Abstract
Store-operated Ca2+ entry (SOCE) is activated following the depletion of internal Ca2+ stores in virtually all eukaryotic cells. Shifted excitation and emission ratioing of fluorescence (SEER) was used to image mag-indo-1 trapped in the tubular (t) system of mechanically skinned rat skeletal muscle fibres to measure SOCE during intracellular Ca2+ release. Cytosolic Ca2+ transients were simultaneously imaged using the fluorescence of rhod-2. Spatially and temporally resolved images of t system [Ca2+] ([Ca2+]t-sys) allowed estimation of Ca2+ entry flux from the rate of decay of [Ca2+]t-sys. Ca2+ release was induced pharmacologically to activate SOCE without voltage-dependent contributions to Ca2+ flux. Inward Ca2+ flux was monotonically dependent on the [Ca2+] gradient, and strongly dependent on the transmembrane potential. The activation of SOCE was controlled locally. It could occur without full Ca2+ store depletion and in less than a second after initiation of store depletion. These results indicate that the molecular agonists of SOCE must be evenly distributed throughout the junctional membranes and can activate rapidly. Termination of SOCE required a net increase in [Ca2+]SR. Activation and termination of SOCE are also demonstrated, for the first time, during a single event of Ca2+ release. At the physiological [Ca2+]t-sys, near 2 mm (relative to t system volume), SOCE flux relative to accessible cytoplasmic volume was at least 18.6 μm s−1, consistent with times of SR refilling of 1–2 min measured in intact muscle fibres.
Store-operated Ca2+ entry (SOCE) is a regulated flow of external Ca2+ into cells to refill depleted internal stores (Parekh & Putney, 2005), which occurs in both non-excitable and excitable cells. Given the diverse range of cells for which SOCE is functional it would be reasonable to expect quite different adaptations of this mechanism, tailored around the specific function and structure of the individual cell. For example, the highly specialized skeletal muscle cell regulates changes in cytoplasmic [Ca2+] ([Ca2+]c) of two orders of magnitude within milliseconds (Rome, 2006) in the process known as excitation–contraction (EC) coupling (Melzer et al. 1995). Consistent with varying functional needs, EC coupling displays different molecular structures and kinetics even among skeletal muscle cells of different taxa (Di Biase & Franzini-Armstrong, 2005). Changes in [Ca2+]c in non-excitable cells are slower and occur over much longer periods (Parekh & Putney, 2005). Skeletal muscle and non-excitable cells may well have evolved SOCE with different features, adequate for different demands. For instance, it is reasonable to expect that Ca2+ entry in response to store depletion will activate more quickly in skeletal muscle cells than in non-excitable cells.
The structure of the interface of the cell with its exterior should also affect the SOCE mechanisms. Most nonexcitable cells are small and compact. In these the endoplasmic reticulum (ER) commands the plasmalemmal store-operated Ca2+ (SOC) channel to open upon store depletion, which may require aggregation of receptors or ER translocation (Wu et al. 2006; Luik et al. 2006; Lewis, 2007). In contrast, skeletal muscle cells are large and elongated. In these cells, the surface membrane consists of a sarcolemma and the tubular (t) system, a network of narrow tubules that invaginate into the cell at regular intervals (Veratti, 1961; Eisenberg, 1983). The large area and close apposition of this membrane system with the Ca2+ store (the sarcoplasmic reticulum, or SR) make the t system a chief candidate locus for SOCE.
SOCE was indeed shown to be functional at the level of the t system in studies with mechanically skinned skeletal muscle fibres (Launikonis et al. 2003). These preparations, which have the sarcolemma mechanically removed causing the t system to reseal, maintain the normal physiological function (including an action potential-induced Ca2+ release from SR at a normal rate; Posterino & Lamb, 2003; Launikonis et al. 2006). In addition to identifying the t system membrane as the locus of Ca2+ entry, the studies with mechanically skinned fibres exhibited a singular advantage: the resealing of t tubules after skinning results in formation of a finite pool of extracellular Ca2+.
A method of imaging Ca2+ in the t system of skinned fibres was developed in the Stephenson laboratory more than a decade ago (Stephenson & Lamb, 1993; Lamb et al. 1995). Following the identification of SOCE in skeletal muscle (Kurebayashi & Ogawa, 2001), this method was modified to study SOCE by trapping the low affinity Ca2+-sensitive dye, fluo-5N, in the t system (Launikonis et al. 2003). This study showed that the resealed t system could be depleted of Ca2+ in less than 30 s following application of caffeine and that depletion failed when the skinned fibres were exposed to high intracellular [Ca2+], a treatment that disrupts the functional coupling between SR and t system (Lamb et al. 1995). These experiments thus confirmed the presence of the store-operated pathway in the t system membrane and validated its assessment from changes in Ca2+-dependent fluorescence in the resealed t system (Launikonis et al. 2003).
Brotto and colleagues also used a non-ratiometric dye trapped in the t system of skinned fibres in attempts to measure SOCE during SR Ca2+ release. In contrast to the results of Launikonis et al. (2003), they reported a t system fluorescence signal that declined only slowly over a period of more than 10 min upon caffeine application (Zhao et al. 2005, 2006; Hirata et al. 2006).
To improve quantification of the decay of [Ca2+] in the t system ([Ca2+]t-sys) as a measure of SOCE during intracellular Ca2+ release, we used a novel technique to image [Ca2+] inside the t system, shifted excitation and emission ratioing of fluorescence, or SEER (Launikonis et al. 2005). SEER improved the quantification because it is ratiometric and has greater sensitivity than previous methods. Additionally, it could be used simultaneously with the cytoplasmic Ca2+ indicator rhod-2.
This combination of techniques in skinned fibres allowed for: (i) control of the composition of the t system lumen and cytoplasmic environment; (ii) recording of [Ca2+] simultaneously in t system and cytoplasm; and (iii) reliably estimating the [Ca2+]SR load by extrapolation from similar experiments where [Ca2+]SR is measured (experiments communicated here and by Fryer & Stephenson, 1996; Herrmann-Frank et al. 1999; Launikonis et al. 2005, 2006).
Voltage-dependent currents were avoided by directly activating the Ca2+ release channel/ryanodine receptor (RyR) of the SR by pharmacological means – rather than by depolarization of the plasma membrane – in solutions that maintain a constant membrane potential (Lamb & Stephenson, 1994). Furthermore, [Ca2+]c was buffered with EGTA and BAPTA to restrict the uptake of cytoplasmic Ca2+ by the t system. The decay in [Ca2+]t-sys during Ca2+ release observed this way was found to be largely due to SOCE. The measurable rate of change of [Ca2+]t-sys thus provides a direct quantification of store-dependent Ca2+ influx simultaneously with intracellular Ca2+ release.
Methods
All experimental methods were approved by the Institutional Animal Care and Use Committee at Rush University. Sprague–Dawley rats (250–300 g) were killed by CO2 asphyxiation and the extensor digitorum longus (EDL) muscles were rapidly excised. Muscles were then placed in a Petri dish under paraffin oil above a layer of Sylgard.
The method of trapping fluorescent dye in the sealed t system has been described (Lamb et al. 1995; Launikonis et al. 2003; Launikonis & Stephenson, 2004). Briefly, small bundles of fibres were isolated and exposed to a ‘dye solution' while still intact. Individual fibres were then isolated and mechanically skinned. Skinned fibres were transferred to a custom built experimental chamber with a coverslip bottom, where they were bathed in an ‘internal solution'.
Solutions
The dye-containing solution applied before skinning of rat fibres contained (mm): NaCl, 145; KCl, 3; CaCl2, 2.5; MgCl2, 2; mag-indo-1 salt, 10; and Hepes 10 (pH adjusted to 7.4 with NaOH). The standard internal solutions are shown in Table 1. High [K+] solutions kept the sealed t system polarized and high [Na+] depolarized it (Lamb & Stephenson, 1994). Reference solutions were used to load SR and t system with Ca2+ and to preequilibrate the preparations to appropriate buffering and membrane potential conditions before Ca2+ release. Solutions with caffeine and low Mg2+ solution were designed to induce SR Ca2+ release (Launikonis & Stephenson, 2000; Lamb et al. 2001).
Table 1.
Composition of internal solutions
| Solution | K+ | Na+ | Ca2+ | Mg2+ | EGTA | BAPTA | Caffeine |
|---|---|---|---|---|---|---|---|
| Reference–0 Ca2+ | 100 | 30 | 0 | 0.65 | 1 | 0 | 0 |
| Reference–100 Ca2+ | 100 | 30 | 0.0001 | 0.65 | 1 | 0 | 0 |
| Reference–800 Ca2+ | 100 | 30 | 0.0008 | 0.65 | 1 | 0 | 0 |
| Reference–0 Ca2+–BAPTA | 100 | 30 | 0 | 0.65 | 0 | 5 | 0 |
| Reference–Na–0 Ca2+ | 0 | 130 | 0 | 0.65 | 1 | 0 | 0 |
| Reference–Na–800 Ca2+ | 0 | 130 | 0.0008 | 0.65 | 1 | 0 | 0 |
| Low Mg2+ | 100 | 30 | 0 | 0.01 | 1 | 0 | 0 |
| Caffeine | 100 | 30 | 0 | 0.01 | 1 | 0 | 30 |
| Low Mg2+–BAPTA | 100 | 30 | 0 | 0.01 | 0 | 5 | 0 |
| Caffeine–BAPTA | 100 | 30 | 0 | 0.01 | 0 | 5 | 30 |
| Caffeine–depol | 0 | 130 | 0 | 0.01 | 1 | 0 | 30 |
All concentrations are in mm. Additionally, solutions contained (mm): glutamate, 100; creatine phosphate, 10; ATP, 5; Hepes, 10; rhod-2, 0.1. Mg2+ was added as MgCl2 and Ca2+ was added as CaCl2. Note that ‘Mg2+' and ‘Ca2+' refer to concentrations of free divalents in solution and that total concentrations of Mg and Ca added were much higher. Osmolality was adjusted to 290 ± 10 mosmol kg−1 with sucrose and pH was set to 7.1 with KOH or NaOH. BTS (SIGMA-Aldrich Co, St Louis, MO, USA; n-benzyl-p-toluene sulphonamide; 50 μm) was added to all solutions to suppress contraction (Cheung et al. 2002). 2-Aminoethyl diphenyl borate (2-APB; 0.1 mM; Sigma-Aldrich) was added to solutions as required from a 100 mm stock in DMSO.
Confocal imaging
Imaging was done as described in Launikonis et al. (2005). Briefly, the experimental chamber was placed above the water immersion objective (40×, NA 1.2) of the confocal laser scanning system (TCS SP2, Leica Microsystems, Exton, PA, USA). Simultaneous acquisition of three images (F1, F2 and F3) was achieved by line-interleaving of three excitation wavelengths (351, 364 and 543 nm) while collecting emitted light in three emission ranges (390–440 nm, 465–535 nm, and 562–666 nm).
Calibration of [Ca2+]t-sys
SEER of mag-indo can be used to image [Ca2+] inside cellular organelles, as previously demonstrated in SR (Launikonis et al. 2005). Equation (1) (Grynkiewicz et al. 1985), with parameters defined in Launikonis et al. (2005), was assumed to describe the relationship between R and [Ca2+]t-sys:
| (1) |
To determine the [Ca2+] inside the t system, dye-loaded preparations were bathed in calibration solutions, which were a standard potassium glutamate internal saline with 0.65 mm Mg2+, 5 μm A23187 (Sigma Aldrich) and 5 μm ionomycin. [Ca2+]c (0–1 mm) was buffered by 15 mm EGTA or nitrilotriacetate in the appropriate range.
[Ca2+]c higher than 1 mm in the bathing solution appeared to cause loss of dye from the preparation, a problem also found in calibrations of mag-indo-1 (fluorscent dye from molecular probes) inside the SR (Launikonis et al. 2005). To circumvent this problem Rmax was determined in conditions that promoted heavy loading of Ca2+ into the t system, namely an internal solution with 800 nm Ca2+ and all K+ replaced with Na+ to depolarize the t system. Rmax was thus determined to be 4.82 ± 0.04 in three fibres. This value was close to the one found by a similar approach in calibrations of mag-indo-1 inside SR in frog muscle fibres (Launikonis et al. 2005). While it is not certain that the concentration reached inside the organelle under these conditions is sufficient to saturate the dye, a nearly identical value of Rmax, 4.98 ± 0.05 (n = 3), was found by the same method for indo-1, a dye that is nearly identical optically, but has a roughly 100 times greater affinity for Ca2+. Fitting eqn (1) to all R versus[Ca2+] data, with Rmax set to 4.82, yielded the following values for the other parameters: γKD= 0.615 mm and Rmin= 0.45. This prediction of Rmin was later confirmed experimentally. When applying the release-inducing low Mg2+–BAPTA to freshly skinned preparations, R in the t system could not fall below 0.45 when inducing store-operated Ca2+ depletion (three fibres), confirming this as the Rmin value.
[Ca2+] in two compartments during release
Simultaneous SEER and rhod-2 images provided spatially and temporally resolved measures of [Ca2+]t-sys and [Ca2+]c during Ca2+ release. Ca2+ was released under conditions leading to known total SR calcium, [Ca2+]SR and cytoplasmic transients (Fryer & Stephenson, 1996; Launikonis & Stephenson, 2000; Launikonis et al. 2005, 2006). Thus, even though we only imaged cytoplasmic and t system [Ca2+] during release, we could estimate that within the SR with some confidence.
SOCE was quantified during Ca2+ release induced by five different solutions (detailed in Table 1). The first was ‘low Mg2+', containing 10 μm Mg2+, and 1 mm EGTA for nominally 0 [Ca2+]. This solution elicited Ca2+ release that was brief and ceased during imaging, leading to immediate reuptake by the SR (Launikonis et al. 2006). Thus, this condition allowed for measurement of transmembrane Ca2+ movements during a complete sequence of SR depletion (which was partial) and recovery. A second condition ‘low Mg2+–BAPTA', suppressed the cytosolic Ca2+ transient. Two other conditions added 30 mm caffeine to the respective low Mg2+ solutions. This enhanced release of SR Ca2+ and kept the RyR in a highly active state, preventing any net reuptake of Ca2+ into the SR. In another series of experiments Na+ replaced all K+ in the solution ‘caffeine–depol', to depolarize the sealed t system. This was in contrast to all the K+-based solutions, which were designed to keep the t system polarized (Lamb & Stephenson, 1994).
It should be noted that in the mammal, Ca2+ release via direct activation of the RyR is faster in the presence of BAPTA than EGTA. We understand this as a consequence of rapid Ca2+ chelation by BAPTA, which suppresses Ca2+-dependent inactivation and has no inhibitory effects, in agreement with the reported absence of Ca2+-induced Ca2+ release in mammalian muscles (Shirokova et al. 1996, 1998; Launikonis & Stephenson, 2000), presumably due to lack of the RyR3 isoform (Zhou et al. 2004; Pouvreau et al. 2007).
Reference solutions with either 100 or 800 nm Ca2+ and Reference–Na–800 Ca2+ (Table 1) were used to load Ca2+ into the t system and SR. There is no precedent study setting conditions for loading Ca2+ to appropriate levels in t system and SR of skinned fibres. So approaches which allowed appropriate levels in [Ca2+]t-sys for experiments to be reached while loading SR close to endogeneous levels were developed. In many cases preparations were left in the order of 5–15 min in Reference–100 Ca2+. We expect this loading protocol to cause Ca2+ in SR to plateau not much beyond endogeneous levels (Launikonis et al. 2005). In some experiments 800 nm Ca2+ was used to load Ca2+ for periods not more than 30 s to avoid overload of SR and reduce the time required to load Ca2+ into the t system. During the course of experiments it was found that Reference–Na–800 Ca2+ loaded the t system at a faster rate than Reference–800 Ca2+. This probably reflects the greater capacity of t system Ca2+-ATPase to translocate Ca2+ in the absence of an electrical gradient. Therefore, Reference–Na–800 Ca2+ was the preferred Ca2+ loading solution. In most cases we expect SR was loaded with Ca2+ at or slightly above its endogeneous level but not ‘heavily' loaded (i.e. close to full; Launikonis & Stephenson, 2000). This is also evident from the moderate time courses of the cytoplasmic Ca2+ transients in the presence of 1 mm EGTA (Figs 4, 7 and 8). However, the final [Ca2+]SR is not precisely known.
Figure 4.
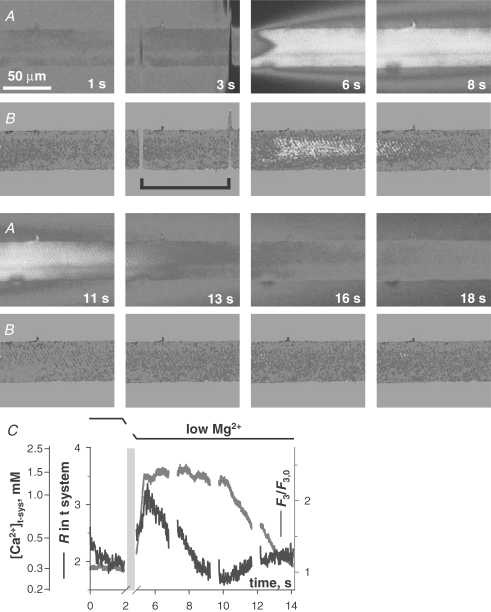
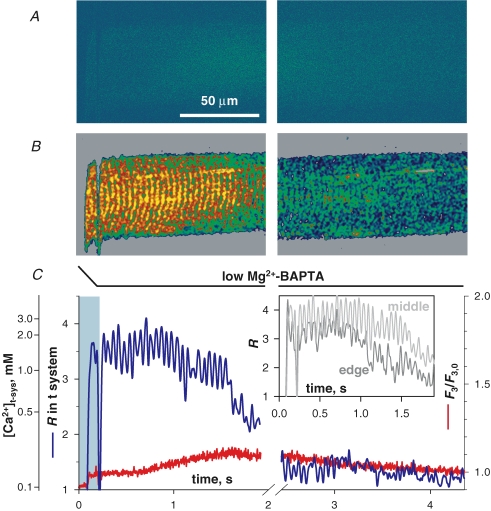
SOCE during Ca2+ release and removal A, selected images of fluorescence F3 of cytosolic rhod-2 and B, ratio R of fluorescence images F1 and F2 of mag-indo-1 in the t system, simultaneously acquired while applying the release-inducing low Mg2+ solution. C, spatially averaged values of F3 (normalized by resting value F3,0, red) and R (blue) versus elapsed time (which maps to the abscissa of x–y scans as described in Methods). The interval of solution change is indicated by the black bracket in image B at 3 s, and by the light blue bar in C, where fluorescence and ratio plots were omitted during solution change. (ID: 072005b_s013.)
Figure 7.
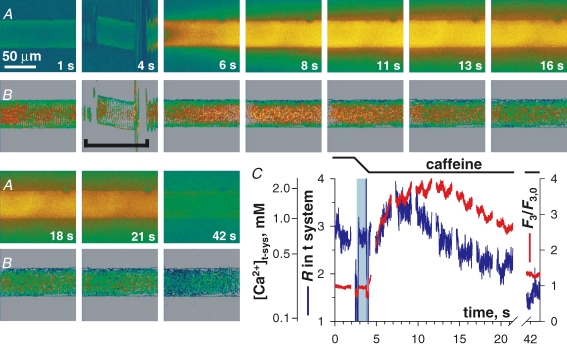
SOCE during complete [Ca2+]SR depletion at resting membrane potential A, images of rhod-2 fluorescence F3 and B, images of mag-indo-1 R in t system, acquired while applying the release-inducing caffeine solution. C, spatially averaged F3/F3,0 (red) and R (blue) versus elapsed time. Other details as in Fig. 4. (ID: 102505e_s010.)
Figure 8.
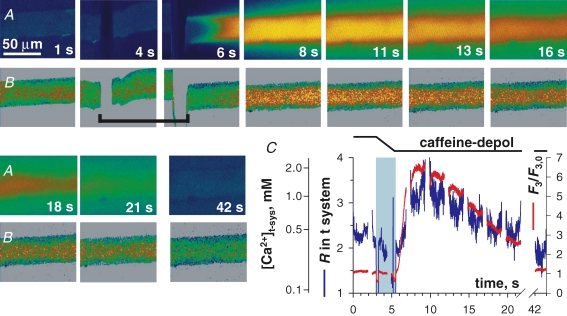
SOCE during complete [Ca2+]SR depletion with a depolarized t system A, F3 and B, R in t system, acquired while applying the release-inducing solution caffeine–depol after transfer from Reference–Na–0 Ca2+, which depolarized the t system. C, spatially averaged F3/F3,0 (red) and R (blue) versus elapsed time. Other details as in Fig. 4. (ID: 092705e_s007.)
Analysis of changes in [Ca2+]t-sys
As the sealed t system is a closed, finite compartment, any change in [Ca2+]t-sys must be due to Ca2+ flux across the membrane (Almers et al. 1981; Friedrich et al. 2001) or changes in the volume of the compartment. In the following we neglect the changes in volume (see below; Launikonis & Stephenson, 2004), and assume that JCa, the net Ca2+ flux across the t system membrane, is proportional to the rate of change, d[Ca2+]t-sys/dt.
When expressed relative to the volume of the t system, the total calcium concentration of the organelle is probably in the tens of millimolar range (Hidalgo et al. 1986; Owen et al. 1997), with most calcium presumably bound to low-affinity sites on membrane proteins. Therefore the net rate of change of total calcium concentration due to entry to the cytosol, d[calcium]c/dt, should be equal to Ad[Ca2+]t-sys/dt, where A is the product of the fractional volume of the t system within the intact fibre (t-sysvol= 0.014; Launikonis & Stephenson, 2002a) and a factor representing the ratio between bound and free Ca2+ in the t system (β), which is expected to be roughly constant but will remain unknown.
We should point out that in the text we refer to Ca2+ current across the sealed t system inferred from net loss of [Ca2+]t-sys as ‘Ca2+ influx' or ‘Ca2+ entry', for consistency with terms describing Ca2+ movements in intact muscle fibres.
SEER imaging of Ca2+ inside the t system
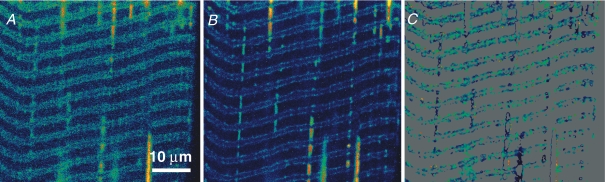
Figure 1 shows confocal xy images of mag-indo-1 in the t system of a mechanically skinned rat EDL fibre. The fibre had been bathed in a physiological solution containing mag-indo-1 while still intact and then mechanically skinned to trap the dye in the t system, as previously described (Lamb et al. 1995; Launikonis et al. 2003; Launikonis & Stephenson, 2004). Figure 1A and B are images F1 and F2, respectively, of a fibre bathed in low Mg2+. The images show two t tubules per sarcomere and infrequent longitudinal connections, which were originally described by Veratti (1961). Note that longitudinal structures appear across the long gap between the t tubules, crossing the M line, and run in series.
Figure 1.
SEER imaging of mag-indo-1 inside the sealed t system of a rat skinned fibre A, image of fluorescence F1 (acquired as defined in Methods); B, image F2; C, R =F1/F2, spatially restricted to the two upper quartiles of the dye distribution (Launikonis et al. 2005). Fibre was bathed in low Mg2+. Image-averaged R was 1.82, corresponding to a [Ca2+]t-sys of 0.28 mm. Average dye concentration in the well-stained region was 12.2 μm. (ID: 101805c_#11.)
The ratio (R) of F1 and F2 is shown in Fig. 1C, only in ‘well-stained' regions, defined as those in the upper quartile of the distribution of the dye (calculated as described by Launikonis et al. 2005). Raw ratioing of F1 and F2 (not shown) does not exhibit a clear arrangement of two t tubules per sarcomere. The dye-based restriction of the image clearly recovers the double t tubular structure. Results presented below (Figs 4, 5, 6, 7 and 8) will show that R computed this way monitors t system [Ca2+] ([Ca2+]t-sys).
Figure 5.
SOCE with suppressed change in [Ca2+]c A, F3 and B, R in t system, acquired while changing from a solution with 5 mm BAPTA, 0 Ca and 0.65 mm Mg2+ to release-inducing low Mg2+–BAPTA. C, spatially averaged F3/F3,0(red) and R (blue) versus elapsed time, which directly correspond to time and y axis in A and B. Inset, average R in central 40 μm (‘middle') and 30 μm slice starting at the lower edge (‘edge') of the fibre image. Note the periodic pattern of [Ca2+]t-sys in C, which results from the regular placement of t-tubules along the fibre. Other details as in Fig. 4. (ID: 072805f_s012.)
Figure 6.
Ca2+ movements following Ca2+ release in caffeine–BAPTA with moderately Ca2+-loaded SR A, F3 and B, R in t system, acquired while changing from a solution with 5 mm BAPTA, 0 Ca and 0.65 mm Mg2+ to releasing-inducing caffeine–BAPTA. C, spatially averaged F3/F3,0(red) and R (blue) versus elapsed time, which directly correspond to time and y axis in A and B. D, spatially averaged F3/F3,0 (red) and [Ca2+]t-sys (green) derived from R in C. Note the abrupt shift in R at the point of introduction of caffeine in the bathing solution. The calibration of R in terms of [Ca2+]t-sys was done using two sets of measured parameters: one for the period without caffeine (before end of blue bar marking solution change), and the other for the period with caffeine (starting at the end of blue bar). Other details as in Fig. 4. (ID: 072805e_s017.)
Furthermore, we can calculate the concentration of mag-indo-1 in the t system, [mag-indo-1]t-sys, in this image (Launikonis et al. 2005). In Fig. 1, [mag-indo-1]t-sys is 12.2 μm. This is much less than the 10 mm mag-indo-1 present in the dye solution applied to the intact fibre, probably due to a combination of factors, including dilution of mag-indo-1 in the normal extracellular fluid surrounding the intact fibre, some ‘squeezing-out' of the dye that entered the intact t system during the skinning process, and slow extrusion from the t system by anion transporters (Launikonis & Stephenson, 2002b, 2004). [mag-indo-1]t-sys was always in the low micromolar range, and therefore no major Ca2+ buffering effects should be expected from the monitoring dye.
Analysis of confocal images
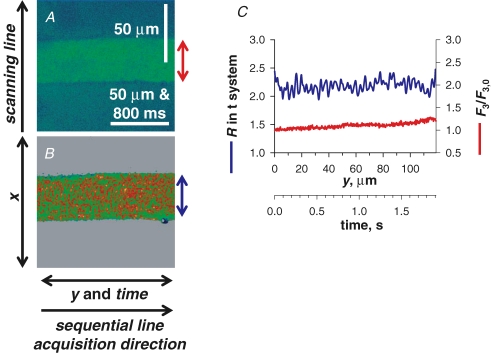
All imaging in this study was performed in xy mode, in most cases at 0.2324 μm pixel distance and 1.25 ms line interval. xy scanning lent itself to a method of image analysis illustrated in Fig. 2. The scanning line, defining the variable x, was transversal to the fibre axis. xy imaging provided information in two dimensions of space and in a temporal dimension as well, along the y axis. The temporal evolution of [Ca2+]t-sys and [Ca2+]c could be derived by averaging, respectively, R(xy) or F3(xy) over x within the borders of the preparation, to obtain a function (R(y) or F3(y)) of y that mapped proportionally to elapsed time. The correspondence factor between t and y, 16.1 ms per μm, is determined by the group scanning speed (3.75 ms per line in each of 3 images, and pixel distance). For the approach to be valid, the fibre must be homogeneous along the y axis, which usually can be decided by inspection of the xy image at rest. Uniformity is considered reasonable when changes of R(y) in the resting fibre are minor relative to the magnitude and rate of the dynamic changes R(y(t)) =G(t) of interest during stimulated release. For instance, in the case of Fig. 2 the sarcomeric structure imposes an oscillatory pattern of period ca 2 μm or 32.2 ms and amplitude ∼20% peak-to-peak. Other minor irregularities add to the variance of R(y). This degree of uniformity is sufficient if the goal is to reveal greater, faster or longer-lasting components in G(t). The events illustrated in Figs 4, 5, 6, 7 and 8 clearly satisfy this criterion. Figure 2 illustrates a degree of homogeneity that is suitable.
Figure 2.
Image acquisition and analysis A, F3 image. B, simultaneously acquired R in t system. The scanning line was transversal to the fibre axis, x, with each line (512 total) sequentially acquired along the longitudinal axis of the fibre, y. C, spatially averaged values of F3 and R from within the borders of the preparation, as indicated by the double arrows of corresponding colour. Note that y maps proportionally to time. (ID: 072805f_s025_z007.)
In experiments represented in Figs 4, 5, 6, 7 and 8R refers to the ratio of F1/F2 of mag-indo-1 in the t system and the normalization of cytoplasmic rhod-2 fluorescence is represented as F3/F3,0. The reference (F0) for normalization of rhod-2 fluorescence was F3(y) within the borders of the preparation under resting conditions immediately prior to exposure of the preparation to release-inducing solution. The F0 value may not represent the minimum F3 value in the F3 image (fluorescence can be lower in the surrounding bathing solution) in Figs 4, 5, 6, 7 and 8. In Fig. 3, a different combination of dyes was used. R refers to the ratio F1/F2 of indo-5F in the cytoplasm and F3/F3,0 represents normalized value of fluo-3 fluorescence in the t system to that under resting conditions.
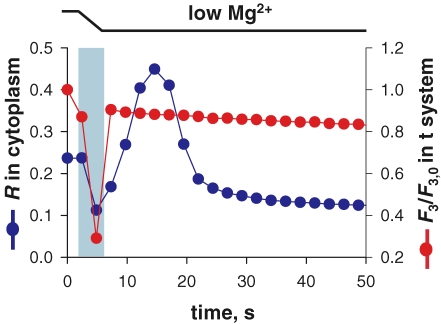
Figure 3.
Ca2+ release is not associated with a change in volume of the sealed t system Image-averaged fluorescence of cytoplasmic indo-5F (blue) and of fluo-3 trapped in t system (red), versus time. At between 3 and 6 s (light blue bar), the bathing solution was changed from reference to the release-inducing low Mg2+. Fluorescence of fluo-3 in the t system, which presumably corresponds to a fully Ca2+-saturated level, decays throughout at a steady low rate. (ID: 012306a_s008.)
Volume changes in the sealed t system during Ca2+ release
In this work store-dependent Ca2+ flux is assessed from the net change in [Ca2+]t-sys following Ca2+ release. Any change in volume of the t system due to water flux during Ca2+ release will confound our derivation of Ca2+ entry. Although the volume of the sealed t system of rat skeletal muscle varies little during osmotic stress (Launikonis & Stephenson, 2004), it was important to check whether this was the case during Ca2+ release. For this purpose fluo-3 was trapped in the t system of rat skeletal muscle. Because fluorescence of fluo-3 is at saturation values, its changes only reflect the evolution of ionic strength, and can be used to derive change in volume (Launikonis & Stephenson, 2004).
The t system and SR were initially loaded with Ca2+ in a reference solution containing 800 nm Ca2+. The preparation was then exposed to a low Mg2+ solution containing 100 μm indo-5F, which induced Ca2+ release and allowed us to image the Ca2+ transient. As shown in Fig. 3, after an artifact caused by the change in solution the fluo-3 fluorescence decreased at a constant rate, both during and after Ca2+ release. This steady decrease is due to bleaching and slow loss of dye from the sealed t system (Launikonis & Stephenson, 2002b, 2004). This result was obtained on all three fibres examined. The result indicates that there were no significant changes in volume of the t system specifically associated with Ca2+ release.
Quenching of fluorescence by caffeine
Caffeine at concentrations greater than 10 mm can quench fluorescence signals of Ca2+ sensitive dyes, inducing a shift in the Rmin and Rmax that has been demonstrated with indo dyes (Muschol et al. 1999; McKemy et al. 2000). To quantify the effect in these preparations, Rmax and Rmin were determined in the presence of 30 mm caffeine.
Rmin was determined by allowing [Ca2+]t-sys to decrease in the presence of caffeine–BAPTA solution. The average thus found in three freshly skinned preparations, 0.73 ± 0.05 (n = 3), is significantly larger than the value (0.45) determined in the same way using low Mg2+–BAPTA as stimulus for Ca2+ release.
Rmax was determined as the maximum reached early when exposing the preparations to 30 mm caffeine and 5 mm BAPTA. This solution causes a rapid release of SR Ca2+ (Launikonis & Stephenson, 2000) which is accumulated rapidly by the t system (data not shown). The maximum R was 5.5 ± 0.07 in three preparations.
Results
This section includes images of the evolution of [Ca2+] within the t system during SR Ca2+ depletion, quantified to derive Ca2+ flux, with simultaneously recorded images of [Ca2+]c at both the normal resting membrane potential and in the chronically depolarized cell. [Ca2+]t-sys is measured by SEER and calibrated in situ. Two types of stimuli were used, leading to Ca2+ release of different duration and extent in conjunction with two cytosolic buffers, to exert different degrees of control of [Ca2+]c.
SOCE during store depletion and recovery
Simultaneously acquired cytoplasmic rhod-2 and t system SEER images are shown in Fig. 4. A summary in Fig. 4C of the temporal evolution of [Ca2+]t-sys and [Ca2+]c was derived by averaging over x within the borders of the preparation, to obtain a function of y that mapped proportionally to elapsed time (see Methods and Fig. 2).
Changing the internal solution from reference with 100 nm Ca2+ and 0.65 mm Mg2+ to low Mg2+ (a nominally Ca2+-free solution with 1 mm EGTA and 10 μm Mg2+) caused the release of SR Ca2+. The drop in internal solution [Ca2+] is shown by the reduction in F3 fluorescence intensity around the preparation. The spatial progression of Ca2+ release (probably timed by diffusion of Mg2+ away from the fibre) results in a V-shaped Ca2+ transient in image A at 6 s and in narrowing of the area of low [Ca2+] surrounding the fibre, as released Ca2+ diffuses into this area. Note that the membrane potential was held at its resting value across the t system throughout the duration of this experiment (Lamb & Stephenson, 1994).
During the initial rise of the Ca2+ transient, [Ca2+]t-sys increased (image B at 6 s). Plasma membrane Ca2+-ATPase and Na+–Ca2+ exchanger have both been shown to colocalize with the dihydropyridine receptor at the junctional membrane domain of the t system (Sacchetto et al. 1996), making translocation of Ca2+ by these proteins the most likely explanation for the increase in [Ca2+]t-sys (Hidalgo et al. 1986, 1991; Donoso et al. 1995). Later, prior to the peak of the Ca2+ transient (B at 6 s; see also graph in Fig. 4C), there was a sharp reversal of the direction of change in [Ca2+]t-sys, i.e. in the sign of net t system flux, which became negative. Later, at about 10 s, there was another reversal in flux, indicating net Ca2+ entry to the t system. This pattern of Ca2+ movements in the presence of low Mg2+ was observed in five fibres.
Using mag-indo-1 in the SR, we previously showed that the SR starts to take up Ca2+ and recover from depletion at the time of RyR closure (Fig. 4 in Launikonis et al. 2006). Therefore the concomitant inversion in sign of the rate of change in [Ca2+]t-sys (which as explained in Methods we interpret as change of net flux) observed here between 11 and 13 s is consistent with reduction of a store-operated inward component of this flux below the level of a presumably small efflux, and validates measurement of [Ca2+]t-sys as a monitor of SOCE.
Attempts were made to block SOCE with the commonly used agent 2-APB. It was found that low Mg2+ was no longer able to elicit Ca2+ release from SR when in the presence of 0.1 mm 2-APB. This result was observed in three preparations. There was no effect of the addition of 0.1% DMSO (2-APB vehicle) to low Mg2+ solution alone on Ca2+ release. Therefore these experiments were abandoned. It is known that 2-APB interacts with a number of proteins, including the inositol trisphosphate receptor (Parekh & Putney, 2005), and therefore an interaction with RyR1 would not be surprising.
SOCE with suppressed changes in [Ca2+]c
In the above experiment, the signal from cytosolic rhod-2 demonstrates a significant increase in [Ca2+]c during release in the presence of 1 mm EGTA (Fig. 4). An exploration of the effects of [Ca2+]c was called for because its increase could directly affect SOCE (Hoth & Penner, 1993; Zweifach & Lewis, 1995) and should promote Ca2+ uptake by the t system, thus complicating the evaluation of SOCE through net flux. To evaluate these points, we performed similar release experiments as those above, with the addition of 5 mm BAPTA to the release-inducing solution.
R and rhod-2 images are shown in Fig. 5A and B for a fibre exposed to low Mg2+–BAPTA. Immediately prior to this exposure, the preparation was passed through reference with 800 nm Ca2+ to increase [Ca2+]t-sys and then equilibrated to a reference solution with BAPTA and no added calcium. R and F3 averaged over x are plotted versus y or time in Fig. 5C. [Ca2+]t-sys was uniform throughout the fibre prior to release (see online supplemental material). A small cytosolic Ca2+ transient, of magnitude 0.1F0, was induced by the stimulus in the presence of BAPTA. Probably because the transient was small, the decay in [Ca2+]t-sys started without any preceding uptake of Ca2+. This result was obtained on the two fibres tested in this manner. Note also that a V-shaped spatiotemporal pattern of Ca2+ release from the SR, similar to that in Fig. 4, should have occurred as Mg2+ diffused away from the preparation. This is not reported by the cytoplasmic rhod-2 due to the presence of BAPTA (Fig. 5).
The inset of Fig. 5C plots the evolution of R restricted to the central or peripheral regions of the preparation, for the first 1.9 s after immersion in low Mg2+–BAPTA. Decay is faster in the periphery, starting in less than 1 s following introduction of low Mg2+–BAPTA, which suggests that SOCE is rapidly activated and determined by depletion at the local level.
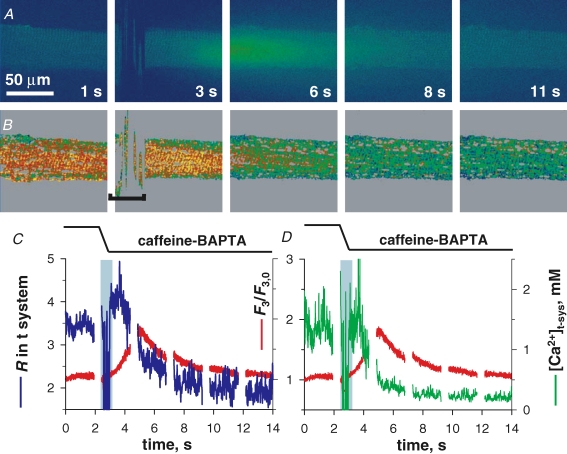
Similar experiments were carried out using caffeine–BAPTA, following the same protocol as in Fig. 5. A decay of [Ca2+]t-sys without recovery, starting within 1–3 s of immersion in caffeine–BAPTA was observed in four preparations. An example is shown in Fig. 6. Note that 15 s in reference solution with BAPTA and 0 Ca did not cause activation of SOCE. Also, there was a small increase in [Ca2+]t-sys preceding the increase in F3. This suggests that the t system devices for Ca2+ uptake respond to a local rise in [Ca2+] in the microdomain of the ‘couplon' (Stern et al. 1997) before the global [Ca2+] increase is reflected in changes in F3.
In Fig. 6 the effect of caffeine quenching of mag-indo-1 fluorescence from the t system can be observed as an abrupt upward shift in R at the point of caffeine introduction to the bathing solution (Fig. 6B and C). The shift constitutes a change in dye properties, which required a different calibration in the presence of caffeine (McKemy et al. 2000). In Fig. 6D the average [Ca2+]t-sys in x has been plotted against y or time. The continuity of [Ca2+]t-sys is maintained, indicating that parameters used to determine [Ca2+]t-sys from R in either the presence or the absence of caffeine were adequate (see Methods).
Following activation of SOCE, [Ca2+]t-sys did not significantly recover in caffeine–BAPTA (Fig. 6). This time course is consistent with the lack of recovery of [Ca2+]SR during continued caffeine exposure (Launikonis & Stephenson, 2000), again indicating that the inward flux of Ca2+ under the conditions described is largely store-operated. Thus, only an exit flux from the t system is observed in low Mg2+–BAPTA (Fig. 5) and in caffeine–BAPTA (Fig. 6) if SR is not heavily loaded with Ca2+. In conclusion, SOCE depletes the sealed t system of Ca2+ in a few seconds when [Ca2+]c is kept low.
SOCE and membrane potential
To examine the effect of t system membrane potential on the store-operated Ca2+ flux, preparations were exposed to K+- or Na+-based caffeine solutions in the presence of 1 mm EGTA. An internal solution with only Na+ will chronically depolarize the t system and a K+-based internal solution with some Na+ will activate the t system Na+-pump and allow the normal [K+] and [Na+] gradients to be reestablished across the t system, resulting in a close to normal resting potential (Lamb & Stephenson, 1990, 1994). To assure and ascertain Ca2+ store depletion under both conditions of membrane potential, 30 mm caffeine and 1 mm EGTA were used in the solution. Indeed, caffeine facilitates SR Ca2+ release under a wide range of conditions and prevents net Ca2+ uptake by the SR (Herrmann-Frank et al. 1999), while 1 mm EGTA buffers released Ca2+ while not hindering the observation of the cytoplasmic Ca2+ transient, thus providing an unambiguous indicator of Ca2+ release, which can in turn be used to assess depletion.
The evolution of [Ca2+]t-sys upon caffeine stimulation in a K+-based glutamate solution is illustrated in Fig. 7. Prior to caffeine, the fibre was loaded in reference with 800 nm Ca2+ (not shown), then immersed in reference with 0 Ca2+ (image A at 4 s). Release initiation is revealed by the increase in F3 intensity and the diffusion of Ca2+ away from the preparation. Note that there is no abrupt shift in R following caffeine introduction to the bathing solution. The reason for this, which is in contrast to that of caffeine in the presence of 5 mm BAPTA (Fig. 6), is not known. However, the shift in R was consistent in all fibres exposed to caffeine–BAPTA. No fibres exposed to caffeine–EGTA displayed the shift. R has been calibrated separately under each condition so that [Ca2+]t-sys can be estimated.
During the rising phase of the caffeine-induced Ca2+ transient there was an increase in [Ca2+]t-sys, indicating uptake of Ca2+ from the cytoplasm (image B at 6 and 8 s). A decrease in [Ca2+]t-sys followed (image B at 13 s), indicating net Ca2+ entry presumably due to SOCE. The depletion of [Ca2+]t-sys continued throughout the recording time, consistent with the lack of recovery of [Ca2+]SR during continued immersion in the same solution (Launikonis et al. 2005, 2006).
In order to assess the consequences of t system depolarization on store-dependent Ca2+ flux, preparations were loaded in sodium glutamate, high [Ca2+]-loading solution and incubated in a Na+ reference solution with 0 Ca immediately prior to exposure to caffeine–depol. An example is shown in Fig. 8. A large release of Ca2+, indicated by the increase in cytosolic rhod-2 fluorescence ensued upon exposure to caffeine–depol, and [Ca2+]t-sys initially increased during the rising phase of the Ca2+ transient. [Ca2+]t-sys began to decrease close to the peak of the Ca2+ transient indicating activation of SOCE.
[Ca2+]t-sys and Ca2+ entry flux
The t system fluxes measured above, in conjunction with present and previous estimates of SR calcium content, confirm the presence of SOCE in this preparation and validate the measurement technique. A qualitative validation consisted of showing a change in direction of the net t system flux concomitant with changes in [Ca2+]SR. Specifically, [Ca2+]t-sys largely fell during release of SR Ca2+ and could only recover as [Ca2+]SR increased. This correspondence was demonstrated most clearly by contrasting Ca2+ release in caffeine (during which there is no recovery of [Ca2+]SR and none was found in [Ca2+]t-sys) with that caused by low Mg2+, where recovery of [Ca2+]SR after brief release (demonstrated by Launikonis et al. 2006) was accompanied by reversal of the net Ca2+ flux in the t system (shown here in Fig. 4). This behaviour is diagnostic of SOCE (Parekh & Putney, 2005).
Whenever there is substantial Ca2+ uptake by the t system, as in initial stages of a Ca2+ transient (e.g. image A at 6 s in Fig. 7), it is impossible to separate SOCE from uptake. It is still possible, however, to provide a lower bound for SOCE as the net entry flux after it becomes inward. As an example, the change in net t system flux to negative or inward in Fig. 7 (B, image at 11 s) indicates that the store-operated inward flux became dominant at this point. The Ca2+ entry flux calculated for the first 1 s after this point was not significantly different from the rate estimated in BAPTA release-inducing solutions at a similar [Ca2+]t-sys (Figs 5 and 6). Later on, [Ca2+]t-sys decreased more slowly (Fig. 7, images at and after 16 s). The lower rate of decay in the EGTA-buffered solution may be due to (i) Ca2+ uptake from the cytoplasm, which would delay the depletion of [Ca2+]t-sys as the driving force for SOCE declined, or (ii) graded deactivation of SOCE as Ca2+ cycled through the SR (Collet & Ma, 2004). It could also be due to a combination of (i) and (ii).
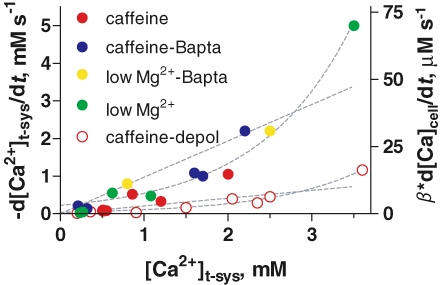
The rate of SOCE can now be determined within this framework and used to establish the influence of [Ca2+]t-sys on store-operated flux. This was done by plotting the rate of decrease of [Ca2+]t-sysversus[Ca2+]t-sys at the initiation of the event. A summary of results obtained with various release-inducing solutions is in Fig. 9. The curves represent best fits with linear or exponential functions. Intriguingly, the exponentials gave better fits at both membrane potentials. This analysis of collected data clearly demonstrates the following features: the rate of Ca2+ flux was significantly greater in normally polarized cells compared to that in depolarized cells. The store-operated Ca2+ flux calculated from each release-inducing solution (a minimum estimate, as it assumes equality of net and unidirectional flux) appeared to define a single-valued function of [Ca2+]t-sys, which is both an indication of a simple mechanism (i.e. not dependent on additional, uncontrolled variables) and an indirect validation of the method of calculating this flux. The apparent nonlinearity of the dependence suggests that [Ca2+]t-sys could have gating (Hofer, 2005), in addition to flux-driving effects, or reflect quantification errors.
Figure 9.
Magnitude of SOCE at different [Ca2+]t-sys and t system membrane potential Symbols plot rate of change of [Ca2+]t-sysversus initial value of [Ca2+]t-sys. Right ordinate axis represents rate of change in terms of total cytosolic Ca2+ concentration. β is the ratio between total and free t system [calcium]. Colours represent different stimuli as indicated, with filled symbols plotting data obtained with a polarized t system and open symbols a depolarized one. Broken lines plot best linear fits passing through the origin or exponential fits. Best fit parameters: slopes of linear regressions were 0.97 ± 0.02 and 0.21 ± 0.01 s−1; and rate constants for exponentials: 0.90 ± 0.05 and 0.92 ± 0.07 s−1, for polarized and depolarized cells, respectively. n = 16 fibres.
The axis on the right side is proportionally calibrated in terms of accessible cytoplasmic volume, assuming a fractional t system volume of 1.4% (Eisenberg, 1983; Dulhunty, 1984; Launikonis & Stephenson, 2002a). It is worth noting that the fractional volume of the t-system (t-sysvol) of intact fibres has been estimated from electron and confocal microscopy, with differing results. The upper estimate for t-sysvol from electron microscopy in mammalian fast-twitch muscle is 0.7% (Dulhunty, 1984), whereas confocal microscopy estimates 1.4% (Launikonis & Stephenson, 2002a). As fibres must be fixed in hypertonic solution for electron microscopy imaging, a correction for shrinkage of the preparation was made from measurements of total fibre volume changes in hypertonic solution, with the assumption that the fibre and t-system volumes shrink proportionally (Eisenberg, 1983). This assumption was found to be incorrect (Launikonis & Stephenson, 2004). Accordingly, fluorescent dyes equilibrated in the t-system of intact, living muscle cells report significantly larger volumes than those estimated from electron microscopy (Endo, 1966; Soeller & Cannell, 1999; Launikonis & Stephenson, 2002a).
The flux rates will scale up after taking into account Ca2+ buffering within the t system wall (see Methods). This buffering power (β=[total calcium]t-sys/[Ca2+]t-sys) has not been determined under the present experimental conditions. Under somewhat comparable conditions, with SOCE activated upon depletion of SR Ca2+ by 30 mm caffeine, assays of total calcium in the t system put β in the range 1–20 (Donoso et al. 1995; Owen et al. 1997; M. Barnes and D. G. Stephenson, unpublished observations).
Discussion
SOCE during Ca2+ release in skinned fibres
By combining confocal measurements of free [Ca2+] within the t system and simultaneous recordings of cytoplasmic Ca2+ we were able to measure for the first time the t system SOCE flux during Ca2+ release from the SR in skeletal muscle.
In this study we build on previous work where it was shown that Ca2+-dependent t system fluorescence is an indicator of SOCE in skinned skeletal muscle fibres (Launikonis et al. 2003). Depletion of SR Ca2+ by application of caffeine caused t system Ca2+ to deplete, showing activation of SOCE. By disrupting the coupling between the t system and SR membranes (Lamb et al. 1995) the signal to open the SOC channel upon store depletion is severed (Launikonis et al. 2003). Because uncoupling interrupts signalling between membranes but does not affect membrane protein function, it is a more precise method of identifying SOCE than the use of a non-specific pharmacological agent, like 2-APB.
It was indeed found that 2-APB interacts with RyR1 in our skinned fibre experiments. This interaction has not been reported in intact fibres. In intact fibre studies the approach is usually to deplete SR Ca2+ in the absence of extracellular Ca2+ and 2-APB, and then introduce extracellular Ca2+ (or the Ca2+ surrogate Mn2+) to evoke SOCE and 2-APB to block it. An interaction of 2-APB and RyR1 would go unnoticed in such an experiment. Alternatively, the diffusional access of 2-APB to the triad could be restricted in intact compared to skinned fibres, consequently preventing 2-APB access to RyR1, together with its interfering with Ca2+ release.
The current results show [Ca2+]t-sys changes during Ca2+ release. Most notably, low Mg2+-induced Ca2+ release resulted in an increase in [Ca2+]t-sys, then an abrupt drop, followed by a recovery as Ca2+ release ended (Fig. 4). This sequence occurred in the order of seconds. These changes in [Ca2+]t-sys can only be due to the net flux of Ca2+ across the t system membrane. Therefore, these changes in [Ca2+]t-sys indicate Ca2+ uptake into the t system during Ca2+ release from SR, SOCE activation in less than 1 s following initiation of Ca2+ release and SOCE termination as the SR refills with Ca2+ at the decline of the Ca2+ release transient (as demonstrated by Launikonis et al. 2006) to allow net reuptake of Ca2+ by the t system. This first demonstration of activation and termination of SOCE during a single Ca2+ release event in muscle shows that activation of SOCE is rapid under these circumstances.
Ca2+ uptake by the t system
A striking observation of this study was that the t system could take up Ca2+ during Ca2+ release from SR at a rapid rate (up to 1 mm s−1 relative to t system volume; Fig. 4). This therefore represents a possible means of Ca2+ depletion in the cell during Ca2+ release. It would be difficult, however, to extrapolate from these observations to a physiological situation, to for instance estimate Ca2+ loss during a train of action potentials that cause fatigue. On the one hand, the present conditions may overestimate the rate of Ca2+ loss, as t system Ca2+ uptake was observed during a very large release of Ca2+ following direct activation of the SR RyR (Figs 4–8). On the other hand, the present measurements amount to a lower limit of Ca2+ loss during a comparable Ca2+ transient, as intact fibres would allow diffusional efflux from tubules to extracellular space, which would reduce the t system concentration buildup and enhance Ca2+ efflux into the t system.
Properties of SOCE in muscle
This study demonstrates three features of SOCE: (i) it does not require complete depletion of the SR, (ii) its activation and rate are approximately independent of the magnitude of the cytoplasmic Ca2+ transient, and (iii) it is activated by local SR depletion. (i) follows from the fact that SOCE was activated prior to the peak of the Ca2+ transients (Figs 4–8), indicating that Ca2+ was still present in the SR at the time of activation. This observation, plus the similarity of fluxes with different degrees of cytosolic buffering, constitutes evidence that [Ca2+]c is not a direct determinant or mediator of SOCE in muscle. The local character of activation of SOCE was shown by the spatially inhomogeneous decrease in [Ca2+]t-sys associated with a non-uniform release of Ca2+ from SR across the fibre (Figs 4–6).
Importantly, activation of SOCE was observed to occur locally in less than 1 s following the introduction of release-inducing solutions containing 5 mm BAPTA. Furthermore, activation of SOCE occurred throughout the whole t system, consistent with the spatiotemporal pattern of SR Ca2+ depletion, which was dependent on the rate of Mg2+ diffusion away from the preparation (Figs 4–6). By comparison with recent studies of SOCE activation in the small, non-excitable Jurkat cell (Wu et al. 2006; Luik et al. 2006), we can further elucidate the mechanism of SOCE activation in skeletal muscle. Following Ca2+ depletion of ER in Jurkat cells there is accumulation of the intrastore Ca2+ sensor STIM1 (stromal interacting molecule 1) at junctional regions of the ER. The subsequent SOC channel activation takes place only at specific sites on the surface membrane (Luik et al. 2006). This process required one-third of junctional ER to relocate to the plasma membrane in an overall process taking 6–10 s to reach 20% maximal activation (Wu et al. 2006). Therefore the time required to reach maximal SOCE activation in mammalian skeletal muscle (Fig. 5) was more than an order of magnitude shorter than that required to reach 20% maximal activation in Jurkat cells.
The contrasting properties of skeletal muscle, where junctional SR is structurally static and activation of SOCE is faster and spatially unrestricted in the t system, suggest that the elementary molecular machinery for SOCE in skeletal muscle is already uniformly distributed and present in the junctional membranes prior to store depletion. A direct physical interaction between the relevant molecules is plausible given that this cell already has the well-defined contacts between the t system and SR that drive EC coupling (Franzini-Armstrong et al. 1999; Paolini et al. 2004). This model is also consistent with the functional disruption of SOCE caused in skeletal muscle by high intracellular [Ca2+] treatment, which severs the protein–protein interactions between the SR and t system at the triad (Launikonis et al. 2003).
In contrast to studies of non-excitable cells (Hoth & Penner, 1993; Zweifach & Lewis, 1995), we found termination of SOCE to be independent of cytoplasmic Ca2+ conditions but fully regulated from the SR. This is consistent with the observation (Kurebayashi & Ogawa, 2001) of a prolonged rise of [Ca2+]c due to SOCE, reaching micromolar levels without inactivation, in an intact skeletal muscle cell in the presence of a SR Ca2+-pump inhibitor. The difference with the inactivation properties described in non-excitable cells may reflect different store-operated currents (ICRAC and ISOC; Parekh & Putney, 2005; Lewis, 2007). In skeletal muscle, an ICRAC with Ca2+ inactivation properties similar to those described in non-excitable cells is yet to be reported. The differences seem teleologically justified. In working muscle, where [Ca2+]c can reach tens of micromolar (Baylor & Hollingworth, 2003), entry flux should be able to proceed independently of the increase in [Ca2+]c in order to maintain a required level of [Ca2+]SR.
Ca2+ entry flux rate
This study provides the first determination of Ca2+ entry flux rates during Ca2+ release in skeletal muscle cells. Ca2+ entry flux during Ca2+ release was found to be dependent on the driving force provided by [Ca2+]t-sys and the membrane potential (Fig. 9), even though the dependence was not linear as simple permeation theory would predict. In any case, the physiological conditions in skeletal muscle of 2 mm[Ca2+]t-sys and a membrane potential of around −90 mV will strongly favour inward Ca2+ flux to the cell when SOC channels are activated in skeletal muscle.
From the results summarized in Fig. 9, the SOCE flux in the resting cell at 2 mm[Ca2+]t-sys (relative to t-system volume) can be estimated at 18.6 μm s−1 (in terms of the accessible cytoplasmic volume) times the buffering power of the t system, a factor in the range 1–20 (see Methods; Hidalgo et al. 1986; Owen et al. 1997). This can be compared with estimates from studies on intact cells, according to which it takes 1–2 min to refill the depleted SR following reintroduction of 2 mm extracellular Ca2+ (Kurebayashi & Ogawa, 2001; Collet & Ma, 2004). The rate of SOCE measured here at physiological [Ca2+]t-sys predicts that 1.12–2.24 mm calcium will enter the cell in 1–2 min, which should be sufficient to refill the SR of mammalian fibres (1.01 mm total calcium concentration; Fryer & Stephenson, 1996) even if graded deactivation of SOCE slowed the influx rate as the SR refilled (Collet & Ma, 2004).
These observations and calculations confirm the presence of SOCE and show that its flux across the t system can fully account for the SR refilling times in intact cells (Kurebayashi & Ogawa, 2001). Recently, though, the existence of SOCE in skeletal muscle fibres has been brought into question by Allard et al. (2006). This group used a combination of voltage- and patch-clamping techniques to monitor electrical activity across the surface membrane during Ca2+ release from SR. In this work the major part of the cell was electrically insulated with silicone grease allowing accurate control of voltage in the exposed area (a 100 μm segment of a ∼30 μm diameter cell). In contrast to the results of others (Vandebrouck et al. 2002), no unitary channel activity was recorded across a patch of the exposed sarcolemma during depleting Ca2+ release. The result was interpreted as evidence for either the absence of SOCE across this membrane, or for it consisting of unitary currents of undetectable size.
Moreover, the macroscopic current recorded by Allard et al. (2006) under voltage-clamp upon repolarization from long Ca2+-depleting pulses did not change in a significant way, suggesting that there was no SOCE across the t system either, and similarly minor changes in current were observed after other treatments that caused cell-wide depletion. These apparently negative results are not inconsistent with the presence of an electrogenic SOCE with the magnitude reported here. Indeed, the Ca2+ current density associated with a flux of 1 mm per minute, about 3.2 A l−1, would result in a macroscopic current of ∼0.3 nA in the cell segments used by Allard et al. (2006). Changes of that magnitude could have passed unnoticed, given the evident presence of other electrophysiological changes upon various treatments (e.g. their Fig. 1). In fact, after 5 min of application of cyclopiazonic acid, which caused a substantial loss of SR calcium, the incremental current in response to a −100 mV pulse increased by ∼0.2 A F−1, or 0.2 nA in a 1000 pF cell (Fig. 5 of Allard et al. 2006). In this framework it is possible to conclude that SOCE is controlled at the triads and not across the sarcolemma in skeletal muscle. However, the experiments of Allard et al. do not fully disprove the presence of SOCE across the sarcolemma, leaving this controversy unresolved.
Other studies of SOCE in skinned fibres (Zhao et al. 2005, 2006; Hirata et al. 2006) suggest a much slower entry flux than observed here, with the t system taking up to 12 min to deplete of Ca2+ upon store depletion. In sharp contrast, all entry fluxes reported in our study ended in seconds, by deactivation of SOCE or t system depletion.
Several technical differences may account for the discrepancies: in the studies referred to above, all cells were probed for their SOCE response in a K+-based internal solution with no Na+, thus rendering the t system Na+ pump inactive and depolarizing the t system. This feature alone does not account for the difference, however, because the depolarized cells of our study still depleted calcium in the t system in less than 1 min following store depletion (Fig. 8). In the previous studies attempts to deplete SR Ca2+ were done with caffeine in the presence of ∼1 mm[Mg2+]c and submillimolar [ATP], without an indicator of SR Ca2+ release. Caffeine is a poor activator of RyR1 in adult skeletal muscle at 1 mm[Mg2+]c (Lamb et al. 2001) and at low [ATP] (Owen et al. 1996; Laver et al. 2001; Dutka & Lamb, 2004). Therefore store depletion, which was not demonstrated under the conditions used, may have been limited, and most significantly, in the previous studies fibres were skinned in a solution with 0.5 mm total calcium and 0.5 mm rhod-5N (Zhao et al. 2005; Hirata et al. 2006), which exposed skinned fibres to micromolar levels of Ca2+. In addition, skinned fibres were further exposed to micromolar levels of Ca2+ for several minutes in order to load the t system and SR (Zhao et al. 2005). The exposure of the intracellular environment to micromolar Ca2+ for a few minutes irreversibly disrupts the coupling between the t system and SR membranes (Lamb et al. 1995; Launikonis et al. 2003; Verburg et al. 2005).
Preliminary results from another laboratory employing skinned fibres to study SOCE in mammalian muscle have also appeared (Duke & Steele, 2006). They report that fluorescence of t system-trapped fluo-5N of rat fibres is lost in less than 1 min following waves of Ca2+ release induced in an internal solution with halothane and low Mg2+. This rate of SOCE is consistent with that presented here and with the levels previously shown in amphibian skeletal muscle (Launikonis et al. 2003).
[Ca2+]SR and activation of SOCE in skeletal muscle
Problems finding a protocol suitable to load Ca2+ into the t system while achieving an endogeneous level of Ca2+ in SR (Fryer & Stephenson, 1996) meant that Ca2+ probably exceeded normal levels in some fibres. Given the fact that SOCE was clearly active prior to complete depletion of Ca2+ in SR (Figs 4–8), the question remains open whether SOCE has a relative or absolute threshold in skeletal muscle. That is, can SOCE start following release of a certain amount of Ca2+ from SR regardless of the initial level or does a certain level of Ca2+ depletion in SR have to be reached for activation?
In summary, we have shown that store-dependent Ca2+ influx across the t system can be measured during Ca2+ release in rat skeletal muscle. SOCE flux determined this way was much higher than in earlier estimates, at values consistent with the rapid refilling of the cellular stores demonstrated in intact cells. SOCE flux was also found to be strongly dependent on the membrane potential and Ca2+ gradient. From our observations of SOCE in these experiments we present a model of SOCE in skeletal muscle where the elementary molecular units of SOCE are evenly distributed throughout the t system and are locally controlled, activated rapidly following Ca2+ release and influenced little by changes in cytosolic [Ca2+] within the physiological range but fully regulated from within the SR.
Acknowledgments
We thank D.G. Stephenson (La Trobe University) for comments on the manuscript. B.S.L. was a C. J. Martin Fellow of the National Health and Medical Research Council (Australia). This work was supported by grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Health to E. Ríos.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.135046/DC1
and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.135046
References
- Allard B, Couchoux H, Pouvreau S, Jacquemond V. Sarcoplasmic reticulum Ca2+ release and depletion fail to affect sarcolemmal ion channel activity in mouse skeletal muscle. J Physiol. 2006;575:68–81. doi: 10.1113/jphysiol.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W, Fink RHA, Palade PT. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Collet C, Ma J. Calcium-dependent facilitation and graded deactivation of store-operated calcium entry in fetal skeletal muscle. Biophys J. 2004;87:268–275. doi: 10.1529/biophysj.103.039305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase V, Franzini-Armstrong C. Evolution of skeletal type e-c coupling: a novel means of controlling calcium delivery. J Cell Biol. 2005;171:695–704. doi: 10.1083/jcb.200503077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso P, Prieto H, Hidalgo C. Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophys J. 1995;68:507–515. doi: 10.1016/S0006-3495(95)80212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AM, Steele D. Store-operated Ca2+ entry following halothane-induced Ca2+ waves in mechanically skinned rat muscle fibres. Proc Physiol Soc. 2006;3:140P. [Google Scholar]
- Dulhunty AF. Heterogeneity of t-tubule geometry in vertebrate skeletal muscle fibres. J Muscle Res Cell Mot. 1984;5:333–347. doi: 10.1007/BF00713111. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Effect of low cytoplasmic [ATP] on excitation-contraction coupling in fast-twitch muscle fibres of the rat. J Physiol. 2004;560:451–468. doi: 10.1113/jphysiol.2004.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, editor. Handbook of Physiology, section 10, Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 73–112. [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966;185:224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong CA, Protasi F, Ramesh V. Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscle. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich O, Ehmer T, Uttenweiler D, Vogel M, Barry PH, Fink RHA. Numerical analysis of Ca2+ depletion in the transverse tubular system of mammalian muscle. Biophys J. 2001;80:2046–2055. doi: 10.1016/S0006-3495(01)76178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–380. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Herrmann-Frank A, Luttgau H-C, Stephenson DG. Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. J Muscle Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Cifuentes F, Donoso P. Sodium-calcium exchange in transverse tubule vesicles isolated from amphibian skeletal muscle. Ann NY Acad Sci. 1991;639:483–497. doi: 10.1111/j.1749-6632.1991.tb17342.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, González ME, García AM. Calcium transport in transverse tubules isolated from rabbit skeletal muscle. Biochim Biophys Acta. 1986;854:279–286. doi: 10.1016/0005-2736(86)90121-5. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Brotto M, Weisleder N, Chu Y, Lin P, Zhao X, Thornton A, Komazaki S, Takeshima H, Ma J, Pan Z. Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes. Biophys J. 2006;90:4418–4427. doi: 10.1529/biophysj.105.076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM. Another dimension to calcium signaling: a look at extracellular calcium. J Cell Sci. 2005;118:855–862. doi: 10.1242/jcs.01705. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:737–749. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarization in skeletal muscle fibres of the rat. J Physiol. 2001;531:715–728. doi: 10.1111/j.1469-7793.2001.0715h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation–contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated calcium entry and the inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effects of Mg2+ on Ca2+ release from sarcoplasmic reticulum of skeletal muscle fibres from yabby (crustacean) and rat. J Physiol. 2000;526:299–312. doi: 10.1111/j.1469-7793.2000.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Tubular system volume changes in twitch fibres from toad and rat skeletal muscle assessed by confocal microscopy. J Physiol. 2002a;538:607–618. doi: 10.1113/jphysiol.2001.012920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Properties of the vertebrate skeletal muscle tubular system as a sealed compartment. Cell Biol Int. 2002b;26:921–929. doi: 10.1006/cbir.2002.0942. [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Osmotic properties of the sealed tubular system of toad and rat skeletal muscle. J Gen Physiol. 2004;123:231–247. doi: 10.1085/jgp.200308946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Ríos E. Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing. J Physiol. 2005;567:523–543. doi: 10.1113/jphysiol.2005.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Ríos E. Depletion ‘skraps' and dynamic buffering inside the cellular Ca2+ store. Proc Natl Acad Sci U S A. 2006;103:2982–2987. doi: 10.1073/pnas.0511252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Lenz GKE, Lamb GD. Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J Physiol. 2001;537:763–778. doi: 10.1111/j.1469-7793.2001.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Luik RM, Wu MW, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Welch W, Airey JA, Sutko JL. Concentrations of caffeine greater than 20 mM increase the indo-1 fluorescence ratio in a Ca2+-independent manner. Cell Calcium. 2000;27:117–124. doi: 10.1054/ceca.1999.0102. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Muschol M, Dasgupta BR, Salzberg BM. Caffeine interaction with fluorescent calcium indicator dyes. Biophys J. 1999;77:577–586. doi: 10.1016/S0006-3495(99)76914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG. Effect of low [ATP] on depolarization-induced Ca2+ release in skeletal muscle of the toad. J Physiol. 1996;493:309–315. doi: 10.1113/jphysiol.1996.sp021385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG, Fryer MW. Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. J Physiol. 1997;498:571–586. doi: 10.1113/jphysiol.1997.sp021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini C, Fessenden JD, Pessah IN, Franzini-Armstrong C. Evidence for conformational coupling between two calcium channels. Proc Natl Acad Sci U S A. 2004;101:12748–12752. doi: 10.1073/pnas.0404836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J Physiol. 2003;524:239–258. doi: 10.1113/jphysiol.2003.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S, Royer L, Yi J, Brum G, Meissner G, Ríos E, Zhou J. Ca2+ sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc Natl Acad Sci U S A. 2007;104:5235–5240. doi: 10.1073/pnas.0700748104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LC. Design and function of superfast muscles: new insights into physiology of skeletal muscle. Annu Rev Physiol. 2006;68:193–221. doi: 10.1146/annurev.physiol.68.040104.105418. [DOI] [PubMed] [Google Scholar]
- Sacchetto R, Margreth A, Pelosi M, Carafoli E. Colocalization of the dihydropyridine receptor, the plasma-membrane calcium ATPase isoform 1 and the sodium/calcium exchanger to the junctional-membrane domain of transverse tubules of rabbit skeletal muscle. Eur J Biochem. 1996;237:483–488. doi: 10.1111/j.1432-1033.1996.0483k.x. [DOI] [PubMed] [Google Scholar]
- Shirokova N, García J, Pizarro G, Ríos E. Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle. J Gen Physiol. 1996;107:1–18. doi: 10.1085/jgp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, García J, Ríos E. Local calcium release in mammalian skeletal muscle. J Physiol. 1998;512:377–384. doi: 10.1111/j.1469-7793.1998.377be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Lamb GD. Visualisation of the transverse tubular system in isolated intact and in mechanically skinned muscle fibres of the cane toad by confocal laser scanning microscopy. J Physiol. 1993;459:15P. [Google Scholar]
- Stern MD, Pizarro G, Ríos E. Local control model of excitation-contraction coupling in skeletal muscle. J Gen Physiol. 1997;110:415–440. doi: 10.1085/jgp.110.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veratti E. Investigations on the fine structure of striated muscle fiber. J Biophys Biochem Cytol. 1961;10:1–59. doi: 10.1083/jcb.10.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg E, Murphy RM, Stephenson DG, Lamb GD. Disruption of excitation–contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol. 2005;564:775–790. doi: 10.1113/jphysiol.2004.082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MW, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Weisleder N, Han X, Pan Z, Parness J, Brotto M, Ma J. Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J Biol Chem. 2006;281:33477–33486. doi: 10.1074/jbc.M602306200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yoshita M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics. 2005;23:72–78. doi: 10.1152/physiolgenomics.00020.2005. [DOI] [PubMed] [Google Scholar]
- Zhou J, Launikonis BS, Ríos E, Brum G. Regulation of Ca2+ sparks by Ca2+ and Mg2+ in mammalian and amphibian muscle. An RyR isoform-specific role in excitation–contraction coupling? J Gen Physiol. 2004;124:409–428. doi: 10.1085/jgp.200409105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.