Abstract
NEMO is an essential regulatory component of the IκB kinase (IKK) complex, which controls activation of the NF-κB signaling pathway. Herein, we show that NEMO exists as a disulfide-bonded dimer when isolated from several cell types and analyzed by SDS-polyacrylamide gel electrophoresis under non-reducing conditions. Treatment of cells with hydrogen peroxide (H2O2) induces further formation of NEMO dimers. Disulfide bond-mediated formation of NEMO dimers requires Cys54 and Cys347. The ability of these residues to form disulfide bonds is consistent with their location in a NEMO dimer structure that we generated by molecular modeling. We also show that pretreatment with H2O2 decreases TNFα-induced IKK activity in NEMO-reconstituted cells, and that TNFα has a diminished ability to activate NF-κB DNA binding in cells reconstituted with NEMO mutant C54/347A. This study implicates NEMO as a target of redox regulation and presents the first structural model for the NEMO protein.
Keywords: NEMO, disulfide bond, NF-kappaB, IKK, hydrogen peroxide, cysteine, molecular modeling
Introduction
The activity of the NF-κB signaling pathway is regulated at several steps and by a variety of protein modifications [1]. The IκB kinase (IKK) complex is composed of two catalytic subunits, IKKα and IKKβ, and the regulatory subunit NEMO, and IKK is a key regulator of NF-κB signaling. Activation of the IKK complex in response to upstream signals enables IKK to phosphorylate the NF-κB inhibitor IκB, which leads to nuclear translocation of NF-κB.
NEMO is essential for activation of the NF-κB signaling cascade. NEMO has multiple protein domains, which are responsible for integrating various signals and binding to adaptor molecules or kinases (IKKα/β) [2]. In addition, NEMO is post-translationally modified by ubiquitination, phosphorylation and SUMOylation in various contexts depending on cell type and stimulus [3]. Using a variety of biochemical techniques, it has been reported that NEMO can form dimers, trimers and tetramers [4-9], and thus, there is controversy about the stoichiometry of NEMO molecules in the IKK complex. In addition, several studies have shown that NF-κB signaling can be activated or inhibited in response to various oxidative stress-inducing compounds, but the targets for reactive oxygen species in the NF-κB pathway are not entirely known [10].
In this study, we characterize a novel protein modification of NEMO—disulfide bond formation in a NEMO dimer---which is enhanced when cells are treated with hydrogen peroxide (H2O2). Furthermore, we have developed a molecular model for the structure of a NEMO dimer, which includes these disulfide bonds.
Materials and methods
Plasmids and site-directed mutagenesis
pcDNA3-based vectors for expression of FLAG-NEMO and Myc-NEMO were obtained from S. Ghosh (Yale University) and S. Miyamoto (University of Wisconsin), respectively. Site-directed mutations in NEMO were generated by overlapping polymerase chain reaction-based mutagenesis and were subcloned into the relevant vectors. Retroviral vectors for wild-type NEMO or NEMO-C54/347A were created in pBABE-puro. All constructs were confirmed by DNA sequencing. Details of plasmid constructions can be found at www.nf-kb.org.
Cell culture and transfection
All cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Biologos) as described [11].
For transfections, cells were seeded such that they were 60% confluent on the following day when transfections were performed using polyethylenimine (PEI) (Polysciences, Inc.). On the day of transfection, cells were incubated with DNA:PEI at a ratio of 3:1 in serum-free DMEM (300 μl for a 60-mm plate) for 15 min at room temperature. The DNA mixture was then added to 4.5 ml of DMEM containing 10% FBS and the final mixture was added to the cells. The next day, the media was replaced with 5 ml of DMEM containing 10% FBS. Cells were harvested or treated 24 h later.
Virus stocks for the expression of NEMO proteins were prepared by cotransfecting 15 μg of a pBABE-puro-NEMO plasmid with 15 μg of the pKAT helper plasmid using 60 μg of PEI into a 60-mm dish of BOSC23 packaging cells as described [12]. Virus stocks were then used to infect NEMO-deficient fibroblasts, and selection was performed using 2.5 μg/ml puromycin (Sigma) for two days [12].
Epoxyquinone monomer (EqM) was synthesized as described [13].
Western blotting
Western blotting was performed essentially as described [14]. Whole cell extracts were prepared in non-ionic detergent AT lysis buffer (lacking DTT) with or without 20 mM N-ethylmaleimide (NEM) (Sigma). Samples were heated in SDS sample buffer (0.625 M Tris, pH 6.8, 2.3% w/v SDS, 10% w/v glycerol) with or without the reducing agent β-mercaptoethanol (5% v/v). Samples containing equal amounts of protein were separated on SDS-polyacrylamide gels, proteins were transferred to nitrocellulose membranes, and filters were incubated overnight at 4°C with anti-NEMO antiserum (#2685, Cell Signaling Technology) at a 1:1000 dilution. Goat anti-rabbit horseradish peroxidase-labeled secondary antiserum was added and immunoreactive proteins were detected by Supersignal Dura West chemiluminescence (Pierce).
Electromobility shift assay (EMSA)
EMSAs were performed using whole-cell extracts from serum-starved cells treated with TNF as described previously [15]. Briefly, equal amounts of cell protein (60 μg) were incubated with a [32P]-labeled κB site probe (κB site: 5′-GGGAAATTCC-3′) at room temperature for 30 min. Reaction mixtures were resolved on 5% nondenaturing polyacrylamide gels and protein-DNA complexes were detected by phosphorimaging.
IKK kinase assay
IKK kinase assays were performed as described previously [11]. Briefly, serum-starved NEMO-reconstituted fibroblasts were treated for 7.5 min with 20 ng/ml of TNFα before harvesting in AT buffer. The IKK complex was immunoprecipitated with a polyclonal antiserum against NEMO (BD Pharmingen) and protein G sepharose beads (Zymed). Immunoprecipitates were then incubated with 2 μg of GST-IκBα (aa 1-55) and 5 μCi [γ-32P]- ATP in kinase reaction buffer for 30 min at 30°C. Denatured samples were electrophoresed on an SDS-polyacrylamide gel, and phosphorylated GST-IκBα was detected by phosphorimaging.
Results
NEMO dimerization is mediated through a disulfide bond
A recent study [8] reported that NEMO could be detected as a dimer on SDS-polyacrylamide gels, but that these NEMO dimers disappeared upon extended heating of protein samples in SDS sample buffer containing β-mercaptoethanaol (β-Me). These results suggested to us that NEMO was forming dimers though a disulfide bond(s) under normal protein isolation conditions. To further investigate NEMO dimer formation, we prepared cell extracts from NEMO-deficient fibroblasts overexpressing FLAG-NEMO using conventional SDS-sample buffer containing β-Me and heated the samples at 95°C for various times from 0 to 10 min. NEMO was then detected by Western blotting. We found that the NEMO dimer that is detected in the absence of heating is progressively reduced to entirely the monomer form with heating for 5 min (Fig. 1A). In the absence of β-Me, NEMO migrates exclusively as a dimer even when samples are heated for 10 min (Fig. 1A, right lane). These results indicate that under standard detergent lysis conditions, NEMO forms disulfide-bonded dimers which can only be fully reduced by heating protein samples in the presence of β-Me for at least 5 min.
Fig. 1.
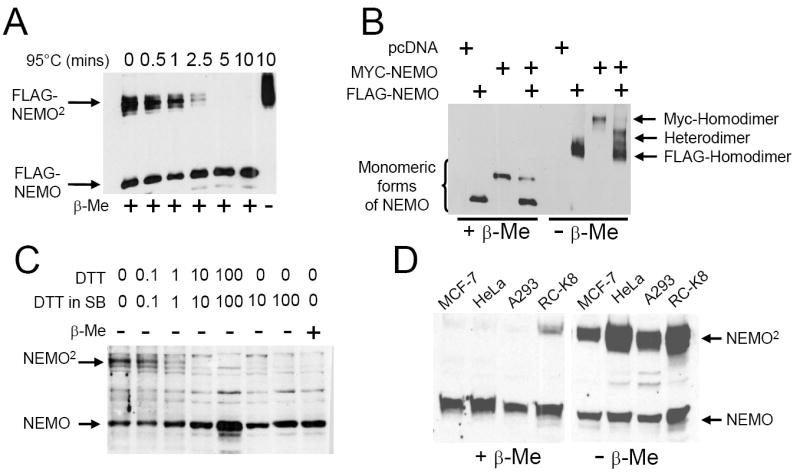
NEMO forms disulfide-bonded dimers. (A) NEMO-deficient fibroblasts were transfected with pcDNA-FLAG-NEMO. Total cell extracts were prepared in AT lysis buffer and samples were resuspended in SDS sample buffer with or without β-mercaptoethanol (β-Me), as indicated. Samples were heated for the indicated amount of time at 95°C. An anti-NEMO Western blot was then performed. FLAG-NEMO is the monomeric form of NEMO (~47 kDa) and FLAG-NEMO2 migrates at approximately twice the molecular weight of FLAG-NEMO. (B) NEMO-deficient fibroblasts were transfected with expression plasmids for vector alone (pcDNA), FLAG-NEMO or Myc-NEMO as indicated. Cell extracts were heated at 95°C in SDS sample buffer with (left four lanes) or without (right four lanes) β-Me. Samples were analyzed by anti-NEMO Western blotting. (C) HeLa cell extracts were prepared in AT lysis buffer containing 20 mM N-ethylmaleimide (NEM) and with the indicated concentration of DTT (top). Samples were then boiled in SDS sample buffer (SB) with the indicated final concentrations of DTT. Samples were analyzed by anti-NEMO Western blotting. (D) Total cell extracts from the indicated cell lines were prepared in AT lysis buffer with 20 mM NEM. Samples were separated by reducing (left panel) or non-reducing (right panel) SDS polyacrylamide gel electrophoresis, and anti-NEMO Western blotting was then performed.
To confirm that the higher molecular weight form of NEMO that we detected under non-reducing conditions was indeed a NEMO homodimer, we transfected NEMO-deficient mouse fibroblasts with plasmids encoding FLAG-tagged NEMO, Myc-tagged NEMO or both. Individually, the FLAG-NEMO and Myc-NEMO proteins migrate as distinct and separable bands under either reducing or non-reducing conditions (Fig. 1B). When both tagged forms of NEMO are co-expressed, intermediate-sized bands are detected under non-reducing conditions (Fig. 1B, far right lane). The simplest interpretation of this result is that these intermediate-sized bands represent NEMO “heterodimers”, composed of Myc-NEMO and FLAG-NEMO, indicating that NEMO forms homodimers in vivo.
Many studies that analyze disulfide bond formation in proteins use N-ethylmaleimide (NEM) to prevent thiol/disulfide exchange [16]. Therefore, we included 20 mM NEM in the lysis buffer to prevent post-lysis disulfide bond formation. In all subsequent immunoblots there is less NEMO dimer than in samples prepared in the absence of NEM (e.g., Figs. 1A,B), which indicates that NEM is efficiently binding to free thiols and preventing them from forming disulfides post-lysis.
We next characterized dimer formation in endogenous NEMO. In extracts from HeLa cells, prepared under non-reducing conditions with NEM in the lysis buffer, a portion of endogenous NEMO migrates as a dimer (Fig. 1C). Even with 1 mM DTT in the lysis buffer (which is used in most standard lysis conditions), some endogenous NEMO protein from HeLa cells is present as a dimer; however, at 10 mM DTT, all NEMO protein is reduced to a monomeric form (Fig. 1C). Moreover, the dimer of endogenous NEMO is detected in a variety of human cell types (MCF-7, HeLa, A293, RC-K8) (Fig. 1D).
Cys54 and Cys347 are required for the formation of NEMO dimers
To identify the Cys residues mediating NEMO dimer formation, we created mutants of NEMO that were mutated at various Cys residues and tested them for dimer formation when expressed in NEMO-deficient mouse fibroblasts. There are 11 Cys residues in human NEMO (Fig. 2A). After analysis of several individual and multiple Cys-to-Ala mutants and deletion mutants, we found that only single mutations of Cys54 or Cys347 showed reduced NEMO dimer formation as compared to wild-type NEMO (Fig. 2B). Mutation of both Cys54 and Cys347 was required for complete loss of dimer formation (Fig. 2B). These results indicate that Cys54 and Cys347 are the primary residues that participate in intermolecular disulfide-bond formation in NEMO and suggest that these disulfide bonds are Cys54/Cys54 and Cys347/Cys347 homotypic interactions.
Fig. 2.
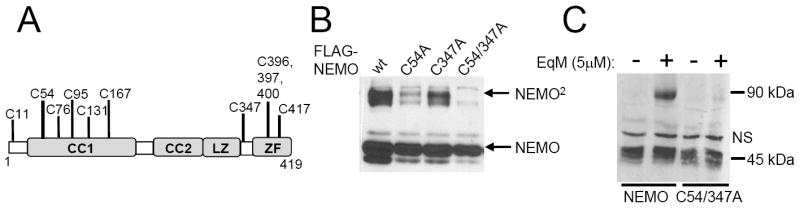
The formation of NEMO dimers requires Cys54 and Cys347. (A) NEMO is a 419 amino acid protein with 11 Cys residues, which are indicated. CC1, CC2, coiled coil domains; LZ, a leucine zipper; ZF, zinc finger. (B) Expression vectors for the indicated FLAG-NEMO proteins (wt, wild-type) were transfected into NEMO-deficient fibroblasts. Whole cell extracts were separated under non-reducing conditions and immunoblotted for NEMO. (C) Stable cell lines expressing NEMO or NEMO-C54/347A (see Fig. 3) were treated for 2 h with the small-molecule EqM. Whole cell extracts were prepared and immunoblotted for NEMO after conventional reducing SDS-polyacrylamide gel electrophoresis. NS, non-specific band.
Epoxyquinone A monomer (EqM), a natural product-derived inhibitor of NF-κB signaling, has been shown to crosslink IKKβ to a dimeric form [11] and EqM can attach to reactive Cys residues. We now show that treatment of cells with EqM crosslinks wild-type NEMO to an SDS- and β-Me-resistant dimer, but EqM does not induce stable dimer formation of NEMO mutant C54/347A (Fig. 2C).
To characterize the functional properties of the relevant Cys mutants of NEMO, we compared the abilities of wild-type and C54/347A mutant NEMO to activate an NF-κB reporter gene after treatment of cells with either TNFα or LPS and to protect NEMO-deficient cells from TNF-induced cell death [17]. In both cases, mutant C54/347A behaved similarly to wild-type NEMO (Fig. S1A, B).
NEMO mutant C54/347A shows delayed kinetics and reduced activation of NF-κB in response to TNFα
To compare further the activities of wild-type NEMO and the dimerization-deficient mutant C54/347A, we created stable cell lines expressing each NEMO protein by infecting NEMO-deficient cell lines with retroviral vectors for wild-type NEMO or mutant C54/347A and then selecting pools of puromycin-resistant cells. Both NEMO proteins were expressed at approximately equal levels in the transduced cells (Fig. 3A), but no NEMO protein was expressed in uninfected or empty vector-infected NEMO knockout cells. We then treated these stable cell lines with TNFα and measured induced NF-κB activity by an EMSA. TNFα induced NF-κB DNA-binding activity more slowly and to a lesser extent in cells reconstituted with NEMO-C54/347A as compared to cells reconstituted with wild-type NEMO (Fig. 3B). That is, induced NF-κB DNA binding is seen within 7.5 min in cells reconstituted with wild-type NEMO, but only at 20 min in cells reconstituted with NEMO-C54/347A, and the overall level of induced NF-κB DNA-binding activity is less in NEMO-C54/347A-reconstituted cells than in wild-type NEMO-reconstituted cells.
Fig. 3.
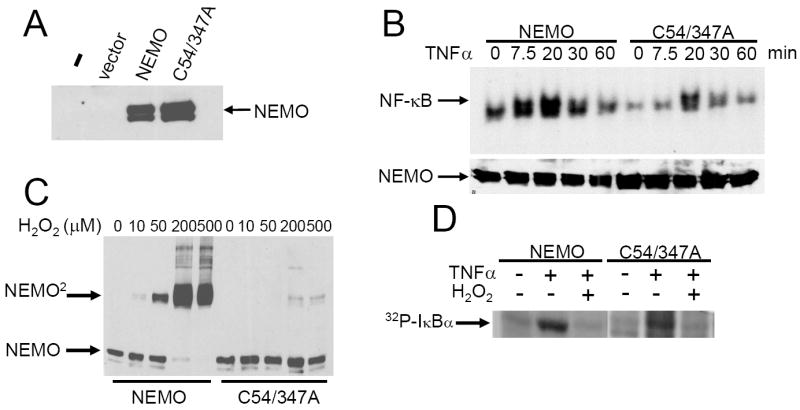
Comparison of wild-type and C54/347A NEMO. (A) Retroviral vectors containing no cDNA (pBABE), wild-type NEMO or C54/347A NEMO and the puromycin resistance gene were used to infect NEMO-deficient mouse fibroblasts. An anti-NEMO Western blot was then performed on stable pools of selected cells. Vector, pBABE. (B) Serum-starved stable cell lines expressing wild-type or C54/347A mutant NEMO were left untreated (0) or were treated with TNFα for the indicated amounts of time and an EMSA was performed using a κB-site probe (upper panel). In the lower panel is an anti-NEMO Western blot taken from the same extracts. (C) Stable cell lines expressing NEMO or NEMO-C54/347A were treated with the indicated concentrations of H2O2 for 10 min. Whole cell extracts were prepared (with 20 mM NEM) and separated by SDS-PAGE under non-reducing conditions. Samples were immunoblotted for NEMO. (D) Serum-starved NEMO-reconstituted cells were untreated or pretreated with H2O2 for 10 min and were then treated with TNFα for 7.5 min. An immune complex IKK kinase assay using GST-IκBα(1-55) was performed. Shown is 32P-labeled GST-IκBα.
Hydrogen peroxide (H2O2) induces NEMO dimerization and inhibits TNF-induced activation of IKK
To determine whether treatment of cells with an oxidizing agent could induce the formation of NEMO dimers, we treated NEMO-deficient cells stably reconstituted with either NEMO or C54/347A with increasing concentrations of H2O2 (10-500 μM) and then subjected protein extracts to anti-NEMO Western blotting after SDS-polyacrylamide gel electrophoresis under non-reducing conditions (Fig. 3C). Reconstituted cells expressing wild-type or C54/347A NEMO show little, if any, dimer in the absence of H2O2. Treatment of cells expressing wild-type NEMO with increasing concentrations of H2O2 induces NEMO dimer formation in a dose-dependent manner, but H2O2 fails to efficiently induce NEMO dimer formation in cells expressing NEMO-C54/347A.
We next investigated whether H2O2 pretreatment could affect IKK kinase activation. Reconstituted cells expressing wild-type NEMO or NEMO-C54/347A were pretreated with 200 μM H2O2 for 10 min prior to stimulating cells with TNF, and induced IKK activity was then measured in an immune complex kinase assay (Fig. 4D). As a control, we show that TNF induces an increase in IKK activity in both cell types in the absence of H2O2 pretreatment. In contrast, there is no TNF-induced IKK activity when cells are pretreated with H2O2. These results indicate that 200 μM H2O2 can prevent activation of IKK by TNF.
Fig. 4.
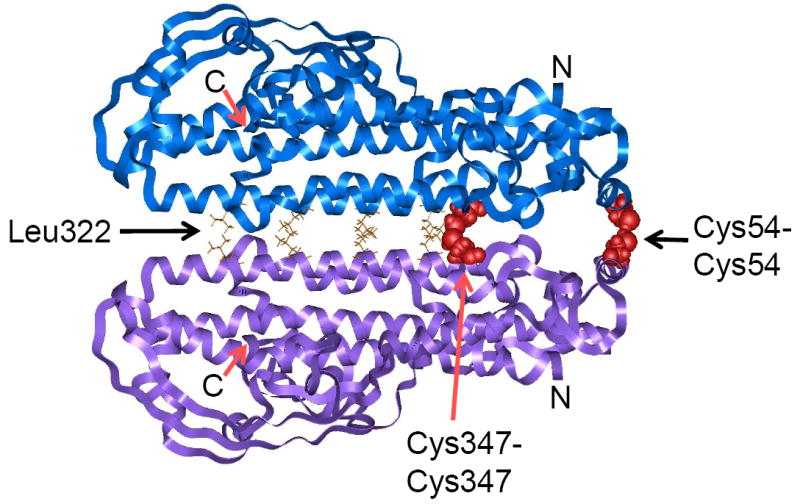
Molecular modeling of the NEMO dimer predicts that Cys54 and Cys347 can form intermolecular disulfide bonds. Light blue and light purple ribbons represent each NEMO monomer. Red, disulfide-bonded cysteines; gold, residues involved in the leucine zipper motif (Leu 322, 329, 336, 343); N, N terminus; C, C terminus.
Molecular modeling of the NEMO dimer
We next modeled the NEMO dimer, based on proteins with similar protein motifs and our biochemical data on disulfide bond formation. Consistent with sequence predictions, our various computational analyses (see Supplementary material) indicate that NEMO monomer adopts a coiled-coil structure, has a C-terminal zinc finger domain, and has an overall high helical content. The NEMO monomer structure was minimized, and docking showed that dimers prefer the head-to-head orientation, which juxtaposes the leucine zipper motifs to hold the two monomers together (Fig. 4A). Furthermore, the leucine zipper brings the two nearby Cys347 residues into proximity for disulfide bonding. Both Cys54-Cys54 and Cys347-Cys347 disulfide bonds are within 4 Å, the distance in which disulfide bonding can occur.
Discussion
Exposure to oxidizing conditions can induce protein disulfides in many cytosolic proteins, which can sometimes affect protein function [18]. In this report, we present evidence that NEMO forms disulfide-bonded homodimers in vivo. In addition, we show that Cys54 and Cys347 of NEMO are required for disulfide bond formation by NEMO, and our predicted structural model of the NEMO dimer indicates that Cys54-Cys54 and Cys347-Cys347 disulfide bonds can form. We cannot distinguish whether there are separate populations of NEMO dimers containing either Cys54-Cys54 or Cys347-Cys347 disulfides or whether both disulfide bonds usually occur in a single dimer. Nevertheless, our analysis of C54A and C347A single mutants shows that NEMO dimers containing either single disulfide bond can exist in cells. Marienfeld et al. [8] also showed that NEMO migrates as a dimer on SDS-polyacrylamide gels; however, they did not characterize the NEMO dimer as a disulfide-bonded species. Similarly, Drew et al. [19] showed that some fragments of NEMO, most of which include either Cys54 or Cys347, form stable high molecular weight species.
Our molecular model of NEMO predicts that each monomer has an elongated α-helical structure and that these monomers are positioned in a head-to-head orientation in the dimer. These structural properties are consistent with gel filtration [6], chemical crosslinking [7] and circular dichroism [19] experiments. Our model positions the leucine zipper to mediate dimerization of NEMO, consistent with mutagenesis studies showing that these leucine residues are required for NEMO oligomerization [5,7]. Our model also places NEMO residues involved in interaction with IKKβ [19] towards the outside of the NEMO dimer.
Fontan et al. [6] reported that NEMO is mainly a monomer in unstimulated mouse fibroblasts. Indeed, in NEMO-deficient mouse cells reconstituted for NEMO expression, we find that most NEMO protein appears as a monomer (Fig. 3C). In contrast, the monomer-to-dimer ratio of endogenous NEMO differs in various human cell types (Fig. 1D). Therefore, we believe that there exists an equilibrium of NEMO monomer-to-dimer, which may vary in different cell types depending on the redox state of the cytosol in a given cell. Furthermore, our results enable us to speculate that formation of the disulfide-bonded NEMO dimer can be modulated by phosphorylation of Ser68 [20].
The NEMO mutant C54/347A, which cannot efficiently form disulfide-bonded dimers, has a delayed and blunted ability to activate NF-κB DNA binding in response to acute one-time treatment with TNF. Nevertheless, there is no difference between NEMO-deficient fibroblasts reconstituted with wild-type vs C54/347A mutant NEMO in sustained reporter gene activation in response to TNF or the long-term survival of cells after treatment with TNF (Fig. S1). Thus, although the C54/347A NEMO mutant is slightly defective in short-term activation, it is not clear if such a mutant would have severe biological consequences. Of note, Marienfeld et al. [8] reported that a NEMO mutant missing amino acids 47-56 (which includes Cys54) also has a minor defect in short-term NF-κB activation in response to TNFα and a reduced ability to form dimers on SDS-polyacrylamide gels. Whether the defects in NEMOΔ47-56 and Cys54/347A mutants for activation of NF-κB are due to their reduced abilities to form disulfide-bonded dimers is not clear.
Activation of NF-κB by oxidative stress appears to be cell type-specific and dose-dependent [10]. Moreover, in several instances, oxidative stress has been reported to impair NF-κB signaling [10,21]. Here we show that H2O2 at a physiological concentration (200 μM) inhibits TNFα-induced IKK activation, and that H2O2 exerts the same effects in cells expressing either wild-type NEMO or NEMO-C54/347A. This result is consistent with H2O2 directly inhibiting IKKβ’s kinase activity, as reported previously [21]. Therefore, although we show that H2O2 can modulate the NEMO monomer vs dimer ratio, H2O2 most likely inhibits IKK activation by directly affecting IKKβ in our system.
Previously, it has been demonstrated that p50, p65, c-Rel and IKKβ are targets of Cys-mediated protein modification [10]. This report is the first demonstration that NEMO also contains redox-sensitive Cys residues. Further characterization of NEMO’s Cys residues may identify novel forms of redox regulation of NF-κB signaling.
Supplementary Material
Acknowledgments
This research was supported by a grant from the NIH (CA47763 to TDG). MH was supported by a Pre-doctoral Fellowship from the Natural Sciences & Engineering Research Council of Canada. SC was supported by NIH grant ES03775 (to E Loechler). WC, TE, KC and SY received funding from the Boston University Undergraduate Research Opportunities Program, and BA was supported by an NSF Undergraduate Research Grant. We thank U Hansen for LPS, S Bardhan and J Porco for EqM, A Hoffmann for NEMO-deficient fibroblasts and E Loechler for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkins ND. Post-translational modifications regulating the activity and function of the NF-κB pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Kim DW, Kwak YT, Prajapti S, Verma U, Gaynor RB. IKKγ/NEMO facilitates the recruitment of the IκBα proteins into the IκB kinase complex. J Biol Chem. 2001;276:36327–36336. doi: 10.1074/jbc.M104090200. [DOI] [PubMed] [Google Scholar]
- 3.Sebban H, Yamaoka S, Courtois G. Posttranslational modifications of NEMO and its partners in NF-κB signaling. Trends Cell Biol. 2006;16:569–577. doi: 10.1016/j.tcb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Israël A, Veron M. NEMO trimerizes through its coiled-coil C-terminal domain. J Biol Chem. 2002;277:17464–17475. doi: 10.1074/jbc.M201964200. [DOI] [PubMed] [Google Scholar]
- 5.Agou F, Traincard F, Vinolo E, Courtois G, Yamaoka S, Israël A, Veron M. The trimerization domain of NEMO is composed of the interacting C-terminal CC2 and LZ coiled-coil subdomains. J Biol Chem. 2004;279:27861–27869. doi: 10.1074/jbc.M314278200. [DOI] [PubMed] [Google Scholar]
- 6.Fontan E, Traincard F, Levy SG, Yamaoka S, Veron M, Agou F. NEMO oligomerization in the dynamic assembly of the IκB kinase core complex. FEBS J. 2007;274:2540–2451. doi: 10.1111/j.1742-4658.2007.05788.x. [DOI] [PubMed] [Google Scholar]
- 7.Tegethoff S, Behlke J, Scheidereit C. Tetrameric oligomerization of IκB kinase γ (IKKγ) is obligatory for IKK complex activity and NF-κB activation. Mol Cell Biol. 2003;23:2029–2041. doi: 10.1128/MCB.23.6.2029-2041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marienfeld RB, Palkowitsch L, Ghosh S. Dimerization of the IκB kinase-binding domain of NEMO is required for tumor necrosis factor α-induced NF-κB activity. Mol Cell Biol. 2006;26:9209–9219. doi: 10.1128/MCB.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinolo E, Sebban H, Chaffotte A, Israel A, Courtois G, Veron M, Agou F. A point mutation in NEMO associated with anhidrotic ectodermal dysplasia with immunodeficiency pathology results in destabilization of the oligomer and reduces lipopolysaccharide- and tumor necrosis factor-mediated NF-κB activation. J Biol Chem. 2006;281:6334–6348. doi: 10.1074/jbc.M510118200. [DOI] [PubMed] [Google Scholar]
- 10.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and NF-κB: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 11.Liang M-C, Bardhan S, Pace EA, Rosman D, Beutler JA, Porco JA, Jr, Gilmore TD. Inhibition of transcription factor NF-κB signaling proteins IKKβ and p65 through specific cysteine residues by epoxyquinone A monomer: correlation with its anti-cancer cell growth activity. Biochem Pharmacol. 2006;71:634–645. doi: 10.1016/j.bcp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Gapuzan M-E, Yufit PV, Gilmore TD. Immortalized embryonic mouse fibroblasts lacking the RelA subunit of transcription factor NF-κB have a malignantly transformed phenotype. Oncogene. 2002;21:2484–2492. doi: 10.1038/sj.onc.1205333. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Bardhan S, Pace EA, Liang M-C, Gilmore TD, Porco JA., Jr Angiogenesis inhibitor epoxyquinol A: total synthesis and inhibition of transcription factor NF-κB. Org Lett. 2002;4:3267–3270. doi: 10.1021/ol026513n. [DOI] [PubMed] [Google Scholar]
- 14.Kalaitzidis D, Davis RE, Rosenwald A, Staudt LM, Gilmore TD. The human B-cell lymphoma cell line RC-K8 has multiple genetic alterations that dysregulate the Rel/NF-κB signal transduction pathway. Oncogene. 2002;21:8759–8768. doi: 10.1038/sj.onc.1206033. [DOI] [PubMed] [Google Scholar]
- 15.Liang M-C, Bardhan S, Li C, Pace EA, Porco JA, Jr, Gilmore TD. Jesterone dimer, a synthetic derivative of the fungal metabolite jesterone, blocks activation of transcription factor nuclear factor B by inhibiting the inhibitor of κB kinase. Mol Pharmacol. 2003;64:123–131. doi: 10.1124/mol.64.1.123. [DOI] [PubMed] [Google Scholar]
- 16.Senkevich TG, White CL, Koonin EV, Moss B. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc Natl Acad Sci USA. 2002;99:6667–6672. doi: 10.1073/pnas.062163799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makris C, Roberts JL, Karin M. The carboxyl-terminal region of IKKγ is required for full IKK activation. Mol Cell Biol. 2002;22:6573–6581. doi: 10.1128/MCB.22.18.6573-6581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghezzi P. Oxidoreduction of protein thiols in redox regulation. Biochem Soc Trans. 2005;330:1378–1381. doi: 10.1042/BST0331378. [DOI] [PubMed] [Google Scholar]
- 19.Drew D, Shimada E, Huynh K, Bergqvist S, Talwar R, Karin M, Ghosh G. Inhibitor κB kinase β binding by inhibitor κB kinase γ. Biochemistry. 2007;46:12482–12490. doi: 10.1021/bi701137a. [DOI] [PubMed] [Google Scholar]
- 20.Palkowitsch L, Leidner J, Ghosh S, Marienfeld RB. The phosphorylation of serine 68 in the IKK-binding of NEMO interferes with the structure of the IKK-complex and TNF-α-induced NF-κB activity. J Biol Chem. 2007 doi: 10.1074/jbc.M708856200. in press. [DOI] [PubMed] [Google Scholar]
- 21.Korn SH, Wouters EFM, Vos N, Janssen-Heininger YMW. Cytokine-induced activation of NF-κB is inhibited by hydrogen peroxide through oxidative inactivation of IκB kinase. J Biol Chem. 2001;276:35693–35700. doi: 10.1074/jbc.M104321200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


