Abstract
In cardiac muscle, intracellular Ca2+ release is controlled by a number of proteins including the ryanodine receptor (RyR2), calsequestrin (CASQ2), triadin-1 (Trd) and junctin (Jn) which form a complex in the junctional sarcoplasmic reticulum (SR) membrane. Within this complex, Trd appears to link CASQ2 to RyR2 although the functional significance of this interaction is unclear. In this study, we explored the functional importance of Trd–CASQ2 interactions for intracellular Ca2+ handling in rat ventricular myocytes. A peptide encompassing the homologous CASQ2 binding domain of Trd (residues 206–230 in the rat; TrdPt) was expressed in the lumen of the SR to disrupt Trd–CASQ2 interactions. Myocytes expressing TrdPt exhibited increased responsiveness of SR Ca2+ release to activation by ICa as manifested by flattened and broadened voltage dependency of the amplitude of cytosolic Ca2+ transients. Rhythmically paced, TrdPt-expressing myocytes exhibited spontaneous arrhythmogenic oscillations of intracellular Ca2+ and membrane potential that was not seen in control cells. In addition, the frequency of spontaneous Ca2+ sparks and Ca2+ waves was significantly increased in TrdPt-expressing, permeabilized myocytes. These alterations in SR Ca2+ release were accompanied by a significant decrease in basal free intra-SR[Ca2+] and total SR Ca2+ content in TrdPt-expressing cells. At the same time a synthetic peptide corresponding to the CASQ2 binding domain of Trd produced no direct effects on the activity of single RyR2 channels incorporated into lipid bilayers while interfering with the ability of CASQ2 to inhibit the RyR2 channel. These results suggest that CASQ2 stabilizes SR Ca2+ release by inhibiting the RyR2 channel through interaction with Trd. They also show that intracellular Ca2+ cycling in the heart relies on coordinated interactions between proteins of the RyR2 channel complex and that disruption of these interactions may represent a molecular mechanism for cardiac disease.
In cardiac muscle, the release of Ca2+ from the sarcoplasmic reticulum (SR) mediates the process of excitation–contraction coupling causing the initiation of the heart beat. SR Ca2+ release is controlled by a multimolecular Ca2+ signalling complex, localized to the junctional SR (Zhang et al. 1997; Franzini-Armstrong & Protasi, 1997; Bers, 2004). The protein components of this complex include the cardiac ryanodine receptor (RyR2), the Ca2+ binding protein calsequestrin (CASQ2), and the junctional SR transmembrane proteins triadin 1 (Trd; Jones et al. 1995) and junctin (Jn; Kobayashi & Jones, 1999). RyR2 operates as a Ca2+ conducting pore (Meissner, 1994; Fill & Copello, 2002) while Trd and Jn have been proposed to anchor CASQ2 to RyR2 (Jones et al. 1995; Kobayashi & Jones, 1999). CASQ2 is a 45 kDa, highly acidic protein that binds Ca2+ with high capacity (40 ions per molecule) and low affinity (KD∼0.6 mm) and is located in the lumen of the SR (Mitchell et al. 1988; Beard et al. 2004). In addition to serving as a major Ca2+ reservoir in the SR (Jones et al. 1998; Terentyev et al. 2003; Knollmann et al. 2006), CASQ2 appears to modulate SR Ca2+ release by influencing the open probability of the RyR2 channel (Ikemoto et al. 1989; Gyorke et al. 2004; Knollmann et al. 2006), possibly via interactions with Trd and Jn (Zhang et al. 1997; Gyorke et al. 2004). Trd (40 kDa) and Jn (30 kDa) are structurally homologous proteins that are each composed of a single membrane spanning domain, a short cytoplasmic N-terminal segment and a long, highly positively charged C-terminal domain extending into the lumen of the SR (Jones et al. 1995; Kobayashi & Jones, 1999; Kobayashi et al. 2000). The luminal domains of both proteins are enriched in alternating positively and negatively charged amino acids, denoted as KEKE motifs, which have been proposed to be involved in protein–protein interactions. These putative protein binding domains are thought to provide the structural basis for coordinated interactions between the Trd and Jn and also with CASQ2 and RyR2 during EC coupling (Zhang et al. 1997). Consistent with this view, the CASQ2 binding domain of Trd was localized to a specific stretch of 25 amino acids (200–224) that encompass a single KEKE motif (Kobayashi et al. 2000), suggesting that the protein–protein interactions that occur between Trd and other components of the junctional complex are highly selective and organized.
Despite significant progress in defining the biochemical properties of the components of the RyR2 complex, our understanding of the functional role of the individual protein components of the junctional complex and the modes of their interactions is limited. In vitro reconstitution studies have revealed that CASQ2 inhibits the functional activity of RyR2 in planar lipid bilayers and these effects of CASQ2 are apparently mediated by Trd and Jn (Beard et al. 2004; Gyorke et al. 2004). As demonstrated by in vitro binding and reconstitution studies, the interaction of CASQ2 with Trd is Ca2+ dependent such that increasing [Ca2+] promotes dissociation of CASQ2 molecules from Trd (and possibly from Jn as well) (Zhang et al. 1997) and relieves the inhibition exerted by CASQ2 on RyR2 complexed with Trd and Jn (Gyorke et al. 2004). Ca2+-dependent communications between RyR2 and the luminal auxiliary proteins have been suggested to play a role in modulation of SR Ca2+ release during EC coupling (Zhang et al. 1997; Gyorke et al. 2004; Rios et al. 2006). In particular, inhibition of the RyR2 channel by CASQ2 at reduced luminal [Ca2+] has been proposed to contribute to SR Ca2+ release termination and release channel refractoriness following SR Ca2+ release in cardiac muscle (Gyorke et al. 2004). Supporting this idea, genetic alterations in CASQ2 that affect interactions of this protein with the RyR2 channel complex can lead to deregulated SR Ca2+ release and cardiac arrhythmia (Houle et al. 2004; Terentyev et al. 2006). However, the specific molecular mechanisms whereby RyR2 activity is regulated by interactions with its luminal binding partners remain to be defined. In the present study we explored the consequences of SR targeted expression of a peptide that mimics the putative CASQ2 binding domain of Trd on SR Ca2+ release in cardiac myocytes. We hypothesized that this peptide would act as a competitive inhibitor of Trd–CASQ2 interactions such that expression of this peptide in the SR should impair any functions that rely on these interactions. Our study shows that inhibiting CASQ2–Trd interactions resulted in hyperactive RyR2 channels and arrhythmogenic Ca2+ oscillations, thereby providing direct experimental evidence that Trd–CASQ2 interactions are required for regulated SR Ca2+ release in cardiac myocytes.
Methods
Construction of recombinant adenoviral vector
The region of the rat cardiac triadin 1 cDNA encoding the homologous CASQ2 binding domain (aas 206–230 for the rat) extending to naturally occurring proline residues (aas 180 and 255 to minimize disruption of protein structure) was amplified from rat heart cDNA using the Pfu thermostable polymerase (Stratagene, La Jolla, CA, USA) using the following primers: sense: GGCGTCGACGAGAAGAAAGCAACTCAC; antisense GCCGCGGCCGCTGGCGTTTCTTTGTCATC). These primers attach a SalI site at the 5′ end and NotI site at the 3′ end of the PCR product. The PCR product was A-tailed and subcloned into pGEM-Teasy vector (Promega, Madison, WI, USA) for sequence verification. The insert was then released with SalI and NotI subcloned in the pCMV/Myc/ER (Invitrogen, Carlsbad, CA, USA) plasmid, digested with the same enzymes. The triadin sequences were amplified along with flanking elements encoding the ER retention signal (KDEL) and the Myc epitope tag with the following primers: sense: GGCTCTAGAGCCATGGGATGGAGCTGTATC; antisense: GGCGGTACCCTACAGCTCGTCCTTCTC, which introduced a 5′XbaI site and 3′KpnI site. This PCR product was again sequence verified and subcloned into the pShuttle vector (Clontech, Mountain View, CA, USA) using the XbaI and KpnI sites. The pShuttle_Trd180–255 expression plasmid was transfected into HEK cells and the expression of the Trd180–255 polypeptide was confirmed by Western blotting using an antibody that recognizes the Myc epitope (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The TrdPt-MycER coding region was transferred into the Adeno-X Viral DNA, and recombinant adenoviruses were generated according to the manufacturers instructions (Clontech). The virus was amplified and purified using the BD Adeno-X virus purification kit (BD Biosciences, San Jose, CA, USA). Rat ventricular myocytes were infected with adenoviruses at a multiplicity of infection of 100 and cultured for 48–56 h (Terentyev et al. 2003).
Electrophysiological recordings
The procedure for cell isolation was approved by the Institutional Animal Care and Use Committee of Ohio State University and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). Adult 200–250 g Sprague–Dawley male rats were anaesthetized with penthobarbital sodium (25 mg kg−1i.p.). Following thoracotomy hearts were quickly excised, mounted and retrogradely perfused on a Langendorff apparatus with collagenase-containing solution at 37°C according to the method previously described (Terentyev et al. 2003). Sixteen hearts were used to isolate ventricular myocytes. Whole-cell patch clamp recordings of transmembrane ionic currents were performed with an Axopatch 200B amplifier (Axon Instruments). The external solution contained (mm): 140 NaCl, 5.4 KCl, 1.0 CaCl2, 0.5 MgCl2, 10 Hepes, and 5.6 glucose (pH 7.3). Patch pipettes (tip resistance of 1–3 MΩ) were filled with a solution that contained (mm): 90 caesium aspartate, 50 CsCl, 3 Na2ATP, 3.5 MgCl2 and 10 Hepes (pH 7.3). The myocytes were stimulated by application of 400 ms-long voltage pulses to specified membrane potentials from a holding potential of −50 mV at 1 min intervals (Viatchenko-Karpinski & Györke, 2001; Terentyev et al. 2003, 2005, 2006). Under these conditions, the SR Ca2+ content can be considered at steady state with respect to resting cytosolic [Ca2+] and the SR is fully loaded to a level set by the balance between SR Ca2+ leak and uptake. For current-clamp experiments Cs+ in the pipette solution was replaced with K+.
Confocal Ca2+ measurements
Intracellular Ca2+ imaging was performed using an Olympus Fluoview 1000 laser scanning confocal microscope equipped with an Olympus 60×, 1.4 NA oil objective. To simultaneously monitor the intra-SR and cytosolic Ca2+ levels, myocytes were loaded with Fluo-5N AM (10 μm for 7–9 h at 37°C, 95% air–5% CO2) as previously described (Shannon et al. 2003; Kubalova et al. 2004). Antibiotic-free Fluo-5N loading media contained: Medium 199M; 5 mm creatine; 5 mm taurine; 25 mm NaHCO3, 5 mm Hepes, pH 7.3. Subsequently, myocytes were permeabilized with saponin to remove the Fluo-5N from the cytoplasm and to introduce Rhod-2 (30 μm) to the internal solution for cytosolic Ca2+ measurements. The control experimental solution contained (mm): 120 potassium aspartate, 20 KCl, 3 MgATP, 10 phosphocreatine, 5 U ml−1 creatine phosphokinase, 0.5 EGTA and 20 Hepes (pH 7.2). Calcium waves were initiated by lowering EGTA in the internal solution from 0.5 to 0.1 mm, free [Ca2+]∼75 nm. Fluo-5N and Rhod-2 were excited sequentially by 488 and 543 nm laser lines, and fluorescence was acquired at a rate of 5 ms per line at wavelengths of 500–530 and > 560 nm, respectively. Ca2+ sparks in permeabilized myocytes and Ca2+ transients in patch-clamped cells were recorded using Ca2+ indicator Fluo-3 in intracellular solution (30 and 50 μm, respectively). Fluo-3 was excited by the 488 nm line of an argon ion laser, and the fluorescence was acquired at wavelengths > 510 nm in the line-scan mode of the confocal system at the rate of 2–5 ms per scan. For quantitative studies, the temporal dynamics in fluorescence were expressed as:
where F represents fluorescence at time t and FCaf represents the fluorescence level of the myocytes upon application of 10 mm caffeine, or:
where F0 stands for baseline fluorescence (Kubalova et al. 2005). All chemicals were from Sigma and all Ca2+ indicators were from Molecular Probes.
Bilayer experiments
Heavy SR microsomes were isolated from canine LV tissue. Canine cardiac RyR2s were purified from SR vesicles and native or purified RyR2s reconstituted into proteoliposomes and fused into planar lipid bilayers for single-channel recordings as previously described (Gyorke et al. 2004). The experimental solutions contained (mm): 350 CsCH3SO3, 3 MgATP, 0.6 MgCl2 (free [Mg2+] 0.9 mm), 0.02 CaCl2, and 20 Hepes, pH 7.4 (cis), and 350 CsCH3SO3, 0.02 CaCl2, and 20 Hepes, pH 7.4 (trans). The holding potential in all experiments was +40 mV. Single-channel currents were recorded with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Union City, CA, USA). Acquisition and analysis of data were performed using pCLAMP 6.01 software (Axon Instruments). A custom peptide corresponding to amino acids 200–224 of canine Trd (sequence: KEERKAKTEEKIKKEVKGGKQEKVK) which contains the putative CASQ2-binding domain (Kobayashi et al. 2000) was synthesized by GenScript Corp., NJ, USA. Recombinant purified canine cardiac calsequestrin (Kobayashi et al. 2000; Gyorke et al. 2004), was obtained as described. CASQ2 was stored and added from a buffer solution containing 20 mm Mops, 150 mm NaCl (pH 7.0), and 3 mg ml−1 of the protein.
Immunocytochemistry
Myocytes were fixed on glass coverslips with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100/citrate buffer (pH 7.2) containing 1% BSA. Specimens were incubated with anti-CASQ2 antibody (rabbit, PA1-913, ABR, 1: 2000) and anti-Myc Tag antibody (mouse, 9B11, Cell Sign. Technol. Inc., 1: 1000); followed by secondary antibodies antirabbit Alexa Fluor 488 and antimouse Alexa Fluor 546 (A11034 and A11003, respectively, 1: 200, Molecular Probes).
Statistical analysis of data
Data are presented as means ±s.e.m. Paired or unpaired data sets were statistically evaluated using Student's t test or by one-way ANOVA test, when appropriate, and significance was defined at P < 0.05.
Results
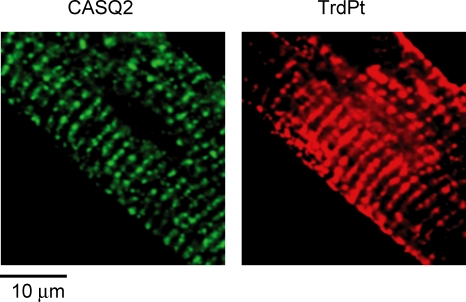
A domain through which Trd interacts with CASQ2 has been localized to a homologous region in the luminal C-terminal of Trd (amino acids 200–224) which contains a single KEKE motif (Kobayashi et al. 2000). To investigate the functional significance of Trd–CASQ2 interactions for calcium handling, we developed a method to inhibit Trd–CASQ2 interactions by expressing a decoy peptide containing a stretch of residues corresponding to the CASQ2 binding domain of Trd (TrdPt) in cardiac myocytes. The appropriate region of Trd cDNA was inserted into an adenoviral vector together with the KDEL ER/SR targeting sequence to generate Ad-TrdPt for subsequent gene transfer into adult rat ventricular myocytes. In addition, a short Myc-tag encoding sequence was added to the transgene to permit verification of expression of the peptide in the ER/SR compartment using a Myc tag-specific antibody (Fig. 1).
Figure 1.
SR localization in ventricular myocytes of adenovirally expressed TrdPt CASQ2 and TrdPt were coimmunostained with CASQ2 and Myc Tag-specific antibodies using green and red fluorescent tagged secondary antibodies, respectively. Images were recorded simultaneously with an Olympus Fluoview-1000 spectral confocal system. Alexa-Fluo 488 was excited with the 488 nm argon laser line and emission was collected at 500–530 nm, while Alexa Fluo-546 was excited at 543 nm and emission recorded at > 570 nm. Measurements were performed 48–56 h after infection.
ICa and intracellular Ca2+ transients
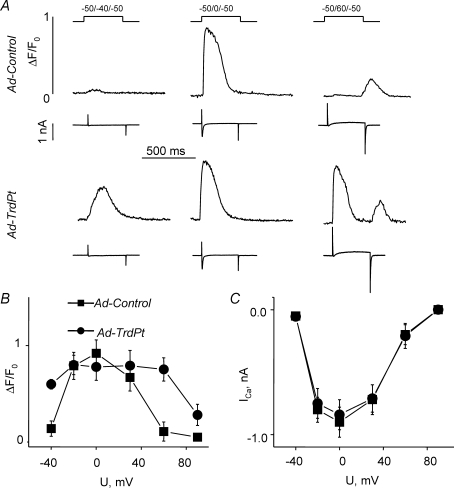
We examined the effects of expression of TrdPt on excitation–contraction coupling in adult rat ventricular myocytes. L-type Ca2+ currents and intracellular Ca2+ transients were simultaneously measured in myocytes depolarized to various membrane potentials (in the range from −40 to +100 mV) (Fig. 2). Expression of TrdPt resulted in a dramatic augmentation of the amplitude of Ca2+ transients at low and high depolarizing steps (where ICa amplitude is small) without a significant change in the amplitude of the maximal Ca2+ transients (at 0 mV) with respect to control (Fig. 2A). Accordingly, the voltage dependence of Ca2+ transients was broadened and flattened in TrdPt myocytes (Fig. 2B). These alterations in Ca2+ transients occurred without changes in the amplitude of ICa, the trigger signal for SR Ca2+ release, at different membrane potentials (Fig. 2C). Therefore TrdPt stimulated SR Ca2+ release by sensitizing it to activation by ICa.
Figure 2.
Loss of voltage dependency of Ca2+ release in cells expressing TrdPt A, traces of ICa (lower traces) and intracellular Ca2+ transients (upper traces) evoked by depolarization steps from −50 mV HP to different voltages in myocytes infected with Ad-TrdPt and Ad-Control vectors. B and C, voltage dependencies of Ca2+ transients (B) and ICa (C) in myocytes infected with Ad-Control (▪), and Ad-TrdPt (•) vectors (n from 6 to12). Average rise times and maximal rates of rise of Ca2+ transients evoked by depolarization to 0 mV were not significantly different for control and TrdPt expressing myocytes (32 ± 4 ms and 0.49 ± 0.12 ms−1 (n = 6) versus 24 ± 4 ms and 0.49 ± 0.16 ms−1 (n = 5), respectively).
TrdPt expression induces cellular arrhythmia
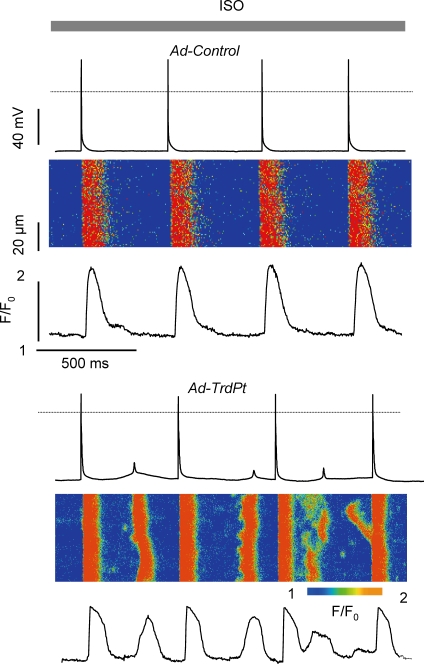
We have previously shown that ectopic expression of specific CASQ2 mutants associated with CPVT or overexpressing WT Trd lead to arrhythmic oscillations in Ca2+ transients and membrane potential in paced cardiac myocytes exposed to ISO (Terentyev et al. 2005, 2006). As both of these interventions may function by disrupting interactions between the components of the RyR2 complex, we next tested whether ectopic expression of TrdPt would cause similar alterations in cytosolic Ca2+ and membrane potential (MP) signals. Therefore, TrdPt-overexpressing and control myocytes were subjected to rhythmic pacing in the presence of ISO. As shown in Fig. 3, rhythmically paced, TrdPt-overexpressing myocytes exhibited profound arrhythmogenic disturbances in Ca2+ cycling and MP manifested by after-Ca2+ transients and delayed afterpolarizations (DADs). Similar results were obtained in four independent TrdPt-overexpressing myocytes (n = 5 from 6) while control myocytes exhibited no such behaviour under identical conditions (n = 0 from 5). Accordingly, average peak diastolic Fluo-3 fluorescence (F/F0) was 1.51 ± 0.20* and 1.08 ± 0.07 in TrdPt (n = 6) and control (n = 5) myocytes, respectively (*significantly different at P < 0.05).
Figure 3.
Arrhythmogenic disturbances in Ca2+ cycling in myocytes expressing TrdPt Whole cell current clamp recordings of membrane potential (upper traces), line-scan images of Ca2+ transients (middle) and time dependent profiles (lower traces) in the presence of 1 μm isoproterenol. Stimulation frequency was 2 Hz.
Spontaneous Ca2+ sparks and Ca2+ waves
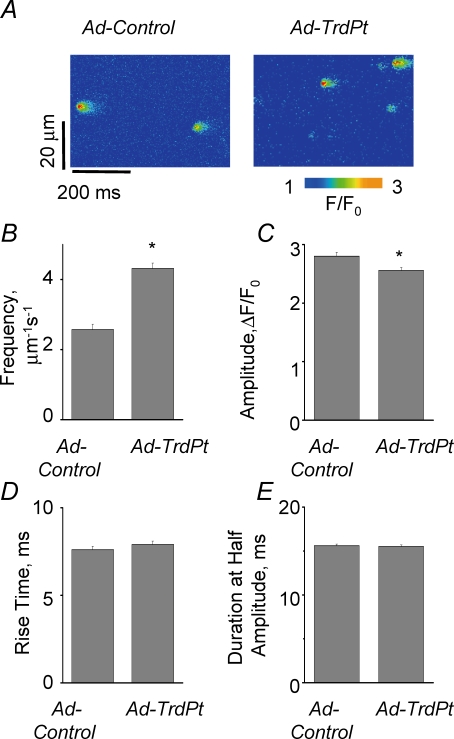
To further investigate the effects of TrdPt-expression on the properties of SR Ca2+ release, we recorded spontaneous Ca2+ sparks in myocytes permeabilized with saponin. In these experiments, [Ca2+] was buffered at ∼75 nm with 0.5 mm EGTA. As shown in Fig. 4, ectopic expression of TrdPt resulted in a significant increase in Ca2+ spark frequency. At the same time, although there was no significant difference for the average amplitude of all detected events, the magnitude of the 5% of the brightest sparks (most likely to be situated at the centre of the line scan) significantly decreased in TrdPt-expressing myocytes compared to control (ΔF/F0 was 2.52 ± 0.05 (n = 29) and 2.80 ± 0.07 (n = 18), respectively; P < 0.05). Thus, the observed effects of TrdPt expression on cell-averaged Ca2+ signals could be ascribed to increased functional activity of individual Ca2+ release sites, i.e. RyR2 clusters.
Figure 4.
Expression of TrdPt leads to increased frequency of spontaneous Ca2+ sparks in permeabilized myocytes A, representative line scan images of Ca2+ sparks in cells infected with adenoviral constructs. B, C, D and E, bar graphs of pooled values of spark frequency (B), amplitude of 5% of brightest events (C), rise time (D) and spark duration at half-amplitude (E) for the same groups of cells. *Significantly different at P < 0.05; one-way ANOVA (n of Ca2+ sparks was 388 and 565; n of cells was 73 and 76 for Ad-Control and Ad-TrdPt-infected myocytes, respectively).
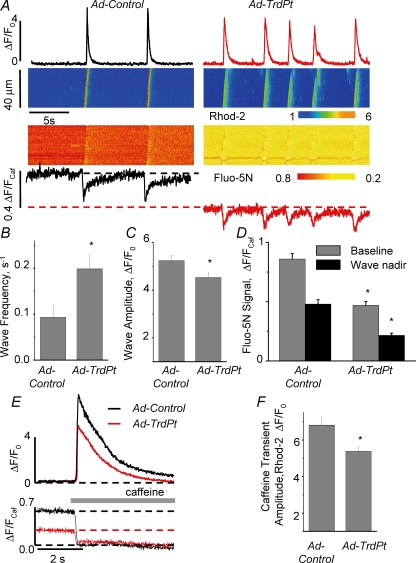
In addition to recording Ca2+ sparks we also measured spontaneous propagating Ca2+ waves by monitoring [Ca2+] in both extra- and intra-SR compartments in saponin-permeabilized myocytes. In these experiments, cytosolic [Ca2+] was monitored with Rhod-2 whereas [Ca2+]SR was tracked with Fluo-5N loaded into the SR (see below). Similar to the effects upon Ca2+ sparks, TrdPt expression increased the frequency of occurrence of Ca2+ waves (Fig. 5A and B). Additionally, the amplitude of the waves was significantly decreased in TrdPt-expressing myocytes with respect to control (Fig 5A and C). These results provide further evidence that TrdPt stimulates SR Ca2+ release making it susceptible to spontaneous activation.
Figure 5.
Effects of expression of TrdPt on properties of cytoplasmic and intra-SR Ca2+ waves in permeabilized myocytes A, line-scan images along with time-dependent profiles of Rhod-2 and Fluo-5N fluorescence. B, C, D and F, pooled data on wave frequency (B), cytosolic wave amplitude measured with Rhod-2 (C), changes in the baseline [Ca2+]SR and intra-SR [Ca2+] wave nadir measured with SR-entrapped Fluo-5N (D) and amplitude of cytosolic caffeine-induced Ca2+ transients (F). E, caffeine-evoked Ca2+ transients (upper panel) and depletion signals (lower panel) recorded in myocytes infected with Ad-Control and Ad-TrdPt vectors. *Significantly different at P < 0.05; one-way ANOVA (n from 8 to 17).
Intra-SR [Ca2+]
Activation of RyR2 by luminal Ca2+ is thought to play an important role in the generation of spontaneous Ca2+ release in cardiac muscle. In various pathological states, increased spontaneous Ca2+ release has been associated with either elevated SR Ca2+ content (Orchard et al. 1983; Stern et al. 1988; Lipp & Niggli, 1993; Diaz et al. 1997) or sensitization of RyR2s to luminal Ca2+ (Kubalova et al. 2005; Jiang et al. 2005). Thus, we investigated the effects of ectopic expression of TrdPt on intra-SR Ca2+ levels in permeabilized myocytes to determine which of these mechanisms contributed to the altered Ca2+ release properties (Fig. 5). Free [Ca2+]SR was monitored with SR-entrapped Fluo-5N (simultaneously with cytosolic [Ca2+]; Fig. 5A), whereas the total SR Ca2+ content was estimated from the amplitude of the cytosolic Ca2+ transients induced by application of caffeine (Fig. 5E). In comparison with control cells, basal free intra-SR [Ca2+] and the extent of [Ca2+]SR depletion (nadir) during waves were significantly reduced in TrdPt myocytes (Fig. 5D). Similarly, the total SR Ca2+ content was significantly lowered in TrdPt myocytes (Fig. 5F). These results suggest that the increased propensity of TrdPt-expressing myocytes for spontaneous Ca2+ release is attributable to sensitization of RyR2 channels to luminal Ca2+ rather than to increased levels of Ca2+ in the SR.
SERCA2a-mediated SR [Ca2+] uptake
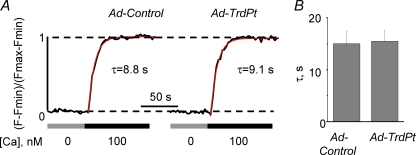
Although designed to specifically inhibit CASQ2 interaction with the RyR2 complex, the TrdPt peptide could produce its effects on myocyte Ca2+ signalling via non-specific interactions with other targets in the SR such as SERCA2a. To test whether TrdPt had an effect on the SERCA2a pump, we performed measurements of SERCA2a-mediated SR [Ca2+] uptake in permeabilized myocytes using SR-entrapped Fluo-5N. In this series of experiments, the SR was first depleted with 10 mm of caffeine, then caffeine was washed out with a [Ca2+]-free solution and ruthenium red (10 μm) was applied to block the RyR2 channels. In the presence of ruthenium red, SR [Ca2+] uptake was initiated by application of 100 nm of [Ca2+] and monitored by measuring the rise of SR [Ca2+] with Fluo-5N. Representative traces and pooled data for SR [Ca2+] uptake kinetics in control and TrdPt-expressing myocytes are shown in Fig. 6. In TrdPt-expressing cells the time constant of SR [Ca2+] was not significantly different from that measured in the control group. These results indicate that intrinsic SERCA2a Ca2+ transport activity was not affected by TrdPt.
Figure 6.
SERCA2a-mediated SR Ca2+ uptake is not significantly altered in TrdPt expressing myocytes A, time course of SR Ca2+ uptake, measured with SR-entrapped Fluo-5N in permeabilized control and TrdPt-expressing myocytes in the presence of 10 μm ruthenium red. Intra-SR [Ca2+] was depleted with 10 mm caffeine; caffeine was washed out with a Ca2+-free solution; then ruthenium red was applied and SR Ca2+ uptake initiated by raising cytosolic [Ca2+] to 100 nm[Ca2+]. B, pooled data for the time constants from single exponential fits of SR Ca2+ uptake. n was 11 and 12 for control and TrdPt expressing cells, respectively.
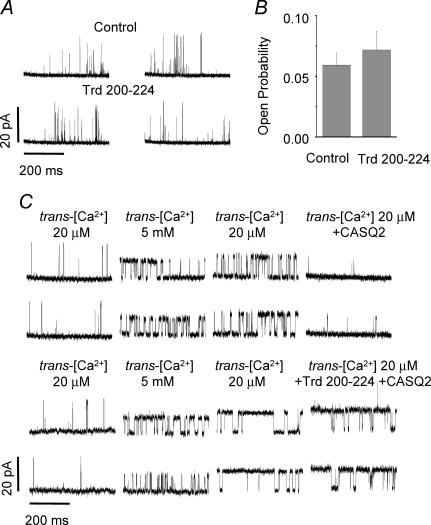
Single RyR2 channel activity
In principle, the TrdPt peptide could exert its effects on SR Ca2+ release by direct activation of the RyR2 rather than by impairing protein–protein interactions between Trd and CASQ2. To examine the ability of TrdPt to directly activate RyR2 through the 200–224 KEKE protein binding motif, we investigated the effects of a synthetic peptide corresponding to this region of Trd on the activity of purified single RyR2 channels in planar lipid bilayers. Single-channel currents were recorded with Cs+ as the charge carrier (Gyorke et al. 2004). Addition of this peptide (5–50 μg per ml) to the luminal (trans) side of the channel did not significantly affect RyR2 open probability (Fig. 7A and B). To directly examine whether TrdPt acted by interfering with protein–protein interactions within the RyR2 complex, we preformed experiments with combined additions of TrdPt and purified CASQ2 to the luminal side of the channel (Fig. 7C). Native RyR2s were first stripped of CASQ2 by increasing trans[Ca2+] from 20 μm to 5 mm (Gyorke et al. 2004). After reverting back to low trans[Ca2+], addition of CASQ2 (5 μg ml−1) resulted in reduction of RyR2 open probability, as previously described (Gyorke et al. 2004; Terentyev et al. 2006). However, when the introduction of CASQ2 was preceded by addition of TrdPt (50 μg ml−1), CASQ2 failed to inhibit the RyR2 channel. Therefore, TrdPt appears to lack the ability to activate the RyR2 channel directly and rather acts by interfering with the ability of CASQ2 to inhibit the RyR2.
Figure 7.
A synthetic decoy peptide corresponding to CASQ2 binding domain of Trd (residues 200–224) interferes with CASQ2 inhibitory action on RyR2 channel complex Trd 200–224 does not modulate activity of purified RyR2 channels. A, representative single RyR2 channel traces. B, pooled data on open probability of purified RyR2s before and after addition to the trans chamber 5–50 μm of the Trd decoy peptide (n = 8). C, representative single-channel traces illustrating the irreversibility of the effects of 5 mm luminal Ca2+ and restoration of initial low activity by CASQ2 added to the trans chamber in native RyR2s (upper panel). Trd 200–224 interferes with the ability of CASQ2 to inhibit native RyR2s (lower panel). The Po values were 0.06 ± 0.01 for low trans[Ca2+] (20 μm); 0.29 ± 0.07 for high trans[Ca2+] (5 mm); 0.41 ± 0.12 reverting to low trans[Ca2+] (20 μm); and 0.04 ± 0.01 (n = 6) versus 0.48 ± 0.08 (n = 4)* after application of 5 μg ml−1 of CASQ2 alone or with 50 μg ml−1 of Trd 200–224. The data are presented as means ±s.e.m.*Significantly different versus CASQ2 alone at P < 0.05 (one-way ANOVA).
Discussion
Growing evidence suggest that interactions of the RyR2 channel with the luminal auxiliary proteins CASQ2 and Trd might be involved in regulation of SR Ca2+ release in cardiac muscle. However, the exact roles of these proteins in cardiac EC coupling remain to be defined. We have studied the functional significance of interactions between CASQ2 and Trd by expression of a peptide that mimics the CASQ2 binding domain of Trd and thus should act as a competitive inhibitor of CASQ2–Trd interactions in cardiac myocytes. Our data demonstrate that this decoy peptide stimulates the functional activity of the Ca2+ release channels, leading to deregulated SR Ca2+ release and arrhythmogenic delayed afterdepolarizations (DADs). These results provide new insights into the molecular events involved in regulation of SR Ca2+ release and show how interference with these events can lead to pathological states such as cardiac arrhythmia.
SR-targeted expression of the TrdPt peptide compromised the ability of the Ca2+ release mechanism to respond to ICa in a graded manner and increased the frequency of spontaneous Ca2+ sparks and Ca2+ waves in intact and permeabilized myocytes. TrdPt expression did not affect SERCA2a Ca2+ transport activity but led to a significant decrease in both free and total SR Ca2+ content consistent with increased Ca2+ leakage from the SR. These effects are indicative of a stimulatory influence of the peptide on the RyR2 channel. Since by design, the sequence of the peptide corresponds to the CASQ2 binding domain of Trd (Kobayashi et al. 2000), we attribute these effects to competitive inhibition by the peptide of CASQ2–Trd interaction in myocytes. This interpretation is strongly supported by the results of our in vitro reconstitution experiments on RyR2s incorporated into lipid bilayers. According to previous studies (Wang et al. 2000; Gyorke et al. 2004), these experiments showed that CASQ2 added to the luminal side of RyR2s stripped of CASQ2 inhibits their open probability. Notably, a synthetic peptide corresponding to the CASQ2 binding domain of Trd (residues 200–224) prevented CASQ2 from inhibiting RyR2s without affecting RyR2 activity directly. Therefore, collectively, our results suggest that the TrdPt affects SR Ca2+ release by altering interactions between CASQ2 and Trd.
The functional effects of TrdPt expression on SR Ca2+ release were qualitatively similar to those observed previously in myocytes overexpressing the full-length wild-type Trd protein (Terentyev et al. 2005). A likely explanation for these results is that expressions of the TrdPt and the full-length protein have similar effects on the function of the junctional complex, presumably involving the disruption of normal protein–protein interactions mediated by the KEKE motif within the Trd decoy peptide. In support of this interpretation, we previously demonstrated that a Trd deletion mutant protein that lacks the fragment encompassing 200–224 failed to exert the functional effects on SR Ca2+ release and RyR2 activity observed with the WT protein or the TrdPt peptide (Terentyev et al. 2005). Although more difficult to interpret due to various adaptive changes, the results obtained with chronic overexpression or ablation of Trd, and Jn in transgenic mouse models also indicated that normal intracellular Ca2+ handling relies on an intricate balance between the components of the RyR2 complex including Jn Trd and CASQ2 (Kirchhefer et al. 2001; Muller et al. 2002; Yuan et al. 2007).
Based on our in vitro reconstitution studies we have previously proposed that CASQ2 modulates the activity of the RyR2 channel in a Ca2+-dependent manner through interaction with Trd and Jn (Gyorke et al. 2004). In particular, CASQ2 inhibited RyR2 complexed with Trd at low luminal [Ca2+] whereas this inhibitory function was not observed at elevated luminal [Ca2+]. The results of the present study, which show that disruption of CASQ2–Trd interactions leads to enhanced and destabilized SR Ca2+ release, are consistent with this mechanism and present, to our knowledge, the first experimental evidence for functionally relevant interactions between CASQ2 and Trd in intact myocytes. These results also predict that genetic defects in CASQ2 and Trd that alter normal communication between these proteins could lead to cardiac arrhythmia. In support of this prediction, we recently showed that a specific CPVT-linked mutation in CASQ2 (CASQ2R33Q) compromised the ability of CASQ2 to inhibit RyR2 activity and produced effects similar to those exerted by the TrdPt peptide when expressed in myocytes (Terentyev et al. 2006).
In summary, our results provide evidence for functionally relevant structure-specific interactions between the SR luminal proteins CASQ2 and Trd in cardiac excitation–contraction coupling. Since disruption of these interactions resulted in potentiation of the Ca2+ release channels, we conclude that CASQ2 exert an inhibitory influence on the RyR2 channel through interaction with the luminal domain of Trd. Thus, our results show that intracellular Ca2+ cycling in heart relies on an intricate interplay between proteins of the RyR2 channel complex and disruption of these interactions may present a molecular mechanism for cardiac disease.
Acknowledgments
This work was supported by NIH grants HL-74045 and HL-63043 (SG) and by American Heart Association SDG grants for D.T. and S.V.-K. We thank Dr Debjani Tripathy for isolation of rat Trd DNA.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.136879/DC1
and
http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.136879
References
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Diaz ME, Trafford AW, O'Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol. 1997;501:3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle T, Ram M, Cala SE. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc Res. 2004;64:227–233. doi: 10.1016/j.cardiores.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Ikemoto N, Ronjat M, Meszaros LG, Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989;28:6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest. 1998;101:1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LR, Zhang L, Sanborn K, Jorgensen AO, Kelley J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J Biol Chem. 1995;270:30787–30796. doi: 10.1074/jbc.270.51.30787. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Neumann J, Baba HA, Begrow F, Kobayashi YM, Reinke U, Schmitz W, Jones LR. Cardiac hypertrophy and impaired relaxation in transgenic mice overexpressing triadin1. J Biol Chem. 2001;276:4142–4149. doi: 10.1074/jbc.M006443200. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi YM, Alseikhan BA, Jones LR. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged β-strand in mediating the protein–protein interaction. J Biol Chem. 2000;275:17639–17646. doi: 10.1074/jbc.M002091200. [DOI] [PubMed] [Google Scholar]
- Kobayashi YM, Jones LR. Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium. J Biol Chem. 1999;274:28660–28668. doi: 10.1074/jbc.274.40.28660. [DOI] [PubMed] [Google Scholar]
- Kubalova Z, Gyorke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, Gyorke S. Modulation of cytosolic and intra-SR calcium waves by calsequestrin in cardiac myocytes. J Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993;65:2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Mitchell RD, Simmerman HK, Jones LR. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988;263:1376–1381. [PubMed] [Google Scholar]
- Muller FU, Kirchhefer U, Begrow F, Reinke U, Neumann J, Schmitz W. Junctional sarcoplasmic reticulum transmembrane proteins in the heart. Basic Res Cardiol. 2002;97(Suppl. 1):I52–I55. doi: 10.1007/s003950200030. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Rios E, Launikonis BS, Royer L, Brum G, Zhou J. The elusive role of store depletion in the control of intracellular calcium release. J Muscle Res Cell Motil. 2006;27:337–350. doi: 10.1007/s10974-006-9082-5. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- Stern MD, Capogrossi MC, Lakatta EG. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988;9:247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Williams SC, Gyorke S. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96:651–658. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Györke S. Modulation of the Ca2+ -induced Ca2+ release cascade by β-adrenergic stimulation in rat ventricular myocytes. J Physiol. 2001;533:837–848. doi: 10.1111/j.1469-7793.2001.t01-1-00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cleemann L, Jones LR, Morad M. Modulation of focal and global Ca2+ release in calsequestrin-overexpressing mouse cardiomyocytes. J Physiol. 2000;524:399–414. doi: 10.1111/j.1469-7793.2000.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, Hahn HH, Zhao W, Waggoner JR, Jones LR, Jones WK, Bers DM, Dorn GW, 2, Wang HS, Valdivia HH, Chu G, Kranias EG. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.