Abstract
Neonatal maternal separation (NMS) affects respiratory control development as adult male (but not female) rats previously subjected to NMS show a hypoxic ventilatory response 25% greater than controls. The paraventricular nucleus of the hypothalamus (PVN) is an important modulator of respiratory activity. In the present study, we hypothesized that in awake rats, altered GABAergic inhibition within the PVN contributes to the enhancement of hypoxic ventilatory response observed in rats previously subjected to NMS. During normoxia, the increase in minute ventilation following microinjection of bicuculline (1 mm) within the PVN is greater in NMS versus control rats. These data show that regulation of ventilatory activity related to tonic inhibition of the PVN is more important in NMS than control rats. Microinjection of GABA or muscimol (1 mm) attenuated the ventilatory response to hypoxia (12% O2) in NMS rats only. The higher efficiency of microinjections in NMS rats is supported by results from GABAA receptor autoradiography which revealed a 22% increase in GABAA receptor binding sites within the PVN of NMS rats versus controls. Despite this increase, however, NMS rats still show a larger hypoxic ventilatory response than controls, suggesting that within the PVN the larger number of GABAA receptors either compensate for (1) a deficient GABAergic modulation, (2) an increase in the efficacy of excitatory inputs converging onto this structure, or (3) both. Together, these results show that the life-long consequences of NMS are far reaching as they can compromise the development of vital homeostatic function in a way that may predispose to respiratory disorders.
During the postnatal period, central nervous system (CNS) development is highly sensitive to environmental perturbations. In rats, experimental conditions that interfere with normal mother–infant interactions are stressful and the long-term consequences of exposure in early life to such adverse conditions are usually associated with abnormal neuroendocrine and behavioural stress responses in adult animals (Plotsky & Meaney, 1993; Rots et al. 1996; Anisman et al. 1998; van Oers et al. 1998a, b; Liu et al. 2000; Mirescu et al. 2004). However, results from our laboratory indicate that experimental interference with mother–pup interaction by neonatal maternal separation (NMS) has consequences that extend beyond anxiety-like behaviour and poor spatial learning because NMS also disrupts basic homeostatic functions such as cardiorespiratory regulation (Genest et al. 2004). This study showed that adult male (but not female) rats previously subjected to NMS (NMS rats) are hypertensive and generate a ventilatory response to hypoxia that is 25% larger than in control animals. As sex-specific (male) hypertension and enhanced hypoxic chemoreflex are important clinical features of respiratory disorders such as sleep disordered breathing (Kara et al. 2003; Smith & Pacchia, 2007), these results raise important questions concerning the impact of stress exposure in early life in the aetiology of respiratory diseases related to neural control dysfunction (McCrimmon & Alheid, 2004; Kinkead et al. 2005a). While our data suggest that both the central and peripheral components of the neural network regulating breathing are affected by NMS (Kinkead et al. 2005a, b, c; Genest et al. 2007), the mechanisms responsible for this respiratory phenotype are not well understood. However, recent results from our laboratory suggest that chronic corticosterone elevation may be involved (Fournier et al. 2006).
Structures from the pons and medulla are key components of the hypoxic chemoreflex (Erickson & Millhorn, 1994; Larnicol et al. 1994; Hirooka et al. 1997; Teppema et al. 1997; Berquin et al. 2000); however, hypoxia and hypercapnia activate groups of neurons in the hypothalamus too, including the paraventricular nucleus of the hypothalamus (PVN) (Yeh et al. 1997; Berquin et al. 2000; Horn et al. 2000; Kc et al. 2002; Mack et al. 2002). In addition to its role in the regulation of hypothalamo-pituitary-adrenal axis (HPA) function, PVN neurons send direct projections onto phrenic motoneurons and share bidirectional connexions between areas involved in cardiorespiratory regulation such as the nucleus tractus solitarius (NTS) and rostral ventrolateral medulla (RVLM) (Sawchenko et al. 2000; Mack et al. 2002, 2007). Based on these observations and the fact that NMS-related disruption of PVN function contributes to the abnormal stress response observed in NMS animals (Anisman et al. 1998; van Oers et al. 1998a; Liu et al. 2000), we propose that the PVN is involved in the enhancement of the hypoxic ventilatory response observed in NMS rats.
The ascending and descending sensory, emotional and cognitive information converging on the PVN is modulated by a local GABAergic inhibitory circuit forming a ring that courses ventrally and laterally around this structure (Boudaba et al. 1996; Herman et al. 2002). GABAergic fibres then reach PVN neurons where they provide tonic inhibition which is mediated mainly via GABAA receptors. The postnatal period is critical for GABAA receptor maturation within the CNS (Liu & Wong-Riley, 2004) and conditions interfering with mother–pup interactions reduce the number and efficacy of GABAA receptors within the brainstem, amygdala and frontal cortex (Caldji et al. 2000); however, the influence of NMS on GABAergic neurotransmission within the PVN has received little attention.
In light of the evidence indicating that GABAergic modulation within the PVN plays a key role in cardiorespiratory control (Haywood et al. 2001; Schlenker et al. 2001; Reddy et al. 2005), we hypothesized that the NMS-related alteration in GABAergic inhibitory neurotransmission within this structure contributes to the enhanced hypoxic ventilatory response observed in NMS rats. To address this issue, we compared the effects of injecting GABAergic agents within the PVN of NMS and control rats on ventilatory activity at rest (normoxia) and during acute stimulation with moderate hypoxia.
Methods
Animals
Experiments were performed on 60 NMS and 60 control (undisturbed) male Sprague–Dawley rats (Charles River Canada, St Constant, Québec, Canada). All animals were born and raised in our animal care facilities. Rats were supplied with food and water ad libitum and maintained in standard laboratory conditions (21°C, 12–12 h dark–light cycle: lights on at 06.00 h and off at 18.00 h). Laval University Animal Care Committee approved all the experimental procedures in this study, and the protocols were in accordance with the guidelines detailed by the Canadian Council on Animal Care.
Mating and NMS procedures
Virgin females were mated and delivered 10–15 pups. Two days after delivery, extra pups were killed, when necessary, to reduce litters to 12 pups, with a roughly equal number of males and females. The NMS protocol, was identical to the one used in our previous studies (Genest et al. 2004, 2007; Kinkead et al. 2005b, c). Briefly, all pups in the litter were separated daily from their mother for 3 h per day (09.00 h to 12.00 h) from day 3–12. Separated pups were placed in an incubator with temperature (35°C) and humidity (45%) controlled, and isolated from each other by a cardboard partition. On day 21, rats were weaned and housed under standard animal care conditions until adulthood (8–10 weeks old; see Table 1 for between group comparison of age and weight data), at which time stereotaxic surgery and ventilatory measurements were performed and/or brains were harvested.
Table 1.
Effects of neonatal maternal separation (NMS) on selected variables including body weight, age and body temperature in male rats during baseline, injection procedure and hypoxia
| Control | NMS | |||||
|---|---|---|---|---|---|---|
| Body weight (g) Age (days) | Baseline | 471 ± 2 72 ± 3 Post injection | Hypoxia | Baseline | 474 ± 17 70 ± 2 Post injection | Hypoxia |
| Body temperature (°C) | ||||||
| PBS | 37.3 ± 0.1 | 37.1 ± 0.1 | 36.8 ± 0.1* | 37.8 ± 0.2 | 37.6 ± 0.2 | 37.2 ± 0.1* |
| GABA | 37.6 ± 0.2 | 37.7 ± 0.2 | 37.0 ± 0.2* | 37.7 ± 0.2 | 37.5 ± 0.1 | 36.9 ± 0.1* |
| Bicuculline | 37.8 ± 0.1 | 37.6 ± 0.2 | 37.1 ± 0.2* | 37.8 ± 0.2 | 37.7 ± 0.2 | 37.3 ± 0.1* |
| Muscimol | 37.7 ± 0.1 | 37.5 ± 0.2 | 37.0 ± 0.2* | 37.7 ± 0.1 | 37.6 ± 0.1 | 37.0 ± 0.1* |
Values are expressed as means ±s.e.m. Baseline values were obtained in quiet but awake rats at least 1 h after the animal acclimated to the plethysmography chamber. Post-injection and hypoxic values were obtained after 10 min of injection and 20 min of exposure to moderate hypoxia (inspired O2 fraction, 0.12), respectively.
Statistically different from baseline (P < 0.05).
The ventilatory and neuroanatomical data obtained from this experimental group were then compared to those of animals not subjected to the NMS procedure (controls) and continuously maintained under standard animal care procedures. Note that for each group, rats originated from at least two different litters to ensure that treatment-related differences were not due to a litter-specific effect.
Series I: functional comparison of GABAergic neurotransmission between NMS and control rats
The first series of experiments used whole-body plethysmography to measure ventilatory activity in the awake, freely moving animal while proceeding with unilateral microinjections of GABAergic agents within the PVN. Measurements were performed at rest (normoxia) and during moderate hypoxia (fraction of inspired O2, 0.12); data were compared between groups (NMS versus controls).
Surgical procedures
Rats (NMS, n = 26; control, n = 25) were anaesthetized with an intraperitoneal injection of a mixture of ketamine (80 mg kg−1) and xylazine (10 mg kg−1). The right PVN was reached stereotaxically (David Kopf Instruments, Tujunga, CA, USA) using the Paxinos and Watson atlas (Paxinos & Watson, 1998) and a 22 gauge stainless steel guide cannula (Plastics One, Australia) was implanted close to the right PVN. Once in place, the guide was secured with screws and cranioplastic cement (Plastics One). With the incisor bar placed at 3.9 mm below the interaural line (horizontal zero), the coordinates from the interaural (anteroposterior, +7.2 mm; lateral, −0.1 mm; dorsoventral, +1.8 mm) and bregma (anteroposterior, −1.8 mm; lateral, +1.8 mm with a 10 deg angle; dorsoventral, −7.2 mm) were averaged for a better accuracy (Paxinos & Watson, 1998). In most (but not all) animals, a telemetry temperature probe (Minimitter, Bend, OR, USA) was inserted into the peritoneal cavity and fixed to the abdominal muscle for continuous monitoring of body temperature during ventilatory measurements according to our standard procedures (Montandon et al. 2006). In the other rats, body temperature was measured with a rectal probe before and after experimentation; continuous tidal volume measurements (and thus minute ventilation) were not obtained in these animals. Ventilatory data were not affected by the method used to measure body temperature (telemetry versus rectal probe). Post-surgery care consisted of three subcutaneous injection of a non-steroid anti-inflammatory (ketoprophene, 2 mg kg−1) mixed in 5 ml lactate-containing Ringer solution immediately after the surgery, and 24 and 48 h later.
Unilateral microinjections and respiratory measurements
Seven days after the surgery, an internal cannula (28 gauge, 1 mm projection beyond the tip of the guide cannula) was inserted into the guide and positioned in the PVN. The animal was placed in the plethysmographic chamber and the cannula was connected to a 10 μl Hamilton glass syringe with a lengthened catheter that swivelled at the top of the chamber allowing free and unrestricted movement of the animal. The cannula and catheter were filled with the drug and phosphate-buffered saline (PBS) prior to the insertion. The rat was allowed approximately 1 h for acclimation to the chamber, and baseline (normoxic) measurements were made when the animal was quiet but awake, and the ventilatory variables were stable. Then, measurements of minute ventilation (V˙E), breathing frequency (f) and tidal volume (Vt) in awake and unrestrained rats were obtained by whole-body flow-through plethysmography as describe in our previous study (Genest et al. 2004). Briefly, the system consisted of a 4.5 l Plexiglas experimental chamber. The flow of air or hypoxic gas mixture delivered to the chamber was kept constant and ranged between 2.0 and 2.5 l min−1. Barometric pressure, chamber temperature and humidity were also measured to express Vt in ml (100 g)−1 (at body temperature and ambient pressure when saturated with water vapour). The baseline values obtained were representative of the data recorded over the preceding 30–45 min. Then, the injection began for 10 min (10 nl min−1) using a microinjection pump (Harvard Apparatus, Holliston, MA, USA). Sham-operated rats were injected with PBS.
In preliminary experiments, GABA-treated rats received either 5 (0.5 mm), 10 (1 mm) or 50 ng (5 mm) GABA (Tocris, Ellisville, MO, USA) diluted in PBS to determine the dose at which the difference in breathing frequency between NMS and controls was the greatest. Based on these results and similar studies using GABAergic agents (e.g. Haywood et al. 2001; Schlenker et al. 2001), GABAA antagonist- and agonist-treated rats were injected with either 10 ng bicuculline methochloride (Tocris) or muscimol (Sigma, St Louis, MO, USA) in PBS, respectively. Animals did not have any seizures or show any signs of discomfort or abnormal behaviour (e.g. excessive grooming) during the microinjection procedure. Ten minutes after the injection, the pump was stopped and a gas mixture of 12% O2 in N2 was delivered to the chamber for 20 min. All measurements were performed between 10.00 h and 12.00 h to minimize changes in endocrine and respiratory activity associated with the circadian rhythm.
At the end of the experiment, the rats were deeply anaesthetized with a ketamine (80 mg kg−1) and xylazine (10 mg kg−1) solution intraperitoneally and perfused with 0.9% saline followed by 4% paraformaldehyde (PFA) in 0.1 m sodium tetraborate buffer (PFA–borax; pH 9.5 at 4°C). Brains were removed from the skull, postfixed for 48 h in 4% PFA–borax, and then placed in 20% sucrose–4% paraformaldehyde solution for 48 h at 4°C. Frozen brains were mounted on a microtome and the brain was cut in 30 μm coronal sections. Slices were collected in a cold cryoprotectant solution (0.05 m sodium phosphate buffer, 30% ethylene glycol and 20% glycerol) and stored at −20°C. The placement of the cannula was verified visually (Nissl staining) and animals with misplaced cannulae were excluded from the analysis.
Series II: neuroanatomical assessment of the effects of NMS on GABAergic neurotransmission
Receptor autoradigraphy
Twenty-five rats (NMS, n = 13; control, n = 12) were used to measure [3H]muscimol (GABAA receptors agonist; NEN, Boston, MA, USA) binding and 27 (NMS, n = 14; control, n = 13) for [3H]flunitrazepam binding (benzodiazepine receptors agonist; NEN). The animals were deeply anaesthetized with isoflurane and decapitated. The brains were immediately removed and frozen with dry ice. Coronal sections (12 μm) were cut through the PVN with a cryostat, thaw-mounted onto gelatin-coated microscope slides and stored at −80°C. The sections were thawed and dried at room temperature (∼20°C), and preincubated for 50 min in the appropriate buffer at room temperature. For the [3H]muscimol assays, a 0.05 m Tris-citrate buffer (pH 7.1) was used, whereas a 0.05 m Tris-HCl buffer (pH 7.1) was used for [3H]flunitrazepam assays. The tissue sections were then incubated in buffer containing various concentrations of tritiated ligands (from 5.5 to 180 nm for 30 min at room temperature for [3H]muscimol; from 0.25 to 15.8 nm for 60 min at 4°C for [3H]flunitrazepam). Non-specific binding was determined by adding GABA (100 μm) and clonazapam (420 nm) to the highest concentration of [3H]muscimol and [3H]flunitrazepam, respectively. After incubation, the sections were rinsed, dried and exposed to Hyperfilm-[3H] (Amersham) for 10 weeks for [3H]muscimol and 5 weeks for [3H]flunitrazepam. The films were developed in Devalex MR developer (Champion Photochemistry Ltd) for 5 min at room temperature and fixed in Redi-fix (Champion Photochemistry Ltd) for 5 min.
In situ hybridization for α1 subunit mRNA
Brains were harvested from 17 rats (NMS, n = 8; control, n = 9) according to the procedure discussed in the ventilatory measurements section (end of series I). Protocols for riboprobe synthesis, hybridization and autoradiographic localization of mRNA signal were previously described (Genest et al. 2004). The α1 mRNA probe (1.5 kb) was generated from the cDNA of the rat (Dr A. J. Tobin and N. Tillakaratne, University of California, Los Angeles, CA, USA). This fragment was subcloned into pBS-Sk+ at the EcoRI site and linearized with XhoI/T3 and SacI/T7 for sense and antisense probes, respectively. Every fourth tissue slice was mounted onto poly-l-lysine-coated slides, desiccated under vacuum overnight, fixed in 4% paraformaldehyde for 20 min, and digested by proteinase K. Brain sections were then rinsed in sterile diethyl pyrocarbonate (DEPC)-water followed by a solution of 100 mm triethanolamine (pH 8.0), acetylated in 0.25% acetic anhydride in 100 mm triethanolamine, and dehydrated through graded concentrations of alcohol. After vacuum drying for a minimum of 2 h, 90 μl hybridization mixture (107 counts min−1 ml−1) was spotted on each slide, sealed under a coverslip and incubated at 56°C overnight (∼15–20 h) in a slide warmer. Coverslips were then removed and the slides were rinsed in 4 × standard saline citrate (SSC; 1 × SSC: 150 mm NaCl and 15 mm trisodium citrate buffer, pH 7.0) at room temperature. Sections were digested by RNAse A (10 mg ml−1, 37°C, 30 min), rinsed in decreasing concentrations of SSC, washed in 0.1 × SSC for 30 min at 60°C, and dehydrated through graded concentrations of alcohol. After being dried for 2 h under vacuum, the sections were exposed to X-ray film (Kodak) overnight. Slide-mounted sections were stained in thionin (0.25%) for 30–90 s to help visualize the limits of each structure during film analysis. Radioisotope-labelled sense (control) cRNA copies were also prepared to verify the specificity of each probe. Hybridization with these probes revealed no signal in the rat brain.
Data analysis and statistics
Respiratory measurements
Basal breathing frequency. Baseline measurements of ventilatory variables were obtained by averaging the last 10 min of stable recording. The effect of drug injection on baseline breathing frequency, tidal volume and minute ventilation were analysed by calculating the percentage change between the ventilatory variables at the end of the injection procedure (t =−10) and the baseline value (t = 0).
Time course of the breathing frequency response. The hypoxic ventilatory response has several time domains that reflect a complex interplay between distinct neural mechanisms. The early phase (onset) of the response is commonly attributed to peripheral chemoreceptor activation whereas the late phase reflects central integration of the sensory afferent signals (Powell et al. 1998). To obtain mechanistic insight into the effects of NMS on the hypoxic ventilatory response, the time course of the frequency response to hypoxia was analysed on a minute-by-minute basis in two distinct segments (early and late phases), according to criteria similar to those established in our previous study (Genest et al. 2004). Briefly, the early phase was defined as the period from the onset of hypoxia (t = 0 min) to the time at which breathing frequency of NMS rats reached steady state, and became similar to that of controls (t = 8 min). The late phase (t > 8 min) was defined as the period during which the frequency response of both groups reached steady state until the end of the hypoxic stimulus (t = 20 min). Measurements of ventilatory variables at the end of the hypoxic exposure (late phase) were obtained by averaging the last 5 min of recording. Note that as NMS augments the breathing frequency response at the onset of hypoxia (Genest et al. 2004), the effects of adding various doses of GABA on this response were obtained by averaging the first 5 min of hypoxia (dose–response curves). This procedure allowed us to determine the dose of GABA active agents used in subsequent experiments.
Dose–effect and change from baseline data were analysed using two-way ANOVA (dose × separation; Statview 5.0 SAS Institute, Cary, NC, USA). Time course data were analysed using two-way ANOVA for repeated measures (hypoxic stimulus × separation).
GABAA receptor autoradiography
Blind quantitative analysis was performed on the film. Transmittance values were measured using a Northern Light Desktop Illuminator (Imaging Research, St Catharines, Ontario, Canada), subtracted from background binding determined from autoradiographs of sections with non-specific binding. The signal was analysed with NIH Image software (version 1.61; W. Rasband, National Institutes of Health, Bethesda, MD, USA) and then converted to fmol (mg tissue)−1 using the specific activity of the tritiated ligand. Densitometric analysis was performed according to a standard scale established using [3H]-polymer standard (American Radiolabelled Chemicals, St Louis, MO, USA). Saturation curves of bound ligand versus concentration were analysed using a non-linear regression analysis of total binding (Sigmaplot 8.0, SPSS Inc., Chicago, IL, USA). Differences in binding parameters were determined by comparing the overall results of the non-linear regression by an unpaired Student's t test.
In situ hybridization
The parvocellular PVN was identified using the atlas of Paxinos & Watson (1998) and sections corresponding to the rostrocaudal coordinate −1.8 from bregma were selected using the thionin-stained slides under bright microscopy. Blind quantitative analysis of hybridization signal for α1 subunit mRNA was performed on X-ray film (Kodak). Transmittance values were measured, subtracted from background noise and the signal was analysed. Densitometric analysis, yielding measures of optical density, was performed according to a standard scale established using 14C standard slides (American Radiolabelled Chemicals, St Louis, MO, USA). The data were analysed using a Student's t test.
All these analyses were followed by a post hoc Student–Neuman–Keuls test when appropriate. Unless otherwise stated, differences were considered significant if P < 0.05. All values are expressed as mean ±s.e.m.
Results
NMS potentiates GABA-mediated attenuation of the breathing frequency response to hypoxia
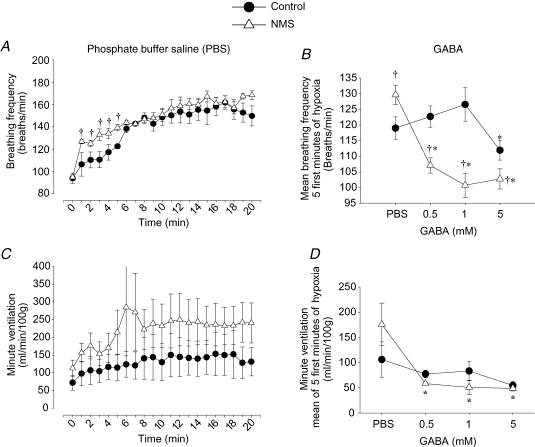
Temporal analysis of the frequency response to hypoxia following PBS microinjection shows that during the initial phase (0–8 min), breathing frequency varied according to stimulus and separation (bi-factorial interaction, P = 0.01; Fig. 1A). During that period, rats subjected to NMS had a breathing frequency response greater than controls (P = 0.02; Fig. 1A and B). These results confirm our previous description of the effect of NMS on the hypoxic chemoreflex and show that the microinjection procedure itself did not affect this response (Genest et al. 2004; Kinkead et al. 2005a). These experiments also confirmed that the frequency response to hypoxia did not differ between NMS and control rats during the late phase (9–20 min) (Fig. 1A). Figure 1C shows the time course of the minute ventilation response to hypoxia measured in PBS-treated rats for which continuous body temperature measurements (with telemetry probes) made it possible to produce corrected tidal volume data (n = 3 for each group). While these results suggest that NMS augments minute ventilation throughout the hypoxic period, this observation could not be confirmed statistically.
Figure 1.
Comparison of the time course of breathing frequency and minute ventilation responses to hypoxia Comparison of the time course of breathing frequency (A) and minute ventilation (C) responses to hypoxia (12% O2) following microinjection of PBS in PVN between rats previously subjected to neonatal maternal separation (NMS; •) and controls (▵). Preliminary experiments comparing the effects of different doses of GABA on the mean breathing frequency (B) and mean minute ventilation (D) measured over the 5 first minutes of hypoxia between NMS and control rats. Values are expressed as mean ±s.e.m.†Statistically different from corresponding control value and *statistically different from PBS (P < 0.05).
The effect of injecting different doses of GABA (0.5, 1 or 5 mm) within PVN before hypoxia was then compared in NMS and control rats to obtain a concentration–response curve. Figure 1B shows that the mean breathing frequency measured during the first 5 min varied according to the dose injected and separation (bi-factorial interaction, P = 0.0001; Fig. 1B). In NMS rats, microinjection of GABA resulted in a dose-dependent attenuation of the mean breathing frequency measured at the onset of hypoxia and a maximum effect was reached at a dose of 1 mm. These data differ from those obtained in control animals in which a 5 mm dose was necessary to observe a significant attenuation of breathing frequency at the onset of hypoxia. Although minute ventilation measurements show that a dose of 0.5 mm is sufficient to attenuate the hypoxic ventilatory response in NMS rats (Fig. 1D), the number of animals with continuous tidal volume (and thus minute ventilation) measurements was not sufficient for adequate (and reliable) statistical analysis. Based on breathing frequency results, we chose to use a GABA dose of 1 mm for the rest of the study. Based on this result and previous studies (e.g. Schlenker et al. 2001; Stocker et al. 2004), this concentration was also used for microinjections of bicuculline and muscimol.
NMS augments PVN-mediated tonic GABAergic inhibition of basal respiratory activity
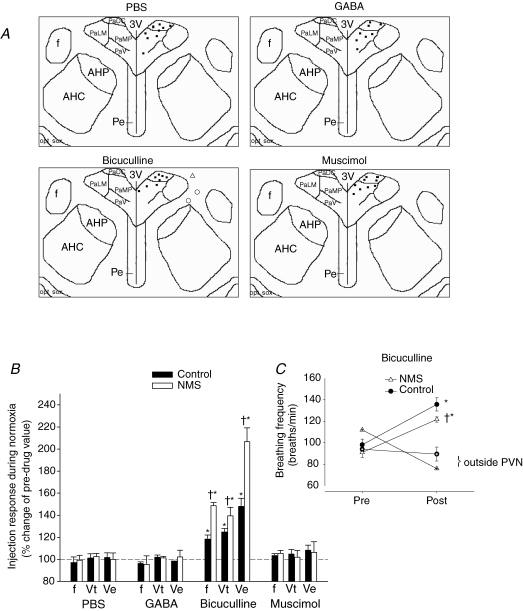
Given that PVN neurons are tonically inhibited via GABAA receptors under basal conditions (Herman et al. 2002), we sought to compare the action of the drugs used on resting ventilatory activity in NMS and control rats. Figure 2A shows successful placement of microinjection cannulae for each pharmacological agent used, and Fig. 2B presents the effects of PBS, GABA, muscimol (selective GABAA agonist) and bicuculline (competitive GABAA antagonist) on basal breathing frequency (f), tidal volume (Vt; volume of air inspired with each breath) and minute ventilation (V˙E=f×Vt). Microinjection of PBS, GABA or muscimol in PVN had no effect on baseline breathing (P > 0.6 for all three variables and drugs; Fig. 2B). In contrast, microinjection of bicuculline increased all ventilatory variables in a manner similar to responses reported previously (Schlenker et al. 2001) (P < 0.0001 for all three variables; Fig. 2B). This increase was greater in NMS rats versus controls (f, P = 0.0006; Vt, P = 0.01; V˙E, P = 0.0006; Fig. 2B). None of the drugs injected affected body temperature during the injection procedure (Table 1). Figure 2C shows data obtained in three rats (two controls, one NMS) in which the cannula was adjacent but outside the PVN. Bicuculline failed to increase breathing frequency in these rats, thus indicating that the effects observed following drug injection are site-specific.
Figure 2.
Microinjection sites and effects of GABAergic agents on resting ventilatory activity in NMS and control rats A, anatomical location of the 34 microinjection sites in the paraventricular nucleus of the hypothalamus (PVN; Bregma −1.8) where phosphate-buffered saline (PBS; vehicle), GABA, bicuculline or muscimol were injected. The bicuculline panel also shows three injection sites outside the PVN (○, control; ▵, neonatal maternal separation (NMS)) for which breathing frequency data are shown in C. B, comparison of the change in basal (normoxic) ventilatory activity (expressed as percentage change from preinjection values) measured 10 min after PBS, GABA, bicuculline or muscimol microinjection (10 ng each) in the PVN between rats subjected to NMS (open bars) and controls (filled bars). Measured ventilatory variables include breathing frequency (f), tidal volume (Vt) and minute ventilation (V˙E). C, comparison of the change in ‘resting’ breathing frequency (expressed in absolute data) following bicuculline microinjection according to cannula placement within the PVN. The symbols with crosses inside show data from three rats (two controls, one NMS) for which the cannula was outside. In this graph, data for the two control rats were averaged. These data show that the effects of the injection are site-specific. Data are expressed as means ±s.e.m.†Statistically different from corresponding control value and *statistically different from PBS (P < 0.05). PaDC, paraventricular hypothalamic nucleus, dorsal cap; PaLM, paraventricular hypothalamic nucleus, lateral magnocellular part; PaMP, paraventricular hypothalamic nucleus, medial parvicellular part; PaV, paraventricular hypothalamic nucleus, ventral part; f, fornix; 3V, third ventricle; AHP, anterior hypothalamic area, posterior; AHC, anterior hypothalamic area, central; Pe, periventricular hypothalamic nucleus; Opt, Optic tract; sox, supraoptic decussation.
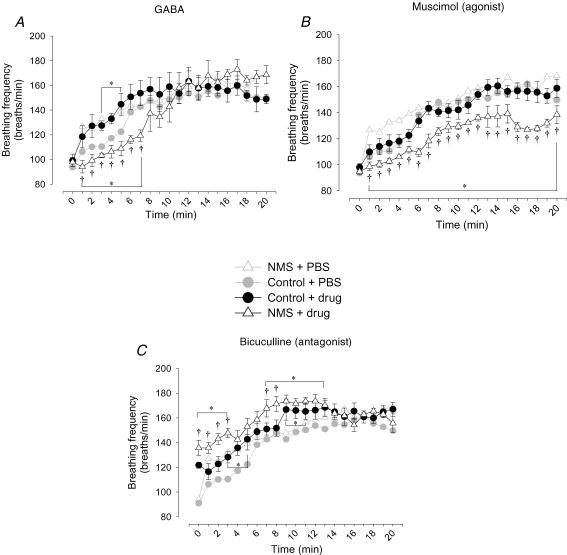
NMS potentiates the effects of GABA agonists on the acute breathing frequency response to hypoxia
Temporal analysis of the frequency response to hypoxia following microinjection of GABA shows that the initial phase of the response (0–8 min) varies according to stimulus, drug and separation (tri-factorial interaction, P = 0.03; Fig. 3A). At the onset of hypoxia, microinjection of GABA (1 mm) reduced the breathing frequency of NMS rats below that of controls (P < 0.0001; Fig. 3A). In contrast, GABA injection in control rats increased the breathing frequency response above the response measured in PBS-treated animals (black versus grey circles, P = 0.04). During the late phase of the response (9–20 min), however, breathing frequency was similar between the groups (P = 0.3).
Figure 3.
Neonatel maternal seperation (NMS) augments the effects of GABA agonists on the breathing frequency response to hypoxia Comparison of the effects of microinjections of GABA (A) muscimol (B) or bicuculline (C) within the PVN on the breathing frequency response to moderate hypoxia (12% O2) between rats previously subjected to neonatal maternal separation (NMS; ▵) and controls (•). Note that the light grey symbols show the data obtained from PBS-treated rats (Figure 1A) for comparison. Data are expressed as means ±s.e.m.†Statistically different from corresponding control value and bars with asterisk indicate a series of values statistically different from corresponding PBS value (P < 0.05).
In NMS rats, injection of the specific GABAA receptors agonist muscimol (1 mm) prior to hypoxia attenuated the initial phase of the breathing frequency response in a manner similar to that observed following microinjection of GABA (tri-factorial interaction, P = 0.07; Fig. 3B). In contrast with the previous series, however, muscimol had no effect on the breathing frequency response of control rats throughout the experiment (Fig. 3B, grey versus black circles). During the late phase of the response, the breathing frequency of NMS rats treated with muscimol remained attenuated in comparison with controls (P < 0.0001; Fig. 3B). As with GABA, attenuation of the breathing frequency response by muscimol was stronger in NMS rats than in controls (P = 0.02).
In both groups of rats, microinjection of bicuculline (1 mm) resulted in a higher breathing frequency response in NMS rats versus controls (P < 0.0001; Fig. 3C) even though baseline breathing frequency was higher (NMS, P = 0.0009; controls, P = 0.01). Over the first 9 min of hypoxia, breathing frequency of bicuculine-treated rats (both NMS and controls) remained above that of PBS-treated animals (shown in light grey) for both groups; breathing frequencies measured in all groups reached the same level during the late phase of hypoxia. Body temperature measurements obtained at the end of hypoxic stimulation revealed a decrease in all groups (Table 1); however, continuous measurement of body temperature with telemetry revealed no effect of drug or treatment (Table 1).
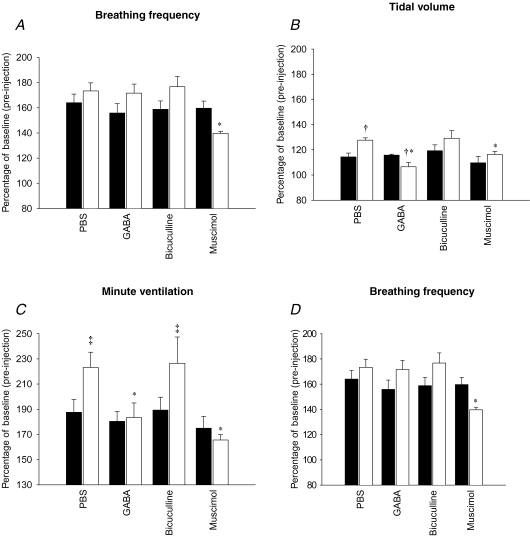
GABA-mediated attenuation of the tidal volume response to hypoxia is greater in NMS rats
During the late phase of hypoxia, the tidal volume, minute ventilation and inspiratory flow (Vt/TI) responses measured in NMS rats were greater than in controls in a manner similar to our previous results (Genest et al. 2004) (Fig. 4B–D, PBS-treated animals). These data indicate that microinjection of PBS alone had no effect on NMS-related enhancement of the hypoxic ventilatory response.
Figure 4.
Comparison of the effects of microinjection of PBS (100 nl), GABA (1 mm), bicuculline (1 mm) or muscimol (1 mm) in the PVN on the ‘late phase’ (last 5 min) of the ventilatory response to moderate hypoxia (12% O2). Ventilatory variables shown are breathing frequency (A), tidal volume (B), minute ventilation (C) and inspiratory flow (D). Results are expressed as a percentage of normoxic (baseline) values obtained 1 min before the injections. Data are shown as means ±s.e.m.†Statistically different from corresponding control value and *statistically different from PBS (P < 0.05). ‡Indicates a value statistically different from corresponding control value (P = 0.06).
The tidal volume response varied according to the drug and separation (bi-factorial interaction, P = 0.02; Fig. 4B). Pre-treatment with GABA or muscimol attenuated the tidal volume response to hypoxia in NMS rats only (P = 0.007 and P = 0.008, respectively). Moreover, NMS rats that received GABA showed a tidal volume response lower than in controls (P = 0.01). Consequently, GABA and muscimol both decreased minute ventilation and inspiratory flow responses of NMS rats to the same level as controls (P = 0.007 and P = 0.005, respectively; Fig. 4C and D). In contrast, bicuculline pretreatment had no effect on the tidal volume, minute ventilation and inspiratory flow responses (drug effect, P = 0.6, 0.9 and 0.3, respectively) and the responses measured in NMS rats were above those of controls (treatment effect, P = 0.02, 0.03 and 0.08, respectively; Fig. 4B–D). Again, the hypoxic ventilatory response of controls was not affected by any of the drugs injected in the PVN. Normalization of breathing frequency data obtained after 20 min of hypoxia showed that, in NMS rats, only muscimol had an inhibitory effect on this response in comparison to PBS (P = 0.008; Fig. 4A). Together, these results indicate that the PVN modulates the tidal volume, minute ventilation and inspiratory flow responses measured during the late phase of hypoxia via GABAergic neurotransmission, and that NMS augments responsiveness to GABAergic agents.
NMS increases GABAA receptor density and alters subunit composition within the PVN
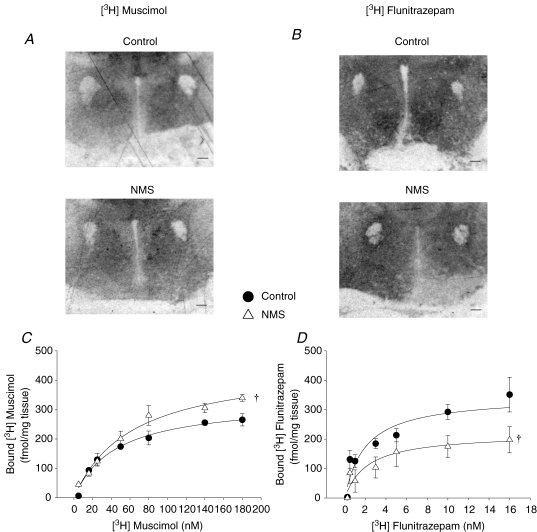
GABAA and benzodiazepine receptor binding sites along with mRNA encoding for the α1 subunit were measured to assess the effects of NMS on GABAergic neurotransmission within the PVN. Analysis of the [3H]muscimol autoradiographic signal shows that the maximum number of GABAA binding sites (Bmax) was 22% higher in NMS rats versus controls (control, 330 ± 26 fmol (mg tissue)−1; NMS, 456 ± 42 fmol (mg tissue)−1, P = 0.03; Fig. 5A and C), but that the binding affinity (Kd) was similar in both groups (control, 44 ± 11 nm; NMS, 62 ± 14 nm).
Figure 5.
Comparison of the [3H]muscimol and [3H]flunitrazepam binding within the PVN between rats previously subjected to neonatal maternal separation (NMS) and controls Representative autoradiographs of [3H]muscimol (A) and [3H]flunitrazepam (B) binding in PVN section for each group of rats. In these panels, the scale bar is 250 μm.C, binding curves for [3H]muscimol obtained in NMS (•) and control rats (▵) showing that NMS augments the number of GABAA recptors within the PVN. D, binding curves for [3H]flunitrazepam obtained in NMS and control rats showing that NMS decreased the number of benzodiazepine binding sites within the PVN. For each concentration, data are expressed as means ±s.e.m.†statistically different from corresponding control value (P < 0.05).
Activation of benzodiazepine binding sites potentiates the inhibitory effects of GABA. Within the PVN, quantification of benzodiazepine binding sites with [3H]flunitrazepam showed that NMS rats had 37% less benzodiazepine binding sites than controls (control, 346 ± 38 fmol (mg tissue)−1; NMS, 219 ± 44 fmol (mg tissue)−1; P = 0.04; Fig. 5B and D); however, binding affinities were similar in both groups (control, 1.9 ± 0.7 nm; NMS, 2 ± 1 nm).
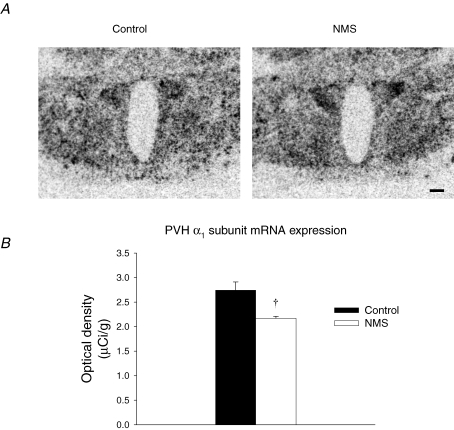
Within GABAA receptors, the α1 subunit is the most widespread GABA binding site and its presence has a profound effect on the outcome following receptor activation (Schousboe & Redburn, 1995). Densitometric analysis of mRNA signal showed that NMS rats had 22% lower α1 subunit mRNA expression relative to controls (P = 0.007; Fig. 6A and B).
Figure 6.
Neonatel maternal seperation (NMS) decreases GABAA receptor α1 subunit mRNA signal in the PVN A, comparison of representative autoradiographs showing basal GABAA receptor α1 subunit mRNA signal in the PVN of NMS and control rats. The scale bar is 250 μm.B, mean optical density of the α1 subunit mRNA signal for NMS (open bars) and controls (filled bars). These data show that, within the PVN, NMS decreased α1 subunit mRNA expression level; this subunit is the most widespread GABA binding site in the CNS. Data are expressed as means ±s.e.m.†Statistically different from corresponding control value (P < 0.05).
Discussion
The neonatal environment has a significant impact on early life programming of PVN function and respiratory control development (Genest et al. 2004). In addition to its role in HPA axis regulation, the PVN is an important modulator of respiratory activity (Yeh et al. 1997; Schlenker et al. 2001; Kc et al. 2002; Mack et al. 2002, 2007; Schlenker, 2005) which prompted us to propose that NMS-related disruption of GABAergic inhibition of PVN function contributes to the enhancement of the hypoxic ventilatory response observed in NMS rats. In this regard, our main findings are that (1) inactivation of ‘resting’ tonic GABAergic inhibition of PVN neurons (via bicuculline injection) results in a greater increase in normoxic ventilatory activity in NMS rats than controls (Fig. 2B), and (2) GABAA receptor activation attenuates the hypoxic ventilatory response of NMS rats only (Figs 3 and 4). (3) In addition, results from autoradiography experiments provide anatomical support for the greater responses observed in NMS rats as they show that NMS augments the number of functional GABAA receptors within the PVN (Fig. 5). Despite such increase in GABAA receptor number, our data clearly show that in NMS rats, the capacity for GABAergic modulation is insufficient to maintain the hypoxic ventilatory response within ‘normal’ range. Together, these results provide new insight into the effects of stress exposure in early life on PVN function and highlight the importance of its role in respiratory regulation. Moreover, this study shows that the life-long consequences of NMS are far reaching as they can compromise the development of vital homeostatic function in a way that may predispose to respiratory disease related to neural control dysfunction.
Basal PVN inhibitory tone and breathing
The region surrounding the PVN forms a ring that courses ventrally and laterally around this structure where it exerts a tonic inhibition via postsynaptic GABAA receptors (Boudaba et al. 1996; Herman et al. 2002). Thus, activation/inactivation of this inhibitory circuit provides an indirect means by which various afferent inputs can modulate PVN function (Herman et al. 2002). This GABAergic circuit ultimately acts on signals relevant to basal respiratory activity as, in the present study, inactivation of tonic inhibition by microinjection of bicuculline within the PVN increases ventilatory activity in both control and NMS rats. However, this tonic GABAergic modulation of the PVN is fully activated under ‘resting’ conditions as microinjection of a GABA agonist (whether GABA or muscimol) had no effect on baseline ventilatory activity in either group. Besides being consistent with the larger number of GABAA receptors measured in NMS rats, the larger response to bicuculline microinjection seen in this group indicates that this tonic GABAergic inhibition from the PVN has a stronger impact on ‘resting’ ventilatory activity than in controls. The larger number of GABAA receptors would act to compensate for a reduced GABA release and/or counteract stronger excitatory inputs converging onto the PVN and respiratory neurons. These hypotheses, which are not mutually exclusive, are discussed in the context of an acute respiratory challenge such as hypoxia.
Modulation of the hypoxic ventilatory response by the PVN
Carotid bodies are the main peripheral O2 sensors, and their activation is responsible for the brisk increase in breathing frequency measured during the early phase of hypoxia (Powell et al. 1998). The afferent signal from the carotid bodies reaches the caudal region of the NTS via the carotid sinus nerve (Housley & Sinclair, 1988; Finley & Katz, 1992) and, during hypoxia, the PVN is activated from noradrenergic pathways originating from the brainstem (Chen et al. 2004). It is important to note that the PVN receives direct excitatory projections from the NTS which bypass the peri-PVN inhibitory ring (Sawchenko et al. 2000; Herman et al. 2002), which explains why the PVN plays an important role in the integration of chemosensory afferent signals (Schlenker, 2005). At the onset of hypoxia, microinjection of GABA tended to increase the breathing frequency response of controls (non-separated rats), whereas the specific GABAA agonist muscimol had no effect (Fig. 3). Assuming that the sphere of action of each drug was similar, these differences are likely to be related to the fact that, unlike muscimol, GABA activates presynaptic GABAB receptors which modulate both GABAergic and glutamatergic inputs to the PVN (Margeta-Mitrovic et al. 1999; Charles et al. 2001; Wang et al. 2003; Liu et al. 2006). In NMS rats, however, both GABA and muscimol microinjections attenuated the breathing frequency response whereas bicuculline seemed to augment it. Unlike GABA, muscimol was effective throughout out the period of hypoxia. Muscimol is a synthetic agonist that remains active within the synaptic cleft for a longer period than GABA because, unlike GABA, it is not taken up by GABAergic nerve terminals (Drolet et al. 1993); however, the fact that attenuation of the minute ventilation response measured at the end of hypoxia was similar for both drugs indicates that, for these experiments, their overall effectiveness is adequate. The effect of bicuculline on the hypoxic response must be interpreted cautiously because, in NMS rats, baseline breathing frequency was elevated to begin with. Nevertheless, the greater effect observed in NMS rats is consistent with the higher number of GABAA receptors within the PVN of these animals (see below). These results suggest that, at the onset of hypoxia, GABAergic modulation of PVN neurons contributes to the regulation of the breathing frequency response and that NMS disrupts this function.
Regulation of GABA release onto PVN neurons is a complex process that varies according to the nature of the simulus presented to the animal. Unlike psychogenic stress (Herman et al. 2003), little is known about the net effect of hypoxia on GABA release within the PVN in control rats. However, hypoxia could inhibit GABAergic neurons projecting onto PVN neurons, and withdrawal of this inhibition would contribute to the increased ventilatory activity by augmenting the impact of PVN on respiratory neurons. This scenario is consistent with the fact that GABAergic modulation of respiratory activity is saturated during normoxia (tonic inhibition). It is also possible that hypoxia further augments GABA release within the PVN as has been shown for the NTS (Tabata et al. 2001). While the present data do not allow us to elucidate the effects of hypoxia on GABA release within the PVN, they clearly show that, in NMS rats, endogenous GABA release within the PVN is insufficient to counter the excitatory afferent inputs converging onto this structure. Our results from GABA agonist injection support this interpretation as additional GABA in NMS rats brings the hypoxic ventilatory response to a level comparable to that of controls. In this case, the higher number of GABAA receptors would try to compensate for the deficit in functional inhibition within this structure. Our previous data strongly suggest (albeit indirectly) that NMS augments carotid body chemoafferent input to the NTS during hypoxia (Kinkead et al. 2005b,c). These results, combined with the privileged (direct) neuranatomical link between the NTS and the PVN, suggest that the greater number of GABAA receptors might match a greater level of PVN activation during hypoxia. However, these interpretations must be viewed cautiously because the present study provides no direct measurement indicating whether NMS increased or decreased GABA release within the PVN in comparison with controls.
While peripheral chemoreceptors are major determinants of the rapid increase in breathing frequency measured at the onset of hypoxia, the late phase (steady state) of the hypoxic ventilatory response is the result of a complex interplay between several sensory and modulatory influences (Powell et al. 1998). Consequently, it is not surprising that disruption of the hypoxic chemoreflex by NMS also involves neural elements beyond chemosensory afferent input (Kinkead et al. 2005a, b). In anaesthetized rats, we used carotid sinus nerve stimulation to activate the hypoxic chemoreflex while bypassing potential NMS-related differences in carotid body chemotransduction. Results from these experiments show that NMS augments the phrenic burst amplitude (but not frequency) response to carotid nerve stimulation (Kinkead et al. 2005b), which is consistent with the increased tidal volume response measured during the late phase of hypoxia in awake animals (see Fig. 4 and Genest et al. 2004). Regulation of the breathing frequency and tidal volume responses to hypoxia involves distinct neural pathways. Given that parts of the PVN send direct projections onto spinal (phrenic) motoneurons where they regulate diaphragmatic activity (Yeh et al. 1997; Kc et al. 2002; Mack et al. 2002), it is not surprising to see that differences in modulation by the PVN of the tidal volume response contribute to the increased hypoxic ventilatory response observed in NMS rats. In these animals, both GABA and muscimol microinjection in the PVN before hypoxia attenuated tidal volume, minute ventilation and inspiratory flow responses to hypoxia whereas no effects were observed in controls (Fig. 4). These data show that GABAergic modulation of the PVN, in addition to its influence on the early frequency response, regulates inspiratory effort, especially during the late phase of hypoxia. Again, it is clear that the capacity for GABA release observed in NMS rats is insufficient to maintain tidal volume (and thus minute ventilation) response to hypoxia similar to that of controls.
GABAA receptor density and composition within the PVN
GABAA receptor development is an important aspect of CNS maturation that is highly influenced by the neonatal environment. The GABAA receptor is a complex assembly of various subunits with specific binding sites that influence the responsiveness and activity of the receptor (Sieghart, 1995). Thus, in addition to the total number GABAA receptors present within a structure, receptor composition in itself will determine the ability of GABA to elicit postsynaptic effects. In the present study, we chose to focus on the α1 subunit because it is the most widespread GABA binding site and its presence has a profound effect on the outcome following receptor activation (Schousboe & Redburn, 1995). Comparison of the effects of NMS with neonatal handling (a procedure which, unlike NMS, stimulates maternal behaviour) indicates that handled rats show (1) higher GABAA receptor levels in the locus coeruleus (LC) and the NTS, (2) more benzodiazepine receptor sites in the central and lateral nucleus of the amygdala, the frontal cortex, the LC and NTS, and (3) higher levels of the mRNA for the γ2 subunit of the GABAA receptor complex, which confers high affinity benzodiazepine binding in the amygdaloid nuclei as well as in the LC and NTS (Caldji et al. 2000). These data are consistent with the reduced startle response and increased exploratory behaviour observed in handled rats. To the best of our knowledge, no study to date has addressed the long-term consequences (whether anatomical or functional) of NMS-related changes in GABAergic modulation of the PVN. At first glance, our results showing that NMS augments GABAA binding sites by 22% in the PVN are in contrast to what we would have predicted from the changes in GABAergic neurotransmission associated with neonatal handling. Although both studies must be compared cautiously given the differences in rat strain, the results reported by Caldji et al. (2000) revealed no significant effect of neonatal environment (short handling procedure versus prolonged maternal separation) on the number of GABAA receptors and benzodiazepine binding sites when results from NMS rats were compared to those from non-handled (control) animals. Conversely, the effects of NMS on GABAA receptor subunit composition reported here are in line with predictions from the behavioural, neuroendocrine and respiratory phenotypes observed in these animals. The net (functional) result of such opposing effects (increased number of receptor versus suboptimal receptor composition) may be difficult to interpret; however, data from microinjection experiments indicate that NMS does not reduce but augments the response to GABA agonists modulating PVN function. The mechanisms underlying this effect are unknown; however, the fact that rats were subjected to NMS during a critical period in the maturation of the GABAergic system probably contributes to this phenotype. For instance, neurons exposed to GABA levels higher than normal during development show an enhanced expression of GABAA receptors via stimulation of transcription and translation processes (Schousboe & Redburn, 1995). However, the effects of NMS on GABA release in the vicinity of PVN neurons during the neonatal period remain unknown. On the other hand, chronic elevation in plasma corticosterone levels observed in NMS rats may be involved as corticosterone produces site- and subunit-specific changes in the mRNA levels of GABAA receptor subunits. In fact, corticosterone attenuates α1 and α2 subunit levels and also alters the sensitivity of GABAA receptors to GABA rather than the receptor number per se (Orchinik et al. 1995). Because chronic elevation of plasma corticosterone alone augments the hypoxic ventilatory response in a manner similar to that observed in NMS rats (Fournier et al. 2006), it is tempting to associate the α1 subunit decrease with the effect of corticosterone.
Conclusion
Although earlier studies emphasized the importance of GABAergic rather than glutamatergic inputs in the modulation of HPA axis activity (Cole & Sawchenko, 2002), our results raise important questions concerning the impact of NMS on the regulation of PVN function. While our data suggest that NMS increases the capacity for inhibitory modulation within the PVN, NMS rats still show a ventilatory response to hypoxia that is 35% higher than in controls (Fig. 4C), which suggests that this respiratory phenotype reflects an imbalance between these opposing modulatory influences at several levels, including the PVN. Because development of GABAergic kinetics is matched by a corresponding development in the glutamatergic system (Ben-Ari et al. 1997; Hutcheon et al. 2000), increased glutamate release is one mechanism by which NMS could disrupt integration of afferent inputs modulating the hypoxic ventilatory response.
The clinical relevance of NMS and its consequences on respiratory control development may seem trivial. However, the enhancement of the hypoxic ventilatory response reported in our studies compares well to the response observed in obstructive sleep apnoea patients, which is approximately 30% greater than that measured in control subjects (Narkiewicz et al. 1999). Moreover, hypersensitivity of the peripheral chemoreceptors independently predicts adverse prognosis in ambulatory patients with chronic heart failure (Ponikowski et al. 2001). Given that in humans abnormally elevated chemoreflexes probably contribute to sleep apnoea and hypertension (Smith & Pacchia, 2007) and predispose to respiratory instability during sleep (Younes et al. 2001; Kara et al. 2003; Younes, 2004), further investigation of the neuroendocrine mechanism underlying the physiological phenotype of NMS rats will provide valuable insight into the pathophysiology of respiratory disorders related to respiratory control dysfunction, such as sleep disordered breathing.
Acknowledgments
This research was supported by the Canada Research Chair in Respiratory Neurobiology (R.K.) and the Canadian Institutes of Health Research (R.K. and G.D.). S.E.G. held a graduate scholarship from the Fonds de la Recherche en Santé du Québec and N.B. held a postdoctoral scholarship from la Fondation de la Recherche sur les Maladies Infantiles. The authors would like to thank Evelyne Vachon for her technical help.
References
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Evans ML, Robbins MJ, Calver AR, Leslie RA, Pangalos MN. Comparative immunohistochemical localisation of GABAB1a, GABAB1b and GABAB2 subunits in rat brain, spinal cord and dorsal root ganglion. Neuroscience. 2001;106:447–467. doi: 10.1016/s0306-4522(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet G, Chalmers J, Blessing W. Vasodepressor neurons in medulla alter cardiac contractility and cardiac output. Hypertension. 1993;21:210–215. doi: 10.1161/01.hyp.21.2.210. [DOI] [PubMed] [Google Scholar]
- Chen X-Q, Du J-Z, Wang YS. Regulation of hypoxia-induced release of corticotropin-releasing factor in the rat hypothalamus by norepinephrine. Regul Pept. 2004;119:221–228. doi: 10.1016/j.regpep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fournier S, Allard M, Gulemetova R, Kinkead R. Chronic elevation of corticosterone increases the ventilatory response to hypoxia in adult rats. FASEB J. 2006;20:A374. [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. J Physiol. 2004;554:543–557. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation induces sex-specific augmentation of the hypercapnic ventilatory response in awake rat. J Appl Physiol. 2007;102:1416–1421. doi: 10.1152/japplphysiol.00454.2006. [DOI] [PubMed] [Google Scholar]
- Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C. γ-Aminobutyric acid (GABA) – a function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension. Hypertension. 2001;37:614–618. doi: 10.1161/01.hyp.37.2.614. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience. 1997;80:1209–1224. doi: 10.1016/s0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Horn EM, Kramer JM, Waldrop TG. Development of hypoxia-induced Fos expression in rat caudal hypothalamic neurons. Neuroscience. 2000;99:711–720. doi: 10.1016/s0306-4522(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, Morley P, Poulter MO. Developmental change in GABAA receptor desensitization kinetics and its role in synapse function in rat cortical neurons. J Physiol. 2000;522:3–17. doi: 10.1111/j.1469-7793.2000.t01-5-00003.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K, Somers VK. Chemoreflexes – physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- Kc P, Haxhiu MA, Trouth CO, Balan KV, Anderson WA, Mack SO. CO2-induced c-Fos expression in hypothalamic vasopressin containing neurons. Respir Physiol. 2002;129:289–296. doi: 10.1016/s0034-5687(01)00321-8. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Genest SE, Gulemetova R, Lajeunesse Y, Laforest S, Drolet G, Bairam A. Neonatal maternal separation and early life programming of the hypoxic ventilatory response in rats. Respir Physiol Neurobiol. 2005a;149:313–324. doi: 10.1016/j.resp.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Gulemetova R, Bairam A. Neonatal maternal separation enhances phrenic responses to hypoxia and carotid sinus nerve stimulation in the adult anesthetised rat. J Appl Physiol. 2005b;99:189–196. doi: 10.1152/japplphysiol.00070.2005. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Joseph V, Lajeunesse Y, Bairam A. Neonatal maternal separation enhances dopamine D2 receptor and tyrosine hydroxylase mRNA expression levels in carotid body of rats. Can J Physiol Pharmacol. 2005c;83:76–84. doi: 10.1139/y04-106. [DOI] [PubMed] [Google Scholar]
- Larnicol N, Wallois F, Berquin P, Gros F, Rose D. c-fos-like immunoreactivity in the cat's neuraxis following moderate hypoxia or hypercapnia. J Physiol (Paris) 1994;88:81–88. doi: 10.1016/0928-4257(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in the rat pre-Botzinger complex. J Appl Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- Liu X, Tribollet E, Raggenbass M. GABAB receptor-activation inhibits GABAergic synaptic transmission in parvocellular neurones of rat hypothalamic paraventricular nucleus. J Neuroendocrinol. 2006;18:177–186. doi: 10.1111/j.1365-2826.2005.01402.x. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Alheid GF. Neonatal stress alters adult breathing. J Physiol. 2004;554:591. doi: 10.1113/jphysiol.2003.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J Appl Physiol. 2007;102:189–199. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABAB receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Montandon G, Bairam A, Kinkead R. Long-term consequences of neonatal caffeine on ventilation, occurrence of apneas, and hypercapnic chemoreflex in male and female rats. Pediatr Res. 2006;59:519–524. doi: 10.1203/01.pdr.0000203105.63246.8a. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJH, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Weiland NG, McEwen BS. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Brain Res Mol Brain Res. 1995;34:29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJS. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol. 2005;289:R789–R797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- Rots NY, de Jong J, Workel JO, Levine S, Cools AR, De Kloet ER. Neonatal maternally deprived rats have as adults elevated basal pituitary-adrenal activity and enhanced susceptibility to apomorphine. J Neuroendocrinol. 1996;8:501–506. doi: 10.1046/j.1365-2826.1996.04843.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Schlenker EH. Integration in the PVN: another piece of the puzzle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R653–R655. doi: 10.1152/ajpregu.00386.2005. [DOI] [PubMed] [Google Scholar]
- Schlenker E, Barnes L, Hansen S, Martin D. Cardiorespiratory and metabolic responses to injection of bicuculline into the hypothalamic paraventricular nucleus (PVN) of conscious rats. Brain Res. 2001;895:33–40. doi: 10.1016/s0006-8993(01)02011-x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Redburn DA. Modulatory actions of gamma aminobutyric acid (GABA) on GABA type A receptor subunit expression and function. J Neurosci Res. 1995;41:1–7. doi: 10.1002/jnr.490410102. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of g-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Smith ML, Pacchia CF. Sleep apnoea and hypertension: role of chemoreflexes in humans. Exp Physiol. 2007;92:45–50. doi: 10.1113/expphysiol.2006.033753. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R719–R725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- Tabata M, Kurosawa H, Kikuchi Y, Hida W, Ogawa H, Okabe S, Tun Y, Hattori T, Shirato K. Role of GABA within the nucleus tractus solitarii in the hypoxic ventilatory decline of awake rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1411–R1419. doi: 10.1152/ajpregu.2001.281.5.R1411. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res. 1998a;111:245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Li C, Levine S. The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology. 1998b;139:2838–2846. doi: 10.1210/endo.139.6.6037. [DOI] [PubMed] [Google Scholar]
- Wang D, Cui LN, Renaud LP. Pre- and postsynaptic GABAB receptors modulate rapid neurotransmission from suprachiasmatic nucleus to parvocellular hypothalamic paraventricular nucleus neurons. Neuroscience. 2003;118:49–58. doi: 10.1016/s0306-4522(02)00906-5. [DOI] [PubMed] [Google Scholar]
- Yeh ER, Erokwu B, LaManna JC, Haxhiu MA. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci Lett. 1997;232:63–66. doi: 10.1016/s0304-3940(97)00579-x. [DOI] [PubMed] [Google Scholar]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]