NdK provided semi-quantitative measurements of their levels throughout the entire course. Cellular pulse-labeled NdK levels decreased over time, as expected, due to its dilution by an increase in cell number during the periods of its cultivation (Fig. S2A). When the percent of released NdK relative to the total was calculated for each time point and graphed over time, the relative level of NdK release was found to rise with the increase in cell number, reaching a maximum of ~40% toward the end (Fig. S2B). Growth phase-dependent increase of NdK protein release is thus indicated. It should be noted thatcultures in these experiments began with 107 cells/ml and thus reached the stationary phase much sooner than those with 106cells/ml shown in Fig. 1A, Fig. 3A and Fig. 5A.
Fig. 3. Nucleoside diphosphate kinase is selectively released from Leishmania cells, which retain a known cytosolic protein, P36.
Spent media were collected daily (see Materials and Methods). The clear supernatants were phosphorylated with gamma[32P]-ATP followed by anti-NdK immunoprecipitation for the presence of NdK [A]; Western blot analysis with anti-P36 antisera/Alexaflour 680 2nd antibodies for the absence of a known cytosolic protein (P36) [B]. Lanes 1–8, Samples collected from days 1–8.
[A] Emergence of immunoprecipitable phosphorylated NdK from the culture supernatants, showing no signal on day 1, increasing intensity from days 2–4 and maintaining equally high levels throughout the stationary phase from days 4–8.
[B] The cytosolic P36 was absent in the same set of samples throughout the entire period of cultivation for eight days. Lane P, Cells (3 × 106) collected on day 8 included here as a positive control.
Cells sedimented from the same set of samples were analyzed for P36 [C–E] and for determining immunodetectability of this cytosolic protein from the lowest cell number [F]. [C–E] Western blot analyses of the samples revealed the presence of P36 in cultured cells [C] and in the cytosolic fraction of their cell lysates [D], but absent in the water insoluble fractions of their lysates collected by centrifugation [E]. These results demonstrate the integrity of the cultured cells for the duration of in vitro cultivation.
[F] A plot of immunofluorescence intensity of Alexafluor 680 versus cell numbers from a Western blot analyses of P36 showing that the lowest limit of detection requires ~3 × 105 cells.
Fig. 4. Release of biosynthetically labeled nucleoside diphosphate kinase increases with he growth of Leishmania promastigotes in vitro.
Cells grown to stationary phase were pulse-labeled for 1 h at 108 cells/ml with [35S]-methionine/cysteine in HBSS and chased in complete culture medium at 107 cells/ml (see Materials and Methods). An aliquot of the culture containing ~108 cells was collected at each time point as indicated (12–60 h) for centrifugation to separate cells and culture supernatant. Both fractions were solubilized in the same volume of 1% Nonidet-P 40 and subjected to immunoprecipitation with anti-Leishmania NdK followed by SDS-PAGE and autoradiography (see Materials and Methods). Arrow, Immunoprecipitated NdK whose intensity increases in the culture supernatants and decreases concomitantly in the cell fractions with the periods of cultivation (Cf. Fig. S2 for quantitative evaluation of cellular and released NdK band intensities with time). Note: The biosynthetically labeled NdK appears larger in this gel due to the use of pre-stained broad range molecular weight markers (NEB), which are known to size proteins less accurately.
Fig. 5. Fluorescent assay for protein release from DsRed transfectants of Leishmania during in vitro cultivation.
Cultures initiated by inoculation of medium with 106 DsRed transfectants/ml were collected and processed as described in Materials and Methods. [A] Fluorescent intensities of Cellular (black bars) and released (Blank bars) samples collected from day 0 to day 6; [B] Fluorescence versus cell number plot showing a linear relationship starting with DsRed from 5 × 104.
Leishmania NdK is a stable enzyme. Its activities remained virtually unchanged after prolonged storage for months at −20 to 4°C. As much as ~80% of its activities were retained after exposure to 25°C for up to 10 days (not shown). Thus, as Leishmania increased in cell density, they released NdK, which accumulated incrementally. The release of NdK from promastigotes in vitro is significant, reaching ~40% of the total at stationary phase, as estimated from biosynthetically labeled NdK proteins (Fig. 4) and its activities associated with cell pellets and spent media (see below and Table 1, NdK Total Units for “released” and “Cytosolic”).
Table 1.
Partial purification of Leishmania amazonensis secreted NdK (1) and cytosolic NdK (2)
| Step | Vol | Ndk | Protein (mg) | Specific Activity | Folds purified | |||
|---|---|---|---|---|---|---|---|---|
| (ml) | a U/ml | Total U | Yield (%) | Per ml | Total | (U/mg protein) | ||
| (1) | ||||||||
| Culture Supernatants | 700 | 2.7 | 1890 | 100 | 3.10 | 2170.00 | 0.87 | 1.0 |
| NH4-Sulfate (45–60%) | 70 | 18.0 | 1281 | 68 | 12.60 | 882.00 | 1.45 | 1.7 |
| Blue Sepharose | 35 | 15.0 | 508 | 27 | 0.07 | 2.45 | 207.00 | 238.0 |
| (2) | ||||||||
| Cell soluble fraction | 10 | 249 | 2537 | 100 | 2.90 | 30.00 | 85.00 | 1.0 |
| NH4-Sulfate (45–60%) | 20 | 92 | 1830 | 72 | 0.14 | 2.70 | 677.00 | 7.9 |
| Blue Sepharose | 7 | 13 | 90 | 4 | 0.002 | 0.014 | 6427.00 | 76.0 |
1 nmol UTP formed from ATP/min under the assay conditions described in Materials and Methods
3.2. Spontaneous release of NdK from cells, independent of their cytolysis
Western blot analyses of the same sample set (as shown in Fig. 3A) for a cytosolic protein (P36) demonstrated that it was retained in the cells, but not co-released with NdK into the culture media (Fig. 3B–E). In all samples examined, a single band of P36 (36 kDa) appeared only in the intact cells (Fig. 3C) and the cytosolic fractions of the cell lysates (D), but not in the culture supernatants (B) or in the insoluble cellular fractions (E). The fluorescent intensity of P36 is linear in relation to the number of cells from ~3 × 105 to 107 (correlation coefficient = 0.985) (Fig. 3[F]). Thus, P36 released from inadvertent cytolysis, if any, cannot exceed ~3% of the total in a given culture at stationary phase. This value is ~13 times less than the ~40% estimated for the release of NdK (Table 1; Fig. S2B), indicating that the latter is a natural spontaneous event, independent of inadvertent cytolysis.
The release of NdK as a natural phenomenon is further supported more conclusively by the results from the sensitive fluorimetric assay for fluorescent DsRed in the culture supernatants of the DsRed transfectants (Fig. 5). Fluorescent microscopy of these and control transfectants revealed cell-associated fluorescence only in the former, as expected. There was an overall cellular fluorescence of DsRed transfectants, indicating that the DsRed from the transgenes is cytosolic (Not shown). Cells and culture supernatants of these transfectants were collected under the same conditions as described for the wild type cells (Fig. 1A and Fig. 4A). Quantitative analyses of these samples by fluorimetry showed that the fluorescent signals increased as the transfectants replicated with the periods of cultivation for up to 5–6 days when the culture reached the stationary phase (Fig. 5A, Black bars). Fluorescence of DsRed was undetectable in the culture supernatants until the stationary phase on days 5 and 6 (Fig. 5A, open bars). The fluorescence intensities from these culture supernatants were very marginal, amounting to ~1% (0.9% and 1.2%) of the signal intensity from the corresponding cell samples. The fluorescence intensities for this value fall within the linear range of DsRed fluorescence-cell number plot where the fluorescence signal for ~5 × 104 cells is the lowest limit, corresponding to only ~0.1% of the stationary phase population (Fig. 5B). The ~40% estimated for the released NdK is thus ~40 times higher than the ~1% calculated for the inadvertent cytolysis of DsRed transfectants grown to the stationary phase.
3.3. Enrichment of released and cytosolic NdK from Leishmania amazonensis
NdK was enriched from both culture filtrates and soluble cellular fractions, as shown in 2 separate experiments, each starting from ~5 × 1010 cells grown in ~700 ml of serum containing medium. Two experimental steps were used: ammonium sulfate fractionation and FPLC on Blue Sepharose followed by stepwise KCl elution (Table 1). Ammonium sulfate fractionation increased specific activities of NdK. The fractionated samples from both culture filtrates and cytosolic fractions step-wise eluted from Blue Sepharose contained the highest NdK specific activities (Table 1), i. e. ~200 and ~6500 U per mg protein, representing 238- and 76-fold enrichment, respectively [Table 1 (1) and (2), Blue Sepharose]. These NdK-enriched fractions from culture filtrates were enzymatically active, producing similar NTP profiles, as observed with the released enzymes in the culture filtrates (Fig. 1B, LNdK, Lanes 1 and 3).
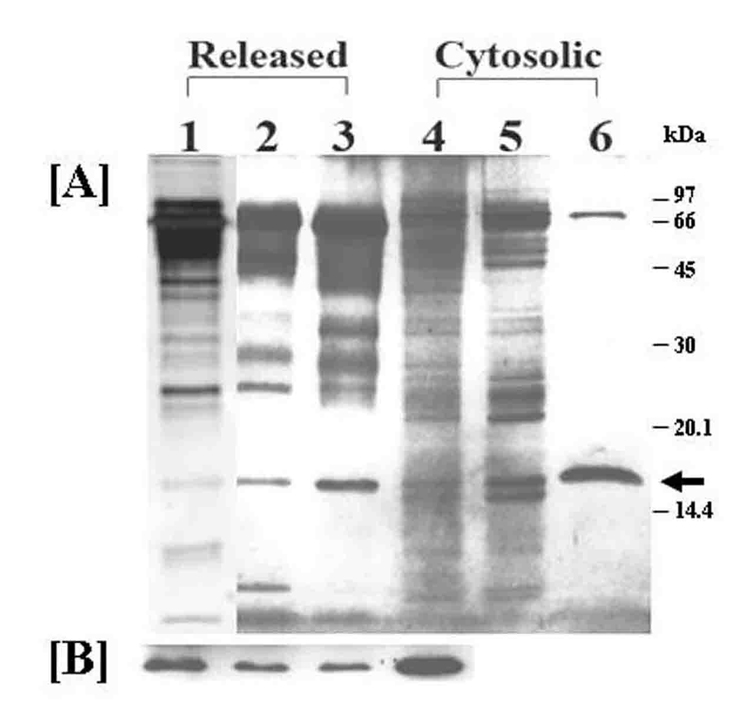
SDS-PAGE analysis of the partially purified materials from both secreted and cytosolic sources revealed a discrete band of ~17 kDa protein (Fig. 6A, lanes 2–3 and 6, arrow), which is the expected size of monomeric NdK (14–18 kDa). The ~17 kDa protein is clearly the dominant species of the only two bands observed in partially purified samples from the cytosolic fractions (Fig. 6A, lane 6). The high molecular weight band of ~68 kDa is not Leishmania NdK tetramer, since it was not co-precipitated with the ~17 kDa protein after immunoprecipitation of autophosphorylated samples with the specific antiserum (Fig. S1). The ~17 kDa band from the culture filtrates is accompanied by other proteins of higher molecular mass, predominantly 65–68 kDa, apparently representing albumin, which is abundant in the serum component of the culture medium (Fig. 6A, Lane 3). Since albumin also binds to Blue Sepharose, its co-elution with NdK is not totally unexpected, accounting in part for the unapparent enrichment of the ~17 kDa protein band seen between the ammonium sulfate precipitates and the Blue Sepharose eluents (Fig. 6 Lane 2 versus Lane 3). There are several possibilities to reconcile this with the large increase of NdK in specific activity observed in the samples after these steps of purification for the culture filtrates (Table 1). Perhaps, albumin degradation products in the Blue Sepharose eluents are charged under the experimental conditions used and thus silver-stained more heavily than expected, but not reflective of their true amounts. Differential sensitivity of the silver stain with different proteins is known and may also account for this, since all lanes were loaded equally with the same amount of proteins determined by BCA assay used for data presented in Table 1.
Fig. 6. SDS-PAGE/silver stain analyses of samples at different steps of Leishmania nucleoside diphosphate kinase enrichment.
[A] Proteins (~1 ug per lane based on BCA assay) from different purification steps (see Table 1) were resolved by SDS-PAGE and visualized with silver-staining. Lanes 1–3, samples from culture supernatants for isolating secreted NdK; Lanes 4–6, samples from soluble fractions of cell lysates for isolating cytosolic NdK. Lanes 1 and 4, crude enzyme preparation; Lanes 2 and 5, 45–60% ammonium sulfate fractionated proteins; Lanes 3 and 6, Ammonium sulfate precipitated proteins with NdK activities eluted from Blue Sepharose column.
[B] Western blot of NdK in the starting materials and enriched fractions from culture supernatants, and from the crude samples of cytosolic fractions.
Lanes 1–3, Loaded were immunoprecipitated samples from 4.3, 0.9 and 0.01 ug proteins from culture supernatants, ammonium sulfate precipitates and blue Sepharose eluates, respectively. Lanes 4, Positive control, i. e. immunoprecipitates from soluble fraction of cell lysates (3 × 107 cells). SDS-PAGE of immunoprecipitated samples revealed faint NdK bands, which were enhanced by Western blot analysis (see Materials and Methods).
The presence of NdK in the starting culture filtrates and their fractions (Fig. 6B, Lanes 1–3), and its identity to that in the cell lysates (Lane 4) was verified by Western Blot analyses with anti-Leishmania NdK antiserum.
3.4. Production and purification of Leishmania recombinant NdK
Leishmania ndk PCR-amplified and cloned into the pET28a was used to produce a 6XHis N-tagged fusion protein in Escherichia coli (Fig. 7). Over-expression of a ~20 kDa protein was evident only after IPTG induction (Fig. 7, Lane 3 versus Lane 2). This is the size expected of the recombinant products, consisting of the 151 aa Leishmania NdK (~17 kDa) and an N-terminal 32 aa peptide composed of a 6XHis tag plus a thrombin-cleavage sequence (= ~3 kDa). The fusion proteins aggregated in the transformants as inclusion bodies, which were solubilized with 0.3% N-lauryl sarcosine for affinity purification (see Materials and Methods). The eluted protein was electrophoretically homogeneous and largely free from extraneous proteins of other molecular weight (Fig 7, lane 4, Tailed arrow). Treatment of the recombinant proteins with thrombin for 4 and 8 hours resulted in partial digestion, but a ~17 kDa band of the NdK was revealed under both conditions (Fig. 7, Lanes 5 and 6, Arrow head). The fusion proteins were catalytically active. The activities increased proportionally with increasing amounts of the recombinant protein used for the assay. Utilization of ATP was indicated by the loss of its intensity concomitant with the emergence of other NTPs in the order of GTP>UTP=CTP (Fig. 1C, rLNdK). A similarly prepared L. major rNdK was also reported recently to be enzymatically active in spite of 6XHis tag at the N-terminal end [7].
Fig. 7. Electrophoretic homogeneity of recombinant Leishmania amazonensis nucleoside diphosphate kinase (6X His-NdK fusion protein).
Fusion proteins of Leishmania ndk in pET28a were isolated from Escherichia coli transformants (see Materials and Methods). Lane 1, molecular weight markers; Lanes 2 and 3, Transformants without (Lane 2) and with (Lane 3) 1 mM IPTG induction, revealing Leishmania rNdK plus a 6XHis tag as a dominant band of ~20 kDa (Tailed arrow); Lane 4, Purified 6X His-NdK (~1 ug); Lanes 5–6, Emergence of the ~17 kDa band (arrow head) after partial thrombin digestion of 6X his-NdK (1 ug) for 4 and 8 h, respectively.
3.5.1. Leishmania NdK-mediated prevention of ATP-induced release of lactate dehydrogenase from J774 macrophages
Exposure of LPS-primed J774 macrophages to eATP resulted in their cytolysis, as indicated by the LDH release. This is eATP concentration-dependent, i. e. ~50% and ~80% LDH release in the presence of 1 mM and 3 mM of eATP, respectively (Fig. 8 [A] and [B] +ATP). Although the extent of LDH-release in relation to the ATP levels used varied with different batches of macrophages used, we observed <10% LDH release from the controls of LPS-pretreated cells in the absence of eATP in all experiments carried out (Fig. 8, First four bars). The eATP-mediated cytolysis of macrophages was reduced when assayed in the presence of Leishmania culture filtrate concentrates (not shown). These observations provided the initial hints for the presence of eATP-utilizing activities in Leishmania culture media. Multiple factors released by Leishmania may contribute to these activities. We focused our attention to the NdK in this study. The NdK in combination with NDPs under the experimental conditions used prevented the eATP-induced cytolysis most significantly, i. e. a decrease of LDH release from ~50% to ~10% and from ~80% to ~40% at 1 mM and 3 mM ATP, respectively (Fig. 8 [A] and [B] +ATP+NDP+NdK vs +ATP). The reversal appeared to be mediated via both sequestration of ATP by NdK and transfer of the phosphate from ATP to NDP, thereby reducing the amounts of ATP for binding to P2X7 receptors. In the presence of ATP at higher level of 3 mM, both actions appeared to be necessary to account for the significant reversal of LDH-release observed, since ~1 U NdK alone in the absence of NDPs was ineffective (Fig. 8B, +ATP, −NDP, +NdK). In presence of 1 mM ATP, NdK alone appeared to significantly reduce the LDH-release from ~50% to ~20% in the absence of NDPs (Fig. 8A, +ATP, −NDP, +NdK), even though it was less effective than when combined with NdK. Clearly, NdK was indispensable under all circumstances, as indicated by the ineffectiveness of NDPs alone at both ATP concentrations (Fig. 8 A and B, +ATP, +NDP, −NdK).
Fig. 8. Extracellular ATP-induced membrane permeabilization of macrophages and its inhibition by Leishmania recombinant NdK.
ATP-mediated cytolysis of LPS-pretreated J774 macrophages was assayed by determining the activities of their cytosolic lactate dehydrogenase (LDH) that was released into the medium (see Materials and Methods). LDH released from Triton X-100-lysed cells was set as 100%. Shown are prevention of LDH release in the presence of 1 mM [A] and 3 mM [B] eATP by NdK [a Blue-Sepharose enriched fraction of enzymatically active NdK (~1 U)] and NDPs (CDP+GDP+UDP, 0.1 mM each). Controls include complete, single- and double-deletions of ATP, NdK and NDPs as shown.
3.5.2. Leishmania recombinant NdK prevents ATP-induced changes in mitochondrial membrane permeability of J774 macrophages
Mitochondria in majority (95±3%) of the control cells, i. e. LPS-primed J774 macrophages fluoresced orange red in the presence of Mitosensor (Fig. 9, Upper Panel), indicative of normal mitochondrial membrane permeability as expected of healthy non-apoptotic cells. Upon exposure to 2 mM ATP for 3 h, 85±5% of these cells fluoresced green and were devoid of the characteristic orange red fluorescence, regardless of whether denatured rNdK was present (Fig. 8, Middle Panel, brNdK). Inclusion of enzymatically active rNdK (~2 U) prevented majority of the cells (90±8.3%) to undergo such changes. Namely, their mitochondria fluoresced orange red (Fig. 8, Bottom Panel), as observed in the normal cells (Fig. 8, Panel LPS). Similar results as described above were also obtained by using enriched NdK fraction from culture supernatants (not shown). These results thus suggest that enzymatically active NdK released by Leishmania interferes in the early event of eATP-induced apoptosis of macrophages.
Fig. 9. Leishmania recombinant NdK prevents ATP-induced changes of macrophages in their mitochondrial membrane permeability.
J774 macrophage cells primed with LPS and treated with 2 mM ATP in presence or absence of enzymatically active recombinant NdK (~2 U) (rNDK) or boiled NDK (brNDK) for 3 h. Cells were subsequently stained with Mitosensor for fluorescence microscopy (see Materials and Methods). LPS, J774 cells treated with LPS alone; LPS+ATP+brNdK and LPS+ATP+rNdK, LPS primed cells treated with eATP in presence of inactive (brNdK) and active rNdK, respectively. Note: Orange granular and diffuse green fluorescence indicates normal and the early apoptotic event of mitochondrial membrane permeability changes, respectively.
4. Discussion
In the present study, nucleoside diphosphate kinase (NdK) from Leishmania amazonensis was analyzed for its potential role in infection in some detail for the first time. The Leishmania NdK activities were initially found to accumulate in the spent media with increasing periods of Leishmania cultivation in vitro (Fig. 1A). Attribution of these activities to NdK from Leishmania was initially complicated by the presence of unknown enzyme(s) from fetal bovine serum in the culture medium, which apparently catalyzes the formation of GTP from GDP under the assay conditions used (Fig. 1A, Lane M). The unknown enzyme(s) is unlikely to be NdK, since no activities of this enzyme were apparent after Leishmania NdK specific-immuno-depletion of culture supernatants (Fig. 2C). The antiserum used is Leishmania NdK-specific, since it does not immunoprecipitate any proteins from J774 macrophages. Nor did it immunoprecipitate any fetal bovine serum protein (see Fig. 3A, Lane 1). The origin of NdK activities in the media from Leishmania was clearly demonstrated immunologically by using this Leishmania NdK-specific antiserum. The Leishmania enzyme activities were lost after immunoprecipitation (Fig. 2C) and the enzyme proteins immunoprecipitable from the spent media after autophosphorylation (Figs. 3A) as well as biosynthetic pulse-labeling (Fig. 4, Released). In addition, this enzyme protein and its activity are both enriched along the steps of partial purification (Fig. 6A; Table 1). With increasing levels of enrichment, a protein of ~17 kDa emerges and is recognized by the Leishmania NdK-specific antiserum (Fig. 6B). Released, cytosolic and recombinant NdKs all have a similar size of ~17 kDa (Fig. 3A, Fig. 4, Fig. 6 and Fig. 7). This is consistent with the size of monomeric Leishmania NdK according to its amino acid composition deduced from the ndk sequence. Evidence presented indicates that Leishmania NdK is not only cytosolic but also released into their extracellular milieu.
All Leishmania NdK preparations have very similar catalytic properties (Fig. 1A–C). The ubiquity and stability of Leishmania NdK reported here are consistent with those reported from related trypanosomes [5, 6], bacteria [17, 18, 44], i. e. utilization of ATP to generate NTPs in different ratios. Leishmania NdK partially purified or as recombinant products catalyzed the formation of GTP as the dominant product (Figs. 1B and 1C), consistent with the known substrate affinity of all NdK in favor of ADP and GDP over CDP and UDP [45]. In this study, Leishmania was found to have only water-soluble cytosolic and secretory NdK (Table 1). In previous studies, NdK of T. brucei was localized to the cytoplasm as well as nucleoplasm [6], whereas the epimastigotes of T. cruzi were found to have water-soluble and membrane-associated NdK (30%) [5]. Membrane association of prokaryotic and eukaryotic NdKs has long been reported [44, 46] with different isoforms targeted to various cellular compartments [47–49]. Immunofluorescent microscopy of Leishmania with the specific anti-Leishmania NdK antiserum revealed neither nuclear localization nor membrane association (unpublished data). Further investigation of Leishmania NdK isoforms in relation to their cellular compartmentalization is needed to unambiguously settle this issue.
Reported here for the first time is that Leishmania secrete NdK, as reported previously for pathogenic bacteria [4] and parasitic nematodes [50, 51]. NdK activity in the Leishmania culture supernatants is far too substantial (~40% of the total NdK activities and proteins) to be accounted for by inadvertent cytolysis. There is no evidence for co-release of a known Leishmania cytosolic protein P36 [40] with NdK (Fig. 3). This is further verified rigorously by the sensitive fluorimetric assay of DsRed transfectants for the virtual absence of DsRed release (Fig. 5) when NdK activities (Fig. 1A) and proteins (Fig. 3A and Fig. 4) are clearly demonstrable in the culture supernatants. Even at the stationary phase beyond day 5, cytolysis of DsRed transfectants is negligible, i. e. ~1%. This is not an underestimation due to acidity known to affect DsRed fluorescence [52], since the pH of the culture supernatants is well above 5. Moreover, the extracellular NdK did not increase beyond the stationary phase (Fig. 3A), as might be expected to occur in the event of cytolysis. Interestingly, the presence of proteins, i. e. heat-inactivated fetal bovine serum or BSA, appears to facilitate Leishmania NdK release in vitro. Pathogenic bacteria, i. e. Mycobacterium bovis, BCG and Pseudomonas aeruginosa also require growth in complex media containing mammalian protein components for the release of this enzyme [17, 18]. In P. aeruginosa, a carboxy terminal motif was identified as important for NdK secretion [8]. None of the Leishmania ndk sequences available contains this motif, i. e. the one cloned here from L. amazonensis and those from the genome database of L. major and L. infantum. Leishmania NdK secretion thus may be based on a hitherto unrecognized motif or via the non-classic protein export mechanisms [53].
The NdK released by Leishmania apparently have functional importance as one of the invasive/evasive determinants crucial for the successful infection of macrophages [54], i. e. decreasing eATP to preserve the integrity of their host cells. Immune cells are thought to release ATP when killed, injured or under stress, leading to its local accumulation in significant amounts at the site of inflammation [12, 13]. Such events occur predictably in microbial infection by pathogens, like Leishmania. eATP is known to suppress inos expression of macrophages [55] as well as to induce cytolysis of these phagocytes via apoptotic and/or necrotic pathways through ATP-binding to the P2X7 purinergic receptors [23, 25, 56]. Both recombinant and native Leishmania NdK are able to substantially lower the ATP-induced LDH release from macrophages (Fig. 8) and their mitochondrial membrane permeability changes (Fig. 9) – an early event of apoptosis [57]. Significantly, promastigotes release NdK progressively, leading to its substantial accumulation at stationary phase when they become the most infective “metacyclic stage”. By microarray analyses of a different species, i. e. L. major for stage-specific transcriptional profiles [58], others have reported that ndk is among those whose expression is up-regulated in the metacyclic and amastigote stages. Although no data were presented in that report for NdK activities and release, we observed these events in both Leishmania stages: promastigotes presented here and axenic amastigotes (not shown). It is proposed that the released NdK plays a role in leishmanial infection of macrophages at both the early phase by promastigotes as well as at the chronic phase by amastigotes. Aside from reducing NO microbicidal activities of macrophages and preventing their cytolysis by decreasing eATP (Fig. 8), intracellular and extracellular Leishmaina NdK can potentially utilize ATP to produce different NTPs. These nucleotides, e. g. GTP and UTP, are known to regulate gene expression in signal pathways and thus may further alter macrophages, rendering them favorable for Leishmania to establish intracellular parasitism.
Supplementary Material
Acknowledgements
We thank Prof. John Keller for his invaluable suggestions in reviewing this manuscript. This work was supported by NIH grant AI 20486 to KPC and by a Sponsored Research Agreement between CDG Therapeutics, Inc., and the University of Illinois at Chicago as well as by NIH grant AI 16790 to AC.
Abbreviations
- eATP
Extracellular ATP
- HBSS
Hank’s Balanced Salt Solution
- LDH
Lactate dehydrogenase
- NdK
Nucleoside diphosphate kinase
- ndk
gene encoding NdK
- NDP
Nucleoside diphosphates
- NTP
Nucleoside triphosphates
- PBS
Phosphate buffered saline
- TM
Tris-magnesium buffer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lascu I, Giartosio A, Ransac S, Erent M. The catalytic mechanism of nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000;32:227–236. doi: 10.1023/a:1005580828141. [DOI] [PubMed] [Google Scholar]
- 2.Postel EH. Multiple biochemical activities of NM23/NDP kinase in gene regulation. J Bioenerg Biomembr. 2003;35:31–40. doi: 10.1023/a:1023485505621. [DOI] [PubMed] [Google Scholar]
- 3.Landfear SM, Ullman B, Carter NS, Sanchez MA. Nucleoside and nucleobase transporters in parasitic protozoa. Eukaryot Cell. 2004;3:245–254. doi: 10.1128/EC.3.2.245-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarty AM. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signaling and polysaccharide synthesis. Mol Microbiol. 1998;28:875–882. doi: 10.1046/j.1365-2958.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 5.Ulloa RM, Muschietti JP, Veron M, Torres HN, Tellez-Inon MT. Purification and characterization of a soluble nucleoside diphosphate kinase in Trypanosoma cruzi. Mol Biochem Parasitol. 1995;70:119–129. doi: 10.1016/0166-6851(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 6.Hunger-Glaser I, Hemphill A, Shalaby T, Hanni M, Seebeck T. Nucleoside diphosphate kinase of Trypanosoma brucei. Gene. 2000;257:251–257. doi: 10.1016/s0378-1119(00)00401-7. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira AH, Ruiz JC, Cruz AK, Greene LJ, Rosa JC, Ward RJ. Expression in E. coli and purification of the nucleoside diphosphate kinase b from Leishmania major. Protein Expr Purif. 2006;49:244–250. doi: 10.1016/j.pep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Kamath S, Chen ML, Chakrabarty AM. Secretion of nucleoside diphosphate kinase by mucoid Pseudomonas aeruginosa 8821: involvement of a carboxy-terminal motif in secretion. J Bacteriol. 2000;182:3826–3831. doi: 10.1128/jb.182.13.3826-3831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melnikov A, Zaborina O, Dhiman N, Prabhakar BS, Chakrabarty AM, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol Microbiol. 2000;36:1481–1493. doi: 10.1046/j.1365-2958.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- 10.Zaborina O, Dhiman N, Chen ML, Kostal J, Holder IA, Chakrabarty AM. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiol. 2000;146:2521–2530. doi: 10.1099/00221287-146-10-2521. [DOI] [PubMed] [Google Scholar]
- 11.Chopra P, Singh A, Koul A, Ramachandran S, Drlica K, Tyagi AK, Singh Y. Cytotoxic activity of nucleoside diphosphate kinase secreted from Mycobacterium tuberculosis. Eur J Biochem. 2003;270:625–634. doi: 10.1046/j.1432-1033.2003.03402.x. [DOI] [PubMed] [Google Scholar]
- 12.Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 13.Di Virgilio F. The P2Z purinoreceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari D, Chiozzi P, Falzoni S, Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 15.Berredo-Pinho M, Peres-Sampaio CE, Chrispim PPM, Belmont-Firpo R, Lemos AP, Martiny A, Vannier-santos MA, Meyer-Fernandes JR. A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys. 2001;391:16–24. doi: 10.1006/abbi.2001.2384. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro CA, Martins-Duarte ES, Ferraro RB, de Souza ALF, Gomes MT, Lopes AHCS, Vannier-Santos MA, Santos ALS, Meyer-Fernandes JR. Leishmania amazonensis: Biological and biochemical characterization of ecto-nucleoside triphosphate diphosphohydrolase activities. Exp Parasitol. 2006;114:16–25. doi: 10.1016/j.exppara.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;31:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- 18.Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, El-Idrissi ME, Prabhakar BS, Chakrabarty AM. P2Z-Independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67:5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty AM. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000;68:4930–4937. doi: 10.1128/iai.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markaryan A, Zaborina O, Punj O, Chakrabarty AM. Adenylate kinase as a virulence factor of Pseudomonas aeruginosa. J Bacterial. 2001;183:3345–3352. doi: 10.1128/JB.183.11.3345-3352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickman SE, Khoury JE, Greenberg S, Schieren I, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–2456. [PubMed] [Google Scholar]
- 22.Coutinho-Silva R, Persechini PM. P2Z purinoreceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- 23.Zambon A, Bronte V, Di Virgilio F, Hanau S, Steinberg TH, Collavo D, Zanovello P. Role of extracellular ATP in cell-mediated cytotoxicity: a study with ATP-sensitive and ATP-resistant macrophages. Cell Immunol. 1994;156:458–467. doi: 10.1006/cimm.1994.1190. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard DK, Wei S, Duan C, Pericle F, Diaz JI, Djeu JY. Role of extracellular adenosine triphosphate in the cytotoxic T-lymphocyte-mediated lysis of antigen presenting cells. Blood. 1995;85:3173–3182. [PubMed] [Google Scholar]
- 25.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 27.Zanovello P, Bronte V, Rosato A, Pizzo P, Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol. 1990;145:1545–1550. [PubMed] [Google Scholar]
- 28.Chiozzi P, Murgia M, Falzoni S, Ferrari D, Di Virgilio F. Role of the purinergic P2Z receptor in spontaneous cell death in J774 macrophage cultures. Biochem Biophys Res Commun. 1996;218:176–181. doi: 10.1006/bbrc.1996.0031. [DOI] [PubMed] [Google Scholar]
- 29.Moore KJ, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 30.Akarid K, Arnoult D, Micic-Polianski J, Sif J, Estaquier J, Ameisen JC. Leishmania major-mediated prevention of programmed cell death induction in infected macrophages is associated with the repression of mitochondrial release of cytochrome c. J Leukoc Biol. 2004;76:95–103. doi: 10.1189/jlb.1001877. [DOI] [PubMed] [Google Scholar]
- 31.Lisi S, Sisto M, Acquafredda A, Spinelli R, Schiavone M, Mitolo V, Brandonisio O, Panaro M. Infection with Leishmania infantum Inhibits actinomycin D-induced apoptosis of human monocytic cell line U-937. J Eukaryot Microbiol. 2005;52:211–217. doi: 10.1111/j.1550-7408.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- 32.Nash PB, Purner MB, Leon RP, Clarke P, Duke RC, Curiel TJ. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J Immunol. 1998;160:1824–1830. [PubMed] [Google Scholar]
- 33.Goebel S, Gross U, Luder CG. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J Cell Sci. 2001;114:3495–3505. doi: 10.1242/jcs.114.19.3495. [DOI] [PubMed] [Google Scholar]
- 34.Hisaeda H, Sakai T, Ishikawa H, Maekawa Y, Yasumoto K, Good RA, Himeno K. Heat shock protein 65 induced by gammadelta T cells prevents apoptosis of macrophages and contributes to host defense in mice infected with Toxoplasma gondii. J Immunol. 1997;159:2375–2381. [PubMed] [Google Scholar]
- 35.Ishikawa H, Hisaeda H, Taniguchi M, Nakayama T, Sakai T, Maekawa Y, Nakano Y, Zhang M, Zhang T, Nishitani M, Takashima M, Himeno K. CD4(+) v(alpha)14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int Immunol. 2000;12:1267–1274. doi: 10.1093/intimm/12.9.1267. [DOI] [PubMed] [Google Scholar]
- 36.Sakai T, Hisaeda H, Ishikawa H, Maekawa Y, Zhang M, Nakao Y, Takeuchi T, Matsumoto K, Good RA, Himeno K. Expression and role of heat-shock protein 65 (HSP65) in macrophages during Trypanosoma cruzi infection: involvement of HSP65 in prevention of apoptosis of macrophages. Microbes Infect. 1999;1:419–427. doi: 10.1016/s1286-4579(99)80045-8. [DOI] [PubMed] [Google Scholar]
- 37.Nasir A, Arora HS, Kaiser HE. Apoptosis and pathogenesis of viral hepatitis C-an update. In Vivo. 2000;14:297–300. [PubMed] [Google Scholar]
- 38.Nakajima-Shimada J, Zou C, Takagi M, Umeda M, Nara T, Aoki T. Inhibition of Fas-mediated apoptosis by Trypanosoma cruzi infection. Biochim Biophys Acta. 2000;1475:175–183. doi: 10.1016/s0304-4165(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 39.Dutta S, Ray D, Kolli BK, Chang K-P. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrob Agents Chemother. 2005;49:4474–4484. doi: 10.1128/AAC.49.11.4474-4484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Chang K-P. Identification by extrachromosomal amplification and overexpression of a zeta-crystallin/NADPH-oxidoreductase homologue constitutively expressed in Leishmania spp. Mol Biochem Parasitol. 1994;66:201–210. doi: 10.1016/0166-6851(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 41.Shankar S, Hershberger CD, Chakrabarty AM. The nucleoside diphosphate kinase of Mycobacterium smegmatis: identification of proteins that modulate specificity of nucleoside triphosphate synthesis by the enzyme. Mol Microbiol. 1997;24:477–487. doi: 10.1046/j.1365-2958.1997.3491724.x. [DOI] [PubMed] [Google Scholar]
- 42.Fong D, Chang K-P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci USA. 1981;78:7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humphreys BD, Dubyak GR. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J Immunol. 1996;157:5627–5637. [PubMed] [Google Scholar]
- 44.Shankar S, Kamath S, Chakrabarty AM. Two forms of the nucleoside diphosphate kinase of Pseudomonas aeruginosa 8830: altered specificity of nucleoside triphosphate synthesis by the cell membrane-associated form of the truncated enzyme. J Bacteriol. 1996;178:1777–1781. doi: 10.1128/jb.178.7.1777-1781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biondi RM, Veron M, Walz K, Passeron S. Candida albicans nucleoside-diphosphate kinase: purification and characterization. Arch Biochem Biophys. 1995;323:187–194. doi: 10.1006/abbi.1995.0025. [DOI] [PubMed] [Google Scholar]
- 46.Kimura N, Shimada N. Membrane-associated nucleoside diphosphate kinase from rat liver. Purification, characterization, and comparison with cytosolic enzyme. J Biol Chem. 1988;263:4647–4653. [PubMed] [Google Scholar]
- 47.Troll H, Winckler T, Lascu I, Muller N, Saurin W, Veron M, Mutzel R. Separate nuclear genes encode cytosolic and mitochondrial nucleoside diphosphate kinase in Dictyostelium discoideum. J Biol Chem. 1993;268:25469–25475. [PubMed] [Google Scholar]
- 48.Kraeft SK, Traincart F, Mesnildry S, Bourdais J, Vernon M, Chen LB. Nuclear localization of nucleoside diphosphate kinase type B (nm23-H2) in cultured cells. Exp Cell Res. 1996;227:63–69. doi: 10.1006/excr.1996.0250. [DOI] [PubMed] [Google Scholar]
- 49.Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000;32:247–258. doi: 10.1023/a:1005584929050. [DOI] [PubMed] [Google Scholar]
- 50.Gounaris K, Thomas S, Najarro P, Selkirk ME. Secreted variant of nucleoside diphosphate kinase from the intracellular parasitic nematode Trichinella spiralis. Infect Immun. 2001;69:3658–3662. doi: 10.1128/IAI.69.6.3658-3662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yatsuda AP, Krijgsveld J, Cornelissen AW, Heck AJ, de Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem. 2003;278:16941–16951. doi: 10.1074/jbc.M212453200. [DOI] [PubMed] [Google Scholar]
- 52.Verkhusha VV, Akovbian NA, Efremenko EN, Varfolomeyev SD, Vrzheshch PV. Kinetic analysis of maturation and denaturation of DsRed, a coral-derived red fluorescent protein. Biochem (Moscow) 2001;66:1659–1670. doi: 10.1023/a:1013325627378. [DOI] [PubMed] [Google Scholar]
- 53.Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 54.Chang K-P, Reed SG, McGwire BS, Soong L. Leishmania model for microbial virulence: the relevance of parasite multiplication and pathoantigenicity. Acta Tropica. 2003;85:375–390. doi: 10.1016/s0001-706x(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 55.Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- 56.Ferrari D, Los M, Bauer MKA, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 57.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1311. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 58.Almeida R, Norrish A, Levick M, Vetrie D, Freeman T, Vilo J, Ivens A, Lange U, Stober C, McCann S, Blackwell JM. From genomes to vaccines: Leishmania as a model. Phil Trans Royal Soc Lond B. 2002;357:5–11. doi: 10.1098/rstb.2001.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.