Abstract
The Kölliker–Fuse nucleus (KF), part of the respiratory network, is involved in the modulation of respiratory phase durations in response to peripheral and central afferent inputs. The KF is immature at birth. Developmental changes in its physiological and anatomical properties have yet to be investigated. Since brain-derived neurotrophic factor (BDNF) is of major importance for the maturation of neuronal networks, we investigated its effects on developmental changes in the KF on different postnatal days (neonatal, P1–5; intermediate, P6–13; juvenile, P14–21) by analysing single neurones in the in vitro slice preparation and network activities in the perfused brainstem preparation in situ. The BDNF had only weak effects on the frequency of mixed excitatory and inhibitory spontaneous postsynaptic currents (sPSCs) in neonatal slice preparations. Postnatally, in the intermediate and juvenile age groups, a significant augmentation of the sPSC frequency was observed in the presence of 100 pm BDNF (+23.5 ± 12.6 and +76.7 ± 28.4%, respectively). Subsequent analyses of BDNF effects on evoked excitatory postsynaptic currents (eEPSCs) revealed significant enhancement of eEPSC amplitude of +20.8 ± 7.0% only in juvenile stages (intermediates, −13.2 ± 4.8%). On the network level, significant modulation of phrenic nerve activity following BDNF microinjection into the KF was also observed only in juveniles. The data suggest that KF neurones are subject to BDNF-mediated fast synaptic modulation after completion of postnatal maturation. After maturation, BDNF contributes to modulation of fast excitatory neurotransmission in respiratory-related KF neurones. This may be important for network plasticity associated with the processing of afferent information.
The respiratory network controls the vital function of breathing and therefore must be fully functional at birth. Maturation within the respiratory network, however, continues during postnatal development (Zhang et al. 2002; Wong-Riley & Liu, 2005; Liu & Wong-Riley, 2005). The synaptic processing of peripheral sensory information in particular requires postnatal maturation, since some synaptic responses to respiratory-related sensory inputs are absent or inactive at the embryonic stage. Compared with medullary regions of the respiratory network that generate the primary respiratory rhythm (see below), the pontine Kölliker–Fuse nucleus (KF) shows substantial functional and histological postnatal immaturity (Dutschmann et al. 2004). The adult KF processes second-order afferent synaptic inputs relevant to breathing (Alheid et al. 2004; Dutschmann et al. 2004; Ezure, 2004; Song & Poon, 2004), so we hypothesize that maturation is of major importance for the development of behaviour- and state-dependent breathing.
There is clear evidence that the neurotrophin brain-derived neurotrophic factor (BDNF), which acts via a specific high-affinity tyrosine kinase B (trkB) receptor, is important for early maturation of neuronal processes that influence the function of the respiratory network. Brain-derived neurotrophic factor knockout (KO) mice show a severe impairment of respiratory motor activity at birth (Erickson et al. 1996). The animals die around postnatal day (P) 5, presumably owing to a disruption of the early postnatal development of the respiratory rhythm (Balkowiec & Katz, 1998; Katz, 2003, 2005). The relevance of BDNF for the respiratory network is further supported by the findings that it modulates neurones located in the pre-Bötzinger complex (Thoby-Brisson et al. 2003, 2004), an essential kernel for rhythmogenesis (Feldman et al. 2003).
The function of BDNF is not, however, restricted to medullary centres involved in primary respiratory rhythm generation. The development of A5 neurones (Errchidi et al. 1991; Viemari et al. 2003) as components of the pontine respiratory group also depends on BDNF (Guo et al. 2005). This suggests that BDNF is essential for the overall maturation and function of the pontomedullary respiratory network.
Furthermore, developmental dysregulation of BDNF expression and secretion is found in animal models (Mecp2−/y KO mice) of the human Rett syndrome (Chang et al. 2006; Wang et al. 2006). These mice exhibit severe breathing disorders in the late stages of postnatal development (Viemari et al. 2005; Stettner et al. 2007). A recent study identified disturbances of glutamatergic synaptic function and processing of vagal sensory inputs in the KF as sources of breathing disorders in Mecp2−/y KO mice (Stettner et al. 2007). Brain-derived neurotrophic factor enhances glutamatergic neurotransmission (Levine et al. 1998; Tyler & Pozzo-Miller, 2001; Yamada et al. 2002; Lu, 2003); therefore, neurodevelopmental dysregulation of BDNF may impair glutamatergic neurotransmission in the KF (Stettner et al. 2007).
In the present study, we investigated developmental changes in the cellular properties of pontine KF neurones in slice preparations. Emphasis was placed on BDNF-mediated changes of spontaneous postsynaptic activity and evoked glutamatergic postsynaptic currents. Brain-derived neurotrophic factor-mediated fast synaptic modulation was observed only in juvenile, mostly mature KF neurones, while in earlier developmental stages it was less pronounced or even absent. Developmental changes were further investigated by measuring respiratory responses to local microinjections of BDNF into the KF in the in situ perfused brainstem preparation.
Methods
Slice preparation
All experiments were performed in accordance with the ethical guidelines of the US National Institutes of Health (NIH) and were approved by the ethics committee of the University Medical Center Göttingen, Georg-August-University.
Sprague–Dawley rats of different ages (postnatal days P1–P21, either sex) were anaesthetized deeply by inhalation of isoflurane (in air) until no reflex responses to noxious pinch of toe or tail were observed. The animals were decapitated during anaesthesia, the brain was removed and the pontomedullary brainstem transected. The brainstem was cut into 200–250 μm sections using a vibratome (752M vibroslice, Campden Instruments, Loughborough; UK). Preparation of slices was performed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl, 125; KCl, 3; KH2PO4, 1.25; CaCl2, 2.5; MgSO4, 1.25; NaHCO3, 25; and d-glucose, 10. The ACSF was equilibrated to pH 7.4 with carbogen (95% O2–5% CO2). All slices were incubated for at least 30 min in oxygenated 35°C ACSF. Slices were transferred into oxygenated ACSF at room temperature and kept for up to 7 h. For recording, slices were placed in a recording chamber, held in place with a platinum-wired nylon grid and continuously superfused at 28°C with oxygenated ACSF.
Localization of the KF in different postnatal stages
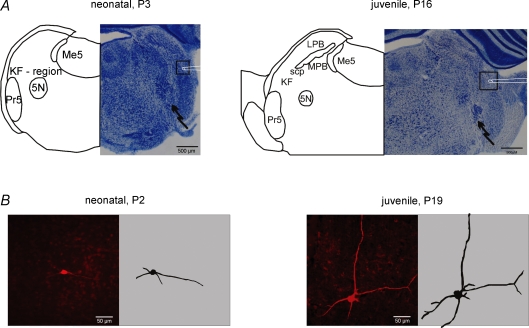
To investigate developmental changes in BDNF-evoked synaptic modulation, we performed experiments on three different age groups: neonatal (P1–5), intermediate (P6–13) and juvenile (P14–21). The KF was identified ventral to the tip of the superior cerebellar peduncle (scp, Fig. 1A) in slices of the intermediate and of the juvenile group. In neonatal slices, the scp is barely detectable. Therefore, we determined the KF region in neonatal slices according to its presumed position between the mesencephalic trigeminal nucleus (Me5), the principal sensory trigeminal nucleus (Pr5) and the motor trigeminal nucleus (5N, Fig. 1A).
Figure 1.
Developmental changes in cell mormphology of Kölliker–fuse neurones and cytoarchitecture of the dorsolateral pons A illustrates recording (square with schematic patch pipette) and stimulation sites (jagged arrow) in pontine slices of different postnatal stages. Please note the developing complexity of the cyto-architecture illustrated by thionin staining (right panels) and semischematic drawings (left panels). B shows photomicrographs of biocytin-stained KF neurones from the neonate and juvenile groups (left panels) and their two-dimensional reconstructions (right panels). Please note the increasing complexity of the dendritic tree during the postnatal maturation. Abbreviations: 5N, motor trigeminal nucleus; KF, Kölliker–Fuse nucleus; LPB, lateral parabrachial nucleus; Me5, mesencephalic trigeminal nucleus; MPB, medial parabrachial nucleus; Pr5, principal sensory trigeminal nucleus; and scp, superior cerebellar peduncle.
Recording spontaneous postsynaptic currents (sPSCs)
Spontaneous postsynaptic currents (sPSCs) of KF neurones were recorded in the whole cell voltage clamp configuration at a holding potential of −70 mV. The intracellular solution contained (mm): KCl, 140; EGTA, 10; Hepes, 10; Na2ATP, 4; MgCl2, 2, CaCl2, 1; and Na2GTP, 0.5. The high chloride concentration of the intracellular solution was chosen to enhance outward chloride currents. The liquid junction potential was experimentally determined around 1 mV and was not corrected. Data were acquired using pClamp acquisition software (version 9.2, Axon Instruments). Signals were amplified (Multiclamp 700B, Axon Instruments), filtered at 1 kHz and digitized at 10 kHz. Before the application of any drug, membrane capacitance and membrane resistance were recorded using the pClamp feature ‘membrane test’ (filtered at 30 kHz).
Brain-derived neurotrophic factor and K252a (trkB inhibitor, both from Calbiochem) were applied systemically via the superfusate. Physiological quantities of BDNF have been reported to peak around 1 ng (g tissue)−1 in the cortex (Das et al. 2001; Gilmore et al. 2003). Therefore, BDNF was bath applied in the picomolar range to approximate to physiological concentrations (e.g. 50 pm BDNF = 1.4 ng ml−1 in the superfusate). Brain-derived neurotrophic factor was applied gradually (1, 10 and 50 pm) to a final concentration of 100 pm. The K252a was applied subsequently at a concentration of 50 nm.
Recording evoked excitatory postsynaptic currents (eEPSCs)
In order to excite ascending projections from medullary regions (e.g. from the nucleus of the solitary tract), a bipolar stimulation electrode was placed ventral to the KF (see Fig. 1A). We used digital stimulus waveforms (pClamp 9.2; Clampex) that were transmitted to a stimulator (custom made). Stimulation trials consisted of 20 sweeps with an intersweep interval of 3 s. Stimulus duration (0.1–0.5 ms) and intensity were adjusted to evoke a submaximal postsynaptic response. To isolate excitatory currents, bicuculline (Sigma, 0.5 μm) and strychnine (Sigma, 0.5 μm) were applied to the bath. We applied bicuculline and strychnine also in early neonatal stages, when chloride currents are reported to be excitatory (Ritter & Zhang, 2000; Balakrishnan et al. 2003). Evoked EPSCs were recorded before (control), 4 min after application of BDNF (50 pm) and 12 min after application of K252a (50 nm). This time range has been reported to be necessary to block the effects of BDNF on inward currents using K252a (Kafitz et al. 1999). The internal solution in this experimental protocol contained (mm): potassium gluconate, 140; CaCl2, 1; EGTA, 10; MgCl2·6H2O, 2; Na2GTP, 0.5; Na2ATP, 4; and Hepes, 10. Liquid junction potentials were between 5 and 7 mV and were not corrected. To identify glutamatergic currents, 2–amino–5–phosphonopentanoic acid (AP5; 20μm) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm) were applied in some of the experiments (n = 11, data not shown). These control experiments were performed randomly in different developmental stages.
Immunocytochemistry
For anatomical verification of the recording site and for morphological analysis, 0.25% biocytin (Sigma) was included in the internal solution of the patch pipette in some of the experiments. Neurones were visualized with biocytin coupled to carbocyanine 3 (Cy3)-conjugated avidin (Rockland; 1:400, overnight at 4°C).
Data analysis
Effects of BDNF application on synaptic activity were analysed off-line using MiniAnalysis (Synaptosoft Inc., Decatur, GA, USA) during control activity and after each application of BDNF or K252a. Responses to each application were analysed over 2 min. Data were transferred to Microsoft Excel, and instantaneous frequency and mean amplitudes of sPSCs were calculated for each cell before and after drug application.
Evoked EPSC amplitudes were analysed using Clampfit. Each trial (20 sweeps) was averaged and the mean amplitudes were calculated. Decay kinetics of eEPSCs were analysed by fitting to a bi-exponential function of the form:
using MiniAnalysis. The fitting range was from peak to end. A weighted τ:
was then calculated, in which A1 and τ1 reflected the amplitude and time constant of the fast component and A2 and τ2 represented the parameters of the slow component.
Working heart–brainstem preparation
The effects of BDNF in the KF in the intact respiratory network were studied using the perfused brainstem preparation (Paton, 1996) of rats at different postnatal stages. The different postnatal stages were pooled into two groups: P6–13 (reflecting the intermediate group) and P16–23 (juvenile group).
In brief, rats were deeply anaesthetized with halothane (in air) and, once respiration was severely depressed and after the animals failed to respond to noxious stimulation, they were transected below the diaphragm, decerebrated at the precollicular level in chilled ACSF (see above), and the left phrenic nerve was cut at the level of the diaphragm. The preparation was transferred to a custom-made recording chamber. In order to maintain colloid osmotic pressure and a flow rate of 28–32 ml min−1, the descending aorta was cannulated and perfused with carbogen (95% O2–5% CO2)-gassed ACSF (osmolarity, 298 ± 5 mosmol l−1; pH 7.35 ± 0.05) containing Ficoll (1.25%) using a peristaltic pump (Watson & Marlow, Cornwall, UK) and a double-lumen catheter. The perfusate (at 31°C) was filtered and passed through bubble traps. Leaking perfusate was collected and recirculated. With the start of perfusion, rhythmic contractions of the respiratory muscles resumed and were abolished with the neuromuscular blocker Norcuron® (2.5 μg l−1, Organon Teknika, Dublin, Ireland).
Perfusion pressure within the aorta was held at 70–90 mmHg by adjusting the flow rate. The inspiratory discharge of the phrenic nerve was recorded (Tektronix, differential amplifier, Berkshire, UK) using a glass suction electrode. Phrenic nerve activity was filtered (8 Hz to 3 kHz) and recorded using a MacLab 8s interface (ADInstruments) and Chart software. Data analysis was performed off-line.
Experimental protocols and data analysis for microinjection studies
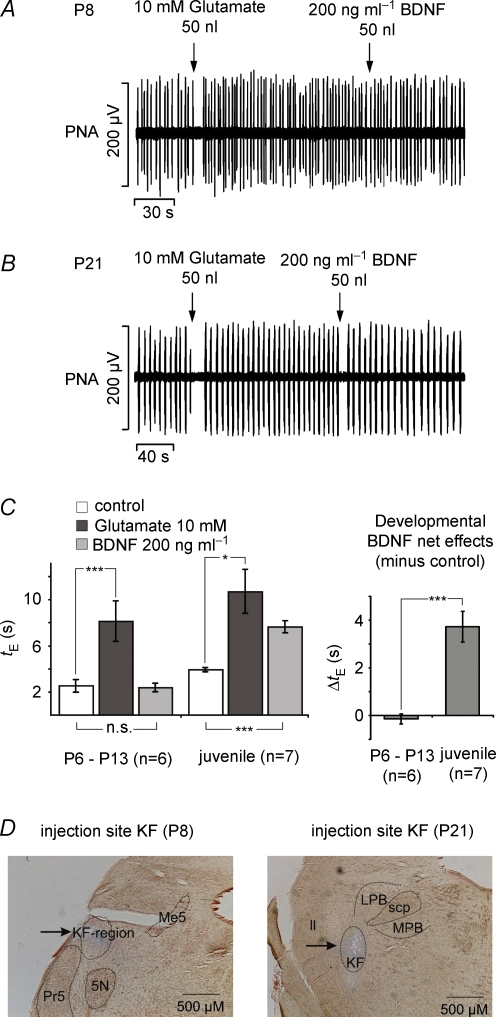
Pressure microinjections of glutamate (10 mm, ICN Biomedicals, CA, USA), of BDNF (200 ng ml−1, 50 nl, Calbiochem) and of Pontamine Sky Blue (Sigma) into the dorsolateral pons were performed with a micromanipulator-driven multibarrelled micropipette (tip diameter, 30–40 μm). In the perfused brainstem preparation, higher BDNF concentrations (7 nm BDNF = 200 ng ml−1) were used to avoid rapid dilution and washout. The injected volumes were measured by observing the movement of the meniscus through a binocular microscope fitted with a calibrated eyepiece graticule. As a first step, the ipsilateral dorsolateral pons (co-ordinates: 0.2–0.5 mm caudal to the caudal end of inferior colliculus and 1.8–2.5 mm lateral to mid-line) was mapped for phrenic nerve responses to glutamate (20–30 nl) injection. After identification of responsive injection sites that evoked transient apnoeas or tachypnoeas and recovery of phrenic nerve activity (PNA) to baseline values, BDNF was injected. At the end of the experiment, the position of the pipette was marked by injecting 40–50 nl of Pontamine Sky Blue. The brainstem was removed and fixed for 1–2 days in 4% paraformaldehyde containing 20% sucrose. For anatomical verification of injection sites, series of 50 μm coronal sections were cut through the pons using a freezing microtome (Reichert and Jung, Bensheim, Germany) and the sections were stained with Neutral Red (Fig. 8D).
Figure 8.
Illustration of respiratory modulation evoked by glutamate and BDNF microinjections into the KF in the perfused brainstem preparation derived from different postnatal stages A Illustrates that microinjections of glutamate in the intermediate group evoked apnoea while subsequent BDNF injection was ineffective. In contrast, at juvenile stages (B) BDNF evoked brief apnoeas similar to those observed after the preceding glutamate injections. C, bar graphs summarize the statistical analysis of the glutamate- and BDNF-evoked prolongation of the expiratory interval (tE) in the intermediate and juvenile age group (left). Developmental comparison of the BDNF-induced effect on tE is shown in the right diagram. *P < 0.05, ***P < 0.001. D, photomicrographs illustrating examples of the localization of injection sites for the intermediate and juvenile groups. Abbreviations: PNA, phrenic nerve activity; 5N, motor trigeminal nucleus; KF, Kölliker–Fuse nucleus; ll, lateral lemniscus; LPB, lateral parabrachial nucleus; Me5, mesencephalic trigeminal nucleus; MPB, medial parabrachial nucleus; Pr5, principal sensory trigeminal nucleus; scp, superior cerebellar peduncle.
Effects on the expiratory interval (tE, the silent phase of PNA) evoked by BDNF were analysed when the previous glutamate injections provoked transient apnoeas (prolongation of tE) or transient tachypnoeas (shortening of tE) for both age groups.
Statistical analyses
Brain-derived neurotrophic factor-evoked changes of respiratory parameters recorded from perfused brainstem preparations were compared using Student's two-tailed t test (Excel, paired for BDNF effects within the age groups, unpaired for differences between the two age groups). The remaining analyses were performed with Systat 11 software (Systat software, Inc. 2004). ANOVA, followed by the Fisher's post hoc LSD test was used to analyse developmental changes in decay kinetics and cellular properties such as membrane capacitance, resistance, sPSC amplitude, instantaneous frequency and current density, as calculated from mean amplitude and capacitance values. ANOVA followed by Fisher's post hoc LSD test was also used to test for significant differences in BDNF-evoked effects between the three different age groups.
The effects of BDNF on sPSC frequency and amplitudes or eEPSC amplitudes within the individual age groups were analysed using the Kolmogorov–Smirnov (K–S) test for multiple comparisons. A P value < 0.05 was considered as significant. All data are presented as means ±s.e.m.
Results
General developmental changes of KF neurones
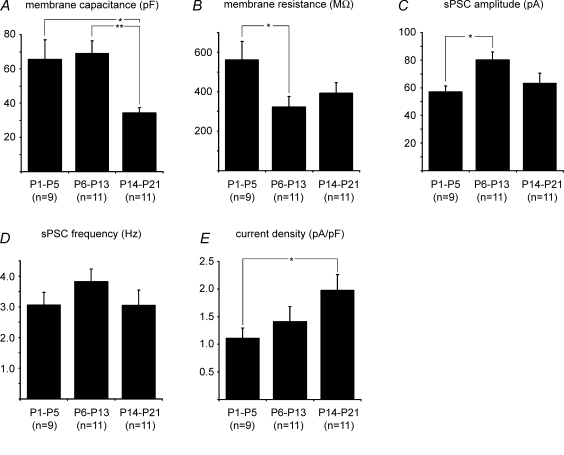
The KF, as an integral part of the mammalian respiratory network, undergoes substantial postnatal maturation (Dutschmann et al. 2004). This is reflected in changes in the cytoarchitectural organization of the dorsolateral pons (see Fig. 1A) and by an increasing complexity of the cell morphology of KF neurones, as illustrated in Fig. 1B. The morphological maturation of KF neurones is accompanied by changes of neuronal properties (Fig. 2). Membrane capacitance decreased significantly from 65.6 ± 11.4 and 68.9 ± 7.5 pF in neonates and intermediates, respectively, to 34.4 ± 2.9 pF in juveniles (P < 0.05 and P < 0.01, Fig. 2A). Membrane resistance showed a strong decline between neonates and intermediates (from 561.7 ± 92.5 to 322.2 ± 53.4 MΩ, P < 0.05, Fig. 2B) but remained stable in juveniles (393.4 ± 52.5 MΩ). Spontaneous PSC amplitude peaked in the intermediate group at 80.3 ± 5.8 pA (P < 0.05), while neonates and juveniles showed similar values (57.1 ± 4.2 and 63.3 ± 7.4 pA, respectively, Fig. 2C). Spontaneous PSC frequency did not change significantly during postnatal development of the KF (3.1 ± 0.4 Hz in neonates, 3.8 ± 0.4 Hz in intermediates and 3.1 ± 0.5 Hz in juveniles, Fig. 2D). Current density increased progressively during postnatal development from 1.1 ± 0.2 pA pF−1 in neonates to 1.4 ± 0.3 pA pF−1 in intermediates to 2.0 ± 0.3 pA pF−1 in juveniles (P < 0.05, Fig. 2E).
Figure 2.
Summary of developmental changes of membrane capacitance (A), membrane resistance (B), sPSC amplitude (C), sPSC frequency (D) and current density E) *P < 0.05, **P < 0.01.
The pronounced decrease in membrane capacitance during postnatal maturation of KF neurones did not correlate with the increasing soma size and dendritic arborization illustrated in Fig. 1B. However, similar changes in the profile of membrane resistance and capacitance have been observed in developing Purkinje cells (Fry, 2006).
Age-dependent modulation of sPSCs after BDNF application
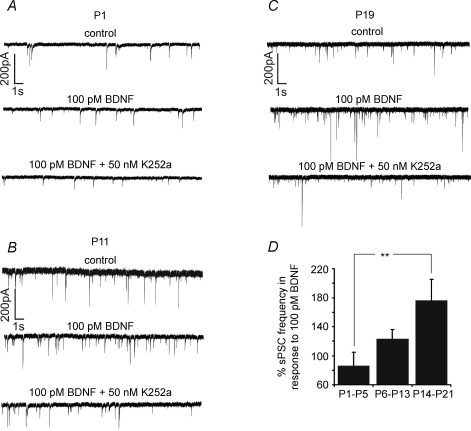
Dose-dependent BDNF modulation of sPSC frequency was strongly age dependent (Compare Fig. 2D for control). Recordings of KF neurones in neonates (P1–5) showed a heterogeneous response pattern to bath application of BDNF. Out of a total of nine neurones, five cells responded with depression of sPSC frequency following the administration of higher concentrations of BDNF (50–100 pm), whereas four neurones showed a mild increase in sPSC frequency (Fig. 3A). Overall, no clear dose-dependent changes in sPSC frequency were observed in response to different concentrations of BDNF (+9.7 ± 11.6% at 1 pm, −12.4 ± 14.8% at 10 pm, −1.6 ± 14.4% at 50 pm and −13.6 ± 18.1% at 100 pm of BDNF, see Fig. 4A). Subsequent bath application of the tyrosine kinase inhibitor K252a resulted in a net increase in sPSC frequency of +14.9 ± 19.1% compared with control levels (Fig. 4A). In the neonate group, no change in sPSC amplitude was found after BDNF application (Fig. 4A).
Figure 3.
Representative recordings of sPSC frequencies of a P1 (A), P11 (B) and P19 neurone (C) in response to BDNF (100 pm) and K252a (50 nm); the diagram (D) summarizes the developmental comparison of the effects of BDNF on sPSC frequency **P < 0.01.
Figure 4.
Illustration of BDNF-mediated effects on sPSC frequency (left panels) and amplitude (right panels) after bath application of the drug at different postnatal stages The left panels of A, B and C summarize the dose–response curves of sPSC frequencies to different concentrations of BDNF. The right panels of A, B and C show that neither BDNF nor K252a had significant effects on the amplitude of sPSCs in the different developmental stages. *P < 0.05, ***P < 0.001.
In the intermediate group (P6–13), sPSC frequency changes were more consistent. The majority (n = 8/11) of the neurones responded to BDNF with an increase in sPSC frequency (Fig. 3B). The lowest concentration (1 pm) of BDNF evoked no effect (−2.2 ± 5.3%). Frequency increased by +3.6 ± 6.9% after 10 pm BDNF, by +20.3 ± 15.6% after 50 pm BDNF and by +23.5 ± 12.6% following application of 100 pm BDNF (P < 0.05, Fig. 4B). Subsequent application of K252a returned sPSC frequency close to control levels (−2.8% compared with baseline; Fig. 4B). Analysis of sPSC amplitude revealed no significant BDNF-induced changes (Fig. 4B).
In the juvenile group (P14–21), a pronounced BDNF-induced increase in sPSC frequency was observed (Fig. 3C). Concentrations of 1 pm BDNF increased sPSC frequency by +27.9 ± 16.1%. Higher concentrations evoked a further augmentation of sPSC frequency to +43.7 ± 21.5% after 10 pm (P < 0.05), +43.0 ± 10.9% after 50 pm (P < 0.001) and +76.7 ± 28.4% after 100 pm BDNF (P < 0.001, Fig. 4C). The effect of BDNF was reversed by subsequent application of 50 nm K252a, and the sPSC frequency returned to 27.7 ± 18.7% above the control level. However, as in the other age groups, sPSC amplitude was not affected by BDNF (Fig. 4C).
Statistical analysis revealed significant differences in BDNF-mediated effects on sPSC frequency modulation between the neonate and the juvenile age groups at concentrations of 10 (−12.4 versus+43.7%, P < 0.05), 50 (−1.6 versus 43.0%, P < 0.05) and 100 pm BDNF (−13.6 versus+76.7%, P < 0.01; Fig. 3D). In contrast, no significant differences were found between neonatal versus intermediate and between intermediate versus juvenile groups (Fig. 3D).
Age-dependent changes in BDNF-mediated modulation of eEPSCs
After the initial screening for effects of BDNF on synaptic activity, eEPSCs were investigated. Dose–response curves revealed stable increases in sPSC frequency at a concentration of 50 pm BDNF in the juvenile group. This concentration was therefore used to produce BDNF-evoked effects in subsequent experiments.
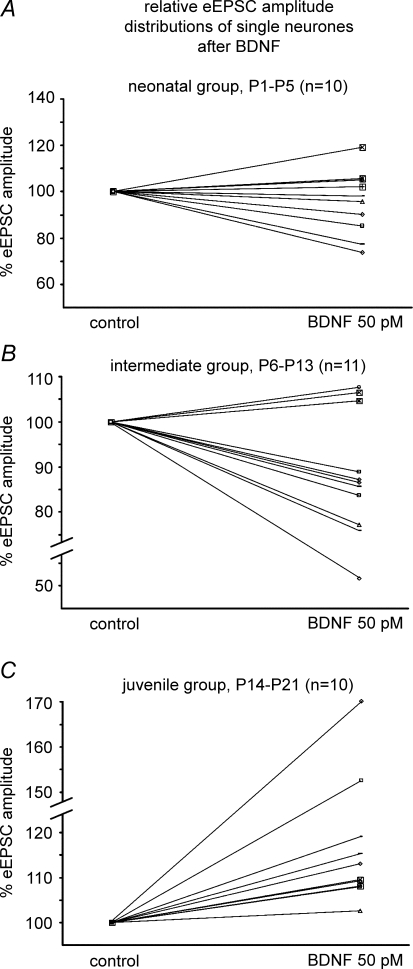
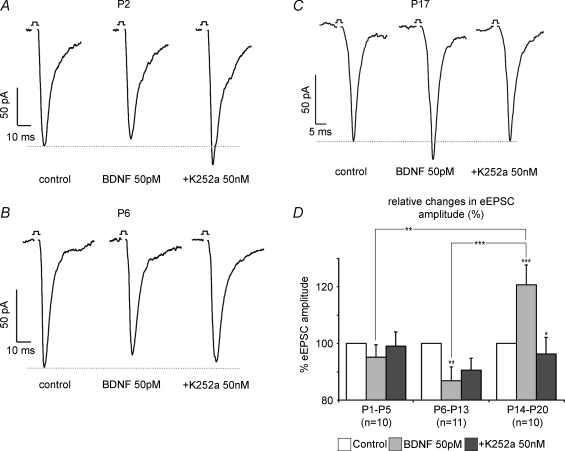
After GABAA and glycine receptors were blocked with 0.5 μm bicuculline and 0.5 μm strychnine, respectively, BDNF-mediated modulation and decay kinetics of eEPSC amplitude were analysed randomly in all age groups. A heterogeneous pattern of BDNF-induced modulation was found in the neonatal group (Fig. 5A). Evoked EPSC amplitude was depressed in six neurones (Fig. 6A), while in four other neurones an augmentation occurred. Overall, BDNF had no statistically significant effect on mean eEPSC amplitude compared with control measurements (−4.8 ± 4.4%). Subsequent application of K252a did not induce any further changes (Fig. 6D).
Figure 5.
Summary of BDNF-mediated effects on eEPSCs at different postnatal stages A, B and C illustrate the BDNF-induced modulation of eEPSC amplitudes from single neurones of the different developmental stages.
Figure 6.
A, B and C show single examples of BDNF-mediated modulation of eEPSC amplitude at the neonate, the intermediate and the juvenile stage, repectively, while D illustrates developmental group data of BDNF and K252a effects on eEPSC amplitudes *P < 0.05, **P < 0.01, ***P < 0.001.
Recordings from KF neurones in the intermediate group (P6–13) revealed a decrease in eEPSC amplitude in response to BDNF in the majority of the recorded neurones (n = 8/11, Fig. 5B). The remaining three neurones responded with a slight increase in eEPSC amplitude. Overall, a moderate depression of eEPSC amplitude of −13.2 ± 4.8% (P < 0.01) was detected, which returned to 90.6 ± 4.2% of the control amplitude after application of K252a (Fig. 6B and D).
In the juvenile group (P14–21, n = 10), BDNF evoked a stable increase in eEPSC amplitude in all neurones tested (Fig. 5C). Overall, we observed an increase of +20.8 ± 7.0% (P < 0.001, Fig. 6C and D). The responses returned to 96.3 ± 5.7% (P < 0.05) of control amplitude with K252a (Fig. 6C and D).
In control experiments (n = 11), application of AP5 (NMDA receptor antagonist; 20 μm) together with CNQX (non-NMDA receptor antagonist; 10 μm) blocked the eEPSCs by 93.2 ± 2%, indicating that the eEPSCs were predominantly glutamatergic (data not shown).
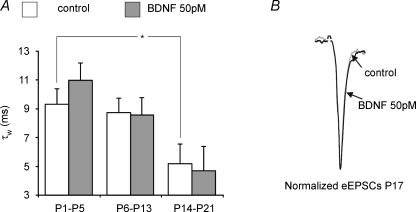
Statistical analyses of developmental changes in the strength and direction of 50 pm BDNF-mediated modulation of eEPSC amplitude revealed significant differences between the three age groups (Fig. 6D). During postnatal development, the weighted time constant τw shortened (9.3 versus 5.2 ms in neonates and juveniles, respectively, P < 0.05, Fig. 7A). Application of BDNF significantly modulated the amplitude of eEPSCs in the intermediate and the juvenile group, whereas the decay kinetics remained unchanged (Fig. 7A and B).
Figure 7.
Developmental changes in weighted time constants and decay kinetics A illustrates BDNF-evoked effects on the weighted time constant (τw) in the different experimental groups. The BDNF had no significant effects on the decay of eEPSCs, but decay kinetics became significantly faster with increasing postnatal age. *P < 0.05. B shows an overlay of normalized currents before and after BDNF application to indicate no BDNF-evoked changes in decay kinetics.
Age-dependent changes in BDNF-evoked modulation of phrenic nerve activity after microinjections into the KF
Experiments focused on those postnatal stages that revealed clear-cut results in the cellular analyses, namely the intermediate (P6–13, n = 8) and the juvenile group (P16–23, n = 9). In the intermediate age group, glutamate microinjections (10 mm, 30–60 nl) into the KF evoked two different forms of PNA modulation: a transient prolongation of tE (transient apnoea) from 2.52 ± 0.547 to 8.11 ± 1.75 s (P < 0.001, n = 6, Fig. 8A and C) and a reduction of tE from 1.89 ± 0.499 to 0.51 ± 0.117 s (transient tachypnoea, not shown). Subsequent injections of equivalent amounts of BDNF (200 ng ml−1, 30–60 nl) did not significantly influence PNA. Statistical analyses revealed only marginal BDNF-evoked changes in tE (from 2.52 ± 0.547 to 2.38 ± 0.358 s, n.s.) at apnoeic injection sites (Fig. 8A and C).
In contrast to the intermediate age group, preparations from the juvenile group showed changes in PNA in response to BDNF that were comparable to the effects of glutamate (Fig. 8B and C). In n = 7 preparations, glutamate (from 3.91 ± 0.193 to 10.68 ± 1.876 s; P < 0.05), as well as BDNF (from 3.91 ± 0.193 to 7.64 ± 0.52 s, P = 0.001), evoked a significant prolongation of tE (Fig. 8B and C). In the remaining two preparations, glutamate injections induced a shortening of tE from 4.17 ± 1.36 to 1.54 ± 0.794 s (data not shown). The effect of glutamate was mimicked by injecting BDNF (tE, 4.17 ± 1.36 versus 1.29 ± 0.798 s, data not shown). Analyses of developmental changes in BDNF-induced prolongation of tE revealed a highly significant difference between the change in tE prolongation in the intermediate versus juvenile age groups (−0.14 ± 0.21 versus 3.72 ± 0.64 s; P < 0.001, Fig. 8C).
Injections of an equivalent volume of Pontamine Sky Blue had no effect on PNA. Effects of volume could therefore be excluded. Histological analyses showed that all injection sites that were associated with apnoea were located in or near the intermediate KF (Fig. 8D).
Discussion
Physiological relevance of BDNF-mediated modulation of glutamatergic inputs in the KF
In the present study, we demonstrate that BDNF-evoked modulation of fast synaptic activity in the KF depends on the degree of neuronal maturation. In neonates, BDNF weakly and heterogeneously modulated spontaneous and evoked excitatory synaptic activity. As postnatal maturation progressed, BDNF significantly modulated synaptic activity. Similar developmental changes in the response to BDNF were obvious for the modulation of the respiratory motor output mediated by the KF.
The BDNF injection sites in the KF correspond to areas known to be involved in the gating of the postinspiratory phase of the breathing cycle (Dutschmann & Herbert, 2006). The postinspiratory activity of the respiratory network largely determines the duration of the inspiratory and, in particular, of the expiratory intervals of the respiratory cycle. The KF is crucial for the modulation of the breathing pattern in response to various afferent inputs (Dutschmann et al. 2004; Song & Poon, 2004). The KF is particularly important for sensory information transmitted by afferent projections from the nucleus of the solitary tract (NTS, Herbert et al. 1990), which densely expresses BDNF (Yan et al. 1997b; Wang et al. 2006).
The finding that BDNF modulates KF neurones only in late postnatal stages is in agreement with our recent hypothesis that only after completion of postnatal maturation does the respiratory network become permissive for activity-dependent fast synaptic plasticity in response to afferent inputs (Dutschmann et al. 2004). We suggest that BDNF is released in an activity-dependent manner in the mature KF during the processing of NTS-related afferent inputs. Release of BDNF may therefore modulate glutamatergic neurotransmission via AMPA and NMDA receptors, which are densely expressed in the KF (Monaghan & Cotman, 1985; Guthmann & Herbert, 1999a, b).
However, this might not account for activity-independent forms of respiratory-related plasticity, such as long-term facilitation of respiratory motor outputs in response to intermittent hypoxia. This form of plasticity also depends on BDNF (Baker-Herman et al. 2004) and is already expressed by the neonatal respiratory network in vivo and in vitro (Bocchiaro & Feldman, 2004; McKay et al. 2004).
Brain-derived neurotrophic factor-mediated effects on synaptic activity in vitro
Brain-derived neurotrophic factor binds to its high-affinity receptor, tyrosine kinase B (trkB), which is widely distributed in the central nervous system (Yan et al. 1997a). The receptors are located pre-, as well as postsynaptically (Wu et al. 1996; Aoki et al. 2000; Yamada & Nabeshima, 2003). At the presynapse, BDNF increases the number of docked vesicles, thereby enhancing transmitter release (Gottschalk et al. 1998; Tyler & Pozzo-Miller, 2001). Moreover, protein kinase C (PKC), as a downstream target of trkB, potentiates spontaneous and evoked transmitter release by different presynaptic mechanisms (Waters & Smith, 2000). At the postsynaptic site, BDNF modulates the amplitude of NMDA receptor-mediated postsynaptic currents (Levine et al. 1995, 1998) and GABAA receptor-mediated currents (Cheng & Yeh, 2003). In our experiments, BDNF induced an increase in sPSC frequency but no change in sPSC amplitude. Furthermore, application of BDNF had no effect on the decay of eEPSCs, suggesting no obvious modulation of postsynaptic response kinetics. In contrast, BDNF modulated eEPSC amplitudes. Thus, our results can be related to either pre- or postsynaptic effects.
Brain-derived neurotrophic factor is thought to enhance excitatory neurotransmission involving both AMPA and NMDA receptors (Levine et al. 1998; Tyler & Pozzo-Miller, 2001; Yamada et al. 2002; Lu, 2003). In contrast, BDNF seems to have a negative influence on inhibitory neurotransmission (Henneberger et al. 2001; Cheng & Yeh, 2003). We have shown that the effects of BDNF on sPSCs and eEPSCs change during the course of postnatal maturation. With on-going maturation, we observed a moderate enhancement in sPSC frequency induced by BDNF, which became more pronounced in juvenile neurones. This might be related to a growing predominance of excitatory synaptic activity within the KF, as it has been shown for medullary parts of the respiratory network (Liu & Wong-Riley, 2005; Wong-Riley & Liu, 2005). Interestingly, an abrupt transition occurs in the medulla oblongata around postnatal day 12. Immunohistochemical studies revealed a transient upregulation of inhibitory neurotransmitters and their receptors, accompanied by a downregulation of glutamate receptors (Liu & Wong-Riley, 2005; Wong-Riley & Liu, 2005). A similar transition may explain the depressing effect of BDNF on eEPSC amplitudes, as seen in the intermediate group. A recent publication demonstrated that BDNF acutely depressed the excitatory input to GABAergic neurones (Jiang et al. 2004). Thus, upregulation of the GABAergic transmitter system during the transition might have caused the depressant effects of BDNF, as revealed in the intermediate age group. The later switch to BDNF-enhanced eEPSC amplitudes and to increased sPSC frequencies corresponds to a substantial upregulation of glutamate receptors (e.g. NMDA receptors) in the respiratory network.
Our findings on the developmental changes of BDNF-evoked synaptic modulation show some contradictions with previously published results, which show that BDNF strongly modulates synaptic activity of spinal motoneurones in neonates, while in later postnatal stages the modulation is blunted or absent (Arvanian & Mendell, 2001a). The different findings can be explained by specific developmental changes in the subunit composition of NMDA receptors located in the spinal cord and the KF. For the KF, these developmental changes in the subunit composition have not been described. However, the NMDA receptor subunit NR2D is expressed predominantly in the adult KF (Guthmann & Herbert, 1999a). NMDA receptors, including the NR2D units, show a low Mg2+ block and are therefore associated with rapid membrane depolarization (Clarke & Johnson, 2006), which may be required for KF-mediated fast adaptation of breathing activity in response to excitatory afferent input (Dutschmann et al. 2004). In contrast, spinal motoneurones are reported to decrease NMDA receptor activity during postnatal development owing to a developing Mg2+ block of NMDA receptors (Arvanian & Mendell, 2001b), suggesting a high expression profile for the NR2A and 2B subunits (see Clarke & Johnson, 2006) in later developmental stages. We conclude that developmental changes in the strength of BDNF-evoked synaptic modulation largely depend on developmental changes in the cytochemical profile of neuronal populations. Therefore, the effects of BDNF during various stages of postnatal development can vary between forebrain, midbrain and hindbrain–spinal cord structures.
Potential clinical implications of the study
The developmental changes in responsiveness to BDNF shown here may be of clinical relevance. Recent publications have described a dysregulation of BDNF expression linked with a neurodevelopmental disorder named Rett's syndrome (RTT; Chang et al. 2006; Wang et al. 2006). Rett's syndrome is associated with severe breathing disorders. In a mouse model for RTT, breathing disorders start to develop 2–3 weeks after birth (Viemari et al. 2005) and thus correlate with the late developmental changes in BDNF function in the KF. Recent data from our laboratory suggest that breathing disturbances in RTT may originate from an impaired excitatory synaptic transmission in the KF (Stettner et al. 2007). The late onset of breathing disorders in the RTT mice, as well as the correlation with late developmental changes in BDNF functions, further underline the physiological importance of BDNF as a neuromodulator in the KF.
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft (SFB 406/C12). We are most appreciative to Anna Bischoff for technical support during the study, to Dr Swen Hülsman for his help during the realization of the project and to Drs Ulrike Dürr and Peter Lalley for valuable suggestions on the manuscript.
References
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci. 2001a;14:1800–1808. doi: 10.1046/j.0953-816x.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Mendell LM. Removal of NMDA receptor Mg2+ block extends the action of neurotrophin-3 on synaptic transmission in neonatal rat motoneurons. J Neurophysiol. 2001b;86:123–129. doi: 10.1152/jn.2001.86.1.123. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice. J Physiol. 1998;510:527–533. doi: 10.1111/j.1469-7793.1998.527bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABAA receptor-mediated responses via postsynaptic mechanisms. J Physiol. 2003;548:711–721. doi: 10.1113/jphysiol.2002.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RJ, Johnson JW. NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J Neurosci. 2006;26:5825–5834. doi: 10.1523/JNEUROSCI.0577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S., Jr Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience. 2001;103:739–761. doi: 10.1016/s0306-4522(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kölliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errchidi S, Montea R, Hilaire G. Noradrenergic modulation of the medullary respiratory rhythm generator in the newborn rat: an in vitro study. J Physiol. 1991;443:477–498. doi: 10.1113/jphysiol.1991.sp018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M. Developmental expression of Na+ currents in mouse Purkinje neurons. Eur J Neurosci. 2006;24:2557–2566. doi: 10.1111/j.1460-9568.2006.05139.x. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Hellard DT, Huang L, Katz DM. Development of pontine noradrenergic A5 neurons requires brain-derived neurotrophic factor. Eur J Neurosci. 2005;21:2019–2023. doi: 10.1111/j.1460-9568.2005.04016.x. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Herbert H. Expression of N-methyl-d-aspartate receptor subunits in the rat parabrachial and Kölliker-Fuse nuclei and in selected pontomedullary brainstem nuclei. J Comp Neurol. 1999a;415:501–517. [PubMed] [Google Scholar]
- Guthmann A, Herbert H. In situ hybridization analysis of flip/flop splice variants of AMPA-type glutamate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei. Brain Res Mol Brain Res. 1999b;74:145–157. doi: 10.1016/s0169-328x(99)00281-8. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Jüttner R, Rothe T, Grantyn R. Postsynaptic action of BDNF on GABAergic synaptic transmission in the superficial layers of the mouse superior colliculus. J Neurophysiol. 2001;88:595–603. doi: 10.1152/jn.2002.88.2.595. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Jiang B, Kitamura A, Yasuda H, Sohya K, Maruyama A, Yanagawa Y, Obata K, Tsumoto T. Brain-derived neurotrophic factor acutely depresses excitatory synaptic transmission to GABAergic neurons in visual cortical slices. Eur J Neurosci. 2004;20:709–718. doi: 10.1111/j.1460-9568.2004.03523.x. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Katz DM. Neuronal growth factors and development of respiratory control. Respir Physiol Neurobiol. 2003;135:155–165. doi: 10.1016/s1569-9048(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Katz DM. Regulation of respiratory neuron development by neurotrophic and transcriptional signaling mechanisms. Respir Physiol Neurobiol. 2005;149:99–109. doi: 10.1016/j.resp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-d-aspartate-sensitive l-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Meth. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Autran S, Fortin G, Champagnat J. BDNF preferentially targets membrane properties of rhythmically active neurons in the pre-Botzinger complex in neonatal mice. Adv Exp Med Biol. 2004;551:115–120. doi: 10.1007/0-387-27023-x_18. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Cauli B, Champagnat J, Fortin G, Katz DM. Expression of functional tyrosine kinase B receptors by rhythmically active respiratory neurons in the pre-Botzinger complex of neonatal mice. J Neurosci. 2003;23:7685–7689. doi: 10.1523/JNEUROSCI.23-20-07685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bévengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chan S, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J Neurosci. 2000;20:7863–7870. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wu K, Xu JL, Suen PC, Levine E, Huang YY, Mount HT, Lin SY, Black IB. Functional trkB neurotrophin receptors are intrinsic components of the adult brain postsynaptic density. Brain Res Mol Brain Res. 1996;43:286–290. doi: 10.1016/s0169-328x(96)00211-2. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997a;378:135–157. [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997b;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Barnbrock A, Gajic S, Pfeiffer A, Ritter B. Differential ontogeny of GABAB-receptor-mediated pre- and postsynaptic modulation of GABA and glycine transmission in respiratory rhythm-generating network in mouse. J Physiol. 2002;540:435–446. doi: 10.1113/jphysiol.2001.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]