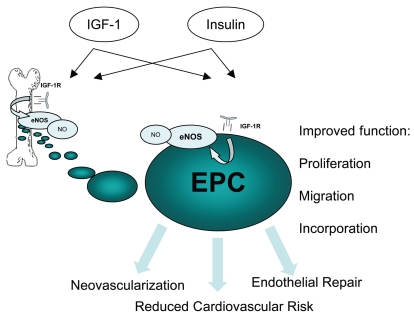
Bone marrow-derived circulating endothelial progenitor cells (EPC) participate in angiogenesis and vascular homeostasis by incorporating into the endothelium of damaged vessels and, perhaps more importantly, exhibiting potent angiogenic paracrine effects (1). EPC are embedded in the microenvironment of bone marrow stromal and endothelial cells and translocate to the circulation upon NO-mediated signaling (2). Additional mobilizing pathways include extracellular signal-regulated kinases (ERK)/matrix metalloproteinase-9 (MMP-9)-mediated release of soluble c-Kit ligand (3). EPC participate in post-natal growth of new blood vessels and/or are home to sites of endothelial damage, maintaining endothelial integrity and function. Circulating EPC numbers are tightly correlated to endothelial function and serve as an independent predictor for cardiovascular diseases (4). Functional properties of EPC may be of equal or greater importance than quantitative alterations. A variety of functional limitations of progenitor cells from patients with coronary artery disease or diabetes, or from aged individuals, have been shown in the past (5–8). Identifying the mechanisms underlying EPC dysfunction in various diseases may lead to the development of treatments for dysfunctional EPC, as well as improved cardiovascular outcome in selected patient groups. This includes drug-mediated improvements of endogenous EPC number and function, as well as ex vivo treatment of autologous dysfunctional EPC before transplantation to ischemic sites after myocardial infarction. In this issue of Molecular Medicine, Humpert and colleagues (9) report on insulin’s beneficial effects on the clonogenic potential of EPCs derived from patients with type 2 diabetes (Figure 1).
Figure 1.
Effects of growth hormone (GH) and insulin supplementation on EPCs; IGF-1 increases overall NO bioavailability that results in EPC release from the bone marrow. IGF1R activation by IGF-1 or insulin on EPC probably leads to enhanced eNOS expression and improvement of cellular functions. This may translate to improvements of vascular repair and overall reduction of cardiovascular risk.
Diabetes is associated with an increased risk for cardiovascular disease, possibly due to impairment of the endogenous vascular repair capacity (6). Under physiological conditions, endothelial nitric oxide synthase (eNOS) mediates anti-arteriosclerotic vascular protection and eNOS-deficient mice display enhanced arteriosclerotic development. In diabetes, eNOS may become “uncoupled,” leading to increased superoxide production and decreased NO bioavailability, both in the vascular wall and in circulating EPC, resulting in EPC dysfunction (6).
In contrast, activation of the insulin-like growth factor-1 (IGF-1) receptor has been shown to increase NO bioavailability systemically (9), resulting in improved EPC number and function (7,8) (Figure 1). Low IGF-1 levels emerged as an independent risk factor for cardiovascular disease. IGF-1 levels are also decreased during aging and are reduced both in type 1 and in type 2 diabetes. Growth hormone (GH) supplementation and subsequent IGF-1 normalization improves cardiovascular outcome in GH-deficient patients. Recently, improvements of EPC function have been shown to be mediated, at least in part, via the IGF-1 receptor (7,8). IGF-1 treatment of EPC increases eNOS expression and activity, as well as telomerase activity in a phosphoinositide-3-kinase/Akt dependent manner. Treatment of mice with IGF-1 improves number and function of EPC, and EPC dysfunction of aged individuals can be rescued by GH treatment in vivo or IGF-1 in vitro (7,8).
Humpert and colleagues (9) report beneficial effects of insulin to the clonogenic potential of EPC derived from patients with type 2 diabetes. This effect was unaltered after silencing the insulin receptor in EPC, but completely prevented after IGF-1 receptor blockade, suggesting insulin’s action to be mainly IGF-1 receptor mediated. Although only investigated in vitro, improvements of EPC function may explain the positive effects of insulin on vascular function in diabetic patients as is seen with intensive insulin-based therapy’s long-term beneficial effects on the risk of cardiovascular disease in diabetic patients (11,12).
When comparing the effects of insulin and IGF-1 on EPC biology, it is important to note that receptor affinity of compounds to the IGF-1 receptor is highly variable. Under normal conditions the binding affinity of insulin to the IGF-1 receptor is ~1000-fold lower than that of IGF-1. Interestingly, drug developmental studies have shown that modulation of insulin’s molecular structure is sufficient to alter its ligand affinity to the IGF-1 receptor (13). For instance, the insulin analogue glargine shows a strong increase in IGF-1 receptor affinity (13). Consequently, this compound was also superior with regard to the clonogenic potential of cultured EPC as investigated in the present study (9). However, whether insulin or insulin analogues with improved IGF-1 receptor affinity would be sufficient to drive IGF-1 receptor activation and subsequent changes in EPC mobilization and function in vivo remains to be determined.
As the endogenous NO synthase inhibitor asymmetric dimethylarginine (ADMA) impairs NO bioavailability and EPCs (14), it is important to note that a tight glycemic control by intensive insulin therapy lowers circulating ADMA levels and improves morbidity and mortality (15). This may aid the beneficial cardiovascular effects of an intensive insulin regime of diabetics. However, hyperinsulinemia is also an independent risk factor for the development of cardiovascular atherosclerosis. Therefore, insulin’s effects on endothelial biology are complex and, in part, dependent on the physiological/pathophysiological environment and, perhaps, changes of the insulin receptor and/or IGF-1 receptor expression. A remaining question is whether IGF-1 receptor expression of EPC is altered in diabetics versus healthy individuals and whether EPC number and function is dependent from alterations of the insulin level in vivo. This would explain why EPC dysfunction from diabetic patients with hypoinsulinemia still remains over time, even when EPCs are cultured under normoglycemic and normoinsulinemic conditions. Long-term effects of insulin analogues on EPC function, number, and subsequent potential cardiovascular protection in diabetics therefore needs future attention. Of note, insulin therapy also decreases levels of the pro-inflammatory tumor necrosis factor-α (TNF-α) in type 2 diabetic patients (16). This is of importance, as TNF-α induces IGF-1 as well as insulin resistance, and, additionally, impairs EPC function and differentiation. The positive effects of insulin on EPC and endothelial function may, therefore, also be mediated in part, by decreasing pro-inflammatory cytokines.
Optimized insulin therapy is of importance in the combat of diabetic complications including cardiovascular diseases (11,12). The interesting observations of Humpert and colleagues may lead to future clinical studies investigating whether insulin or insulin analogues with high IGF-1 receptor affinity may enhance EPC function and number to support the endogenous vascular repair capacity especially in diabetics.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Bauersachs J, Thum T. Endothelial progenitor cell dysfunction: mechanisms and therapeutic approaches. Eur J Clin Invest. 2007;37:603–6. doi: 10.1111/j.1365-2362.2007.01833.x. [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–6. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 3.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2003;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner N, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 5.Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HD. Platelet receptor inhibition in ischemic syndrome management (PRISM) investigators. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2005;110:3206–12. doi: 10.1161/01.CIR.0000147611.92021.2B. [DOI] [PubMed] [Google Scholar]
- 6.Thum T, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–74. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 7.Thum T, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–43. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 8.Thum T, et al. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I. J Clin Endocrinol Metab. 2007;92:4172–9. doi: 10.1210/jc.2007-0922. [DOI] [PubMed] [Google Scholar]
- 9.Humpert PM, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor dependent signaling. Mol Med. 2008;14:301–308. doi: 10.2119/2007-00052.Humpert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böger RH, et al. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest. 1996;98:2706–13. doi: 10.1172/JCI119095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin VL, Khan F, Kennedy G, Belch JJ, Greene SA. Intensive insulin therapy improves endothelial function and microvascular reactivity in young people with type 1 diabetes. Diabetologia. 2008;51:353–6. doi: 10.1007/s00125-007-0870-2. [DOI] [PubMed] [Google Scholar]
- 12.Nathan Cleary PA, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtzhals P, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- 14.Thum T, et al. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46:1693–701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 15.Ellger B, et al. Glycemic control modulates arginine- and ADMA-levels during critical illness by preserving DDAH-activity. Endocrinology. 2008 Feb 21; doi: 10.1210/en.2007-1558. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Bogdanski P, et al. Influence of insulin therapy on expression of chemokine receptor CCR5 and selected inflammatory markers in patients with type 2 diabetes mellitus. Int J Clin Pharmacol Ther. 2007;45:563–7. doi: 10.5414/cpp45563. [DOI] [PubMed] [Google Scholar]