Abstract
It is now widely accepted, given the current weight of experimental evidence, that reactive oxygen species (ROS) contribute to cell and tissue dysfunction and damage caused by glucolipotoxicity in diabetes. The source of ROS in the insulin secreting pancreatic β-cells and in the cells which are targets for insulin action has been considered to be the mitochondrial electron transport chain. While this source is undoubtably important, we provide additional information and evidence for NADPH oxidase-dependent generation of ROS both in pancreatic β-cells and in insulin sensitive cells. While mitochondrial ROS generation may be important for regulation of mitochondrial uncoupling protein (UCP) activity and thus disruption of cellular energy metabolism, the NADPH oxidase associated ROS may alter parameters of signal transduction, insulin secretion, insulin action and cell proliferation or cell death. Thus NADPH oxidase may be a useful target for intervention strategies based on reversing the negative impact of glucolipotoxicity in diabetes.
Introduction
Under conditions of elevated metabolism or enhanced mitochondrial activity many tissue specific cells are continuously subject to insult from reactive oxygen species (ROS). The damage inflicted by ROS has been implicated in conditions of inflammation, diabetes mellitus, age-related degeneration and tumour formation (Evans et al. 2002; Brownlee, 2005; Greenman et al. 2007). A better understanding of the mechanisms responsible for ROS generation and reactivity may result in new intervention strategies leading to reduction in damage associated with oxidative stress.
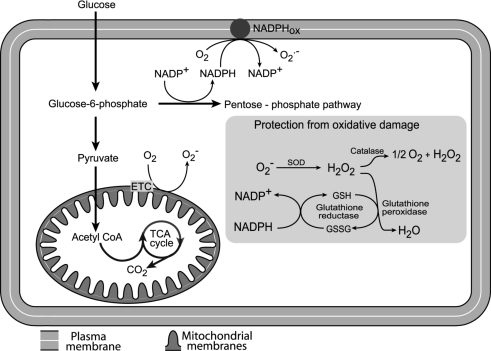
Overproduction of ROS or a failure in intracellular defences against ROS will result in pathogenesis of disease (Droge, 2002; Turrens, 2003; Fridlyand & Philipson, 2005) including diabetes, which is the focus of this review. Some of the important mechanisms related to production of ROS and intracellular defence mechanisms are summarized in Fig. 1. Mitochondria generate cellular energy through TCA cycle activity and the associated electron transport chain of the inner membrane. The reducing equivalents (NADH and FADH2) that are produced from the TCA cycle are reoxidized via a process that involves transfer of electrons through the electron transport chain (ETC) and associated translocation of protons across the mitochondrial inner membrane, creating the transmembrane electrochemical gradient (estimated as 150–200 mV negative to the cytosol). This gradient provides the electrochemical potential to make ATP from ADP and Pi, driven by proton movement back through the ATP synthase complex. Under normal conditions, the proton gradient is also diminished by H+‘leak’ to the matrix. The ‘leak’ occurs either via non-protein membrane pores, protein–lipid interfaces (H+ leak), or by proton channels known as uncoupling proteins (UCPs).
Figure 1.
Relevant sites of production of reactive oxygen species (ROS) and antioxidant systems in a generic cell type ROS can be generated through glucose metabolism in mitochondria (by electron transport chain (ETC) activity) and in the plasma membrane (through NADPH oxidase – NADPHox). The main antioxidant enzymes are superoxide dismutase (SOD), glutathione reductase, glutathione peroxidase and catalase.
However, mitochondria can generate significant ROS and reactive nitrogen species because of unavoidable oxidative phosphorylation chemistry. Superoxide anions (O2−) are a byproduct of single electron reduction of ubiquinone. Furthermore, these anions are the major contributors to other reactive species inside the mitochondrion (Turrens, 2003), for example the reaction of O2− with nitric oxide produces peroxynitrite. Superoxide anions, peroxynitrite and other reactive species are very powerful chemical oxidants (Evans et al. 2002; Turrens, 2003)
The electron transport chain-dependent movement of protons across the inner mitochondrial membrane establishes an electrochemical gradient of which mitochondrial membrane potential (ΔΨm) is an important component. An increase in ΔΨm will result in elevated ATP production but reduced electron transport capability, thus leading to increased ROS production (Korshunov et al. 1997). Uncoupling agents (for example, UCPs) reduce the proton gradient across the mitochondrial inner membrane and decrease ΔΨm, causing decreased ATP and ROS production but increased ADP concentration.
Phagocytic cells of the immune system, such as macrophages and neutrophils, require a plasma membrane/phagosome associated enzyme complex, termed NADPH oxidase, to generate O2−, which is subsequently used to damage and kill pathogenic organisms. However, it has now become clear that NADPH oxidase is not restricted to the immune system but alternative isoforms may be active in may other cell types as an essential component of redox signalling mechanisms (see below for further details).
Cells require antioxidant systems to neutralize ROS (Fig. 1). For example, superoxide anions are enzymatically converted to hydrogen peroxide by a manganese superoxide dismutase (MnSOD) within mitochondria. Hydrogen peroxide can then be rapidly removed by the mitochondrial enzyme glutathione (GSH) peroxidase. The inner mitochondrial membrane also contains vitamin E, which is a powerful antioxidant as it can accept unpaired electrons to produce a stable product. A further antioxidant enzyme, catalase, is the major hydrogen peroxide detoxifying enzyme found exclusively in peroxisomes (Fig. 1) (Turrens, 2003).
However, while cells have a number of antioxidant mechanisms available, it is still possible for ROS to evade antioxidant defence mechanisms, resulting in a slow accumulation of chronic damage. Both the mitochondrion and nucleus contain a variety of DNA repair enzymes to correct oxidant-induced modifications (Evans et al. 2004; Turrens, 2003), but damage most likely occurs when the endogenous antioxidant network and repair systems are overwhelmed (Hutter et al. 2007; Maiese et al. 2007; Rachek et al. 2007). However, it is essential to repair oxidant-induced damage to DNA or mutations may result in impaired transcription. Damaged mitochondria may be removed by autophagy, but many aspects of this process are obscure (Evans et al. 2004). However, it is known that mitochondrial biogenesis is regulated by some specific transcriptional activators and coactivators as well as by hormones (Goffart & Wiesner, 2003). Any imbalance in these processes will lead to cell dysfunction and possibly death resulting in oxidative stress-related diseases.
However, the molecular and biochemical mechanisms that link oxidative stress-related processes and diseases remain elusive. We will specifically discuss oxidative stress in pancreatic β-cells and insulin responsive cells in this review because they are likely to reflect the pathogenic mechanisms associated with the onset of type 2 diabetes mellitus (T2DM) a disease of considerable socio-economic impact.
ROS associated cell damage in diabetes
T2DM is a metabolic disease characterized by elevation of blood glucose concentration, lipid abnormalities and vascular complications. Of importance to this article, insulin resistance and pancreatic β-cell insufficiency with respect to insulin production are major features in the progression of T2DM (Bell & Polonsky, 2001; Kahn, 2003). The molecular basis for excessive mitochondrial oxidative damage in diabetes has been expertly reviewed elsewhere (Green et al. 2004)
Chronic exposure to elevated glucose and fatty acid concentrations can cause damage in different types of cells by a variety of mechanisms (‘glucolipotoxicity’), but oxidative stress may be a common link in cell dysfunction (Kahn, 2003; Kajimoto & Kaneto, 2004). Insulin resistance seems to precede and predict the development of T2DM and is common to the major metabolic tissues and organs including muscle, adipose tissue and liver. Recent studies suggest that insulin-stimulated muscle glycogen synthesis is the major metabolic pathway for disposing of excess glucose in healthy adults after a meal (Petersen & Shulman, 2002), and thus diverting glucose into anabolic rather than catabolic pathways. Increased plasma concentration of free fatty acid (FFA) leads to intramyocellular lipid accumulation in humans, and this has also been proposed to play a critical role in initiating and developing insulin resistance and also pancreatic β-cell death (McGarry, 2002; Azevedo-Martins et al. 2006). Fatty acids were first shown to be oxidized by isolated cardiac and skeletal muscles, so inhibiting glucose utilization, in 1963 (Randle et al. 1963). Increased oxidation of fatty acids resulted in an increase in the intramitochondrial NADH/NAD+ ratio, so reducing pruvate dehydrogenase activity and thus glucose oxidation. Thus increased FFA metabolism may also lead to increased ROS production. It has been reported that glucose or FFAs initiate the formation of ROS in muscle, adipocytes, pancreatic β-cells and other cells (Talior et al. 2003; Brownlee, 2005; Haber et al. 2006).
Interestingly, compared to many other cell types, the β-cell may be at high risk for oxidative damage with an increased sensitivity for apoptosis. This high risk may be due to (i) excessive levels of mitochondrial ROS generation, (ii) additional ROS generation through elevated β-cell NADPH oxidase activity (see below), and (iii) failure of antioxidant defence. With respect to T2DM, β-cell dysfunction and associated depressed insulin secretion must be evident before hyperglycaemia develops (Kahn, 2003).
It is important to emphasize at this point that damage induced by ROS and/or the failure of antioxidant defence, repair and biogenesis in insulin-secreting and insulin target cells can contribute to the onset of T2DM and its complications. However, in contrast to other scholarly reviews, which have emphasized the important role for mitochondrial derived ROS, we wish to present an additional explanation for ROS generation (NADPH oxidase dependent) and subsequent interference in insulin signalling and signal transduction.
Oxidative stress and β-cell dysfunction
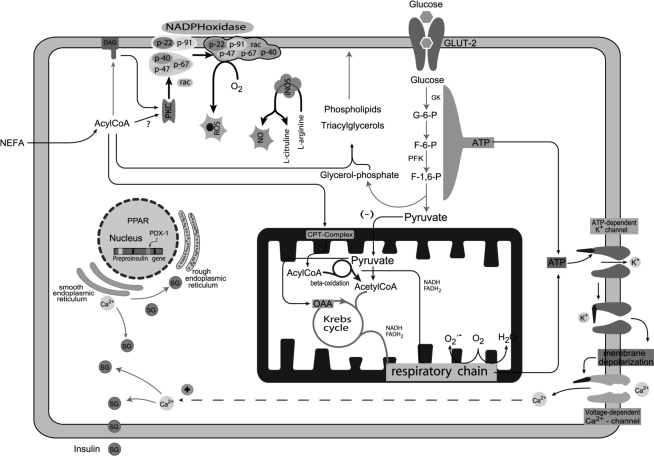
Glucose-stimulated insulin secretion (GSIS) as currently understood is summarized in Fig. 2. Glucose is transported across the plasma membrane (via specific transporters, GLUT 1 and GLUT2) and is rapidly phosphorylated by a specific glucokinase with high Km for glucose. The combination of transport and phosphorylation determines metabolic flux through glycolysis in the β-cell. Increased glycolytic flux in β-cells results in a rapid increase in the production of reducing equivalents, increased activity of shuttle mechanisms responsible for transferring electrons to the mitochondrial matrix, and TCA cycle activity leading to increased ATP production in mitochondria (Fig. 3) and in an enhanced ratio of ATP to ADP in the cytoplasm. This will result in closure of the ATP-sensitive K+ channels (KATP), decreasing the hyperpolarizing outward K+ flux. This results in depolarization of the plasma membrane, influx of extracellular Ca2+, a rapid increase in intracellular Ca2+, and activation of protein kinases, which then mediate exocytosis of insulin (Newsholme et al. 2006, 2007). In contrast to most other mammalian cell types, in β-cells increased glucose concentration stimulates a rapid and proportional increase in glycolytic flux followed by a robust stimulation in the production of reducing equivalents, due to channelling of glucose carbon into the TCA cycle, which can lead to an enhancement of ROS production. An elevation of intracellular Ca2+ induced by increased Ca2+ influx through voltage-gated Ca2+ channels is a primary driver of the GSIS mechanism. However, further increases in intracellular Ca2+ can stimulate mitochondrial generation of ROS while Ca2+, via protein kinase C (PKC) activation, may enhance NADPH oxidase-dependent generation of ROS (see below) and thus induce oxidative stress and/or apoptosis (Kruman et al. 1998; Yu et al. 2006; Morgan et al. 2007). It is also known that β-cells have relatively low levels of free radical detoxifying and redox-regulating enzymes, such as superoxide dismutase, glutathione peroxidase, catalase and thioredoxin. The consequence of limited scavenging systems is that that upon Ca2+ stimulation of mitochondrial and NADPH oxidase systems, ROS concentrations in β-cells may increase rapidly and so easily reach damaging levels.
Figure 2.
Mechanism of insulin secretion stimulated by glucose and fatty acids in pancreaticβ-cells Glucose and fatty acids generate ATP, which promotes closure of the ATP-dependent K+ channel leading to cell membrane depolarization. As a consequence, voltage-dependent Ca2+ channels are opened, increasing intracellular Ca2+ concentration leading to insulin secretion. The NADPH oxidase complex in the plasma membrane is activated through protein kinase C (PKC), which is activated by fatty acid derived signalling molecules. The production of nitric oxide (NO) by inducible nitric oxide synthase (iNOS) is up-regulated by cytokines and fatty acids subsequently impacting on pancreatic β-cell function. CPT-complex, carnitine palmitoyl transferase complex; F-1,6-P, fructose-1,6-diphosphate; F-6-P, fructose-6-phosphate; GLUT-2, glucose transporter-2; GK, glucokinase; G-6-P, glucose-6-phosphate; OAA, oxaloacetic acid; PDX-1, pancreatic duodenal homeobox gene-1; PFK, phosphofructokinase; PKC, protein kinase C; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; SG, secretory granule.
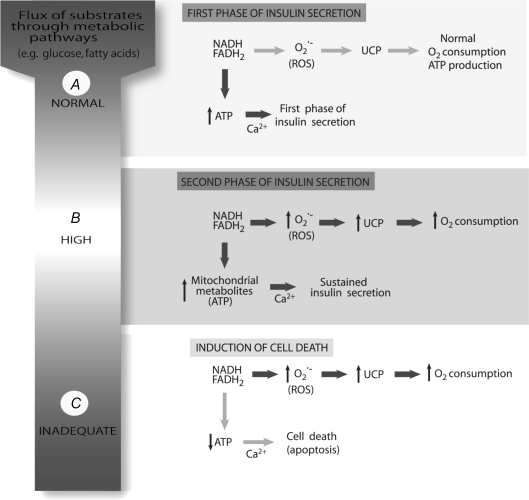
Figure 3.
The central role of reactive oxygen species (ROS) and uncoupling protein (UCP) for the first and second phases of insulin secretion or induction of cell death The normal flux of metabolites (e.g. glucose and fatty acids) through metabolic pathways (e.g. TCA cycle) generates NADH and FADH2 that are used by the electron transport chain for proton translocation and ATP synthesis. An increased ATP/ADP ratio leads to elevation of intracellular Ca2+ and the peak of insulin secretion in the first phase (A). The production of ROS and activation of UCP are associated with high metabolic flux required to maintain the ATP/ADP ratio and to sustain insulin secretion for a prolonged period (B). In this situation the O2 consumption is elevated. However, as a consequence of sustained ROS production and UCP activation causing excessive H+ leak, ATP levels will fall resulting in cell death by apoptosis (C).
Mechanisms of NADPH oxidase ROS production and antioxidant defences in β-cells
We have described the role of mitochondrial metabolism and Ca2+ in stimulation of ROS production in the β-cell mitochondria above. This area has additionally been expertly reviewed elsewhere (Green et al. 2004). However, there is an independent mechanism responsible for generation of ROS in β-cells, which involves activation of a membrane associated enzyme known as NADPH oxidase, which may contribute to oxidative stress under physiological conditions. The production of reactive oxygen species for antimicrobial action by professional phagocytic cells (e.g. neutrophils and macrophages) mainly occurs through NADPH oxidase activation. The ‘classical’ NADPH oxidase, which has been traditionally associated with cells of the immune system, may be an isoform from what is now recognized as a large family, e.g. NOX1, NOX2 or NOX3. These isoforms depend on the presence of various activator or organizer subunits for activity. The enzyme complex catalyses the one electron reduction of oxygen to generate superoxide using NADPH as the electron donor (Babior, 1999; Pithon-Curi et al. 2002; Babior, 2004). In the inactive state, the integral membrane proteins gp91phox and p22phox constitute the catalytic core of the ‘classical’ enzyme along with the heterodimeric flavocytochrome b558. The essential element gp91phox contains haeme and flavine adenine dinucleotide (FAD), which are involved in the electron transfer activity of the enzyme. The additional proteins, p67phox, p47phox and p40phox as well as the small GTPases (Rac 1 or Rac 2), are required for regulation of the NADPH oxidase activity and are located in the cytosol during the resting state (Babior, 1999, 2002, 2004). Enzyme activation is initiated by phosphorylation of several serine or threonine residues of the p47phox subunit, mainly by PKC, promoting the subsequent translocation of the cytosolic subunits to the membrane (Inoguchi et al. 2000; Babior, 2002; Fontayne et al. 2002; Bey et al. 2004). Upon activation, the six hetero-subunits of the ‘classical’ NADPH oxidase (or fewer subunits associated with alternative isoforms) form an active oxidase complex in a stimulus-dependent manner, which produces large amounts of superoxide using NADPH as the electron donor (Hashida et al. 2004). Tight regulation of the enzyme activity is achieved by two mechanisms: (i) separation of the oxidase subunits into different subcellular locations during the resting state (cytosolic and membrane-bound), and (ii) modulation of reversible protein–protein and protein–lipid interactions. These interactions can either promote the resting state or allow translocation of specific subunits to the membrane in response to appropriate stimuli. In contrast, NOX4 is an enigmatic member of the NOX family of ROS-generating NADPH oxidases. NOX4 has a wide tissue distribution (kidney, endothelial cells, osteoclasts, smooth muscle cells, fibroblasts, mesangial cells, adipocytes, pancreatic islets and embryonic stem cells), but the physiological function and activation mechanisms are largely unknown. It is thought that cytoplasmic acitivator subunits are not associated with this isoform and H2O2 is the major form of ROS generated by this NOX isoform (Serrander et al. 2007)
Thus specific isoforms of the O2− generating NADPH oxidase family (i.e. NOX1–3) are an important source of ROS in non-phagocytic cells including pancreatic islets. It has been reported that nutrients (such as high levels of glucose and palmitate) stimulated cultured aortic smooth and endothelial cell phagocyte-like NADPH oxidase via PKC-dependent activation (Inoguchi et al. 2000), while a more recent study demonstrated increased production of the NADPH oxidase components gp91phox and p22phox in β-cells obtained from animal models of type 2 diabetes (Nakayama et al. 2005). Of relevance to this review Oliveira et al. (2003) reported the expression of NADPH oxidase (NOX1, 2 or 3) components in rat islets. RT-PCR analysis revealed mRNA expression of gp91phox, p22phox and p47phox in β-cells of isolated rat islets. Immunohistochemistry of pancreatic sections showed positive staining for p47phox in β-cells from the islets. p47phox expression was also demonstrated in a clonal rat pancreatic β-cell line, BRIN BD11 (Morgan et al. 2007). Glucose-dependent ROS production was partially suppressed by GF109203X, a PKC-specific inhibitor (Morgan et al. 2007), suggesting that glucose stimulated ROS production mainly occurred through a PKC-dependent mechanism. Interestingly, glucose also stimulated ROS production in cultured vascular cells and ROS production occurred through PKC-dependent activation of NADPH oxidase (Inoguchi et al. 2000). The precise mechanisms for participation of PKC in the activation of NADPH oxidase and the physiological role of this enzyme in pancreatic β-cells still remain to be fully established. However, low levels of ROS have recently been shown to be essential for optimal GSIS (Pi et al. 2007).
Further evidence in support of the rat islet NOX studies published originally by Oliveira et al. (2003) and Morgan et al. (2007) was provided in a recent paper that described the detection of mRNA for the NADPH oxidase components from NOX1, NOX2, NOX4 including p22phox as a membrane associated components and p47phox, Noxo1 (homologue of p47phox), Noxa1 (homologue of p67phox) and p40phox as cytosolic components of rat islets and an insulinoma derived cell line, RINm5F (Uchizono et al. 2006). p47phox expression was confirmed by immunohistochemistry in rat islets.
Excessive levels of reactive oxygen species not only directly damage cells by oxidizing DNA, protein and lipids, but indirectly damage cells by activating a variety of stress-sensitive intracellular signalling pathways such as NF-kB, p38 MAPK, JNK/SAPK, hexosamine and others. Activation of these pathways results in the increased expression of numerous gene products that may cause cellular damage and play a major role in the aetiology of the late complications of diabetes. In addition, recent data in vitro and in vivo suggest that activation of the same or similar stress pathways results in insulin resistance and impaired insulin secretion. Accordingly, it has been proposed that there is a link among the hyperglycaemia- and FFA-induced increases in ROS and oxidative stress, activation of stress-sensitive pathways and the eventual development of not only the late complications of diabetes but also insulin resistance and β-cell dysfunction. Although our understanding of how hyperglycaemia-induced oxidative stress ultimately leads to tissue damage has advanced considerably in recent years (Vincent et al. 2005; Rolo & Palmeira, 2006), effective therapeutic strategies to prevent or delay the development of this damage remain limited.
The involvement of ‘non-damaging’ levels of ROS in signal transduction is now firmly accepted and examples include roles in cell growth or programmed cell death (apoptosis) (Burdon, 1995; Irani et al. 1997; Irani, 2000), kinase activation (Lu, 1993; Lo et al. 1996), immune responses (Valko et al. 2007), cell calcium signalling (Bedard & Krause, 2007) and gene expression (Schoonbroodt & Piette, 2000). For example, increased inducible nitric oxide synthase (iNOS) expression in response to redox-dependent transcription factor NFκB activation is a specific example of ROS regulated gene expression. Vascular tone and inhibition of platelet adhesion is regulated by a nitric oxide- and hydrogen peroxide-dependent activation of guanylate cyclase. Angiotensin II, Thrombin, platelet-derived growth factor (PDGF) and tumour necrosis factor-α (TNF-α) are known to increase ROS production in vascular smooth muscle cells through activation of an isoform of the NOX family NADPH oxidase.
However, since expression levels of antioxidant enzymes such as catalase, and glutathione peroxidase are very low in β-cells compared to other tissues (Lenzen et al. 1996; Tiedge et al. 1997), β-cells are thought of as targets for oxidative stress-mediated tissue damage (Maechler et al. 1999; Tanaka et al. 1999; Evans et al. 2002; Tanaka et al. 2002; Robertson et al. 2003). Thus it is likely that production of ROS and subsequent oxidative stress is involved in β-cell deterioration in type 2 diabetes.
There is strong evidence for oxidative stress-dependent changes in intracellular signalling, resulting in chronic inflammation and insulin resistance in vivo as reported by others (Brownlee, 2005; Fridlyand & Philipson, 2005; Katakam et al. 2005). While mitochondrial ROS generation may be important for regulation of mitochondrial UCP activity and thus cellular energy metabolism (see below), the NADPH oxidase associated ROS may specifically alter parameters of signal transduction, insulin secretion, insulin action and cell proliferation or cell death. From a mechanistic perspective, an increase in reactive molecules can trigger the activation of stress-sensitive serine/threonine kinase signalling pathways such as JNK, NF-kB, p38 MAPK (and others) that in turn phosphorylate multiple targets, including the insulin receptor and IRS proteins. Increased serine phosphorylation of IRS reduces its ability to undergo tyrosine phosphorylation and may accelerate the degradation of IRS-1, offering a plausible explanation for the molecular basis of oxidative stress-induced insulin resistance. There are convincing data to support an important role for the activation of JNK, IKK, PKC, and perhaps other stress- and inflammation-activated kinases in the pathogenesis of oxidative stress-induced insulin resistance, and suggest that they might be attractive pharmacological targets to increase insulin sensitivity. The use of antioxidants and pharmacological inhibitors in suppressing the chronic activation of these pathways is consistent with this idea. Moreover, identification of the molecular basis and sites of action for the protection afforded by a variety of antioxidants against oxidative stress-induced damage might lead to the discovery of additional pharmacological targets for novel therapies to prevent, reverse, or delay the onset of oxidative stress-induced insulin resistance.
β-Cell protection through suppression of NADPH oxidase
Culture of the clonal rat pancreatic β-cell line BRIN BD11 for 24 h with 10 mm alanine resulted in substantial changes in gene expression (Cunningham et al. 2005). Sixty-six genes were up-regulated > 1.8-fold, including many involved in cellular signalling, metabolism, gene regulation, protein synthesis, apoptosis and the cellular stress response. Subsequent functional experiments confirmed that alanine provided protection of BRIN BD11 cells from pro-inflammatory cytokine-induced apoptosis (Cunningham et al. 2005). Protection from apoptosis was mimicked by NMA or DPI suggesting alanine enhances intracellular antioxidant generation. These observations indicate important long-term effects of alanine in regulating gene expression, secretory function and the integrity of insulin-secreting cells. Indeed specific amino acids or their intracellular targets may play a key role in β-cell function in vivo and identification of their mechanism of action may lead to the development of new antidiabetic drugs.
Additional metabolic considerations in cellular oxidative damage
Four metabolic pathways activated in hyperglycaemic conditions have been reported to be involved in cell damage: (1) increased polyol pathway flux, (2) increased advanced glycation end product (AGE) formation, (3) activation of PKC isoforms, and (4) increased hexosamine pathway flux. Hyperglycaemia elevates the enzymatic convertion of glucose to the polyalcohol sorbitol, which is metabolized to fructose by sorbitol dehydrogenase increasing the NADH/NAD+ ratio. This metabolic pattern favours the triose phosphate oxidation and promotes de novo synthesis of diacylglycerol (DAG) (Brownlee, 2001), which is a potent PKC activator.
Glucose metabolism may give rise to fatty acid synthesis and generation of lipid derived signalling molecules such as LC-FA acyl CoA and DAG. The increase of O2− production in pancreatic β-cells in response to elevated glucose metabolism may result in activation of NADPH oxidase via increased lipid derived signalling molecules, such as DAG, and subsequent activation of PKC as described above (Morgan et al. 2007).
Reducing sugars (glucose, glucose-6-phosphate, and fructose) can react with a free amino group to generate a Schiff base. Formation of the Schiff base is relatively fast being highly reversible. However, the rearrangement of the Schiff base is much faster, originating an Amadori product (Ulrich & Cerami, 2001). The Amadori glycation product tends to be accumulated in proteins leading to advanced glycation. Formation of intracellular AGE precursors reduces target cell integrity by modifying protein function or by inducing receptor-mediated production of reactive oxygen species (Yan et al. 1994).
The hexosamine biosynthesis is an additional pathway of glucose metabolism that may mediate some of the toxic effects of this monosaccharide (Du et al. 2000). Under usual metabolic conditions, 2–5% of glucose entering the cells is directed to the hexosamine pathway, starting from the conversion of fructose 6-phosphate to glucosamine 6-phosphate by glutamine: fructose-6-phosphate amidotransferase (James et al. 2002). Hyperglycaemia leads to overproduction of superoxide that significantly inhibits glyceraldehyde-3-phosphate dehydrogenase activity (Du et al. 2000), and activates the pathways related to hyperglycaemia-induced damage by diverting glycolytic metabolites to hexosamine synthesis. The end product of this pathway, UDP-N-acetylglucosamine, is the substrate for glycosylation of intracellular proteins (McClain & Crook, 1996), including transcription factors and so affecting the expression of several genes (Gabriely et al. 2002; Goldberg et al. 2002). It is possible that glucose-induced overproduction of superoxide (Fig. 1) can regulate metabolic fluxes through these pathways.
Mitichondrial superoxide generation and the role of uncoupling proteins
The UCPs, which have an approximate mass of 32 kDa, are members of the mitochondrial anion transporter family including adenine nucleotide transporters. UCPs are located at the internal membrane of the mitochondria and link the intermembrane space with the matrix. The uncoupling protein family is characterized by five UCP homologues (UCP1–UCP5), but UCP2 and UCP3 have a high sequence identity with UCP1. It is generally accepted that UCP1 expression and activity is restricted to brown adipose tissue (BAT) and its physiological role in thermogenesis is well understood, whereas UCP2 and UCP3 are more widely distributed but their physiological function is yet to be fully explained (Hirabara et al. 2006; Nicholls, 2006).
In pancreatic β-cells UCP2 expression has been reported and its importance for metabolic regulation identified (Saleh et al. 2002). However, not all of the physiological functions of this uncoupling protein are known. It is possible that high flux through the respiratory chain results in high levels of O2− production. As O2− are activators of UCP2 activity, then H+ translocation across the mitochondrial inner membrane is stimulated, dissipating the H+ gradient, lowering O2− production and ATP synthesis. Initially insulin secretion is not impaired as only the first phase of insulin secretion is fully dependent on the ATP/ADP ratio. However, Ca2+ and mitochondrially derived coupling factors (such as glutamate, citrate, acyl-CoA and NADPH) are required for the sustained second phase of insulin secretion, which is much less responsive to ATP/ADP ratio change (Krausz et al. 1987). In this way UCP2 has a pivotal role in β-cell function controlling the ATP/ADP ratio, O2− production and, indirectly, TCA cycle flux (Fig. 3). TCA cycle activity will be high when UCP2 is active as reducing equivalents produced in the cycle are quickly re-oxidized by complexes I and II but the electron-dependent generation of a proton gradient is quickly dissipated by UCP2 activity. However, this mechanism may fail after prolonged exposure to high glucose levels and sustained O2− production due to a reduction in ATP generation (Fig. 3). Several studies have indicated that chronic hyperglycaemia impaired insulin secretion and was associated with excessive O2− production, UCP2 activation, decreased mitochondrial membrane potential and ultimately GSIS (Leahy et al. 1992; Hribal et al. 2003; McQuaid et al. 2006). Pancreatic islets from UCP2 knockout mice maintained GSIS following chronic hyperglycaemia, while chemical removal of endogenous O2− prevented the hyperglycaemia-induced loss of GSIS (Chan et al. 2004; Chan & Kashemsant, 2006; Saleh et al. 2006).
Effect of free fatty acids and glucose on β-cell apoptosis
Glucose and fatty acids have a synergistic effect on pancreatic β-cells. After acute exposure both stimulate insulin secretion. In contrast, after long-term exposure, both impair β-cell function and may also affect β-cell survival. Chronic exposure of normal rat pancreatic islets to high circulating levels of FFA or glucose, known to occur in type 2 diabetes, may increase β-cell apoptosis (Piro et al. 2002; Rachek et al. 2006; Newsholme et al. 2007). High fat deposition in β-cells that occurs in Zucker diabetic fatty (zdf) rats, an animal model of type 2 diabetes and obesity, is associated with apoptosis of these cells. The β-cell impairment in this type of diabetes may involve induction of ceramide synthesis, a key component of a signal-transduction pathway that leads to apoptosis. Ceramide induces DNA fragmentation, and suppression of its synthesis prevents FFA-induced DNA fragmentation. Maechler & Wollheim (2001) reported that in normal rats, an elevation of saturated but not monounsaturated fatty acids increased pancreatic β-cell apoptosis. Impairment of β-cell function may involve excessive generation of ROS through increased NADPH oxidase activity (Morgan et al. 2007). This would subsequently affect mitochondrial function, reducing ATP production and so insulin secretion (Brownlee, 2003; Morgan et al. 2007). Additionally palmitate metabolism may give rise to ceramide synthesis and ceramide is a key component of the signal transduction pathway for ROS-induced apoptosis (Cacicedo et al. 2005). Ceramide may also induce apoptosis by inactivation of pro-survival pathways. This lipid can inhibits phosphatidyl inositol 3-kinase (PI3K), which, in turn, results in a block in protein kinase B (PKB also known as Akt/PKB) activation (Beeharry et al. 2004). Downstream targets of the PI3K/PKB pathway involved in survival include GSK-3 (Pap & Cooper, 1998), caspase-9 (Cardone et al. 1998), and the Bcl-2 family member Bad (Datta et al. 1997). Ceramide generation is further discussed in the context of insulin resistance as described below.
The mechanism for the effect of high glucose on pancreatic β-cell apoptosis remains to be elucidated but several studies have been carried out to obtain further information on this subject. Exposure of islets from diabetes-prone Psammomys obesus (the fat sand rat, an animal model of type 2 diabetes) to high glucose levels resulted in a dose-dependent increase in β-cell DNA fragmentation (Donath et al. 1999). Addition of high glucose resulted in apoptosis of pancreatic β-cells from ob/ob mice and normal wistar rats in vitro (Efanova et al. 1998). Oxidative stress due to elevated ROS and peroxinitrite generation may damage DNA by promoting single-strand DNA break formation leading to initiation of the apoptotic cascade.
Insulin resistance and ROS – physiological adaptation to protection from oxidative stress
Insulin resistance plays a central role in the development of several metabolic abnormalities and diseases such as obesity, type 2 diabetes mellitus and the metabolic syndrome (Petersen & Shulman, 2006; Hirabara et al. 2007). In these conditions there is an elevation of both glucose and FFA levels in the blood and an increase in oxidative stress in exposed cells and tissues (Boden, 1997; Evans et al. 2003; Le Marchand-Brustel et al. 2003, Kaneto et al. 2006; Taylor & Schmitz-Peiffer, 2006). The high degree of oxidative stress has been postulated to play an important role in decreasing insulin responsiveness (Evans et al. 2003; Urakawa et al. 2003). Normally glucose flux through glycolysis is stimulated following insulin receptor activation in insulin sensitive tissues such as muscle and adipose tissue; this leads to increased ROS production when the reducing equivalent supply to the respiratory chain is increased by glucose metabolism. However, insulin resistance should lead to an inhibition of insulin action and to decreased insulin-dependent glucose uptake. Insulin resistance at the molecular level may be mediated by inhibition of signal transduction at the apex of the signalling pathway, which involves the insulin receptor (IR) β-subunit, which contains an intrinsic tyrosine kinase activity, undergoes tyrosyl autophosphorylation and is activated after insulin binding. The major early steps of the insulin signalling pathway involve tyrosine phosphorylation of IR substrates 1 and 2 (IRS-1 and IRS-2) (Sun et al. 1991; Burks & White, 2001). Tyrosine phosphorylation of IRS proteins activates signalling pathways that regulate essential cellular processes including intermediary metabolism, gene expression, growth and differentiation of pancreatic islets (Hirayama et al. 1999; Paris et al. 2004). The main docking proteins to bind to the IR is the IRS family, of which IRS1 and IRS2 are the main isoforms in insulin-sensitive tissue. The binding of IRSs to the IR triggers phosphorylation on multiple tyrosine residues within the C-terminal region of IRS, leading to the generation of highly specific binding sites of a number of SH2 domain-containing signalling molecules. Those molecules include phosphatidylinositol 3-kinase (PI3K), Nck, and Grb-2, of which PI3K seems to be a central insulin signalling molecule in mediating the metabolic effect of insulin. PI3K is composed of a catalytic and a regulatory subunit (p110 and p85, respectively). As a result of IRS tyrosine phosphorylation, the p85 subunit of PI3K binds to the PH domain of IRS1/2, leading to an increase in the catalytic activity of p110. This activation results in a subsequent rise in intracellular phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate content (PIP3). A wide range of downstream targets of PI3K have been identified. Among them are serine/threonine kinases such as phosphoinositide-dependent protein kinase (PDK1), PKB, PKC, p70 S6 kinase, and glycogen synthase kinase 3 (GSK3).
Exposure of different cell lines to micromolar concentrations of hydrogen peroxide leads to the activation of stress kinases such as c-Jun N-terminal kinase, p38, IκB kinase, and extracellular receptor kinase 1/2. This activation is accompanied by a down-regulation of the cellular response to insulin (insulin resistance), leading to a reduced ability of insulin to promote glucose uptake, and glycogen, lipid and protein synthesis (Tanti et al. 1994; Kanety et al. 1995; Hotamisligil et al. 1996; Tirosh et al. 1999; Maddux et al. 2001; Evans et al. 2003; Ogihara et al. 2004; Bloch-Damti et al. 2006). There are several major stress-sensitive kinases that, when activated, are likely to be involved in attenuating insulin signalling via effects on IRS proteins or downstream kinase pathways such as NF-κB-activating kinases, p38 MAPK, JNK/SAPK, PKC (Fig. 4).
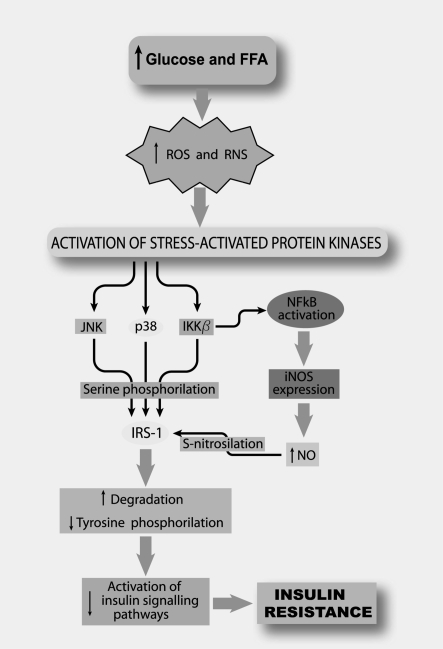
Figure 4.
Induction of insulin resistance by oxidative stress Prolonged high plasma levels of glucose and free fatty acids (FFA) lead to an increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which induce activation of various stress-activated protein kinases such as JNK, p38, and IKKβ. These kinases have been suggested to phosphorylate serine insulin receptor substrate-1 (IRS-1). In addition, IKKβ leads to activation of NFκB, a transcriptional factor that increases iNOS expression and nitric oxide (NO) production. NO can induce IRS-1 S-nitrosylation. Both serine phosphorylation and S-nitrosylation of IRS-1 have been associated with increased proteosome-dependent degradation of signal transduction associated proteins and suppressed insulin signalling. These effects result in insulin resistance in the liver, skeletal muscle and adipose tissue.
The oxidative stress associated mechanisms leading to impaired insulin signalling in cells are complicated, involving increased serine/threonine phosphorylation of IRS1, impaired insulin-stimulated redistribution of IRS1 and PI3K between cytosol and low-density microsomal fraction, followed by a reduced Akt/PKB phosphorylation. The serine/threonine phosphorylated forms of IRS molecules are less able to associate with the insulin receptor and downstream target molecules, especially PI3K (Gual et al. 2005; Evans et al. 2005), resulting in impaired insulin action, including Akt/PKB activation. In addition, the serine/threonine phosphorylated forms of IRS molecules are more susceptible to proteasome mediated degradation (Haruta et al. 2000; Gual et al. 2005; Hiratani et al. 2005; Greene et al. 2003).
The activation of the JNK pathway reduces insulin gene expression and interferes with insulin action. Suppression of this pathway in obese diabetic mice can protect β-cells from oxidative stress, and thus could be a potential therapeutic target for diabetes. (Kaneto et al. 2002). There are three isozymes of c-Jun N-terminal kinase, JNK1, JNK2 and JNK3, and only JNK1 has been shown to be implicated in type 2 diabetes (Hirosumi et al. 2002). Thus, it is likely that JNK1 is a crucial mediator of the progression of both insulin resistance and β-cell dysfunction found in type 2 diabetes. It has been reported that serine phosphorylation of IRS-1 inhibits insulin-stimulated tyrosine phosphorylation of IRS-1, leading to an increase in insulin resistance (Aguirre et al. 2000, 2002). IRS-1 serine 307 phosphorylation was markedly decreased in Ad-DN-JNK-treated mice. An increase in IRS-1 tyrosine and Akt/PKB serine 473 phosphorylation was also observed in Ad-DN-JNK-treated mice (Nakatani et al. 2004). Therefore, an increase in IRS-1 serine phosphorylation may be closely associated with the development of insulin resistance induced by JNK overexpression. These results indicate that suppression of the JNK pathway enhances insulin signalling, which leads to amelioration of glucose tolerance. Similar effects were observed in high-fat/high-sucrose diet-induced diabetic mice.
Additional stress-sensitive kinases that are reported to be involved in IRS-mediated insulin resistance include the mammalian target of rapamycin (mTOR) (Mussig et al. 2005), several isozymes of PKC, including PKCβ and PKCε (Ishizuka et al. 2004; Dey et al. 2005; Greene et al. 2006), and the IKKβ–NFκB signalling cascades (Gao et al. 2002). Once activated, these kinases are able to phosphorylate multiple targets, including the insulin receptor and IRS proteins such as IRS-1 and IRS-2. To date, only one published study has directly evaluated the effects of oxidative stress on IRS serine phosphorylation and IRS protein content, in the context of cellular insulin resistance (Bloch-Damti et al. 2006). Consistent with the molecular basis of oxidative stress-induced insulin resistance proposed here, these investigators found that oxidative stress (H2O2) caused an increase in serine phosphorylation of IRS-1 and IRS-2, decreased content of IRS-1, and insulin resistance in 3T3-L1 adipocytes.
The reduction of insulin gene expression and secretion by oxidative stress has been correlated with changes in the DNA-binding activity of pancreatic and duodenal homeobox factor-1 (PDX-1). PDX-1 is a member of the homeodomain containing transcription factor family (Miller et al. 1994; Ohlsson et al. 1993). It is expressed in the pancreas and the duodenum and plays a crucial role in pancreatic development and differentiation (Jonsson et al. 1994; Stoffers et al. 1997; Dutta et al. 1998; Ferber et al. 2000; Horb et al. 2003; Miyatsuka et al. 2003; Taniguchi et al. 2003; Cao et al. 2004; Kaneto et al. 2005), and in maintaining normal β-cell function by regulating multiple important β-cell genes, including insulin, GLUT2 and glucokinase (Peers et al. 1994; Petersen et al. 1994; Waeber et al. 1996; Watada et al. 1996; Ahlgren et al. 1998; Brissova et al. 2002; Chakrabarti et al. 2002; Kulkarni et al. 2004). When HIT cells or isolated rat islets were exposed to oxidative stress, PDX-1 binding to the insulin gene was markedly reduced (Matsuoka et al. 1997; Kaneto et al. 2001). In addition, as a potential mechanism for JNK-mediated PDX-1 inactivation, it has been demonstrated that PDX-1 is translocated from the nucleus to the cytoplasm of β-cell-derived HIT cells in response to oxidative stress (Kawamori et al. 2003).
Increased plasma concentration of FFA and increased intramyocellular lipid content are typically associated with insulin resistant states, including T2DM. Uptake of long chain fatty acids can lead to stimulation of the synthesis of a variety of lipid derived metabolites including triacylglycerol, cholesterol esters and ceramide. Palmitoyl-CoA and serine are required for the initial rate determining step for ceramide synthesis catalysed by serine palmitoyltransferase (SPT). Ceramide can be utlised for sphingomyelin synthesis but is an important signalling molecule in its own right. It may inhibit insulin signal transduction by inhibiting Akt/PKB translocation to the plasma membrane from the cytosol, phosporylation and activation of Akt/PKB and promoting dephosphorylation (see Zierath, 2007 for comment) Indeed evidence for ceramide as a common intermediate linking nutrient excess to the induction of insulin resistance in rodents has recently been published (Holland et al. 2007). Alternatively lipid-induced insulin resistance could be due to FFA providing reducing equivalents for the electron transport chain, leading to increased ROS production. However, it is not clear how mitochondrially derived ROS could influence signal transduction at the plasma membrane. We suggest that saturated fatty acids can stimulate both the expression and the activity of NADPH oxidase (Newsholme et al. 2007; Morgan et al. 2007). Subsequently elevated ROS production at the plasma membrane may lead to inhibition of signalling at the level of IRS protein phosphoylation and thus result in a ROS-dependent protection mechanism. An elevation in FFA leads to an increase in intracellular fatty acid metabolites, which may also activate a serine/threonine kinase cascade leading to phosphorylation of serine/threonine sites on IRS-1 and IRS-2 (Le Marchand-Brustel et al. 2003; Powell et al. 2004). In addition, diet-induced obesity in mice increases the expression of inducible nitric oxide synthase (iNOS) in skeletal muscle which may provoke S-nitrosylation of the insulin receptor, IRS-1 and Akt/PKB in rat soleus muscle. S-Nitrosylation was associated increased degradation of IRS-1, impaired insulin signalling and subsequent responses (Carvalho-Filho et al. 2005) (Fig. 4). Indeed elevated levels of S-nitrosylation associated with increased expression and activity of iNOS has emerged as an important player in the development of insulin resistance in muscle. This interesting development is beyond the scope of this review and has been recently reviewed elsewhere (Kaneki et al. 2007)
Concluding remarks
ROS are now accepted a major factor in the onset and development of T2DM and other diseases. ROS can induce inactivation of the signalling pathway between the insulin receptor and the glucose transporter system leading to the onset of insulin resistance in T2DM. Comparing metabolic pathways of GSIS and ROS production in the β-cell suggests that secretagogues causing increased insulin secretion by the GSIS mechanism can also lead to increased ROS production, via mitochondrial and NADPH oxidase mechanisms. This should lead to activation of oxidative stress concomitantly with stimulation of insulin secretion. As NADPH oxidase is expressed at relatively high levels in the islet β-cell, relative to other islet cells, any future therapies based on targeting NADPH oxidase in the islet and ROS production may be beneficial for maintaining β-cell integrity in the difficult environment of nutrient oversupply and immune challenge.
Acknowledgments
We thank The Health Research Board of Ireland, Enterprise Ireland, Wellcome Trust, FAPESP, CNPq and CAPES for providing research funding to our respective laboratories.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. β-Cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo-Martins AK, Monteiro AP, Lima CL, Lenzen S, Curi R. Fatty acid-induced toxicity and neutral lipid accumulation in insulin-producing RINm5F cells. Toxicol Vitro. 2006;20:1106–1113. doi: 10.1016/j.tiv.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Babior BM. The activity of leukocyte NADPH oxidase: regulation by p47PHOX cysteine and serine residues. Antioxid Redox Signal. 2002;4:35–38. doi: 10.1089/152308602753625834. [DOI] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Beeharry N, Chambers JA, Green IC. Fatty acid protection from palmitic acid-induced apoptosis is lost following PI3-kinase inhibition. Apoptosis. 2004;9:599–607. doi: 10.1023/B:APPT.0000038039.82506.0c. [DOI] [PubMed] [Google Scholar]
- Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in β-cell function. Nature. 2001;414:788–791. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C δ is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- Bloch-Damti A, Potashnik R, Gual P, Le Marchand-Brustel Y, Tanti JF, Rudich A, Bashan N. Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia. 2006;49:2463–2473. doi: 10.1007/s00125-006-0349-6. [DOI] [PubMed] [Google Scholar]
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Brownlee M. A radical explanation for glucose-induced β cell dysfunction. J Clin Invest. 2003;112:1788–1790. doi: 10.1172/JCI20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Burks DJ, White MF. IRS proteins and β-cell function. Diabetes. 2001;50(Suppl. 1):S140–S145. doi: 10.2337/diabetes.50.2007.s140. [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ. High glucose is necessary for complete maturation of Pdx1-VP16-expressing hepatic cells into functional insulin-producing cells. Diabetes. 2004;53:3168–3178. doi: 10.2337/diabetes.53.12.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ. S-Nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem. 2002;277:13286–13293. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006;34:802–805. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- Chan CB, Saleh MC, Koshkin V, Wheeler MB. Uncoupling protein 2 and islet function. Diabetes. 2004;53(Suppl. 1):S136–S142. doi: 10.2337/diabetes.53.2007.s136. [DOI] [PubMed] [Google Scholar]
- Cunningham GA, McClenaghan NH, Flatt PR, Newsholme P. L-Alanine induces changes in metabolic and signal transduction gene expression in a clonal rat pancreatic β-cell line and protects from pro-inflammatory cytokine-induced apoptosis. Clin Sci (Lond) 2005;109:447–455. doi: 10.1042/CS20050149. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Dey D, Mukherjee M, Basu D, Datta M, Roy SS, Bandyopadhyay A, Bhattacharya S. Inhibition of insulin receptor gene expression and insulin signaling by fatty acid: interplay of PKC isoforms therein. Cell Physiol Biochem. 2005;16:217–228. doi: 10.1159/000089847. [DOI] [PubMed] [Google Scholar]
- Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia-induced β-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Bonner-Weir S, Montminy M, Wright C. Regulatory factor linked to late-onset diabetes? Nature. 1998;392:560. doi: 10.1038/33311. [DOI] [PubMed] [Google Scholar]
- Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic β-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC α, β II, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- Fridlyand LE, Philipson LH. Oxidative reactive species in cell injury: Mechanisms in diabetes mellitus and therapeutic approaches. Ann N Y Acad Sci. 2005;1066:136–151. doi: 10.1196/annals.1363.019. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Yang XM, Cases JA, Ma XH, Rossetti L, Barzilai N. Hyperglycemia induces PAI-1 gene expression in adipose tissue by activation of the hexosamine biosynthetic pathway. Atherosclerosis. 2002;160:115–122. doi: 10.1016/s0021-9150(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κ B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Goffart S, Wiesner RJ. Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp Physiol. 2003;88:33–40. doi: 10.1113/eph8802500. [DOI] [PubMed] [Google Scholar]
- Goldberg HJ, Whiteside CI, Fantus IG. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-β I and -δ. J Biol Chem. 2002;277:33833–33841. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
- Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53(Suppl. 1):S110–S118. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- Greene MW, Ruhoff MS, Roth RA, Kim JA, Quon MJ, Krause JA. PKCδ-mediated IRS-1 Ser24 phosphorylation negatively regulates IRS-1 function. Biochem Biophys Res Commun. 2006;349:976–986. doi: 10.1016/j.bbrc.2006.08.158. [DOI] [PubMed] [Google Scholar]
- Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003;278:8199–8211. doi: 10.1074/jbc.M209153200. [DOI] [PubMed] [Google Scholar]
- Greenman IC, Gomez E, Moore CE, Herbert TP. Distinct glucose-dependent stress responses revealed by translational profiling in pancreatic β-cells. J Endocrinol. 2007;192:179–187. doi: 10.1677/joe.1.06898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Haber EP, Procopio J, Carvalho CR, Carpinelli AR, Newsholme P, Curi R. New insights into fatty acid modulation of pancreatic β-cell function. Int Rev Cytol. 2006;248:1–41. doi: 10.1016/S0074-7696(06)48001-3. [DOI] [PubMed] [Google Scholar]
- Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- Hashida S, Yuzawa S, Suzuki NN, Fujioka Y, Takikawa T, Sumimoto H, Inagaki F, Fujii H. Binding of FAD to cytochrome b558 is facilitated during activation of the phagocyte NADPH oxidase, leading to superoxide production. J Biol Chem. 2004;279:26378–26386. doi: 10.1074/jbc.M309724200. [DOI] [PubMed] [Google Scholar]
- Hirabara SM, Silveira LR, Abdulkader FR, Alberici LC, Procopio J, Carvalho CR, Pithon-Curi TC, Curi R. Role of fatty acids in the transition from anaerobic to aerobic metabolism in skeletal muscle during exercise. Cell Biochem Funct. 2006;24:475–481. doi: 10.1002/cbf.1327. [DOI] [PubMed] [Google Scholar]
- Hirabara SM, Silveira LR, Abdulkader F, Carvalho CR, Procopio J, Curi R. Time-dependent effects of fatty acids on skeletal muscle metabolism. J Cell Physiol. 2007;210:7–15. doi: 10.1002/jcp.20811. [DOI] [PubMed] [Google Scholar]
- Hiratani K, Haruta T, Tani A, Kawahara J, Usui I, Kobayashi M. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun. 2005;335:836–842. doi: 10.1016/j.bbrc.2005.07.152. [DOI] [PubMed] [Google Scholar]
- Hirayama I, Tamemoto H, Yokota H, Kubo SK, Wang J, Kuwano H, Nagamachi Y, Takeuchi T, Izumi T. Insulin receptor-related receptor is expressed in pancreatic β-cells and stimulates tyrosine phosphorylation of insulin receptor substrate-1 and -2. Diabetes. 1999;48:1237–1244. doi: 10.2337/diabetes.48.6.1237. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang L-P, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated fat- and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Hribal ML, Perego L, Lovari S, Andreozzi F, Menghini R, Perego C, Finzi G, Usellini L, Placidi C, Capella C, et al. Chronic hyperglycemia impairs insulin secretion by affecting insulin receptor expression, splicing, and signaling in RIN β cell line and human islets of Langerhans. FASEB J. 2003;17:1340–1342. doi: 10.1096/fj.02-0685fje. [DOI] [PubMed] [Google Scholar]
- Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F, Jansen-Durr P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6:245–256. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Kajita K, Natsume Y, Kawai Y, Kanoh Y, Miura A, Ishizawa M, Uno Y, Morita H, Yasuda K. Protein kinase C (PKC) β modulates serine phosphorylation of insulin receptor substrate-1 (IRS-1) – effect of overexpression of PKCβ on insulin signal transduction. Endocr Res. 2004;30:287–299. doi: 10.1081/erc-120039580. [DOI] [PubMed] [Google Scholar]
- James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor κB-dependent promoter activation. Diabetes. 2002;51:1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- Kajimoto Y, Kaneto H. Role of oxidative stress in pancreatic β-cell dysfunction. Ann N Y Acad Sci. 2004;1011:168–176. doi: 10.1007/978-3-662-41088-2_17. [DOI] [PubMed] [Google Scholar]
- Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, Yamasaki Y. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic β-cell dysfunction and insulin resistance. Int J Biochem Cell Biol. 2006;38:782–793. doi: 10.1016/j.biocel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Xu G, Song KH, Suzuma K, Bonner-Weir S, Sharma A, Weir GC. Activation of the hexosamine pathway leads to deterioration of pancreatic β-cell function through the induction of oxidative stress. J Biol Chem. 2001;276:31099–31104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor α-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- Katakam AK, Chipitsyna G, Gong Q, Vancha AR, Gabβ J, Arafat HA. Streptozotocin (STZ) mediates acute upregulation of serum and pancreatic osteopontin (OPN): a novel islet-protective effect of OPN through inhibition of STZ-induced nitric oxide production. J Endocrinol. 2005;187:237–247. doi: 10.1677/joe.1.06411. [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH2-terminal kinase. Diabetes. 2003;52:2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Krausz Y, Eylon L, Cerasi E. Calcium-binding proteins and insulin release. Differential effects of phenothiazines on first- and second-phase secretion and on islet cAMP response to glucose. Acta Endocrinol (Copenh) 1987;116:241–246. [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y, Gual P, Gremeaux T, Gonzalez T, Barres R, Tanti JF. Fatty acid-induced insulin resistance: role of insulin receptor substrate 1 serine phosphorylation in the retroregulation of insulin signalling. Biochem Soc Trans. 2003;31:1152–1156. doi: 10.1042/bst0311152. [DOI] [PubMed] [Google Scholar]
- Leahy JL, Bonner-Weir S, Weir GC. β-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992;15:442–455. doi: 10.2337/diacare.15.3.442. [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- Lu D, Maulik N, Moraru II, Kreutzer DL, Das DK. Molecular adaptation of vascular endothelial cells to oxidative stress. Am J Physiol Cell Physiol. 1993;264:C715–C722. doi: 10.1152/ajpcell.1993.264.3.C715. [DOI] [PubMed] [Google Scholar]
- Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of α-lipoic acid. Diabetes. 2001;50:404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic β cells. J Biol Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic β-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest. 1997;99:144–150. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes. 1996;45:1003–1009. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- McQuaid TS, Saleh MC, Joseph JW, Gyulkhandanyan A, Manning-Fox JE, MacLellan JD, Wheeler MB, Chan CB. cAMP-mediated signaling normalizes glucose-stimulated insulin secretion in uncoupling protein-2 overexpressing β-cells. J Endocrinol. 2006;190:669–680. doi: 10.1677/joe.1.06723. [DOI] [PubMed] [Google Scholar]
- Miller CP, McGehee RE, Jr, Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994;13:1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310:1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal β cell line. Diabetologia. 2007;50:359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- Mussig K, Fiedler H, Staiger H, Weigert C, Lehmann R, Schleicher ED, Haring HU. Insulin-induced stimulation of JNK and the PI 3-kinase/mTOR pathway leads to phosphorylation of serine 318 of IRS-1 in C2C12 myotubes. Biochem Biophys Res Commun. 2005;335:819–825. doi: 10.1016/j.bbrc.2005.07.154. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Inoguchi T, Sonta T, et al. Increased expression of NAD(P)H oxidase in islets of animal models of Type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun. 2005;332:927–933. doi: 10.1016/j.bbrc.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Brennan L, Bender K. Amino acid metabolism, β-cell function, and diabetes. Diabetes. 2006;55(Suppl. 2):S39–S47. [Google Scholar]
- Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions of the pancreatic β-cell: the role of fatty acids. Clin Sci (Lond) 2007;112:27–42. doi: 10.1042/CS20060115. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. The physiological regulation of uncoupling proteins. Biochim Biophys Acta. 2006;1757:459–466. doi: 10.1016/j.bbabio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Asano T, Katagiri H, Sakoda H, Anai M, Shojima N, Ono H, Fujishiro M, Kushiyama A, Fukushima Y, et al. Oxidative stress induces insulin resistance by activating the nuclear factor-κ B pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia. 2004;47:794–805. doi: 10.1007/s00125-004-1391-x. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic β-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52:1457–1463. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Paris M, Bernard-Kargar C, Vilar J, Kassis N, Ktorza A. Role of glucose in IRS signaling in rat pancreatic islets: specific effects and interplay with insulin. Exp Diabesity Res. 2004;5:257–263. doi: 10.1080/15438600490905169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers B, Leonard J, Sharma S, Teitelman G, Montminy MR. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- Petersen HV, Serup P, Leonard J, Michelsen BK, Madsen OD. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci U S A. 1994;91:10465–10469. doi: 10.1073/pnas.91.22.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14(Suppl. 1):34S–40S. doi: 10.1038/oby.2006.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007 doi: 10.2337/db06-1601. in press. [DOI] [PubMed] [Google Scholar]
- Piro S, Anello M, Di Pietro C, Lizzio MN, Patane G, Rabuazzo AM, Vigneri R, Purrello M, Purrello F. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism. 2002;51:1340–1347. doi: 10.1053/meta.2002.35200. [DOI] [PubMed] [Google Scholar]
- Pithon-Curi TC, Levada AC, Lopes LR, Doi SQ, Curi R. Glutamine plays a role in superoxide production and the expression of p47phox, p22phox and gp91phox in rat neutrophils. Clin Sci (Lond) 2002;103:403–408. doi: 10.1042/cs1030403. [DOI] [PubMed] [Google Scholar]
- Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachek LI, Musiyenko SI, LeDoux SP, Wilson GL. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology. 2007;148:293–299. doi: 10.1210/en.2006-0998. [DOI] [PubMed] [Google Scholar]
- Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006;55:1022–1028. doi: 10.2337/diabetes.55.04.06.db05-0865. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Saleh MC, Wheeler MB, Chan CB. Uncoupling protein-2: evidence for its function as a metabolic regulator. Diabetologia. 2002;45:174–187. doi: 10.1007/s00125-001-0737-x. [DOI] [PubMed] [Google Scholar]
- Saleh MC, Wheeler MB, Chan CB. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J Endocrinol. 2006;190:659–667. doi: 10.1677/joe.1.06715. [DOI] [PubMed] [Google Scholar]
- Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-κ B activation pathways. Biochem Pharmacol. 2000;60:1075–1083. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause K-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007 doi: 10.1042/BJ20061903. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-δ activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab. 2003;285:E295–E302. doi: 10.1152/ajpendo.00044.2003. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic β cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Yamato E, Tashiro F, Ikegami H, Ogihara T, Miyazaki J. β-Cell neogenesis induced by adenovirus-mediated gene delivery of transcription factor pdx-1 into mouse pancreas. Gene Ther. 2003;10:15–23. doi: 10.1038/sj.gt.3301846. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Gremeaux T, van Obberghen E, Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994;269:6051–6057. [PubMed] [Google Scholar]
- Taylor AJ, Ye JM, Schmitz-Peiffer C. Inhibition of glycogen synthesis by increased lipid availability is associated with subcellular redistribution of glycogen synthase. J Endocrinol. 2006;188:11–23. doi: 10.1677/joe.1.06381. [DOI] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Tirosh A, Potashnik R, Bashan N, Rudich A. Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. A putative cellular mechanism for impaired protein kinase B activation and GLUT4 translocation. J Biol Chem. 1999;274:10595–10602. doi: 10.1074/jbc.274.15.10595. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H, Iida M, Sumimoto H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006;80:133–139. doi: 10.1016/j.lfs.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Stevens MJ, Backus C, McLean LL, Feldman EL. Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antioxid Redox Signal. 2005;7:1494–1506. doi: 10.1089/ars.2005.7.1494. [DOI] [PubMed] [Google Scholar]
- Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327–1334. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- Watada H, Kajimoto Y, Miyagawa J, Hanafusa T, Hamaguchi K, Matsuoka T, Yamamoto K, Matsuzawa Y, Kawamori R, Yamasaki Y. PDX-1 induces insulin and glucokinase gene expressions in αTC1 clone 6 cells in the presence of betacellulin. Diabetes. 1996;45:1826–1831. doi: 10.2337/diab.45.12.1826. [DOI] [PubMed] [Google Scholar]
- Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- Yu JH, Kim KH, Kim H. Role of NADPH oxidase and calcium in cerulein-induced apoptosis: involvement of apoptosis-inducing factor. Ann N Y Acad Sci. 2006;1090:292–297. doi: 10.1196/annals.1378.031. [DOI] [PubMed] [Google Scholar]
- Zierath JR. The path to insulin resistance: paved with ceramides? Cell Metab. 2007;5:161–163. doi: 10.1016/j.cmet.2007.02.005. [DOI] [PubMed] [Google Scholar]