Abstract
An attenuated fever response to pathogens during late pregnancy is a phenomenon that has been described in several mammalian species, and although mechanisms are not completely understood, decreased prostaglandin E2 (PGE2) synthesis has been implicated. Upstream of PGE2, there is evidence to suggest that anti-inflammatory cytokines such as interleukin-1 receptor antagonist (IL-1ra) could play a significant role. In the present study we addressed the role of pro-inflammatory cytokines during late pregnancy, specifically interleukin-6 (IL-6), an important circulating mediator in fever. Turpentine oil (TURP), a very potent pyrogen and activator of IL-6, was injected into the hind-limb muscle of rats at the 18th day of pregnancy (GD 18) or in non-pregnant (NP) age-matched female controls. As expected, TURP injection induced a highly significant fever in the NP animals, which peaked 11 h post-injection and lasted for over 24 h. This was accompanied by a significant rise in circulating IL-6 levels, which correlated with changes in PGE2 synthesizing enzymes expression in the hypothalamus. In complete contrast, TURP-induced fever was totally absent in GD 18 animals whose body temperature did not deviate from basal values. The lack of response was additionally reflected by the absence of change in IL-6 concentration and by the significant attenuation of PGE2 synthesizing enzymes expression, which correlated with the suppressed expression of SOCS3, a hypothalamic marker of IL-6 activity. Contrary to the changes in circulating IL-6 levels at GD 18, IL-1ra was induced to levels comparable to those of NP females, suggesting that the influence of this anti-inflammatory cytokine on the fever response to TURP is at best minimal. These data further confirm the importance of IL-6 in fever generation and provide evidence that it may be a key component of the attenuated fever response in late pregnancy.
Fever is a hallmark response to injury or infection with a well-established adaptive value (Kluger & Rothenburg, 1979; Kluger, 1991; Roth, 2006); its induction, duration, magnitude and relapse are tightly regulated by the balance between the members of a family of endogenous mediators known as cytokines that can act as pro-inflammatory/pyrogenic (interleukin (IL)-1β; tumour necrosis factor α (TNFα) and IL-6) or anti-inflammatory/cryogenic intermediaries (the endogenous IL-1 receptor antagonist (IL-1ra) and IL-10 among others) (Kluger, 1991; Leon, 2002; Roth, 2006). In the central nervous system, pyrogenic cytokines induce prostaglandin E2 (PGE2) synthesis, the ultimate step in fever induction (Ivanov et al. 2002; Ivanov & Romanovsky, 2004; Rummel et al. 2006) by the transcriptional induction of cyclooxygenase-2 (COX-2) and microsomal PGE synthase-1 (mPGES-1), the rate-limiting enzymes of this pathway (Lacroix & Rivest, 1998; Ivanov & Romanovsky, 2004; Matsumura & Kobayashi, 2004). Disruption of this tightly controlled process could result in an abnormal fever response, such as that observed during late pregnancy where an attenuated response to exogenous pathogens has been documented in several mammalian species. In these studies experimental animals including mice, rats, sheep and guinea pigs (Kasting et al. 1978; Martin et al. 1995), exhibited a reduction in the fever response to infusion of either the viral mimic polyinosinic: polycytidylic acid (poly I:C) (Cooper et al. 1988), endotoxin lipopolysaccharide (LPS) (Kasting et al. 1978; Zeisberger et al. 1981; Martin et al. 1995) or endogenous pyrogens such as IL-1β (Simrose & Fewell, 1995) and PGEs (Stobie-Hayes & Fewell, 1996; Eliason & Fewell, 1997). Several studies have linked this to a reduction in central PGE2 synthesis (Imai-Matsumura et al. 2002), probably as a result of blunted hypothalamic COX-2 induction (Imai-Matsumura et al. 2002; Mouihate et al. 2002). Recently, the involvement of central nitric oxide synthase suppressing LPS-induced fever at late pregnancy has been suggested (Begg et al. 2007). What remains uncertain is the nature of the peripheral signals responsible for the reduced COX-2 expression and, ultimately, the fever response. We have recently reported (Ashdown et al. 2006b) that higher than normal circulating levels of IL-1ra in LPS-treated pregnant rats at the later stages of pregnancy, could be partly responsible for these effects. In support of these findings we further demonstrated a causal link between increased levels of circulating IL-1ra and hypothalamic COX-2 induction. Unlike IL-1ra, however, no consistent differences were reported on the ability of animals at late stages of pregnancy to synthesize pro-inflammatory/pyrogenic cytokines in response to LPS. In these animals the LPS-induced production of IL-1β and IL-6 were reported to be reduced when compared with virgin females (Fofie et al. 2005), but not when compared with earlier stages of pregnancy or in lactating dams, whose febrile response is higher in magnitude than dams in late pregnancy (Mouihate et al. 2005).
It has been also suggested that near-term pregnancy suppression of fever may stem from the inability to mount an appropriate/full response to circulating pro-inflammatory cytokines (Chen et al. 1999). This, however, has not been supported by the finding that intracellular signalling pathways triggered by LPS/IL-1β or LPS-induced IL-6 to induce COX-2 remained intact with no differences reported between pregnant dams at early and late stages of pregnancy (Mouihate et al. 2005; Harre et al. 2006). Particularly surprising about these findings is in regard to IL-6, which we and others have demonstrated to be a critical component of the fever response (Kozak et al. 1997, 2006; Cartmell et al. 2000; Rummel et al. 2006). The circulating levels of IL-6 increase dramatically following a systemic inflammatory challenge and correlate significantly with the fever response. During late pregnancy the levels of IL-6 appear to remain unchanged when compared with GD 15 controls (Harre et al. 2006). This observation argues against the importance of this cytokine in the regulation of the fever response. One possibility for this anomaly may lie in the type of stimulus being used. For instance, the majority of studies investigating this aspect (Cooper et al. 1988; Fofie & Fewell, 2003; Fofie et al. 2005; Mouihate et al. 2005; Harre et al. 2006), including ours (Ashdown et al. 2006b), have used a generalized systemic challenge namely LPS or poly I:C. These immunogens tend to act by triggering a robust immune response, which involves the targeting of multiple organs/systems to induce cytokine' release (Givalois et al. 1994; Cartmell et al. 2001; Turrin et al. 2001). Such an overwhelming response may be a factor masking any intricate changes that may occur in the levels of circulating pyrogens such as IL-6. We have previously described a model of localized inflammation, namely the intramuscular (i.m.) injection of turpentine oil (TURP), that induces fever by the activation of febrile effectors in a more defined serial fashion (Luheshi et al. 1997; Leon, 2002). The mode of action of this potent pyrogen involves the production of localized necrotic damage (Wusteman et al. 1990), which results in the sequential induction of TNFα and IL-1β at the site of injury (Zheng et al. 1995; Luheshi et al. 1997). The locally increased cytokines, particularly IL-1β, induce IL-6 synthesis and release into the circulation (Luheshi et al. 1997; Turnbull et al. 2003). A particular advantage of this approach is that it facilitates the study of IL-6 without the influence of other circulating cytokines. Given this property, we sought to characterize the particular role of IL-6 in fever generation in late-pregnant rats (gestational day 18 (GD 18)) by measuring the changes in its levels in the circulation and how they correlate with the fever response, and by investigating changes in brain mechanisms regulating the fever response to this important circulating pyrogen.
Methods
Adult female Sprague–Dawley rats (Charles River, Saint Constant, Quebec, Canada) were used in all experiments; two kinds of animals were employed: randomly cycling non-pregnant (NP) females (250–300 g) and primiparous pregnant females at gestational day 18 (GD 18; 250–340 g). All the individual experimental procedures for this study were approved by the Animal Care Committee of McGill University. Care throughout the duration of the experiment was provided according to the Canadian Council of Animal Care guidelines. Animals were housed individually in a controlled environment at an ambient temperature of 21 ± 2°C; they had free access to food and water and were handled for at least 7 days prior to experimentation. Observations were carried out in a 12 h:12 h light–dark cycle (lights on from 08:00 to 20:00 h).
In all studies, animals received a single intramuscular (i.m.) injection of 100 μl of purified turpentine oil (TURP) (Riedel-deHaën, Sleeze, Germany) or 100 μl of sterile physiological saline (SAL) into the gastrocnemius muscle of the left hind limb. Animals were killed (see details below) 11 h after either treatment.
Measurement of body temperature
Changes in core body temperature were measured using remote radio-biotelemetry (Data Sciences, St Paul, MN, USA) as previously described (Sachot et al. 2004). Briefly, anaesthetized animals (i.m.; 50 mg kg−1 ketamine hydrochloride, 5 mg kg−1 xylazine hydrochloride, 0.5 mg kg−1 acepromazine maleate; 1 μl (g body weight)−1) were implanted intraperitoneally (i.p.) with pre-calibrated temperature-sensitive radio transmitters (TA10TA-F40, Data Sciences). The level of anaesthesia was assessed by the withdrawal reflex to a toe pinch. Pregnant females were implanted on GD 7 and allowed to recover until GD 18. Transmitter output frequency (Hz) was monitored, at 10 min intervals, by an antenna mounted in a receiver board, situated beneath the cage of each animal. The output data from each transmitter were transformed into degrees centigrade by Dataquest software (Data Sciences). On the day of the experiment, NP and GD 18 females were separated into SAL or TURP groups (n = 5–7 per group). Injections were administered between 08:00 and 09:00 h and the changes in core body temperatures monitored up to 11 h post-treatment. Body temperature data were analysed by calculating the area under the temperature-versus-time curve (AUC or fever index) for each animal; mean AUC per group was used for statistical analysis.
Plasma cytokine measurements
Plasma concentrations of IL-6, IL-1β and IL-1ra were determined from animals killed at the peak of the febrile response, 11 h after TURP injection, in order to measure the maximum humoral responses to the TURP challenge. Blood was collected from animals deeply anaesthetized with a lethal dose of pentobarbital sodium (i.p.; 60 mg (kg body weight)−1) via cardiac puncture (n = 5–7 per group) using sterile heparinized syringes and placed into sterile tubes. Samples were then centrifuged (5300 g, 15 min at 4°C), aliquoted and stored at −80°C until assays were performed. Sandwich enzyme-linked immunosorbent assays (ELISA) for IL-6, IL-1β and IL-1ra (NIBSC, Potters Bar, UK) were performed as previously described (Rees et al. 1999), except that plasma samples and secondary antibodies (NIBSC) were diluted in a buffer containing 0.5 m NaCl, 2.5 mm NaH2PO4, 7.5 mm Na2HPO4, 0.1% Tween 20, 1% ovalbumin and 1: 100 normal sheep serum (Sigma-Aldrich, St Louis, MO, USA). Intra-assay and inter-assay variations were below 10%. The detection limit for IL-6 and IL-1β was 40 pg ml−1, and 78 pg ml−1 for IL-1ra. All samples were assayed in duplicate.
Hypothalamus dissection
Killed animals designated for plasma collection were perfused with sterile physiological saline (prepared in DEPC-treated H2O); brains were dissected, frozen on dry ice and stored at −80°C until used. Hypothalami were microdissected from frozen tissue and divided into right and left hemispheric portions; the left hemisphere was used for RNA extraction and the right for protein extraction.
RNA extraction and RT–PCR in the hypothalamus
In order to asses the changes in transcription of SOCS3, IL-1β, COX-2 and mPGES-1 genes in the hypothalamus, reverse transcription (RT), followed by PCR were performed as previously reported (Rummel et al. 2006). Briefly, total RNA was extracted by disaggregating tissues in 1 ml of TRIzol (Invitrogen, Burlington, ON, Canada). Total RNA was isolated according to the manufacturer's protocol and the air-dried RNA pellet dissolved in 50 μl of DEPC-treated water. One microgram of total RNA was transcribed into DNA. PCR reactions were performed for COX-2, mPGES-1, SOCS3, IL-1β and β-actin using 1.8 μl of cDNA and 6 pmol of specific primers (Alpha DNA, Montreal, QC, Canada) for COX-2 (forward: 5′-TGATAGGAGAGACGATCAAGA-3′; reverse: 5′-ATGGTAGAGGGCTTTCAACT-3′), mPGES-1 (forward: 5′-TTTCTGCTCTGCAGCACACT-3″; reverse: 5′-CATGGAGAAACAGGTGAACT-3′), SOCS3 (forward: 5′-CCAGCGCCACTTCTTCAC-3′; reverse: 5′-GTGGAGCATCATACTGGTCC-3′), IL-1β (forward: 5′-CCCAAGCACCTTCTTTTCCTTCATCTT-3′, reverse: 5′-CAGGGTGGGTGTGCCGTCTTTC-3′) and β-actin (forward: 5′-GCCGTCTTCCCCTCCATCGTG-3′; reverse: 5′-TACGACCAGAGGCATACAGGGACAAC-3′) using a Gene Amp PCR system 9700 Thermocycler (Applied Biosystems, Foster City, CA, USA). The following parameters were used: (1) 5 min at 94°C (all primer pairs); (2) 30 s at 94°C, 30 s at 60°C (for SOCS3, IL-1β and β-actin) or 57°C (for mPGES-1 and COX-2), followed by 45 s at 72°C for 20, 28, 34, 36 or 40 cycles (for β-actin, COX-2, mPGES-1, IL-1β and SOCS3, respectively); and (3) 72°C for 10 min (for all primer pairs). PCR products were separated by gel electrophoresis (1.5% agarose) and band densities were obtained using GeneTool image analysis software (Syngen, Frederick, MD, USA). Each cDNA data were expressed as a ratio of β-actin optical density and then relative to measurements obtained from the SAL group of NP females ((gene X/β-actin mRNA)/(mean of gene X/β-actin from SAL-NP group)) in order to pool data from different gels. Initial PCR experiments using total RNA were performed for each pair of primers to ensure that products did not result from genomic DNA amplification. Additionally, the linear phase of PCR amplification of cDNA was determined by performing RT–PCR on a sample from each treatment group for an increasing number of cycles (20–50 cycles).
Protein extraction and hypothalamic COX-2 Western blot analysis
The right side of the hypothalamus was disaggregated in lysis buffer (50 mm Tris-HCl, 2 mm EDTA and 1% Nonidet) that included a protease inhibitor cocktail for general use (Sigma-Aldrich). Protein content was quantified using Bradford's reagent (Sigma-Aldrich) following manufacturer's instructions, aliquoted and frozen at −80°C until used.
On the day of the analytical procedure the protein was mixed (1: 1) with Laemmli running buffer (Bio-Rad Laboratories, Mississauga, ON, Canada) mixed with β-mercaptoethanol (50 μl of β-mercaptoethanol per millilitre of Laemmli buffer; Sigma-Aldrich) and incubated at 95°C for 5 min. This (50 μg) was then loaded into pre-cast acrylamide gels (4–20% Tris-glycine gel, Invitrogen) and electrophoresed for 2 h at 125 V. The protein was then transferred (overnight at 15 V) to nitrocellulose membranes (Hybond ECL, Amersham Biosciences Corp., Piscataway, NJ, USA) using Xcell II Blot Module (Invitrogen), following the manufacturer's instructions for liquid transference.
For immunodetection, membranes were blocked with 1 × Tris-buffer saline (TBS), 10% non-fat dry milk (Bio-Rad Laboratories) and 0.1% Tween 20 for 2 h at room temperature. Membranes were then incubated overnight (at 4°C) with a mixture of antibodies raised against murine COX-2 (1: 1000; Cayman Chemical, Hornby, ON, Canada) and actin (1: 10 000, Sigma-Aldrich) diluted in 1 × TBS, 5% milk and 0.1% Tween 20. After, antibody excess was washed (1 × for 15 min and 4 × for 5 min) with 1 × TBS, 0.1% Tween 20, membranes were incubated with a detection antibody (1: 2000; donkey anti-rabbit IgG-HRP, Santa Cruz Biotechnology, Santa Cruz, CA, USA; diluted in 1 × TBS, 1% milk and 0.1% Tween 20) for 1 h at ambient temperature. After a similar series of washing steps, the membranes were incubated with ECL Western blotting detection reagents (0.125 ml cm−2; Amersham Biosciences Corp.) for 1 min and then exposed to a chemoluminescence sensitive film (Hyperfilm ECL, Invitrogen) for 1–5 min. Films were digitized and optical density was determined using GeneTool image analysis software (Syngen, Frederick, MD, USA). Two bands were detected, one at approximately 42 kDa corresponding to actin and another at ∼72 kDa corresponding to COX-2. Levels of COX-2 expression were expressed as a ratio of the β-actin signal and then, as for PCR data, as relative amounts of the measurements obtained for the SAL-NP females in order to pool data from different membranes.
Data analysis
All data are presented as mean values ±s.e.m. and were analysed using StatView software (version 4.57, Abacus Concepts Inc., Berkeley, CA, USA). One-way ANOVA was used to analyse the data from the temperature, ELISA, PCR and Western blot studies. In cases where comparisons using ANOVA were significant, Newman–Keuls multiple comparisons test was performed. Correlations between parameters were analysed and expressed as fitness to linear curve (r2); statistical significance was determined by ANOVA. In all cases, P values less than 0.05 were deemed statistically significant.
Results
TURP-induced fever is abolished in late pregnancy
Basal core-body temperature (Tc) was generally lower in pregnant animals compared with NP females (Fig. 1). This finding is consistent with previous reports that show reduced light-phase basal Tc during late pregnancy (Fewell et al. 2002; Fofie & Fewell, 2003). Similar to previous reports (Cooper & Rothwell, 1991; Luheshi et al. 1997), i.m. injection of 100 μl of TURP into the left rear-limb of NP females (n = 7), induced a significant increase in Tc that started 2.5 h after injection and peaked between 10 and 11 h post-injection (∼39.5°C), with a maximum increase in temperature of 2.4°C (Fig. 1). Fever persisted during the light phase of the second day but was no longer evident during that day's dark phase (data not shown). In contrast, and somewhat surprising given the potency of the TURP stimulus, rats at a late pregnancy stage (GD 18) exhibited no change in body temperature, which did not deviate from basal over the time course of the study (n = 6; Fig. 1). As would be expected given the lack of response in GD 18 animals, the comparison of the fever index showed that NP females injected with TURP had significantly higher fever index than NP-SAL (n = 5) and GD 18-TURP groups (one-way ANOVA: F3,22= 8.72, P = 0.0008; Newman–Keuls test versus SAL-injected NP females: P < 0.001 and versus TURP-injected GD 18 females: P < 0.01; inset Fig. 1), while that of GD 18–TURP did not differ from the one of GD 18–SAL (n = 5; Newman–Keuls test: P > 0.05). Interestingly, and despite the absence of the fever response in the pregnant females, TURP-induced oedema of the hind-limb (a hallmark sign of localized inflammation) was present in GD 18 animals, and was similar in severity to that exhibited by the counterpart cycling females. None of these animals showed signs of extreme discomfort that necessitated the premature termination of the study.
Figure 1.
TURP-induced fever is attenuated in late pregnancy Injection of TURP (100 μl; i.m.) induced a significant rise in core body temperature in NP females (Tc,°C). Animals in the 18th day of pregnancy (GD 18) were completely resistant to the febrile effects of TURP. The arrow indicates the time of injection (animals were injected between 08:00 and 09:00 h). Inset, fever indexes (or area under the curve, AUC) were calculated for each group. AUC in the NP females injected with TURP was significantly greater than both SAL-injected NP females and TURP-injected GD 18 dams. NP females versus SAL-injected NP females: **P < 0.01 and versus TURP-injected GD 18 females: *P < 0.05.
Circulating levels of cytokines: dampened IL-6 induction is associated with the absence of fever response
At the peak of the fever response, 11 h after TURP injection, NP females showed a 5.4-fold increase in the concentration of IL-6, from 27.4 ± 9.2 pg ml−1 in the SAL group (n = 5) up to 146 ± 29 pg ml−1 in the TURP-injected animals (n = 7; one-way ANOVA: F3,19= 6.42, P < 0.01; Newman–Keuls test versus SAL-injected NP females: P = 0.0012; Fig. 2A). IL-6 levels in the SAL-injected GD 18 group (n = 5; 38.6 ± 2.2 pg ml−1) did not differ from those of the SAL-injected NP females (Newman–Keuls test: P = 0.74). Late-pregnant rats did not show a significant induction of IL-6 after TURP injection (n = 6; 60.5 ± 21.9 pg ml−1; Newman–Keuls test versus SAL-injected GD 18 dams: P = 0.5), and compared with TURP-injected NP-females, these levels were significantly lower (Newman–Keuls test: P = 0.0094; Fig. 2A).
Figure 2.
Circulating IL-6 and IL-1ra levels after TURP injection are differentially modulated in late pregnant dams A, 11 h after the injection of TURP (100 μl; i.m.), IL-6 concentration was significantly increased in NP females; this increase was not evident in late-pregnant dams (GD 18), whose levels did not differed from those found in the corresponding SAL-injected controls. TURP-injected NP females versus SAL-injected NP females: **P < 0.01 and versus TURP-injected GD 18 females: *P < 0.05. B, top panel, correlation analysis between plasma IL-6 levels (x axis) and temperature 11 h after injection (y axis) from NP females revealed a significant relationship between these parameters (n = 12 including SAL- and TURP-injected animals; r2= 0.74, P = 0.0003). Bottom panel, the same analysis showed that in GD 18 dams this correlation was not evident (n = 11; r2= 0.08, P = 0.07). C, IL-1ra concentration was significantly increased in both NP and GD 18 females after TURP injection; there was no significant difference in the induced levels of the anti-inflammatory cytokine between these two groups. TURP-injected versus SAL-injected NP females: **P < 0.01; TURP-injected versus SAL-injected GD 18 females: *P < 0.05.
NP females showed a significant correlation between plasma IL-6 concentration and Tc (r2= 0.74, P = 0.0003; top panel Fig. 2B) which is consistent with the proposed role of this cytokine in driving the fever response after localized infection or inflammation (Cartmell et al. 2000; Rummel et al. 2006). In contrast, no such correlation was observed in the GD 18 pregnant dams (bottom panel Fig. 2B) in concordance with their suppressed febrile response.
Circulating IL-1β and IL-1ra levels were also assayed. IL-1β levels were found to be below detection limits of the assay in all animals tested (NP and GD 18 females; data not shown). TURP-injected groups had a similar rise in IL-1ra concentrations (one-way ANOVA: F3,17= 9.86, P = 0.0005; Newman–Keuls test: P > 0.05), from 406 ± 49 pg ml−1 and 500 ± 55 pg ml−1 under the SAL treatment up to 3151 ± 668 pg ml−1 and 2067 ± 386 pg ml−1 11 h after TURP injection in NP and GD 18 animals, respectively (Newman–Keuls test SAL- versus TURP-injected NP females: P < 0.01; and SAL- versus TURP-injected GD 18 dams: P < 0.05; Fig. 2C).
Changes in circulating IL-6 levels correlate with hypothalamic SOCS3 mRNA expression
To further confirm that circulating IL-6 was acting on the hypothalamus, SOCS3 mRNA was measured in the same animals used for cytokine determination. SOCS3, which is induced by the IL-6-activated intracellular signalling pathway (Lebel et al. 2000), is part of a negative feedback mechanism that limits the pro-inflammatory actions of this cytokine (Croker et al. 2003).
In NP females, 11 h after TURP injection, SOCS3 mRNA was induced ∼1.8-fold compared with SAL-injected females (one-way ANOVA: F3,19= 8.79, P = 0.0007; Newman–Keuls test versus SAL-injected NP group: P < 0.001; Fig. 3A); these changes correlated significantly with circulating IL-6 levels and Tc (r2= 0.53, P = 0.0074 for IL-6; r2= 0.55, P = 0.0059 for Tc; top panel of Fig. 3B and C). In contrast, pregnant females did not show any significant increase in SOCS3 mRNA (Newman–Keuls test versus SAL-injected GD 18 dams: P > 0.05; Fig. 3A) nor a correlation between this mRNA and Tc or IL-6 levels (bottom panels Fig. 3B and C). Similar to plasma IL-6, SOCS3 mRNA levels in TURP-injected GD 18 dams were significantly lower compared with those of TURP-injected NP females (Newman–Keuls test: P = 0.019; Fig. 3A).
Figure 3.
Hypothalamic SOCS3 mRNA, a marker of IL-6 activity, is not induced after TURP challenge during late pregnancy A, in NP females, SOCS3 mRNA expression in the hypothalamus was significantly induced 11 h after TURP injection (100 μl; i.m.); in contrast, this increase was blunted in GD 18 dams. TURP-injected NP females versus SAL-injected NP females: **P < 0.01 and versus TURP-injected GD 18 females: *P < 0.05. Each band in the gel represents one animal; two animals per group are shown. B, top panel, correlation analysis between hypothalamic SOCS3 mRNA levels (x axis) and temperature 11 h after injection (y axis) from NP females revealed a significant relationship between these parameters (n = 12 including SAL- and TURP-injected animals; r2= 0.55, P = 0.0059). Bottom panel, the same analysis showed that in GD 18 dams this correlation was not evident (n = 11; r2= 0.0001, P = 0.89). C, top panel, in NP females, correlation analysis as in B for plasma IL-6 levels (x axis) and hypothalamic SOCS3 mRNA levels (y axis) revealed a significant link between these parameters (n = 12 including SAL- and TURP-injected animals; r2= 0.53, P = 0.0074). Bottom panel, in contrast, in GD 18 dams this correlation (IL-6 versus hypothalamic SOCS3 mRNA) was not evident (n = 11; r2= 0.14, P = 0.25).
Fever pathways in the hypothalamus: evidence of blunted prostaglandin synthesis and reduced induction of IL-1β
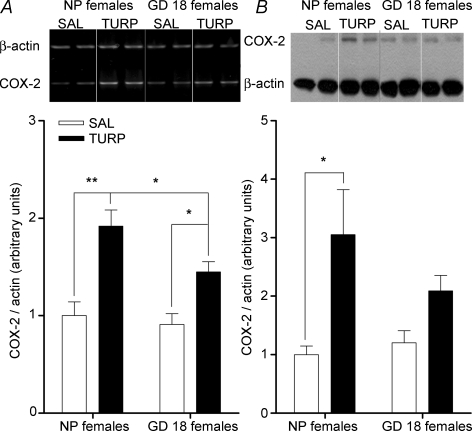
Expression of COX-2 was determined by RT-PCR; 11 h after TURP injection, hypothalamic COX-2 mRNA was significantly induced (1.9-fold) in NP females compared with SAL-injected ones (one-way ANOVA: F3,19= 11.39, P = 0.0002; Newman–Keuls test: P < 0.001; Fig. 4A). In GD 18 females TURP injection also caused a significant induction of COX-2 mRNA (1.4-fold, Newman–Keuls test versus SAL-injected GD 18 dams: P < 0.05), although this was significantly lower compared with the induction in NP females (Newman–Keuls test: P < 0.05; Fig. 4A).
Figure 4.
COX-2 expression, at the level of mRNA and protein, is differentially regulated in NP and GD 18 females after TURP injection A, TURP injection (100 μl; i.m.) induced a significant increase of COX-2 mRNA expression in the hypothalamus; this induction was reduced, but still present, in GD 18 dams. TURP-injected NP females versus SAL-injected NP females: **P < 0.01; TURP-injected GD 18 females versus SAL-injected GD 18 females and versus TURP-injected NP females: *P < 0.05. Each band in the gel represents one animal; two animals per group are being shown. B, COX-2 protein levels (measured by Western blot) in the hypothalamus of NP females were increased 11 h after TURP injection (100 μl; i.m.); this increase was not present in GD 18 dams. TURP-injected NP females versus SAL-injected NP females: *P < 0.05. Each band in the gel represents one animal; two animals per group are shown.
In order to confirm the RT-PCR data, COX-2 expression was assayed by Western blot. NP females had 3-fold more COX-2 levels after TURP injection (one-way ANOVA: F3,18= 4.6, P = 0.015; Newman–Keuls test versus SAL-injected NP group: P < 0.01; Fig. 4B). In contrast, pregnant females injected with TURP did not show such an increase compared with their own control group (Newman–Keuls test: P > 0.05; Fig. 4B). Although induced levels of COX-2 were greater in NP females than in GD 18 dams (3.0 ± 0.77 arbitray units (a.b.) versus 2.1 ± 0.29 a.b., respectively) this difference was not statistically significant (Newman–Keuls test: P = 0.15).
Similar to the changes in COX-2, mPGES-1 mRNA levels were significantly up-regulated in NP (2.15-fold; one-way ANOVA: F3,19= 11.8, P = 0.0001; Newman–Keuls test versus SAL-NP females: P < 0.0001) and GD 18 females (1.7-fold; Newman–Keuls test versus SAL-GD 18 dams: P < 0.05; Fig. 5). mPGES-1 mRNA levels differed significantly between the two TURP-injected groups (Newman–Keuls test: P < 0.05; Fig. 5).
Figure 5.
Attenuated mPGES-1 mRNA expression in the hypothalamus of GD 18 females RT-PCR analysis of mPGES-1 mRNA levels in the hypothalamus revealed a significant induction in NP females after TURP injection (100 μl; i.m.); in comparison, levels of this mRNA were increased to a lesser extent in GD 18 dams. TURP-injected NP females versus SAL-injected NP females: **P < 0.01; TURP-injected GD 18 females versus SAL-injected GD 18 females and versus TURP-injected NP females: *P < 0.05. Each band in the gel represents one animal; two animals per group are being shown.
In NP females, expression levels of COX-2 (measured as mRNA or as protein) and mPGES-1 correlated significantly with circulating IL-6 levels (Table 1). SOCS3 mRNA levels correlated with the expression levels of both enzymes (Table 1). In late-pregnant rats no correlation was found between IL-6 and COX-2 or mPGES-1 (Table 1). However, similar to cycling females, SOCS3 mRNA levels correlated with those of COX-2 and mPGES-1 (Table 1).
Table 1.
Correlation of hypothalamic COX-2 and mPGES-1 expression levels with Tc, plasma IL-6, hypothalamic IL-1β and SOCS3 mRNAs
| Core temperature (Tc) | Plasma IL-6 | Hypothalamic IL-1β mRNA | Hypothalamic SOCS3 mRNA | |||||
|---|---|---|---|---|---|---|---|---|
| NP | GD 18 | NP | GD 18 | NP | GD 18 | NP | GD 18 | |
| COX-2 mRNA | r2 = 0.68** | r2 = 0.01 | r2 = 0.47** | r2 = 0.09 | r2 = 0.79*** | r2 = 0.09 | r2 = 0.70** | r2 = 0.72** |
| COX-2 protein levels | r2 = 0.39* | r2 = 0.02 | r2 = 0.33* | r2 = 0.12 | r2 = 0.26 | r2 = 0.26 | r2 = 0.70** | r2 = 0.33 |
| mPGES-1 mRNA | r2 = 0.69** | r2 = 0.005 | r2 = 0.5 ** | r2 = 0.05 | r2 = 0.84*** | r2 = 0.07 | r2 = 0.64** | r2 = 0.41* |
r2 denotes goodness of fit to a linear curve and significance was evaluated by ANOVA. n = 12 for NP group and 11 for GD 18 group.
P < 0.05
P < 0.01
P < 0.0001.
Although IL-1β was not detected in the circulation, it has been proposed that centrally it plays a significant role in TURP-induced fever (Luheshi et al. 1997). Therefore we decided to measure the expression of its mRNA levels within the hypothalamus. In TURP-injected NP females IL-1β mRNA was significantly induced (2.4-fold, one-way ANOVA: F3,19= 13.26, P = 0.0001; Newman–Keuls test versus SAL-injected NP group: P < 0.0001; Fig. 6A) and correlated with Tc (r2= 0.77, P = 0.0002; top panel Fig. 6B), IL-6 concentration in plasma (r2= 0.45, P = 0.017; top panel Fig. 6C) and SOCS3 mRNA (r2= 0.84, P < 0.0001; figure not shown). Similarly, GD 18 females showed a significant induction of hypothalamic IL-1β (1.7-fold, Newman–Keuls test versus SAL-injected GD 18 females: P < 0.05), although these values were significantly lower than those in TURP-injected NP females (Newman–Keuls test: P < 0.05; Fig. 6A) and did not correlate with Tc (r2= 0.16, P = 0.22; bottom panel Fig. 6B) or with SOCS3 mRNA (r2= 0.18, P = 0.19; figure not shown). However, hypothalamic IL-1β mRNA showed a low but significant correlation with circulating IL-6 levels (r2= 0.6, P = 0.046; bottom panel Fig. 6C). Tc and IL-1β mRNA only correlated with expression levels of COX-2 and mPGE-1 in NP females, and not in GD 18 dams (Table 1).
Figure 6.
Hypothalamic IL-1β mRNA induction after TURP is attenuated GD 18-females A, similar to other central markers of inflammation, IL-1β mRNA expression increased in the hypothalamus of NP females, 11 h after TURP injection (100 μl; i.m.). In TURP-injected GD 18 dams the levels of this mRNA were lower, but still significantly induced. TURP-injected NP females versus SAL-injected NP females: **P < 0.01; TURP-injected GD 18 females versus SAL-injected GD 18 females and versus TURP-injected NP females: *P < 0.05. Each band in the gel represents one animal; two animals per group are being shown. B, top panel, hypothalamic IL-1β mRNA levels (x axis) correlated significantly with temperature 11 h after TURP injection (y axis) in NP females (n = 12 including SAL- and TURP-injected animals; r2= 0.77, P = 0.0002). Bottom panel, the same analysis showed that no such correlation existed in GD 18 dams (n = 11; r2= 0.16, P = 0.22). C, top panel, as in B, in NP females hypothalamic IL-1β mRNA levels (y axis) showed a low, but significant correlation with plasma IL-6 levels (x axis) (n = 12 including SAL- and TURP-injected animals; r2= 0.45, P = 0.017). Bottom panel, in GD 18 dams this correlation was still present (n = 11; r2= 0.46, P = 0.046). The expression of microsomal PGE synthase-1 is attenuated in GD 18 dams after TURP injection.
Discussion
In the present study we demonstrated that a TURP challenge, which would normally induce a highly significant and prolonged fever response in non-pregnant rats, was almost totally ineffective during late pregnancy (Fig. 1). The absence of this response appeared to be the result of an attenuated IL-6 release into the circulation (Fig. 2A), reflected in a diminished hypothalamic SOCS3 mRNA induction (Fig. 3A) and a reduction in COX-2 and mPGES-1 synthesis in this brain structure (Figs 4 and 5). A functional link between fever, peripheral IL-6 and hypothalamic expression of SOCS3, COX-2 and mPGES-1 was confirmed in the cycling females treated with TURP by the induction of these markers and more crucially by the significant correlation between them and the fever response (Figs 2B, 3B and C and Table 1). Importantly, this relationship was absent in the GD 18 females, where these molecules were scantily induced by TURP. The levels of the anti-inflammatory cytokine IL-1ra were comparable in both NP and GD 18 females injected with TURP (Fig. 2C). This contrasts with our earlier observations made in pregnant females injected with LPS (Ashdown et al. 2006b) where it was evident that rather than IL-6, the main factor regulating the attenuated fever response to LPS was in fact a higher than normal production of IL-1ra in the pregnant females. To understand this discrepancy the differences between systemic challenges like i.p. LPS injection and localized activation of the immune system, as occurs with TURP, must be considered. LPS could target directly several tissues that produce IL-1ra (Turrin et al. 2001), such as white adipose tissue (WAT), the amount of which increases during pregnancy (Dayer et al. 2006), most probably resulting in the over-production of this cytokine during late pregnancy. In contrast, TURP effects are confined to the local site of inflammation thus diminishing the possibility of stimulating fat deposits which are more accessible to a circulating stimulus.
Our current observations demonstrating drastically reduced levels of IL-6 (and consequently hypothalamic SOCS3 mRNA) and COX-2 after TURP injection during late pregnancy, provide a possible mechanism underlying the reported reduction in PGE2 levels in the brain, the ultimate step in fever generation (Fewell et al. 2002; Imai-Matsumura et al. 2002; Mouihate et al. 2002). These findings are similar to those reported previously in IL-6 ‘knock out’ mice, which were shown to be resistant to TURP-induced fever (Kozak et al. 1997), and exhibit significantly attenuated COX-2 induction (Turnbull et al. 2003). These results, coupled with ones made in the present study, strongly support our recent observations demonstrating a direct link between circulating IL-6 and brain COX-2 expression (Rummel et al. 2006). In those experiments we clearly demonstrated that IL-6 injection induces COX-2 expression in endothelial cells lining the microvasculature of the brain (Rummel et al. 2006) and that neutralization of LPS-induced circulating IL-6 almost totally abolishes the expression of this enzyme in the same structures.
In addition to the changes in the levels of COX-2 in the current study, we have observed a significant reduction in the levels of mPGES-1 expression after TURP in late-pregnant animals. This enzyme is involved in the last step of PGE2 synthesis, downstream of COX-2, and is fundamental in TURP-induced fever (Saha et al. 2005). In the central nervous system, regulation of mPGES-1 expression has been shown to be linked with that of COX-2 after LPS challenge (Yamagata et al. 2001; Ivanov et al. 2002; Ivanov & Romanovsky, 2004). Our findings support the hypothesis of overlapping mechanisms of transcriptional regulation for both enzymes (Yamagata et al. 2001; Ivanov et al. 2002; Ivanov & Romanovsky, 2004), which is likely controlled by IL-6 in the case of TURP-induced inflammation.
Traditionally, a central role in the regulation of COX-2 and mPGES-1 expression has been ascribed to IL-1β and other molecules classically involved in NF-κB activation (Laflamme et al. 1999; Kojima et al. 2004; Nadjar et al. 2005; Sooranna et al. 2006). The observations made in the current study do not negate this hypothesis since we have demonstrated that hypothalamic IL-1β is induced in both groups of TURP-treated animals but are reduced significantly in pregnant females 11 h after TURP injection (Fig. 6). However, at earlier time points, 4 and 6 h after TURP injection, when fever and increased COX-2 and mPGES-1 expression were already evident (data not shown), hypothalamic IL-1β mRNA was not induced, while circulating IL-6 and hypothalamic SOCS3 mRNA levels were already significantly up-regulated (data not shown). At the later time point tested (11 h), however, there was a high degree of correlation between COX-2 and mPGES-1 expression levels and those of IL-1β mRNA in the hypothalamus (Table 1) similar to the one found for IL-6 and SOCS3 mRNA (Table 1). Given the established role of IL-1β in COX-2 and mPGES-1 regulation, it is tempting to suggest that its central induction may contribute to regulation of PGE2-synthesizing enzymes expression in a sustained febrile response as occurs after TURP injection. The anomaly of this hypothesis is that there are no data suggesting a direct link between peripheral IL-6 and brain IL-1β, and our preliminary observations (data not shown) do not support the existence of such an association.
The observation that pregnant animals responded to the TURP challenge with a significant induction of both hypothalamic COX-2 and mPGES-1 mRNAs (Figs 4A and 5), although this was not reflected in changes in core temperature (Fig. 1), is paradoxical. There are two possible explanations for this finding: (i) The existence of an additional regulatory step that uncouples transcription from translation of COX-2, by which COX-2 protein levels are not increased even with augmented transcription of the COX-2 gene; accordingly, our results showed that hypothalamic COX-2 protein levels were not significantly elevated in late-pregnant dams (Fig. 5B); (ii) The sensitivity to the pyrogenic effect of central PGE2 could be attenuated in near-term pregnant rats. This, in fact, has been previously demonstrated by infusing PGE2 directly into the lateral ventricles of near-term pregnant rats, which resulted in an attenuated pyrogenic effect (Eliason & Fewell, 1998; Chen et al. 1999; Eliason & Fewell, 1999). The mechanisms underlying the attenuated febrile response to PGE2 are not completely clear, although they may involve alteration in PGE receptor expression (Mouihate et al. 2002), changes in brain prostaglandin clearance and/or catabolism (Ivanov & Romanovsky, 2003), or increased expression of the antipyretic arginine–vasopressin (Eliason & Fewell, 1998, 1999; Chen et al. 1999).
In previous reports, the inability to find altered molecular fever pathways in the central nervous system after LPS during late pregnancy, upstream of PGE2 synthesizing enzymes (Mouihate et al. 2005; Harre et al. 2006), could stem from the nature of the given stimulus. As mentioned earlier, LPS is capable of stimulating several organs to produce fever mediators that act in a highly redundant fashion, as is further evidenced by the lack of effect of targeted mutations (‘knock out’) of cytokines on LPS-febrile response (Kopf et al. 1994; Zheng et al. 1995; Kozak et al. 1997; Leon, 2002). Several of these mediators could activate similar intracellular signalling pathways, responsible for COX-2 induction, which could have masked our ability to detect alterations in such pathways. In contrast, in TURP-induced fever, the respective situation is such that elimination of any of the identified components in the cytokine pathway (TNFα, IL-1β and IL-6) abolishes this response (Zheng et al. 1995; Kozak et al. 1997; Luheshi et al. 1997; Leon, 2002) and this is readily reflected in the activation state of downstream mediators, as we report in the present study. Additionally, the use of different controls groups (NP females in the current study and GD 15 and lactating females in Mouihate et al. 2005 and Harre et al. 2006) could explain some of these inconsistencies.
The mechanisms underlying the blunted IL-6 production during pregnancy were not directly addressed in our study; however, several interesting possibilities arise. TURP-induced fever depends largely on IL-6 induction and release into the circulation, as a direct result of localized release of other pyrogenic cytokines such as TNFα and IL-1β (Luheshi et al. 1997; Turnbull et al. 2003). Our previous demonstration that IL-1ra induction is enhanced after LPS injection in late-pregnant rats (Ashdown et al. 2006b), does not extend to all inflammation models such as the one used in the current study; however, it is still feasible that IL-1ra induction in the local site of inflammation (where subcutaneous adipose tissue may be activated) may be enhanced, thus indirectly preventing IL-6 synthesis by inhibiting the action of IL-1β at the level of the necrotic tissue. Other plausible candidates for the reduced IL-6 production during near-term pregnancy are progesterone and oestrogen, that act as generalized modulators of both innate and adaptive immunity (Beagley & Gockel, 2003; Doria et al. 2006). Oestrogen levels rise towards the end of pregnancy (from GD 17 onwards), while progesterone levels, that are maintained at a very high level throughout most of the duration of pregnancy, diminish around GD 19 (Mann & Bridges, 2001). Oestrogen has been shown to decrease the inflammatory effects of carrageenan (Cuzzocrea et al. 2001), IL-1β (Ospina et al. 2004) and LPS (Vegeto et al. 2001; Mouihate & Pittman, 2003). Its anti-inflammatory effect seems to stem from its ability to arrest NF-κB-mediated transcription (Ghisletti et al. 2005) of genomic targets that include IL-6 (Galien & Garcia, 1997) and COX-2 genes (Mouihate & Pittman, 2003; Ospina et al. 2004). This could account for the abolished effects of TURP in dams at GD 18 described in our study. Mouihate & Pittman (2003) showed that oestrogen and progesterone replacement reduced circulating IL-6 induction by IL-1β, but not after LPS injection, although fever and COX-2 expression were modulated in the opposite direction. However, the effect of different doses of oestrogen, in the presence of low levels of progesterone, has not been determined.
The physiological significance of reduced fever or immune activation during pregnancy is generally regarded as a protective mechanism against the detrimental effects of an abnormal rise in core body temperature or cytokines on fetal development. This is based on a growing body of evidence suggesting that immune activation during mid- or late-pregnancy is a risk factor strongly associated with increased incidence of psychiatric disorders such as cerebral palsy, autism and schizophrenia (Boksa, 2004; Fortier et al. 2004; Meyer et al. 2005, 2006; Ashdown et al. 2006a). Accordingly, prenatal exposure to IL-6 was shown to induce abnormalities in hippocampal structure, deficits in spatial working memory, hypertension and alterations in stress response in the adult offspring (Samuelsson et al. 2004; Samuelsson et al. 2006). Based on these observations and the results from the current study, it would appear that this cytokine, which has been shown to cross the blood–placental barrier (Dahlgren et al. 2006), plays a significant role in the reported neurodevelopmental defects associated with maternal infection, stress or injury during gestation and could provide a promising pharmacological target to help meliorate these effects.
Acknowledgments
This work was supported by Canadian Institutes of Health Research and National Alliance for Research on Schizophrenia and Depression. A.A.V. is a recipient of a PhD scholarship from the Canadian Bureau of International Education.
References
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006a;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Ashdown H, Poole S, Boksa P, Luheshi GN. Interleukin-1 receptor antagonist as a modulator of gender differences in the febrile response to lipopolysaccharide in rats. Am J Physiol Regul Integr Comp Physiol. 2006b;292:R1667–R1674. doi: 10.1152/ajpregu.00274.2006. [DOI] [PubMed] [Google Scholar]
- Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Begg DP, Kent S, McKinley MJ, Mathai ML. Suppression of endotoxin-induced fever in near-term pregnant rats is mediated by brain nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2174–R2178. doi: 10.1152/ajpregu.00032.2007. [DOI] [PubMed] [Google Scholar]
- Boksa P. Animal models of obstetric complications in relation to schizophrenia. Brain Res Brain Res Rev. 2004;45:1–17. doi: 10.1016/j.brainresrev.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Hopkins SJ, Rothwell NJ, Poole S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J Physiol. 2001;531:171–180. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000;526:653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hirasawa M, Takahashi Y, Landgraf R, Pittman QJ. Suppression of PGE2 fever at near term: reduced thermogenesis but not enhanced vasopressin antipyresis. Am J Physiol Regul Integr Comp Physiol. 1999;277:R354–R361. doi: 10.1152/ajpregu.1999.277.2.R354. [DOI] [PubMed] [Google Scholar]
- Cooper AL, Rothwell NJ. Mechanisms of early and late hypermetabolism and fever after localized tissue injury in rats. Am J Physiol Endocrinol Metab. 1991;261:E698–E705. doi: 10.1152/ajpendo.1991.261.6.E698. [DOI] [PubMed] [Google Scholar]
- Cooper KE, Blahser S, Malkinson TJ, Merker G, Roth J, Zeisberger E. Changes in body temperature and vasopressin content of brain neurons, in pregnant and non-pregnant guinea pigs, during fevers produced by Poly I:Poly C. Pflugers Arch. 1988;412:292–296. doi: 10.1007/BF00582511. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L, Calabro G, Caputi AP, Maggi A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7:478–487. [PMC free article] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Dayer JM, Chicheportiche R, Juge-Aubry C, Meier C. Adipose tissue has anti-inflammatory properties: focus on IL-1 receptor antagonist (IL-1Ra) Ann N Y Acad Sci. 2006;1069:444–453. doi: 10.1196/annals.1351.043. [DOI] [PubMed] [Google Scholar]
- Doria A, Iaccarino L, Sarzi-Puttini P, Ghirardello A, Zampieri S, Arienti S, Cutolo M, Todesco S. Estrogens in pregnancy and systemic lupus erythematosus. Ann N Y Acad Sci. 2006;1069:247–256. doi: 10.1196/annals.1351.022. [DOI] [PubMed] [Google Scholar]
- Eliason HL, Fewell JE. Influence of pregnancy on the febrile response to ICV administration of PGE1 in rats studied in a thermocline. J Appl Physiol. 1997;82:1453–1458. doi: 10.1152/jappl.1997.82.5.1453. [DOI] [PubMed] [Google Scholar]
- Eliason HL, Fewell JE. AVP mediates the attenuated febrile response to administration of PGE1 in rats near term of pregnancy. Am J Physiol Regul Integr Comp Physiol. 1998;275:R691–R696. doi: 10.1152/ajpregu.1998.275.3.R691. [DOI] [PubMed] [Google Scholar]
- Eliason HL, Fewell JE. Arginine vasopressin does not mediate the attenuated febrile response to intravenous IL-1β in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R450–R454. doi: 10.1152/ajpregu.1999.276.2.R450. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Eliason HL, Auer RN. Peri-OVLT E-series prostaglandins and core temperature do not increase after intravenous IL-1β in pregnant rats. J Appl Physiol. 2002;93:531–536. doi: 10.1152/japplphysiol.01036.2001. [DOI] [PubMed] [Google Scholar]
- Fofie AE, Fewell JE. Influence of pregnancy on plasma cytokines and the febrile response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2003;88:747–754. doi: 10.1113/eph8802594. [DOI] [PubMed] [Google Scholar]
- Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2005;90:95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucl Acids Res. 1997;25:2424–2429. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- Harre EM, Mouihate A, Pittman QJ. Attenuation of fever at near term: is interleukin-6-STAT3 signalling altered? J Neuroendocrinol. 2006;18:57–63. doi: 10.1111/j.1365-2826.2005.01393.x. [DOI] [PubMed] [Google Scholar]
- Imai-Matsumura K, Matsumura K, Terao A, Watanabe Y. Attenuated fever in pregnant rats is associated with blunted syntheses of brain cyclooxygenase-2 and PGE2. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1346–R1353. doi: 10.1152/ajpregu.00396.2002. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1104–R1117. doi: 10.1152/ajpregu.00347.2002. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Near-term suppression of fever: inhibited synthesis or accelerated catabolism of prostaglandin E2? Am J Physiol Regul Integr Comp Physiol. 2003;284:R860–861. doi: 10.1152/ajpregu.00618.2002. author reply R861–865. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- Kasting NW, Veale WL, Cooper KE. Suppression of fever at term of pregnancy. Nature. 1978;271:245–246. doi: 10.1038/271245a0. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Rothenburg BA. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979;203:374–376. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther. 2004;6:R355–R365. doi: 10.1186/ar1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kozak W, Poli V, Soszynski D, Conn CA, Leon LR, Kluger MJ. Sickness behavior in mice deficient in interleukin-6 during turpentine abscess and influenza pneumonitis. Am J Physiol Regul Integr Comp Physiol. 1997;272:R621–R630. doi: 10.1152/ajpregu.1997.272.2.R621. [DOI] [PubMed] [Google Scholar]
- Kozak W, Wrotek S, Kozak A. Pyrogenicity of CpG-DNA in mice: role of interleukin-6, cyclooxygenases, and nuclear factor-κB. Am J Physiol Regul Integr Comp Physiol. 2006;290:R871–R880. doi: 10.1152/ajpregu.00408.2005. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1β in mediating NF-κB activity and COX-2 transcription in cells of the blood–brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19:10923–10930. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel E, Vallieres L, Rivest S. Selective involvement of interleukin-6 in the transcriptional activation of the suppressor of cytokine signaling-3 in the brain during systemic immune challenges. Endocrinology. 2000;141:3749–3763. doi: 10.1210/endo.141.10.7695. [DOI] [PubMed] [Google Scholar]
- Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- Luheshi GN, Stefferl A, Turnbull AV, Dascombe MJ, Brouwer S, Hopkins SJ, Rothwell NJ. Febrile response to tissue inflammation involves both peripheral and brain IL-1 and TNF-α in the rat. Am J Physiol Regul Integr Comp Physiol. 1997;272:R862–R868. doi: 10.1152/ajpregu.1997.272.3.R862. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Lactogenic hormone regulation of maternal behavior. Prog Brain Res. 2001;133:251–262. doi: 10.1016/s0079-6123(01)33019-4. [DOI] [PubMed] [Google Scholar]
- Martin SM, Malkinson TJ, Veale WL, Pittman QJ. Fever in pregnant, parturient, and lactating rats. Am J Physiol Regul Integr Comp Physiol. 1995;268:R919–R923. doi: 10.1152/ajpregu.1995.268.4.R919. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci. 2004;9:2819–2826. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Clerget-Froidevaux MS, Nakamura K, Negishi M, Wallace JL, Pittman QJ. Suppression of fever at near term is associated with reduced COX-2 protein expression in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2002;283:R800–R805. doi: 10.1152/ajpregu.00258.2002. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Ellis S, Harre EM, Pittman QJ. Fever suppression in near-term pregnant rats is dissociated from LPS-activated signaling pathways. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1265–R1272. doi: 10.1152/ajpregu.00342.2005. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Pittman QJ. Neuroimmune response to endogenous and exogenous pyrogens is differently modulated by sex steroids. Endocrinology. 2003;144:2454–2460. doi: 10.1210/en.2002-0093. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Tridon V, May MJ, Ghosh S, Dantzer R, Amedee T, Parnet P. NFκB activates in vivo the synthesis of inducible Cox-2 in the brain. J Cereb Blood Flow Metab. 2005;25:1047–1059. doi: 10.1038/sj.jcbfm.9600106. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1β-mediated induction of COX-2 pathway in rat cerebral blood vessels. Am J Physiol Heart Circ Physiol. 2004;286:H2010–H2019. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- Rees GS, Ball C, Ward HL, Gee CK, Tarrant G, Mistry Y, Poole S, Bristow AF. Rat interleukin 6: expression in recombinant Escherichia coli, purification and development of a novel ELISA. Cytokine. 1999;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- Roth J. Endogenous antipyretics. Clin Chim Acta. 2006;371:13–24. doi: 10.1016/j.cca.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1316–R1326. doi: 10.1152/ajpregu.00301.2006. [DOI] [PubMed] [Google Scholar]
- Sachot C, Poole S, Luheshi GN. Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J Physiol. 2004;561:263–272. doi: 10.1113/jphysiol.2004.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Engstrom L, Mackerlova L, Jakobsson PJ, Blomqvist A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1100–R1107. doi: 10.1152/ajpregu.00872.2004. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABAA dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Ohrn I, Dahlgren J, Eriksson E, Angelin B, Folkow B, Holmang A. Prenatal exposure to interleukin-6 results in hypertension and increased hypothalamic-pituitary-adrenal axis activity in adult rats. Endocrinology. 2004;145:4897–4911. doi: 10.1210/en.2004-0742. [DOI] [PubMed] [Google Scholar]
- Simrose RL, Fewell JE. Body temperature response to IL-1β in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1179–R1182. doi: 10.1152/ajpregu.1995.269.5.R1179. [DOI] [PubMed] [Google Scholar]
- Sooranna SR, Grigsby PL, Engineer N, Liang Z, Sun K, Myatt L, Johnson MR. Myometrial prostaglandin E2 synthetic enzyme mRNA expression: spatial and temporal variations with pregnancy and labour. Mol Hum Reprod. 2006;12:625–631. doi: 10.1093/molehr/gal061. [DOI] [PubMed] [Google Scholar]
- Stobie-Hayes KM, Fewell JE. Influence of pregnancy on the febrile response to intracerebroventricular administration of PGE1 in rats. J Appl Physiol. 1996;81:1312–1315. doi: 10.1152/jappl.1996.81.3.1312. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Prehar S, Kennedy AR, Little RA, Hopkins SJ. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology. 2003;144:1894–1906. doi: 10.1210/en.2002-220964. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salaman CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wusteman M, Wight DG, Elia M. Protein metabolism after injury with turpentine: a rat model for clinical trauma. Am J Physiol Endocrinol Metab. 1990;259:E763–E769. doi: 10.1152/ajpendo.1990.259.6.E763. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberger E, Merker G, Blahser S. Fever response in the guinea pig before and after parturition. Brain Res. 1981;212:379–392. doi: 10.1016/0006-8993(81)90470-4. [DOI] [PubMed] [Google Scholar]
- Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann KJ, Conn CA, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1β-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]