Abstract
Store-operated channels (SOCs) are plasma membrane Ca2+-permeable cation channels which are activated by agents that deplete intracellular Ca2+ stores. In smooth muscle SOCs are involved in contraction, gene expression, cell growth and proliferation. Single channel recording has demonstrated that SOCs with different biophysical properties are expressed in smooth muscle indicating diverse molecular identities. Moreover it is apparent that several gating mechanisms including calmodulin, protein kinase C and lysophospholipids are involved in SOC activation. Evidence is accumulating that TRPC proteins are important components of SOCs in smooth muscle. More recently Orai and STIM proteins have been proposed to underlie the well-described calcium-release-activated current (ICRAC) in non-excitable cells but at present there is little information on the role of Orai and STIM proteins in smooth muscle. In addition it is likely that different TRPC subunits coassemble as heterotetrameric structures to form smooth muscle SOCs. In this brief review we summarize the diverse properties and gating mechanisms of SOCs in smooth muscle. We propose that the heterogeneity of the properties of these conductances in smooth muscle results from the formation of heterotetrameric TRPC structures in different smooth muscle preparations.
In smooth muscle cells agonists of G-protein-coupled receptors (GPCRs) produce contraction by increasing cytosolic calcium concentration ([Ca2+]c). This increase in [Ca2+]c results from release of Ca2+ ions from intracellular stores, mainly the sarcoplasmic reticulum (SR), and influx of Ca2+ ions from the extracellular medium. A component of this Ca2+ influx is mediated by voltage-dependent Ca2+ channels (VDCCs) but a significant contribution of Ca2+ influx occurs independently of VDCCs (Large, 2002). Moreover the contribution of Ca2+ influx through voltage-independent channels varies markedly according to the type of smooth muscle. This is part of the explanation underlying the observation that blockers of L-type Ca2+ channels are more effective in blocking Ca2+ influx and contraction of some smooth muscle preparations (e.g. resistance blood vessels and intestine) compared to other tissues (e.g. tracheal smooth muscle and many large blood vessels; see Bolton, 1979). Thus, during cell stimulation in the former preparations most of the Ca2+ influx occurs through VDCCs while in the latter tissues Ca2+ enters the cell mainly through voltage-independent Ca2+ channels. There has been much research recently into voltage-independent Ca2+ channels in smooth muscle not only because of their physiological importance but also because these pathways represent realistic targets for therapeutic intervention in important diseases involving excessive smooth muscle contraction such as hypertension and asthma.
Similar to many other cell types there is significant evidence for the expression of Ca2+-permeable cation channels in smooth muscle which are classified as receptor-operated (ROCs) and store-operated channels (SOCs; see reviews by Large, 2002; McFadzean & Gibson, 2002; Albert & Large, 2003; Beech et al. 2004). Moreover there is accumulating evidence that members of the canonical subgroup of transient receptor potential (TRPC) channels constitute both ROCs and SOCs in smooth muscle. ROCs are activated in response to cell surface receptor (usually GPCR) stimulation which is independent of depletion of internal Ca2+ stores. For example, the native TRPC6-like channel in rabbit portal vein is activated by noradrenaline acting on α-adrenoceptors but this channel is not stimulated by agents that directly release Ca2+ from the SR (Wang & Large, 1991; Inoue et al. 2001). Generally ROCs are activated by GPCRs coupled to phospholipase C (PLC) although in rabbit ear artery myocytes ROCs may be constitutively driven by phospholipase D (Albert et al. 2005). In contrast SOCs are plasmalemmal ion channels which are proposed to be stimulated by depletion of internal Ca2+ stores. The strict definition of a SOC is a channel which is activated by a decrease in the Ca2+ concentration within the SR ([Ca2+]SR, or endoplasmic reticulum, ER) and not by the subsequent rise (or reduction) in [Ca2+]c (Parekh & Putney, 2005). Further it is sometimes stated that SOCs are not activated by second messengers generated by PLC (Lewis, 2007). An important question is whether a single cellular mechanism activates SOCs and several hypotheses have been tested over the years (see Parekh & Putney, 2005 for a detailed review). In the present review we will briefly summarize the properties of SOCs in smooth muscle where they have been studied at the single channel level. It is intended to highlight the fact that SOCs in smooth muscle have diverse biophysical properties and multiple activation mechanisms and to indicate possible molecular explanations for these different characteristics.
Single channel properties of SOCs in smooth muscle
In physiological conditions SOCs are evoked by stimulation of plasmalemmal GPCRs (e.g. α-adrenoceptors in vascular smooth muscle) coupled to PLC with subsequent formation of inositol-1,4,5-trisphosphate (IP3) which causes release of Ca2+ ions from the SR and subsequent opening of SOCs. However, receptor agonists are rarely used to study SOCs because they are expected to simultaneously activate ROCs which would complicate interpretation of data from such experiments. Consequently the usual method to record SOC activity in isolation is to study responses evoked by the selective SR Ca2+-ATPase (SERCA) inhibitors thapsigargin and cyclopiazonic acid (CPA). In smooth muscle the first recording of a store-operated conductance was measured with whole-cell recording from a single mouse anococcygeus myocyte treated with CPA (Wayman et al. 1996). This type of experiment has been carried out in several laboratories (see Albert & Large, 2003) but often the [Ca2+]c is not buffered to a fixed concentration and it is possible that the observed current is therefore evoked by changes in [Ca2+]c.
The gold standard method of recording SOC activity is to measure whole-cell currents evoked by depleting intracellular Ca2+ stores, e.g. with SERCA inhibitors, while clamping the [Ca2+]c to approximately resting levels with high (10 mm) concentrations of a calcium chelator such as BAPTA or EGTA. It is important to point out that these conditions are unlikely to be achieved physiologically and cell stimulation will normally be accompanied by an increase in [Ca2+]c. Nevertheless such experiments have demonstrated that CPA induces a whole-cell current in freshly dispersed rabbit portal vein myocytes (Albert & Large, 2002a; Liu et al. 2005b) and human airway smooth muscle (Peel et al. 2006). In these conditions it is assumed that the channel underlying the whole-cell current is a SOC according to the strict definition of SOC activation.
However, there is a great advantage in recording SOC activity at the single channel level because it is possible to be more confident of studying a single molecular mechanism which may not be the case with whole-cell recording or measurement of [Ca2+]c with dyes. There have been several studies which demonstrate that single Ca2+-permeable cation channels with unitary conductances of 2–5 pS can be recorded in the cell-attached configuration in isolated smooth muscle cells in response to application of CPA or thapsigargin (Table 1, mesangial cells are included because they possess a similar phenotype to smooth muscle). In contrast the widely studied Ca2+-release-activated current (ICRAC) has not been studied at the single channel level but a unitary conductance has been estimated to be approximately 0.02 pS from stationary noise analysis (Table 1). The disparity in unitary conductances highlights important differences between SOCs in smooth muscle and ICRAC in non-excitable cells and indicates that these channels are likely to possess different molecular structures (see later).
Table 1.
Comparison of the biophysical properties of store-operated channel current (Isoc) in vascular myocytes with calcium-release-activated current (ICRAC) in non-excitable cells
| Cell type | Single channel conductance | PCa/PNa | Activation mechanisms | Molecular components of channel | |
|---|---|---|---|---|---|
| Isoc | Aortaa,b | ∼3 pS | 1: 1 | CIF, iPLA2 and LPLs | — |
| Portal veinc,d,e | ∼2 pS | 50: 1 | PKC, CaM | TRPC1/C5f | |
| Mesenteric arteryg | ditto | ditto | PKC | TRPC1g/C5f | |
| Pulmonary arteryh | ∼5 pS (20 mm[Ca2+]o) | — | — | — | |
| Mesangial cellsi,j,k | ∼3 pS | — | PKC | TRPC4 | |
| Icracl,m | Mast cells | ∼0.02 pS (110 mm[Ca2+]o) | 1000: 1 | STIM1 | Orai1 |
| RBL-1/2H3 | |||||
| Jurkat T cells |
—, information not available; CIF, calcium influx factor; iPLA2, calcium-independent phospholipase A2; LPLs, lysophospholipids; PKC, protein kinase C; CaM, calmodulin. aTrepakova et al. (2001); bSmani et al. (2004); cAlbert & Large (2002a); dAlbert & Large (2002b); eAlbert et al. (2006b); fauthors' unpublished data, see Fig. 4; gSaleh et al. (2006); hGolovina et al. (2001); iMa et al. (2000); jMa et al. (2002); kWang et al. (2004); lParekh & Putney (2005); mLewis, 2007). Unless indicated in parentheses, the conductance values were obtained in 1.5 mm[Ca2+]o.
Some of these studies were carried out with cell-attached recording but this configuration is limited in that it is likely that SERCA inhibitors raise [Ca2+]c which may be the stimulus to activate the assumed SOCs rather than depletion of [Ca2+]SR. However, it has been shown that the cell-permeable Ca2+ chelator BAPTA-AM, which will decrease [Ca2+]c and hence passively deplete [Ca2+]SR, also activates single channels with identical properties to those induced by thapsigargin in aortic myocytes (Trepakova et al. 2001) and CPA in rabbit portal vein (Albert & Large, 2002a) and mesenteric artery (Saleh et al. 2006). Hence it seemed reasonable to conclude that the single channels recorded with the cell-attached configuration were genuine SOCs.
Diverse biophysical properties and activation mechanisms of SOCs in vascular smooth muscle
Parekh & Putney (2005) have discussed the variety of store-operated calcium entry mechanisms in different cell types but it also seems that there are at least two classes of SOCs in smooth muscle. This issue is described in detail by Albert & Large (2003) but Table 1 illustrates the two main different biophysical characteristics of SOCs in rabbit aortic myocytes compared to portal vein/mesenteric artery myocytes. The unitary conductances are similar in all three preparations in 1.5 mm extracellular Ca2+ concentration ([Ca2+]o) but they have significantly different Ca2+ permeabilities. SOCs in portal vein/mesenteric artery myocytes are approximately 50 times more permeable to Ca2+ ions than the SOC in aortic myocytes when estimated from reversal potentials in different [Ca2+]o (Table 1).
It is apparent that the relative Ca2+ permeability of SOCs in smooth muscle is considerably smaller than ICRAC (see Table 1) and thus SOCs in smooth muscle are non-selective cation channels with a degree of Ca2+ permeability. It seems that ICRAC is well suited for refilling intracellular Ca2+ stores whereas in smooth muscle SOCs may also represent a depolarizing mechanism as well as a Ca2+ influx pathway. These differences in Ca2+ permeability between SOCs in smooth muscle and ICRAC in non-excitable cells provide further evidence that these channels are likely to possess different molecular structures (see later).
In addition there appears to be at least two activation mechanisms of SOCs in smooth muscle. In aortic myocytes it has been proposed that depletion of [Ca2+]SR releases a calcium influx factor (CIF) from the stores which displaces calmodulin (CaM) bound to membrane-delimited Ca2+-independent phospholipase A2 (iPLA2) which produces lysophospholipids to open SOCs (Smani et al. 2004). This represents a classical store-dependent activation mechanism. In contrast, in rabbit portal vein and mesenteric artery myocytes, SOCs evoked by both CPA and BAPTA-AM are almost completely blocked by inhibitors of protein kinase C (PKC) indicating a pivotal role for this kinase in SOC activation (Albert & Large, 2002b; Saleh et al. 2006). Moreover the diacylglycerol (DAG) analogue 1-oleoyl-sn-glycerol (OAG), and phorbol esters which stimulate PKC, also induced SOC activity in portal vein and mesenteric artery myocytes (Albert & Large, 2002b; Saleh et al. 2006). However, it is not clear how a reduction of [Ca2+]SR leads to activation of PKC and subsequent SOC opening. Therefore the SOCs in aorta versus portal vein/mesenteric artery differ in both biophysical properties and gating mechanisms.
Store-independent activation of SOCs in rabbit portal vein myocytes
A significant observation was that the sympathetic neurotransmitter noradrenaline activated SOCs in outside-out patches of portal vein myocytes in which CPA had no effect (Fig. 1A). These channels had identical characteristics to SOCs evoked by CPA and BAPTA-AM in cell-attached patches which were blocked by PKC inhibitors and therefore this noradrenaline-induced activity can be termed store-independent activation of SOCs (Albert & Large, 2002b). Presumably noradrenaline activates membrane-delimited PKC which is mediated by the production of DAG. In addition the phosphatase inhibitor calyculin A also evoked SOCs (Fig. 1B) in outside-out patches. This result not only supports the notion that a kinase may be involved in SOC activation but also suggests that there is a constitutively active phosphorylating pathway working independently of intracellular stores which can open SOCs. This store-independent pathway is in contrast to the established definition of SOC activation.
Figure 1.
Store-independent activation of store-operated channel (SOC) activity by noradrenaline and calyculin A in rabbit portal vein myocytes A, bath application of cyclopiazonic acid (CPA) did not evoke SOC activity whereas subsequent bath application of noradrenaline did induce SOC activity in an outside-out patch held at −70 mV. B, bath application of calyculin A stimulated SOC activity in a quiescent outside-out patch at −70 mV. Figures reproduced from Albert & Large (2002b).
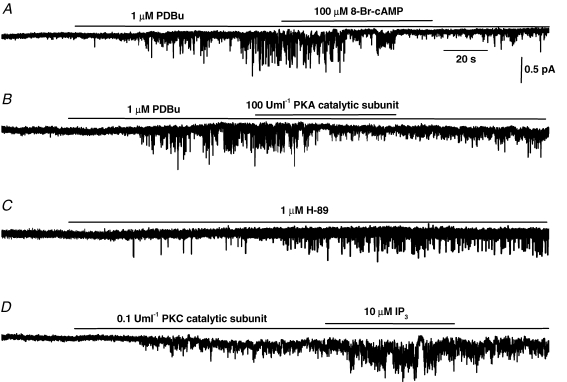
Figure 2A, B and D illustrates SOC activation in inside-out patches of portal vein myocytes by the phorbol ester PDBu, a PKC catalytic subunit and inhibition by a PKA catalytic subunit. These data reinforce the idea that store-independent activation of SOCs is mediated by PKC and also demonstrate that the channels may be inhibited by protein kinase A (PKA)-dependent mechanisms (Liu et al. 2005b). Therefore the channels can be stimulated and inhibited in excised membrane patches where there appears to be no functional Ca2+ stores and hence channel activity is not related to [Ca2+]SR. It was shown that β-adrenoceptor stimulation inhibits SOC activity via PKA in portal vein myocytes and therefore this mechanism may contribute to the vasodilatation mediated by β-adrenoceptors (Liu et al. 2005b). An interesting finding was that although IP3 applied on its own to the cytoplasmic surface did not induce SOC activity it did markedly potentiate the probability of channel opening of SOCs evoked by PKC catalytic subunit (Fig. 2D) and by PDBu, CPA and BAPTA-AM (Liu et al. 2005a). Therefore the intracellular mediator which releases Ca2+ from the SR also potentiates the influx mechanism for refilling the store. Previously one hypothesis for SOC activation is the conformational coupling mechanism whereby IP3 receptors in the SR couple to the plasmalemmal SOC on depletion of [Ca2+]SR to cause channel opening (Parekh & Putney, 2005). However, in our experiments there is no evidence for the presence of SR in inside-out patches (due to lack of effect of CPA) and therefore this effect may be due to IP3 itself acting on or close to the SOC. Application of the PKA inhibitor H-89 also induced channel activity (Fig. 2C), which supports the hypothesis that there is a tonically active stimulation mechanism which is independent of [Ca2+]SR and also that there is a constitutive inhibitory pathway involving PKA.
Figure 2.
Role of PKC and PKA in SOC activation in rabbit portal vein myocytes A, bath application of PDBu activated SOC activity in an inside-out patch at −80 mV which was reversibly inhibited by co-application of 8-Br-cAMP. B shows that PDBu-induced SOC activity is inhibited by co-application of a PKA catalytic subunit in an inside-out patch at −80 mV. C, bath application of H-89 induced SOC activity in a quiescent cell-attached patch at −80 mV. D shows that SOC activity evoked by a PKC catalytic subunit was potentiated by co-application of IP3 in an inside-out patch at −80 mV. Figures reproduced from Liu et al. (2005a, b).
It should also be noted that in human glomerular mesangial cells epidermal growth factor-activated SOCs have been shown to be mediated via a store-independent mechanism which does not involve IP3 receptors or release of Ca2+ ions from internal Ca2+ stores (Li et al. 2004).
Calmodulin stimulates SOCs in rabbit portal vein myocytes
The hypothesis that there is a constitutive driver causing SOC opening, which is counteracted by constitutive inhibitory PKA stimulation, provoked us to investigate whether calmodulin (CaM) stimulates SOCs as this Ca2+-binding protein is known to modulate many types of ion channel, including Ca2+-permeable channels. Application of 100 nm CaM to the intracellular surface of an inside-out patch stimulates a 2 pS channel in portal vein myocytes (Fig. 3Aa and b) which has identical properties to SOCs (Albert et al. 2006a). This stimulation of SOCs by CaM is Ca2+ dependent and SOCs are stimulated in 1 nm[Ca2+]c with a maximal effect around 100 nm[Ca2+]c. In higher [Ca2+]c the excitatory effects of CaM decrease (Fig. 3Ac). It should be noted that direct application of Ca2+ ions (up to 2 μm) alone to the cytoplasmic surface of inside-out patches never evokes these channels (Albert & Large, 2002b).
Figure 3.
CaM activates SOC activity and CaM kinase II has an inhibitory effect on SOC activity in rabbit portal vein myocytes Aa, bath application of CaM induces SOC activity in an inside-out patch at −80 mV; b shows that CaM-activated SOCs had a slope conductance of 1.8 pS and an extrapolated reversal potential of +28 mV; and c illustrates that CaM-evoked SOC activity is modulated by changing [Ca2+]i. B shows that BAPTA-AM-induced SOC activity was blocked by co-application of the CaM antagonist calmidazolium (CMZ) in a cell-attached patch at −80 mV. C, bath application of CMZ to a quiescent cell activated SOC activity that subsequently declined in a cell-attached patch at −80 mV. D, bath application of the CaM kinase II inhibitor KN-93 evoked sustained SOC activity in a cell-attached patch at −80 mV. E shows that CaM-evoked SOC activity in 100 nm[Ca2+]i was reversibly inhibited by co-application of purified CaM kinase II in an inside-out patch at −80 mV. Aa and b, B, C, D and E were reproduced from Albert et al. (2006b). Ac is authors' previously unpublished data.
Further support for a role of CaM in activating SOCs comes from the observation that the CaM antagonist calmidazolium (CMZ) completely inhibits SOC activity evoked by BAPTA-AM (Fig. 3B). However, the involvement of CaM seems to be complicated since application of CMZ to quiescent cells initially stimulates SOCs which were subsequently inhibited with continued application of CMZ (Fig. 3C). The initial stimulation by CMZ seems to result from removal of an inhibitory action of CaM kinase II since addition of CaM kinase II inhibitors produce sustained SOC activation (Fig. 3D). Therefore CaM appears to have dual actions: it directly activates SOCs but may also produce inhibition by stimulating CaM kinase II and these dual actions are illustrated in Fig. 3E where addition of purified CaM kinase II inhibits SOCs evoked by CaM (see Albert et al. 2006a for full details). Therefore in rabbit portal vein myocytes both PKC and CaM appear to have pivotal roles in SOC activation although the precise conditions for the involvement of PKC and CaM remain to be elucidated.
In summary, different gating mechanisms exist for SOCs in different smooth muscle types (aorta versus portal vein/mesenteric artery in rabbit) and, in addition, studies in the portal vein indicate that SOCs can be activated by diverse pathways which do not require depletion of intracellular Ca2+ stores with one pathway involving activation by PKC and the other utilizing CaM. Two noteworthy points are that, first, CaM evokes SOC activity in physiological concentrations ([CaM]c in many cell types is about 1–10 μm; Saimi & Kung, 2002) and in [Ca2+]c between 1 and 100 nm providing a possible basis for a constitutive driver mechanism. Secondly, it is possible that SOCs may be activated by increasing [Ca2+]c via CaM (or perhaps a Ca2+-dependent PKC). Additionally SOCs may also be stimulated by a decrease in [Ca2+]c due to the removal of the inhibitory influence of CaM kinase II. With respect to these points it should be remembered that in an intact cell, as when recording from a cell-attached patch, it is likely that CPA will increase [Ca2+]c whereas BAPTA-AM will reduce [Ca2+]c. Hence the effect of these agents on [Ca2+]c should always be taken into account when appraising experimental approaches used to investigate SOC activity.
Molecular composition of SOCs
The discovery of mammalian homologues of the Drosophila transient receptor potential (TRP) gene which encode 28 non-selective cation channel proteins with varying permeabilities to Ca2+ ions, has lead to a plethora of studies on the possible role of TRP channels underlying SOCs. Many of these studies have focused on the canonical transient receptor potential (TRPC) subfamily which comprises seven channel proteins (TRPC1–C7, C2 is a pseudogene in humans). Over-expression studies in cultured cell lines have shown that almost all TRPC channel proteins can be activated by SERCA inhibitors and by dialysing the intracellular medium with high concentrations of Ca2+ chelators, which suggests that TRPCs may represent SOCs (Parekh & Putney, 2005).
An important point when considering the molecular composition of TRPCs underlying SOCs is the general opinion that the majority of functional TRPCs in vivo consist of different TRPC subunits associated together in complex heterotetrameric structures. A variety of techniques including co-immunoprecipitation, fluorescence resonance energy transfer (FRET) and expression of dominant-negative TRPC subunits have shown that TRPC1 can form interactions with TRPC4/C5 and that TRPC3/C6/C7 channel proteins can also interact with one another (Goel et al. 2002; Hofmann et al. 2002). Moreover in embryonic rat brain microsomes associations between TRPC1 and TRPC4/C5 and between TRPC3/C6 have also been described (Strübing et al. 2003). Functional studies have shown that TRPC1 and TRPC3 associate to form distinct channels in human parotid gland ductal cells (HSY) cells which were activated by store depletion using thapsigargin and also by the DAG analogue 2-acetyl-sn-glycerol indicating a possible store-independent activation pathway (Liu X. et al. 2005). In rat H19-7 hippocampal cell lines TRPC1/TRPC3 have also been proposed to mediate store-operated Ca2+ entry (Wu et al. 2004). Moreover TRPC1 and TRPC4 proteins are thought to form a heterotetrameric SOC in endothelial cells (Brough et al. 2001; Freichel et al. 2001; Ahmmed et al. 2004) and TRPC1/C3/C7 are thought to form endogenous SOCs in HEK-293 cells (Zagranichnaya et al. 2005). In summary, it is apparent that numerous TRPC subunits can interact in a highly complex manner to form SOCs in several cell types.
A recent advance in the understanding of the potential molecular composition of SOCs has been the discovery of two families of transmembrane proteins, STIM and Orai, which have been proposed to mediate ICRAC in non-excitable cells with STIM1 acting as an ER Ca2+ sensor/activator of Orai1 and Orai1 constituting the CRAC channel/ion transport mechanism (see Lewis, 2007 for review and references). As there are vast differences between the biophysical properties of ICRAC and many SOCs (see above and Table 1) it seems unlikely that Orai proteins alone mediate the pore-forming subunits of all SOCs. However, recent studies have suggested that STIM1 and Orai1 may interact with TRPC proteins to modify their function. Huang and colleagues showed that over-expression of the cytosolic terminus of STIM1 increased TRPC1 activity and also demonstrated that STIM1 and TRPC1 proteins can associate with one another (Huang et al. 2006). In addition over-expression of Orai proteins was shown to enable thapsigargin to activate TRPC3 and TRPC6 activity through a STIM1-mediated mechanism which was not present in the absence of Orai proteins (Liao et al. 2007). These results indicate that STIM proteins may act as store-operated regulators of SOCs and also that Orai proteins may combine with TRPCs to produce functional store-operated channels either through acting as a pore-forming subunit or as a regulatory β-subunit (Huang et al. 2006). STIM1 has also been shown to regulate agonist-evoked activity of all TRPC subunits, except TRPC7, through either directly binding to TRPC1, TRPC4 and TRPC5 proteins or mediating heteromultimerization of TRPC3 and TRPC6 proteins with these STIM1-binding TRPC subunits (Yuan et al. 2007). Importantly this study proposed a new definition of SOCs, as channels that are regulated by STIM1 and require store-depletion-mediated clustering of STIM1 for activation, and concluded that all TRPCs, except TRPC7, can function as SOCs (Yuan et al. 2007). Furthermore TRPC1 has been suggested to produce a ternary complex with STIM and Orai1 which is important for activating SOCs in human salivary glands (Ong et al. 2007).
To date there is little information on the role of STIM and Orai in smooth muscle although human airway myocytes have been shown to express STIM1/2 mRNA and siRNA targeted at STIM1 markedly reduced Ca2+ influx and whole-cell currents evoked by CPA (Peel et al. 2006) indicating a potentially important role for STIM in mediating SOCs in these myocytes. In light of the proposed roles of STIM1 in regulating TRPC activity (see above) and the increasing evidence indicating that TRPCs mediate SOCs in smooth muscle (see below) an important area of future research will be to investigate the roles/mechanisms of STIM proteins in regulating SOC activity in smooth muscle.
The diverse molecular compositions of ICRAC (by Orai proteins) and SOCs (possibly a heterotetrameric TRPC structures) provides a possible explanation as to why there are many types of SOCs with different biophysical properties, permeabilites to Ca2+ and activation mechanisms. Furthermore this potential diversity in the make-up of SOCs poses the question against the strict definition of SOCs: is it probable that activation of these channels is governed only by a single store-operated mechanism?
Molecular identity of SOCs in vascular smooth muscle
Recently several groups using different experimental approaches have presented evidence for TRPC1 being an essential component of SOCs in smooth muscle. In rabbit cerebral artery myocytes anti-TRPC1 antibodies raised against a putative extracellular epitope of TRPC1 reduced thapsigargin-induced Ca2+ influx (Xu & Beech, 2001) and in pulmonary artery myocytes inhibition of endogenous TRPC1 expression by specific antisense oligonucleotides reduced CPA-evoked whole-cell currents (Sweeney et al. 2002). In addition, in the aorta A7r5 cell line knockdown of endogenous TRPC1 expression with siRNA and antisense methods reduced endogenous whole-cell currents induced by thapsigargin (Brueggemann et al. 2006). Moreover Fig. 4Aa and Ba illustrates work from our laboratory with single channel studies which show that bath application of anti-TRPC1 antibodies, raised against a putative intracellular epitope, to the cytoplasmic surface of inside-out patches produced marked inhibition of SOC activity evoked by CaM in portal vein myocytes (AP Albert, SN Saleh, CM Peppiatt-Wildman & WA Large unpublished data) and of SOCs stimulated by angiotensin II in mesenteric artery (see Saleh et al. 2006).
Figure 4.
TRPC1 and TRPC5 are important components of SOCs in rabbit portal vein and mesenteric artery myocytes Aa and b, bath application of, respectively, anti-TRPC1 and anti-TRPC5 antibodies reversibly inhibited CaM-evoked SOC activity in inside-out patches from portal vein myocytes at −80 mV. Ba and b, bath application of, respectively, anti-TRPC1 and anti-TRPC5 antibodies inhibited Ang II-induced stimulation of SOC activity in inside-out patches from mesenteric artery myocytes at −80 mV. Ba was reproduced from Saleh et al. (2006). Aa and b, and Bb are authors' previously unpublished data.
In addition it has been shown that an anti-TRPC5 antibody raised against a putative extracellular epitope inhibited thapsigargin-evoked Ca2+ influx in rabbit pial arterioles (Xu et al. 2006). Furthermore we have demonstrated that anti-TRPC5 antibodies generated against putative intracellular domains and bath applied to the intracellular surface of inside-out patches from portal vein and mesenteric artery myocytes also reduced SOC activity (Fig. 4Ab and Bb, AP Albert, SN Saleh, CM Peppiatt-Wildman & WA Large unpublished data). It should be noted that these antibodies display a high degree of selectivity when used in combination with inside-out patch recording of single channel activity since anti-TRPC1 and -TRPC5 antibodies do not inhibit the activity of constitutively active native TRPC3 channels in rabbit ear artery (Albert et al. 2006a) or receptor-operated TRPC6 (mesenteric artery, Saleh et al. 2006) or heterotetrameric TRPC3/C7 channels (coronary artery, Peppiatt-Wildman et al. 2007). Moreover TRPC1 and TRPC5 proteins have been shown to co-localize and associate with one another in human saphenous vein (Xu et al. 2006). These data suggest that TRPC1/TRPC5 heterotetramers may mediate SOCs in vascular smooth muscle which is supported by the observation that expression of TRPC1/TRPC5 channel proteins produced single channel currents with a unitary conductance of about 5 pS (Strübing et al. 2001) which is close to the conductance value of SOCs found in vascular smooth muscle (2–5 pS, Trepakova et al. 2001; Golovina et al. 2001; Albert & Large, 2002a).
These data provide strong evidence that TRPC1, possibly as a heterotetramer with TRPC5, is an essential component of SOCs in vascular smooth muscle. However, it is likely that other TRPC proteins are involved and the difference in biophysical properties between SOCs in aorta myocytes versus portal vein/mesenteric myocytes results from different heterotetrameric structures.
Multiple activation mechanisms of TRPCs proposed to mediate SOCs in vascular smooth muscle
We have shown that PKC and CaM can activate SOCs in vascular myocytes and there is support for TRPC1 and TRPC5 being components of these channels. Therefore it is interesting to consider the evidence for activation of expressed TRPC1 and TRPC5 by PKC and CaM.
Stimulation of PKC activates TRPC1
A unique property of TRPC1 is that stimulation of PKC has been shown to be required for its activation by store depletion (Ahmmed et al. 2004) whereas the activity of all other TRPCs have been shown to be inhibited by stimulation of this kinase (Soboloff et al. 2007). In cultured human umbilical vein endothelial cells pharmacological inhibitors of PKC, expression of a PKCα-defective mutant and an anti-TRPC1 antibody, reduced Ca2+ influx and whole-cell currents induced by thrombin, thapsigargin and intracellular application of IP3. Moreover this study showed that thrombin and thapsigargin produced PKCα-dependent phosphorylation of TRPC1 proteins (Ahmmed et al. 2004). These data provide compelling evidence for PKCα-mediated phosphorylation of TRPC1 proteins having a critical role in activating SOC activity. The DAG analogue and PKC activator, OAG, has also been shown to activate expressed TRPC1 (Lintschinger et al. 2000). In addition, stimulation of PKCα has also been shown to be essential in the activation of SOCs in mesangial cells (Ma et al. 2002) although these channels are proposed to consist of TRPC4 proteins (Wang et al. 2004).
It is tempting to speculate that TRPC1 subunits confer PKC sensitivity to SOC activation previously described in portal vein/mesenteric artery myocytes (Fig. 5Ba, Albert & Large, 2002b; Saleh et al. 2006).
Figure 5.
Schematic diagram of multiple activation mechanisms of TRPC1/TRPC5-mediated SOCs in rabbit portal vein myocytes A, CPA may activate SOCs by depleting Ca2+ levels within the SR ([Ca2+]SR) leading to stimulation of PKC/CaM by an unknown pathway (dashed lines) which induces channel opening. However, CPA may also induce SOC activity by evoking a rise in cytosolic Ca2+ levels ([Ca2+]c) which leads to activation of a Ca2+-sensitive PKC and CaM. B, BAPTA-AM may also activate SOCs by depleting [Ca2+]SR but this agent may produce a reduction in [Ca2+]c to remove the inhibitory action of CaM kinase II (CaMKII) on SOCs leading to stimulation of the channels via the presence of a constitutive driver, e.g. CaM. C, noradrenaline (NA) acting at α1-adrenoceptors activates SOC activity via both store-dependent and -independent pathways involving stimulation of PKC. In addition lysophospholipids (LPLs) may also activate SOC opening.
Effect of CaM on TRPC1 and TRPC5
It is well established that TRPC proteins contain multiple CaM binding sites with all members of the TRPC family containing a conserved region at the carboxyl (C) terminus which binds both CaM and IP3 receptors (CIRB domain) and some TRPCs also exhibit non-conserved CaM binding sites (Tang et al. 2001; see Zhu, 2005 for a detailed review). Functional studies have shown that CaM has mainly an inhibitory action on TRPC channel activity, including TRPC1, by competing with an excitatory effect of IP3 at the CIRB domain (Zhu, 2005). However, in contrast to these inhibitory actions of CaM on TRPCs, a characteristic feature of TRPC5 channel activity is that it can be facilitated by CaM. Application of intracellular CaM facilitated the peak amplitude and rate of onset of expressed TRPC5-mediated whole-cell currents through a novel non-CIRB binding site, and in addition CaM has also been shown to be important for maintaining agonist-induced TRPC5 activity through the CIRB domain (Ordaz et al. 2005). Moreover the Ca2+/CaM-dependent enzyme myosin light chain kinase (MLCK) has also been shown to be essential for initiating activation of TRPC5 channels through a constitutively active trafficking process which regulates transport of TRPC5 channels to the plasma membrane (Kim et al. 2006; Shimizu et al. 2006).
Therefore these facilitatory actions of CaM on TRPC5 activity may indicate that the excitatory effect of CaM on SOCs in rabbit portal vein is conferred by TRPC5 subunits (see above, Albert et al. 2006a) and involves a direct interaction with TRPC5 proteins since inhibitors of CaM-dependent enzymes, e.g. MLCK, CaM kinase II and calcineurin, do not inhibit CaM-evoked SOC activity (Albert et al. 2006a).
In addition, expressed TRPC5 activity has also been shown to be activated by lysophospholipids, including lysophosphatidylinositol (LPI), via a relatively direct interaction with the channel proteins (Flemming et al. 2005). This may relate to the proposed role of iPLA2-mediated production of LPIs in activating native SOCs in aorta myocytes (Smani et al. 2004) and suggest that TRPC5 subunits are also components of these SOCs.
Conclusion
Experimental evidence indicates that it is unlikely that a single mechanism is involved in activating SOCs in smooth muscle and Fig. 5 summarizes our proposal for the activation of SOCs in rabbit portal vein myocytes. We suggest that TRPC1 and TRPC5 proteins are components of SOCs but it is probable that other TRPC subunits and possibly Orai and STIM proteins are involved. Figure 5A and B shows that CPA and BAPTA-AM may activate SOC activity in portal vein according to the strict definition of SOCs whereby these agents produce a reduction of [Ca2+]SR to activate SOCs. Evidence shows that both CaM and PKC are involved in this process (see earlier) but it is not clear how a reduction in [Ca2+]SR stimulates PKC and CaM. Alternatively it is possible that activation of SOCs by CPA results from an increase of [Ca2+]c which stimulates Ca2+-sensitive PKC and/or CaM which evoke SOCs (Fig. 5A). Also, BAPTA-AM may activate SOCs by reducing [Ca2+]c to remove the inhibitory effect of CaM kinase II with subsequent opening of channels through constitutive driver activity involving PKC and/or CaM. Finally Fig. 5C shows that physiological agonists such as noradrenaline acting on a GPCR linked to the classical phosphoinositol biochemical cascade activates SOCs via two separate pathways: (i) a store-dependent pathway involving production of IP3 which releases Ca2+ from internal stores leading to a rise in [Ca2+]c and stimulation of PKC/CaM to cause channel opening, and (ii) a store-independent/membrane-delimited pathway involving generation of DAG which stimulates PKC to induce SOC activity. Our evidence suggests that the primary mechanisms of activation of SOCs in smooth muscle in physiological conditions involve PKC and CaM mechanisms, perhaps mediated by changes in [Ca2+]c. In addition it is possible that several cellular mechanisms contribute to the whole-cell current or intracellular Ca2+ signal produced by agents that deplete intracellular Ca2+ stores.
In summary there is strong evidence that vascular smooth muscle expresses diverse SOCs that differ in biophysical properties and activation mechanisms. This heterogeneity in channel properties is likely to be mediated by TRPC proteins assembled together into different heterotetrameric channel structures.
Acknowledgments
The work in the laboratory of the authors was supported by The Wellcome Trust and The British Heart Foundation.
Note added in proof
Recently Dietrich et al. (2007) have demonstrated that whole-cell currents induced by CPA and IP3 are similar in vascular myocytes from wild-type and TRPC1−/− mice. Thus it appears that in these experiments TRPC1 subunits do not contribute to the SOC signal in contrast to the CPA-evoked and PKC-mediated single channel currents described in the present review which do seem to involve TRPC1 proteins. These data further support our hypothesis that diverse molecular components and activation mechanisms contribute to cellular signals that are activated by agents that deplete intracellular Ca2+ stores.
References
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Cα phosphorylates transient receptor potential channel-1 (TRPC1) and regulates store-operated Ca2+ entry. Role in signalling increased endothelial permeability. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Albert AP, Large WA. A Ca2+-permeable non-selective cation channel activated by depletions of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002a;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J Physiol. 2002b;544:113–125. doi: 10.1113/jphysiol.2002.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. doi: 10.1016/s0143-4160(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Albert AP, Piper AS, Large WA. Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes. J Physiol. 2005;566:769–780. doi: 10.1113/jphysiol.2005.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Liu M, Large WA. Dual effects of calmodulin on store-operated cation channels in rabbit portal vein myocytes. Br J Pharmacol. 2006a;148:1001–1011. doi: 10.1038/sj.bjp.0706797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Pucovsky V, Prestwich SA, Large WA. TRPC3 properties of a native Ca2+-permeable cation channel in rabbit ear artery myocytes. J Physiol. 2006b;571:361–369. doi: 10.1113/jphysiol.2005.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophilia TRP. J Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–1738. [PubMed] [Google Scholar]
- Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos Y, Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Archiv. 2007 doi: 10.1007/s00424-007-0314-3. in press, doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu S-Z, Li J, Fanning Z, Naylor J, Benham CD, Bateson AN, Maraki K, Beech DJ. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2005;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weibgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX-J. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Harea Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–337. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Kim MT, Kim BJ, Lee JH, Kwon SC, Yeon DO, Yang DK, So I, Kim KW. Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol. 2006;290:C1031–C1040. doi: 10.1152/ajpcell.00602.2004. [DOI] [PubMed] [Google Scholar]
- Large WA. Receptor-operated Ca2+-permeable non-selective cation channels in vascular smooth muscle: a physiologic perspective. J Cardiovasc Electrophysiol. 2002;13:493–501. doi: 10.1046/j.1540-8167.2002.00493.x. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Li W-P, Tsiokas L, Sansom SC, Ma R. Epidermal growth factor activates store-operated Ca2+ channels through an inositol 1,4,5-trisphosphate-independent pathway in human glomerular mesangial cells. J Biol Chem. 2004;279:4570–4577. doi: 10.1074/jbc.M304334200. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol and Ca2+-sensitive cation channels. J Biol Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- Liu M, Albert AP, Large WA. Facilitatory effect of Ins(1,4,5)P3 on store-operated Ca2+-permeable cation channels in rabbit portal vein myocytes. J Physiol. 2005a;566:161–171. doi: 10.1113/jphysiol.2005.088260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Large WA, Albert AP. Stimulation of β-adrenoceptors inhibits store-operated channel currents via a cAMP-dependent protein kinase mechanism in rabbit portal vein myocytes. J Physiol. 2005b;562:395–406. doi: 10.1113/jphysiol.2004.077602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay BC, Singh BB, Groscher K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- Ma R, Kudlacek PE, Sansom SC. Protein kinase Cα participates in activation of store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol Cell Physiol. 2002;283:C1390–C1398. doi: 10.1152/ajpcell.00141.2002. [DOI] [PubMed] [Google Scholar]
- Ma R, Smith S, Child A, Carmines PK, Sansom SC. Store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2000;278:F954–F961. doi: 10.1152/ajprenal.2000.278.6.F954. [DOI] [PubMed] [Google Scholar]
- McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;35:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Chen KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium-release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC channel activity. J Biol Chem. 2005;280:30788–30796. doi: 10.1074/jbc.M504745200. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119–127. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annu Rev Physiol. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol. 2006;577:479–495. doi: 10.1113/jphysiol.2006.119305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Yoshida T, Wakamori M, Ishii M, Okada T, Takahashi M, Seto M, Sakurada K, Kiuchi Y, Mori Y. Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J Physiol. 2006;570:219–235. doi: 10.1113/jphysiol.2005.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Hewavitharana T, He LP, Luncsford P, Xu W, Venkatachalam K, van Rossum D, Patterson RL, Gill DL. TRPC channels: Integrators of multiple cellular signals. Handb Exp Pharmacol. 2007;179:575–597. doi: 10.1007/978-3-540-34891-7_34. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunovas S, Birnbaumer L, Zhu MX. Identification of common minding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of Trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Large WA. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pluznick JL, Wei P, Padanilam BJ, Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004;287:C357–C364. doi: 10.1152/ajpcell.00068.2004. [DOI] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anoccygeus. Br J Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J Biol Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- Xu S-Z, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Xu S-Z, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2653–H2659. doi: 10.1152/ajpheart.00495.2006. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3 and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- Zhu MX. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–111. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]