Abstract
Bacterial infections and proinflammatory cytokines induce behavioral and physiological responses associated with withdrawal and sickness behavior. These effects in rodents are often exacerbated by stressful environmental contexts. To model this synergistic effect of arousal and stress, low doses of lipopolysaccharide (LPS), 4 or 40 ng/kg, were administered to rhesus monkeys in different environmental contexts. Activity, emotional and social behaviors, cortisol, interleukin-6 (IL-6), and peripheral leukocyte trafficking were assessed in 4 experiments: an initial 3 hour time-course in a novel cage (Experiment 1); an extended 24 hour time-course (Experiment 2); a highly arousing context in which an animal was engaged in the Human Intruder Paradigm (HIP, Experiment 3); and finally in an undisturbed context in their home cage (Experiment 4). The moderately arousing novel cage potentiated leukocyte, neutrophilic, IL-6, and cortisol changes as compared to the home cage. Unlike the social withdrawal seen in rodents, monkeys engaged in a marked increase in social behavior. IL-6 levels were positively correlated with proximal contact, which was induced to a greater degree by the higher dose of LPS. In contrast, the high arousal HIP condition appeared to obviate the effects of LPS. Thus, the rhesus monkey provides an excellent animal model for investigating the behavioral and physiological actions of endotoxemia, which are profoundly influenced by the situational context in which the individual is evaluated.
Keywords: endotoxin, lipopolysaccharide, monkey, interleukin-6, cortisol, arousal, leukocyte
1. Introduction
The release of proinflammatory cytokines (PIC) during the initial mobilization of innate immunity initiates widespread physiological alterations (Dinarello, 1991), including an activation of the hypothalamic-pituitary-adrenal (HPA) axis (Dunn, 1992) and a massive efflux of leukocytes from tissue to blood (Redl et al., 1991). As a part of this coordinated shift, PIC released within the brain mediate profound behavioral changes, which appear to be designed primarily to maximize internal resources to fight an infection, generate a fever, and repair damaged tissue (Hart, 1988; Maier & Watkins, 1998). This constellation of responses has been called sickness behavior and can include lethargy, an inability to concentrate, anhedonia, depression, and withdrawal from social interaction (Dantzer, 2004). Administration of PIC or bacterial cell wall components (i.e., endotoxin) can induce this behavioral reaction (Kent et al., 1992; Dantzer 2001a, 2001b; Bluthe et al., 1995), whereas central infusion of a cytokine antagonist such as interleukin-1 receptor antagonist attenuates PIC activity (Dantzer, 2004) and HPA activation (Dunn, 1992), as well as abolishes sickness behavior (Bluthe et al., 1995).
Behavioral and physiological responses to PIC and endotoxin are not invariant, however. The sequelae can be markedly influenced by the dose and route of administration, as well as the context in which the host is challenged (Aubert, 1999). For example, the maternal drive to care for pups or the sexual motivation of male rats can override the somnolescent and withdrawal effects typically instigated by IL-6 or lipopolysaccharide (LPS) (Aubert et al., 1997; Yirmiya et al., 1995). It has also been reported that the social rank of group-living mice influences whether withdrawal or defensiveness is induced (Cohn & de Sa-Rocha, 2006). Similarly, the somnogenic effects of IL-1 in monkeys can be supplanted by the threatening presence of a person during the Human Intruder Paradigm (HIP) (Friedman et al., 1996; Kalin & Shelton, 2003). Conversely, pairing endotoxin treatment with certain exogenous stressors, such as tail shock or restraint, can synergistically increase PIC expression beyond the effect of endotoxin or stress induction alone (Anisman et al., 2005; Johnson et al., 2004; Maier & Watkins, 1998). The potential influence of several environmental contexts on the response to LPS was investigated further in the following research using nonhuman primates. Their cortisol levels were also determined given that adrenal hormones can moderate the effect of administered cytokines or endotoxin and alter the course of disease (Besedovsky et al., 1986; Munck et al., 1992; Raison & Miller, 2003).
While this type of research in rats typically employs high doses of endotoxin, there have been a number of human studies exploring the effects of low-level inflammatory responses on behavior and mood (Lin et al., 2005; Grippo et al., 2005). Doses as low as 0.8 ng/kg endotoxin in humans can elicit increased anxiety and depression symptoms with minimal upregulaton of thermogenic and sympathetic activity (Reichenberg et al., 2001). Animal species vary greatly in their sensitivity to endotoxin, from the extreme responsiveness in lagomorphs and horses to the relative resistance seen in mice and rats (Opal, 1999). Nonhuman primates, including the rhesus monkey, are usually described as not being very susceptible to LPS, although this conclusion stems from assessments of the higher microgram concentrations of LPS needed to induce septic shock in macaques and baboons (Veloso et al., 1995; Redl et al., 1999; Zurovsky et al., 1987). Therefore, it was of additional interest to investigate if monkeys would respond to the lower nanogram doses of endotoxin employed in human studies, both to gauge the effect on behavior and physiology, as well as to determine how environmental context influenced these changes. The aims of our 4 experiments were to establish a low dose endotoxemia paradigm in the rhesus monkey, as well as to evaluate the potentially additive effects of psychological disturbance after administration of LPS. Specifically, we assessed peripheral interleukin-6 (IL-6), cortisol and degree of neutrophilia, as well as social and non-social behaviors in familiar and novel environments. Our findings on LPS extend prior research in the rhesus monkey, which showed that administration of IL-1 activates the HPA axis and stimulates a large release of IL-6 into the bloodstream and central nervous system (Reyes & Coe, 1996). However, the current results indicate that the responses can be enhanced or abrogated depending on the context in which the host experiences the challenge to its immune system.
2. Methods
2.1. Animals
Twenty-three rhesus monkeys (Macaca mulatta) between 14-to-23 months of age were utilized in four experiments. Six males and seventeen females were assessed. All were born and reared similarly at the Harlow Primate Laboratory. The monkeys were housed with familiar peers in small social groups under constant conditions. Room temperature was maintained at 21°C. The light/dark cycle was 14:10 h, where lights came on at 0600. Commercial monkey chow (Purina) was provided between 0630−0730 and was supplemented with fresh fruit 3 times a week. Water was available ad libitum. The husbandry conditions were identical for the social pairs and the individuals temporarily housed in a test cage for experiments 1−3. To minimize the influence of daily caretaking activities, all LPS injections were scheduled at a quiet time of day in the early afternoon (1200−1300). All experimental procedures were approved by the Institutional Animal Care and Use Committee and the Office of Biological Safety at the University of Wisconsin – Madison.
2.2. Lipopolysaccharide: Preparation and Administration
Endotoxin derived from E. coli (0:113) was reconstituted with sterile water at a concentration of 1000 ng/ml from sterile lyophilized powder (USP, Rockville, MD). This type of LPS was similar to the form purified for intravenous infusion in humans, which is used to minimize the chance that extraneous endotoxin contaminants will influence the immunologic response (Suffredini et al., 1999). On the day of intravenous (i.v.) injection, single vials were thawed, vortexed, and diluted with sterile saline, to achieve doses of either 4 or 40 ng/kg body weight in a volume of 0.2 ml. Administration of the same volume of sterile saline into the saphenous vein was used as the control condition to assess the nonspecific effect of handling disturbance on the monkeys. Monkeys were then returned to the appropriate housing condition, which was either a familiar or novel cage.
2.3. Blood Collection and Physiological Measures
Blood (3−4.0 ml) was collected via femoral or saphenous venipuncture at 1, 3, or 24 h post-injection depending on the experiment. Samples were rapidly obtained through procedures that minimize handling disturbance and to which the subjects were accustomed. EDTA-treated blood (1 ml) was utilized to determine a leukocyte count and cell differential for quantifying the degree of neutrophilia (General Medical Laboratories, Madison, WI). For determination of cortisol and IL-6 levels, the remaining blood was centrifuged at 3200 rpm. The plasma was then frozen at −60°C until assay. Cortisol levels were assayed in duplicate by iodinated radioimmunoassay (Gammacoat, Stillwater, MN). All cortisol was run in two assays, where the intra- and inter-assay coefficients of variation were 4.18% and 4.62%, respectively. The lower sensitivity of the kit for cortisol was 0.2 ng/ml. IL-6 levels were quantified with an enzyme-linked immunosorbent assay (ELISA) known to cross-react with monkey IL-6 (ELISA; R&D Quantakine kits, Minneapolis, MN). This assay had been validated previously in our laboratory, and monkey and human IL-6 are >98% homologous (Reyes and Coe, 1996). Samples were diluted to fall within the standard curves of either a single high sensitivity (baseline; 0.16−10 pg/ml) or a single standard kit (Post-LPS; 3−300 pg/ml). Our focus on the 1−3 h response was based on human studies indicating that the gene expression of blood leukocytes to endotoxin is maximally active at 2 and 4 h post-injection (Calvano et al., 2005). Reichenberg and colleagues (2001) observed significant increases in IL-6 concentrations, anxiety and depression at various times between 1−4 h post-injection. Krabbe et al. (2005) later confirmed that IL-6 was maximally expressed at 3 h after administration.
2.4. Behavioral Analyses
Behavior was recorded using a digital camcorder (Sony), to which monkeys had been adapted on two prior days. Behavioral responses were monitored via closed circuit television and the records were then scored on a laptop computer (Apple). Scorers were blind to experimental condition. For the purposes of this report, the focus is on the duration and type of affiliative contact between the social pairs in Experiments 1 and 2, and the agitated behavior expressed by isolated animals responding to both being alone and the HIP test in Experiment 3. Close social contact ('Contact Cling') was defined as the focal animal pressing its ventral surface to its companion, arms typically clasped around the animal and hips parallel. Proximal Contact, by contrast, was less interactive and largely consisted of the focal animal purposefully sitting near its companion, passively positioning itself to be groomed with little reciprocal grooming. Anxious and hostile behaviors unique to the HIP in Experiment 3 have been described elsewhere (Kalin & Shelton, 2003). Study 4 focused solely on the physiological responses to LPS to preclude any disturbance evoked by the presence of an observer or camera.
2.5. Experimental Series
2.5.1. Experiment 1
The initial 3 h dose-response study sought to establish the lowest nanogram dose of LPS capable of: 1) significantly affecting IL-6 levels and inducing neutrophilia, and 2) changing the expression of social, active, and/or passive behaviors. The experimental test condition was designated as being one of moderate arousal due to the periodic blood sampling, as well as recent housing of the test animals in a novel room away from the main monkey colony. Fifteen monkeys were originally assigned to one of three treatment groups: 1) saline; 2) 4 ng/kg LPS; 3) 40 ng/kg LPS. One monkey in the saline and another in the 4 ng/kg LPS condition was not included in the final data analyses because their baseline leukocyte profile indicated signs of a subclinical bacterial infection.
At the beginning of the experiment phase, two animals were removed from their home cage, weighed, and placed into a test cage in a quiet room away from the colony area. Monkeys were allowed to acclimate for approximately one week. On the experiment day, a predetermined subject was administered either saline, 4 ng/kg LPS, or 40 ng/kg LPS. The other animal was not manipulated. Animals were then left undisturbed for 1 h, during which time behavior was recorded. At 1 h post-injection, blood was collected from the test subject, after which time the monkey was returned to the test cage. Animals were left undisturbed for another 2 h while behavior was recorded. At 3 h post-injection, a second blood sample was then taken from the same monkey. The other monkey in the pair was tested after an interval of 1−2 days.
2.5.2. Experiment 2
After it was determined that 4 ng/kg LPS was sufficient to elicit both behavioral and physiological changes, the reaction to 4 ng/kg was evaluated up to 24 h post-injection in an extended time-course study. Eight naïve monkeys, pair-housed as in Experiment 1, were injected i.v. with either 4 ng/kg LPS or saline. Behavior was observed at baseline and during the following time periods: 1−2, 3−4, 5−6, and 23−24 h post-injection. Blood was collected under baseline conditions and at 24 h post-injection to measure IL-6, cortisol and leukocyte trafficking. After a 1−2 day period without experimental manipulations, the companion animal was injected with either 4 ng/kg LPS or saline and assessed in the same manner.
2.5.3. Experiment 3
This experiment evaluated the effect of a highly arousing stress context on the LPS response, where a subject was isolated away from their familiar social group during the 3 h assessment period. Four monkeys that had received only saline in Exp. 1 were assigned to the 4 ng/kg LPS condition, and in a counterbalanced manner four formerly LPS treated animals served as saline controls. This HIP experiment included an 'alone' condition, during which the behavior of the monkey was observed for 2.5 h. The investigator then entered the room and stood approximately 3 ft from the animal's cage. The first 15 min involved a No Eye Contact (NEC) condition, followed by a Stare Challenge (SC) condition for another 15 min. This paradigm has been shown to elicit behavioral agitation and activation of the HPA axis (Kalin & Shelton, 2003; Friedman et al., 1996). Blood was obtained immediately afterwards at the 3 h time point post-injection.
2.5.4. Experiment 4
To gauge the typical response to LPS without environmental potentiation, 10 monkeys were injected i.v. with 4 ng/kg LPS while housed normally in their familiar home cage. Only physiological measures were obtained in this experiment. Following a brief separation to inject the LPS (< 5 min.) these animals remained in this undisturbed home cage living condition, and blood was then collected at 3 h post-injection. Six of these subjects had previously been given either 4 ng/kg LPS (n = 4) or 40 ng/kg LPS (n = 2) several months earlier, which allowed us to determine if endotoxin tolerance would be evident (West & Heagy, 2002). Given the lack of physiological differences between those animals previously given 4 or 40 ng/kg LPS, both groups were combined and compared to the LPS-naïve animals.
2.5.5. Across Experiment Comparisons
Finally, to compare the effects of the 3 different environmental contexts, the physiological responses to 4 ng/kg LPS at 3 h were compared across Experiment 1 (Novel + Companion), Experiment 3 (Alone + Intruder), and Experiment 4 (Home Cage).
2.6. Statistical Analyses
Data were analyzed primarily using a mixed Analysis of Variance repeated measures (ANOVA RM) analysis, with LPS Dose as the between group measure and Time as the within subject variable. No effect of Gender was found across physiological or behavioral parameters. Multivariate Analysis of Variance (MANOVA) was used for non-repeated measures data in Experiment 3 to protect against experiment-wise error rate (Hummel & Sligo, 1971). Fisher's LSD, ANOVAs, and t-tests were used as post-hoc tests to explore main effects and interactions. Appropriate square root or base log 10 transformations were used to transform variables that violated univariate normality, homoscedasticity of variance, or sphericity. Alpha level was set to .05. Behavior was generally analyzed by first sampling a one hour observation period in 4 trial blocks of 5 min each, with an equal amount of time elapsing between each block. Given the consistency of behavior, all 4 trial blocks for a given hour were combined. For Experiment 3, the entire 30 min observation period during the HIP was used for data analysis. Baselines values for all variables were comparable unless otherwise indicated.
3. Results
3.1. Experiment 1: 3h LPS Time Course in Novel Cage (Moderate Arousal)
3.1.1. Physiology
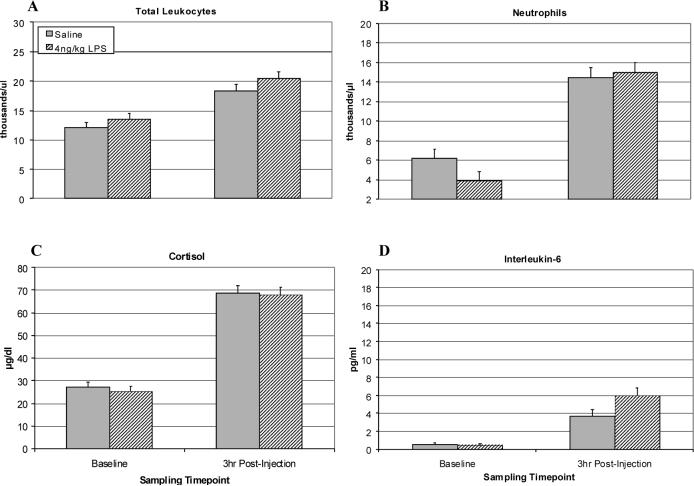
For cortisol, an ANOVA RM revealed a main effect of LPS [F(2,10) = 4.22, p < .05], indicating that LPS induced a significant activation of the HPA axis at 1 and 3 h. Post hoc tests showed that at 3 h the 40 ng/kg condition differed from the saline control [p = .05] and 4 ng/kg dose [p < .05], whereas the 4 ng/kg group did not differ from saline (see Figure 1C). IL-6 release was also significantly upregulated at 1 and 3 h for both 4 and 40 ng/kg subjects as compared to the controls [F(2,10) = 73.03, p < .001]. Post hoc testing indicated a dose-dependent difference between all groups at both 1 and 3 h [p < .001] (see Figure 1D). A follow-up analysis of the interaction using contrasts revealed significant linear [p < .001] and quadratic [p < .05] trends, with the 40 ng/kg group exhibiting peak IL-6 values at 1 h followed by a decline at 3 h—perhaps in response to the rising cortisol at 3 h (see Figure 1C). The LPS-induced changes in leukocyte trafficking were similar for both LPS doses, with both 4 and 40 ng/kg inducing an increase in neutrophils and total leukocyte count. When comparing 4 and 40 ng/kg LPS animals to saline subjects, there was an LPS Dose × Time interaction for the number of leukocytes [F(1,11) = 6.91, p < .05], primarily driven by the increase in neutrophils [F(1,11) = 7.16, p < .05]. Post hoc tests indicated that cell counts were highest at 3 h for leukocytes [t(11) = 2.19, p = .05] and neutrophils [t(11) = 2.34, p < .05] (see Figures 1A and 1B). Monocyte counts were also higher in the 4 ng/kg LPS monkeys at 1 and 3 h post-injection than for controls or 40 ng/kg animals (F(2,10) = 4.503, p < .05).
Figure 1.
Physiological effects of 4 or 40 ng/kg LPS as compared to saline in juvenile rhesus macaques (n = 4, 5, and 4, respectively) at 3 h post-injection in a novel cage. Monkeys were tested while living as a pair after being recently transferred to a novel cage. (A) Total leukocyte number per μl. (B) Number of neutrophils per μl. (C) Plasma cortisol levels (μg/dl). (D) Plasma IL-6 (pg/ml). Data are expressed as Mean ± SEM. *p<.05.
3.1.2. Behavior
Following LPS injections, there were significant effects on affiliative behavior [F(2,10) = 9.71, p < .01] (see Figure 2). In contrast to the social withdrawal seen in rodents (Kent et al., 1992a, 1992b), post hoc testing revealed that monkeys administered either 4 or 40 ng/kg LPS exhibited far more social behavior than the saline controls [p = .001 and p < .05, respectively]. At the higher LPS dose, 40 ng/kg animals tended to engage in less affiliation than the 4 ng/kg subjects [p = .064]. Both of the LPS groups manifested qualitatively different kinds of social behavior (Table 1). At 1 h and 3 h IL-6 values strongly correlated with the degree of Proximal Contact throughout the observed 3 h time course [r(13) = .59 to .88, p < .05].
Figure 2.
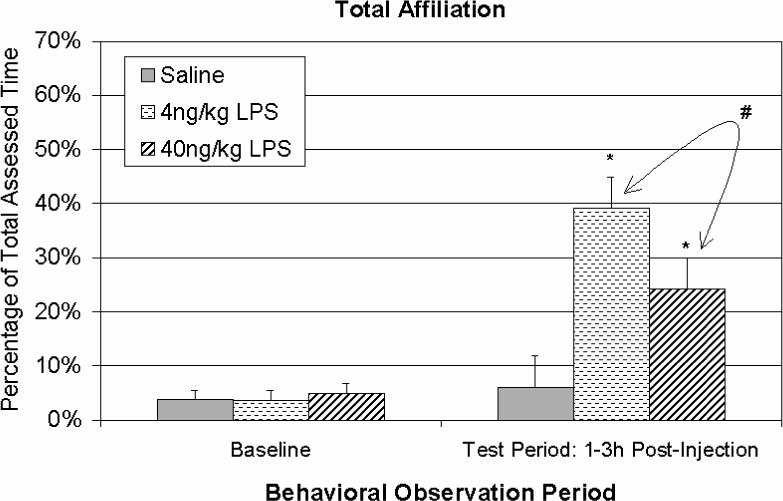
Percentage of time spent engaged in affiliative behavior over a 3h period after saline, 4, or 40 ng/kg LPS (n = 4, 4, and 5, respectively) in a novel cage. LPS-treated animals expressed more affiliation, with a marginal decrease among 40 ng/kg subjects compared to 4 ng/kg. Data are expressed as Mean ± SEM. *p<.05; #p=.064.
Table 1.
Percentages of total time spent engaging in either Contact Cling or Proximal Contact following saline or LPS in Experiments 1 and 2. Proximal Contact is a more somnogenic-like and less interactive form of affiliation than Contact Cling.
| Experiment 1 (3 Hour Time Course) | |||
|---|---|---|---|
| Saline | 4 ng/kg LPS | 40 ng/kg LPS | |
| Contact Cling | 3.5% | 36.4%*** | 5.8% |
| Proximal Contact | 2.5% | 2.7% | 18.5%*** |
| Experiment 2 (24 Hour Time Course) | ||
|---|---|---|
| Saline | 4 ng/kg LPS | |
| Contact Cling | 2.0% | 29.0%** |
| Proximal Contact | 13.0% | 10.0% |
p < .01, 4 ng/kg vs. Saline
p <.001 4 or 40 ng/kg vs. Saline
3.2. Experiment 2: 24h Extended Time Course in Novel Cage
3.2.1. Physiology
Assessment of cortisol levels at 24 h post-LPS indicated that the values were not significantly different than basal values or from those of monkeys injected with saline. IL-6 sampled at 24 h was still slightly elevated over baseline, as reflected by a significant LPS × Time interaction [F(1,6) = 7.51, p < .05] and a comparison of basal vs. 24 h IL-6 in the LPS-treated monkeys [t(6) = −2.39, p = .05]. Total leukocyte numbers including neutrophils in circulation were no longer elevated at 24 h for LPS subjects.
3.2.2. Behavior
Observations of behavioral responses across the first 6 h indicated that there were sustained effects of LPS that grew over time. The 4 ng/kg monkeys evinced significantly more affiliative contact than controls throughout the 6 h assessment period [F(1,6) = 6.61, p < .05] (see Figure 3), specifically displaying more Contact Cling [F(1,6) = 12.96, p < .05] (Table 1). This finding extended the LPS effect seen at 1 h and 3h in Experiment 1. By 24 h, however, the behavioral changes had subsided.
Figure 3.
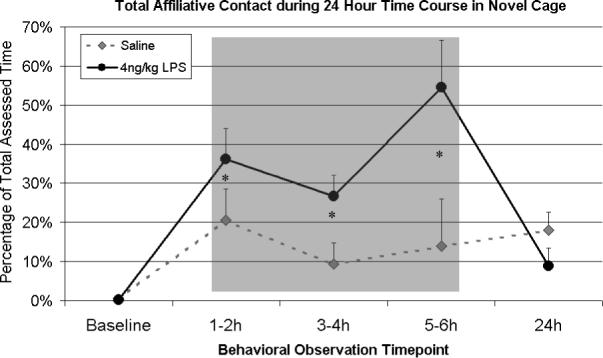
Affiliative behavior observed between focal animal and untreated companion over 24 h time course after either saline or 4ng/kg LPS (n = 4/group) in a novel cage. Data are expressed as Mean ± SEM. Monkeys showed significantly more affiliative behavior throughout the 6 h during the LPS time course (see Figure 2). *p<.05.
3.3. Experiment 3: High Arousal (Alone + HIP) Condition
3.3.1. Physiology
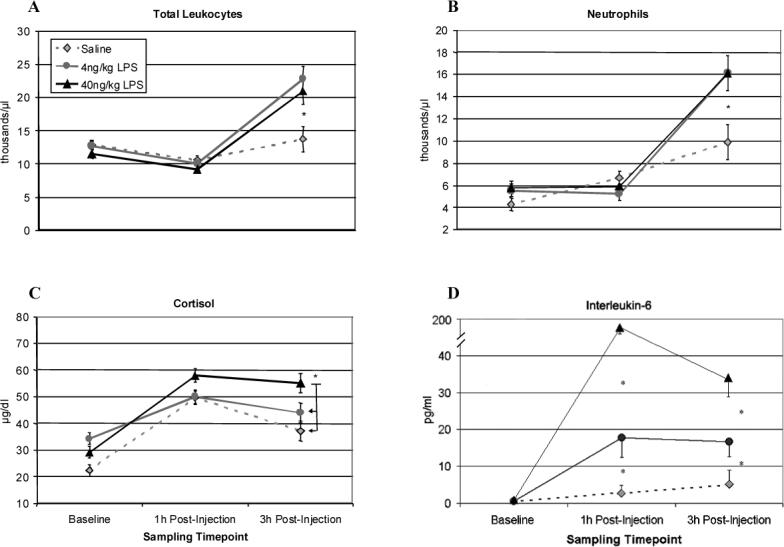
A comparison of cortisol, IL-6 and leukocyte numbers after the HIP condition indicated there was not a differential effect of administering a low dose of LPS versus saline (see Figure 4A-D). When comparing saline and LPS treatments to basal levels, however, the highly arousing context increased cortisol [F(1,7) = 557.65, p < .001] and IL-6 [F(1,7) = 16.97, p < .05] concentrations, as well as neutrophil [F(1,7) = 39.05, p = .001] and total leukocyte numbers [F(1,7) = 294.82, p < .001]. The HPA activity and these aspects of the immune response were thus not potentiated further by 4 ng/kg LPS. However, there were significantly more immature neutrophils (bands) in circulation as compared to saline animals [F(1,6) = 6.04, p < .05)].
Figure 4.
Effect of saline or 4 ng/kg LPS on physiological responses during stress of relocation and exposure to the Human Intruder Paradigm (HIP) (n = 4/group). Data are expressed as Mean ± SEM. (A) Total leukocyte count per μl. (B) Number of neutrophils per μl. (C) Plasma cortisol levels (μg/dl). (D) Plasma levels of IL-6 (pg/ml). LPS did not significantly augment the arousing effects of the HIP.
3.3.2. Behavior
The individually housed LPS monkeys exhibited less locomotion [F(2,12) = 10.08, p < .01] throughout the 'alone' period as compared to saline controls, with manifestations of agitated behavior occurring exclusively during the NEC and SC of the HIP protocol for all animals. These responses confirmed the highly arousing nature of the 'alone' and HIP conditions. However, MANOVAs indicated that during the HIP manipulation LPS did not significantly modify locomotor activity, the expression of anxious or hostile behaviors, or any other behaviors as compared to monkeys injected with saline. Thus, while a low dose of LPS affected a change in behavior during moderate arousal ('alone' condition), it did not appear to potentiate behavioral changes in the highly aroused monkey.
3.4. Experiment 4: Undisturbed Home Cage Condition
3.4.1. Physiology
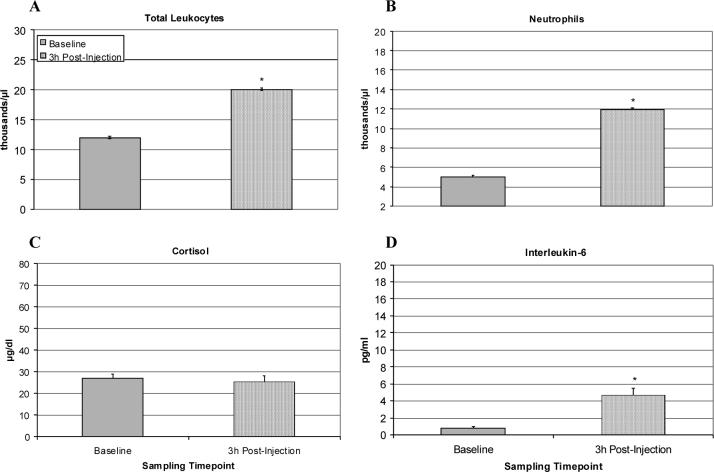
Administration of the low LPS dose (4 ng/kg) to undisturbed animals in their home cage caused a significant increase in IL-6 levels at 3 h post-injection [F(1,7) = 13.89, p < .01] (see figure 5D). Cortisol levels remained within the basal range, however, indicating that the HPA axis was not stimulated (see figure 5C). This attenuated response was not due to the prior exposure of six of the ten animals to 4 or 40 ng/kg LPS. All monkeys also showed increases in total leukocyte [F(1,7) = 24.82, p = .001] and neutrophil numbers [F(1,7) = 13.02, p < .01], respectively (see Figures 5A & B). However, the overall responses were lower than seen in the previous experiments and not mediated by signs of an endotoxin tolerance.
Figure 5.
Physiological effects of saline or LPS in the home cage at 3 h post-injection (n = 10). Data are expressed as Mean ± SEM. (A) Total leukocyte number per μl. (B) Number of neutrophils per μl. (C) Plasma cortisol (μg/dl). (D) Plasma IL-6 levels (pg/ml). There was a significant shift in the cell differential due to LPS, but the resulting neutrophilia and IL-6 changes were more modest than in Experiment 1. Cortisol levels were not elevated above baseline. *p<.05.
3.5. Across Experiments: Effects of 4ng/kg LPS in Different Contexts
3.5.1. Physiology
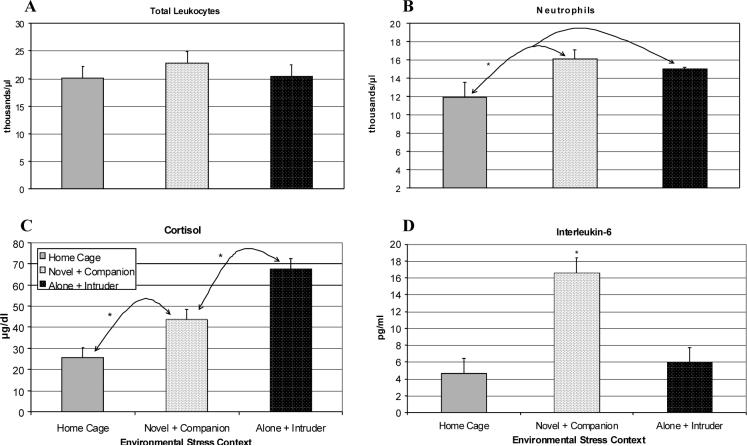
A comparison of IL-6 responses to the 4 ng/kg LPS dose at 3 h across Experiments 1, 3 and 4 revealed a significant effect of environmental context [F(2,15) = 5.27, p < .05]. Post hoc tests showed that the highest levels occurred in Experiment 1 (Novel + Companion condition) as compared to Experiment 4 (Home Cage, p < .01) and Experiment 3 (Alone + Intruder, p < .05) (see figure 6D). Similarly, there was a significant difference in the degree of adrenal activation post-LPS across the three housing conditions [F(2,15) = 27.38, p < .001]. Post hoc testing indicated that the Novel + Companion context evoked higher levels than the Home Cage [p = .003], and the highest responses were seen in the Alone + Intruder condition [p < .001] (see Figure 6C). The concentrations of IL-6 and cortisol across all 3 experiments were highly correlated [r(31) = .75, p < .001], with a maximal relationship seen in the Novel + Companion condition [r(13) = .86, p < .001]. Although context affected the neutrophilia as well [F(2,15) = 5.82, p < .05], post hoc tests indicated that Novel + Companion (p < .05) and Alone + Intruder (p < .01) differed from Home Cage but not each other (see Figure 6B). Context also affected circulating levels of monocytes [F(2,15) = 4.11, p < .05] and lymphocytes [F(2,15) = 7.19, p < .01). The effect on the total leukocyte count did not vary across the 3 experiments (see Figure 6A).
Figure 6.
Comparison of the physiological effects of 4 ng/kg LPS at 3 h post-injection during different environmental contexts (Novel + Companion and Alone + Intruder, n = 4; Home Cage, n = 10). Data are expressed as Mean ± SEM. (A) Total leukocyte number per μl. (B) Number of neutrophils per μl. (C) Plasma cortisol (μg/dl). (D) Plasma IL-6 levels (pg/ml). *p<.05.
4. Discussion
In this series of four studies, we established that low dose endotoxemia can be modeled in the rhesus monkey and that its response to LPS follows a similar time course to that of humans (Reichenberg et al., 2001; Krabbe et al., 2005). Rather than exhibiting the endotoxin insensitivity commonly described in septic shock studies (Veloso et al., 1995), the monkeys were responsive to LPS doses as low as 4 ng/kg. While Haudek et al. (2003) were not able to induce comparable IL-6 responses with a 4 ng/kg dose in the baboon, their animals were continuously anesthetized with ketamine. In addition to this observation of sensitivity to LPS in the rhesus monkey, the subject's environment proved to be a critical factor in exacerbating or curtailing the physiological and behavioral responses to LPS.
Specifically, the degree of contextual stress strongly influenced the magnitude and nature of changes induced by LPS, similar to studies using much higher doses of LPS in rodents (Maier & Watkins, 1998; Johnson et al., 2004; Carobrez et al., 2002). The most marked synergy between environmental context and LPS was evident in the moderately aversive condition (Experiments 1 and 2), in which two monkeys had been acclimated to a novel test cage for only one week before injections. In this case, the extent of leukocyte release and neutrophilia was comparable to that seen in monkeys with bacterial infections. While neutrophil numbers and IL-6 concentration were also upregulated in the Home Cage (Experiment 4), it was to a much smaller degree. Finally, while moderate arousal exacerbated the effects of LPS in Experiments 1 and 2 and during the 'alone' condition of Experiment 3, administration of a low dose of 4 ng/kg LPS did not further influence the effect of a highly stressful condition. In this case, the high cortisol levels induced by HIP and social separation may have interfered with the actions of LPS. Low doses of endotoxin may only synergize with moderate—but not severe—exogenous stress, unless higher doses are utilized and paired with potent stressors such as inescapable shock (Johnson et al., 2003, 2004), restraint (Quan et al., 2001; Mekaouche et al., 1994), social aggression (Carobrez et al., 2002), or social isolation (Tuchscherer et al., 2004). It is also of interest that all of the physiological systems that respond to endotoxin will also respond to psychological challenges (Fleshner et al., 1996; Zhou et al., 1993).
Perhaps one of our most novel findings was that LPS injections in Experiments 1 and 2 stimulated a profound increase in affiliative behavior, rather than the social withdrawal typically described in rodents (Kent et al., 1992a; Kent et al., 1992b; Dantzer, 2004). Given that low doses of endotoxin can induce mild fever in humans (Reichenberg et al., 2001), such affiliation may be motivated by thermoregulatory drive rather than psychological needs. But that explanation is not as likely because Veloso and colleagues (1995) typically found no febrile responses among rhesus monkeys given even milligrams of endotoxin. The duration of this behavioral change was striking even at the low dose of 4 ng/kg, and persisted for at least 6 h—which extends beyond the time period of behavioral perturbation seen in humans (Reichenberg et al., 2001). It was not until 24 h later that behavioral and physiological responses largely returned to baseline. Given that sociality among rhesus monkeys is necessary for normal development and hedonic well-being throughout the lifespan (Suomi and Harlow, 1975), a mildly aversive dose of 4 ng/kg LPS could motivate young animals to seek out comfort from a familiar companion. Indeed, it has recently been emphasized that powerful motivations or needs can temporarily overcome or influence sickness behavior (Aubert, 1999), such as maternal retrieval of scattered rat pups, social rank in mice, or male sexual behavior in rats (Aubert et al., 1997; Cohn & de Sa-Rocha, 2006; Yirmiya et al., 1995). Ultimately, these drives may be overcome by higher doses that induce the physiology of endotoxemia and eventually septic shock. Our 40 ng/kg monkeys, for instance, exhibited marginally less affiliative behavior than the 4 ng/kg group. The affiliation of these high dose monkeys was primarily characterized by a proximity behavior, Proximal Contact, that appeared to be less interactive and more somnogenic, and it was correlated with the level of IL-6 in circulation. These differential effects of dose and context are in keeping with prior findings on the somnogenic actions of 25μg IL-1alpha administered to monkeys in the presence of a companion (Friedman et al., 1996). Increasingly higher concentrations of PIC may override a psychological motivation to affiliate, which coincides with Hart's hypothesis about social withdrawal in sick animals (1988).
While we confirmed that LPS has the potential to markedly stimulate peripheral IL-6 release (Turnball et al., 1998), it too could be markedly affected by the environmental context. In general, when looking across all 4 studies, IL-6 concentrations were highly correlated with the level of HPA activity as measured by cortisol. These findings concur with the prevailing view that the adrenal response is a common concomitant of immune activation, and probably plays a role in modulating and curtailing an immune over-reaction during the initial phases of infection (Del Rey & Besedovsky, 2000; Munck & Guyre, 1986). As an extension of this relationship, the highly elevated cortisol levels induced by the HIP in Experiment 3 may have contributed to the lack of any additional actions of the LPS in that context. Similarly, the high cortisol response induced by the 40 ng/kg dose in Experiment 1 may partially explain the appearance of a more rapid decline in IL-6 levels at 3 h post-injection.
Although the LPS in the stressful context of Experiment 3 (Alone + Intruder) did not augment behavioral and physiological reactions during the HIP, the nature of the physiological changes in the saline control condition warrant some discussion. Simply relocating an animal to a novel cage without a companion was sufficient to induce cellular, IL-6 and cortisol changes similar to effects seen during the acute phase response to pathogen invasion (Berczi, 1998), as well as during low dose endotoxemia in humans (Krabbe et al., 2005). Many papers have now reported that both central and peripheral PIC are released during arousing situations, and may contribute to elevations in body temperature and other neural changes associated with stress (Hansen et al., 2000; Johnson et al., 2003, 2004). In summary, this research has demonstrated that very low LPS doses can greatly alter behavioral and biological parameters in rhesus monkeys, and that exogenous stressors can to varying degrees either potentiate or decrease the biological actions of endotoxin.
Acknowledgements
This research was supported in part by grants from the National Institute of Allergy and Infectious Disease to CLC (AI46521, AI607517). A.A.Willette was supported by predoctoral fellowships from the University of Wisconsin-Madison and the Ford Foundation. Special appreciation is due to Heather Crispen and Alla Slukvina for their invaluable help with the sample collection and assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963–72. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11(2):107–18. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Aubert A. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev. 1999;23(7):1029–36. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Berczi I. The stress concept and neuroimmunoregulation in modern biology. Ann N Y Acad Sci. 1998;851:3–12. doi: 10.1111/j.1749-6632.1998.tb08969.x. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Beaudu C, Kelley KW, Dantzer R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995;677(1):171–176. doi: 10.1016/0006-8993(95)00194-u. [DOI] [PubMed] [Google Scholar]
- Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Carobrez SG, Gasparotto OC, Buwalda B, Bohus B. Long-term consequences of social stress on corticosterone and IL-1beta levels in endotoxin-challenged rats. Physiol Behav. 2002;76(1):99–105. doi: 10.1016/s0031-9384(02)00694-7. [DOI] [PubMed] [Google Scholar]
- Cohn DW, De Sa-Rocha LC. Differential effects of lipopolysaccharide in the social behavior of dominant and submissive mice. Physiol Behav. 2006;87(5):932–7. doi: 10.1016/j.physbeh.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N.Y. Acad. Sci. 2001a;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 2001b;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500(13):399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Besedovsky HO. The cytokine-HPA axis circuit contributes to prevent or moderate autoimmune processes. Z Rheumatol. 2000;59(Suppl 2):II/31–5. doi: 10.1007/s003930070015. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- Dunn AJ. The role of interleukin-1 and tumor necrosis factor alpha in the neurochemical and neuroendocrine responses to endotoxin. Brain Res Bull. 1992;29(6):807–12. doi: 10.1016/0361-9230(92)90148-q. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Reyes TM, Coe CL. Context-dependent behavioral effects of interleukin-1 in the rhesus monkey (Macaca mulatta). Psychoneuroendocrinology. 1996;21(5):455–68. doi: 10.1016/0306-4530(96)00010-8. [DOI] [PubMed] [Google Scholar]
- Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267(1 Pt 2):R164–70. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84(5):697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Daniels S, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Subdiaphragmatic vagotomy does not block intraperitoneal lipopolysaccharide-induced fever. Auton Neurosci. 2000;85(13):83–7. doi: 10.1016/S1566-0702(00)00224-1. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123–37. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Haudek SB, Natmessnig BE, Furst W, Bahrami S, Schlag G, Redl H. Lipopolysaccharide dose response in baboons. Shock. 2003;20(5):431–6. doi: 10.1097/01.shk.0000090843.66556.74. [DOI] [PubMed] [Google Scholar]
- Hummel TJ, Sligo JR. Empirical comparison of univariate and multivariate analysis of variance procedures. Psychological Bulletin. 1971;76(1):49–57. [Google Scholar]
- Johnson JD, O'Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R422–32. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Watkins LR, Maier SF. The role of IL-1β in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992a;13(1):24–8. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992b;89(19):9117–20. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005;19(5):453–60. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Lin YH, Liu AH, Xu Y, Tie L, Yu HM, Li XJ. Effect of chronic unpredictable mild stress on brain-pancreas relative protein in rat brain and pancreas. Behav Brain Res. 2005;165(1):63–71. doi: 10.1016/j.bbr.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Brennan FX, Nguyen K, Watkins LR, Maier SF. RU-486 blocks differentially suppressive effect of stress on in vivo anti-KLH immunoglobulin response. Am J Physiol. 1996;271(5 Pt 2):R1344–52. doi: 10.1152/ajpregu.1996.271.5.R1344. [DOI] [PubMed] [Google Scholar]
- Mekaouche M, Givalois L, Barbanel G, Siaud P, Maurel D, Malaval F, Bristow AF, Boissin J, Assenmacher I, Ixart G. Chronic restraint enhances interleukin-1-beta release in the basal state and after an endotoxin challenge, independently of adrenocorticotropin and corticosterone release. Neuroimmunomodulation. 1994;1(5):292–9. doi: 10.1159/000097179. [DOI] [PubMed] [Google Scholar]
- Munck A, Naray-Fejes-Toth A. The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol Cell Endocrinol. 1992;90(1):C1–4. doi: 10.1016/0303-7207(92)90091-j. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM. Glucocorticoid physiology, pharmacology and stress. Adv Exp Med Biol. 1986;196:81–96. doi: 10.1007/978-1-4684-5101-6_6. [DOI] [PubMed] [Google Scholar]
- Opal SM. The value of animal models in endotoxin research. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. Marcel Dekker; New York: 1999. pp. 809–816. [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115(1−2):36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Redl H, Schlag G, Bahrami S, Scahde U, Ceska M, Stutz P. Plasma neutrophil activating peptide-1/interleukin-8 and neutrophil elastase in a primate bacteremia model. J Infect Dis. 1991;164:383–388. doi: 10.1093/infdis/164.2.383. [DOI] [PubMed] [Google Scholar]
- Redl H, Schalg G, Bahrami S. Endotoxemia in primate models. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in health and disease. Marcel Dekker; New York: 1999. pp. 809–816. [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Coe CL. Interleukin-1 beta differentially affects interleukin-6 and soluble interleukin-6 receptor in the blood and central nervous system of the monkey. J Neuroimmunol. 1996;66(1−2):135–41. doi: 10.1016/0165-5728(96)00038-0. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis. 1999;179(5):1278–82. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF. Effects of differential removal from group on social development of Rhesus monkeys. J Child Psychol Psychiatry. 1975;16(2):149–64. doi: 10.1111/j.1469-7610.1975.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann N Y Acad Sci. 1998;840:434–43. doi: 10.1111/j.1749-6632.1998.tb09582.x. [DOI] [PubMed] [Google Scholar]
- Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A, Stabenow B. Effects of postnatal social isolation on hormonal and immune responses of pigs to an acute endotoxin challenge. Physiol Behav. 2004;82(2−3):503–11. doi: 10.1016/j.physbeh.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Veloso D, Denny TM, Cosgriff HD. Differential susceptibility of rhesus monkeys to high doses of endotoxin. J. Endotoxin Res. 1995;2:411–420. [Google Scholar]
- West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30(1 Suppl):S64–73. [PubMed] [Google Scholar]
- Yirmiya R, Avitsur R, Donchin O, Cohen E. Interleukin-1 inhibits sexual behavior in female but not in male rats. Brain Behav Immun. 1995;9(3):220–33. doi: 10.1006/brbi.1995.1021. [DOI] [PubMed] [Google Scholar]
- Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133(6):2523–30. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- Zurovksy Y, Laburn H, Mitchell D, MacPhail AP. Responses of baboons to traditionally pyrogenic agents. Can J. Physiol. Pharmaocol. 1987;65:1402–1407. doi: 10.1139/y87-220. [DOI] [PubMed] [Google Scholar]