Abstract
HIV-1 Tat protein is one of the neurotoxins involved in the pathogenesis of HIV-1-associated neuronal disorders. Combined electrophysiological and optical imaging experiments were undertaken to investigate whether HIV-1 Tat30-86, herein referred to as Tat30-86, acted directly or indirectly via the release of glutamate or both and to test its effect on the properties of spontaneous quantal events in cultured cortical neurons. Whole-cell patch recordings were made from cultured rat cortical neurons in either current- or voltage-clamp mode. Tat30-86 (50-1000 nM) induced in a population of cortical neurons a long-lasting depolarization, which was accompanied by a decrease of membrane resistance and persisted in a Krebs solution containing tetrodotoxin (TTX, 0.5 μM). Depolarizations were slightly reduced by pretreatment with glutamate receptor antagonists CNQX (10 μM) and AP-5 (50 μM), and were markedly reduced in a Ca2+-free Krebs solution; the differences were statistically significant. Tat30-86-induced inward currents had a reversal potential between -30 and 0 mV. While not causing a noticeable depolarization, lower concentrations of Tat30-86 (10 nM) increased membrane excitability, as indicated by increased numbers of neuronal discharge in response to a step depolarizing pulse. Tat30-86 (10 nM) increased the frequency of spontaneous miniature excitatory postsynaptic currents (mEPSCs), while not significantly affecting their amplitude. Tat30-86 (10 nM) moderately increased the frequency as well as the amplitude of spontaneous miniature inhibitory postsynaptic currents (mIPSCs). Ratiometric Ca2+ imaging studies showed that Tat30-86 produced three types of Ca2+ responses: 1) a fast and transitory increase, 2) Ca2+ oscillations, and 3) a fast increase followed by a plateau; the glutamate receptor antagonists eliminated the late component of Ca2+ response. The result suggests that Tat30-86 is an active fragment and that it excites cortical neurons directly and indirectly via releasing glutamate from adjacent neurons.
Keywords: calcium imaging, electrophysiology, postsynaptic currents
Infection of the central nervous system (CNS) with the human immunodeficiency virus 1 (HIV-1) may lead to cognitive, motor and behavioral dysfunctions, collectively termed HIV-associated dementia (HAD) or AIDS dementia complex (Kaul et al., 2001; Gonzales-Scarano and Martin-Garcia, 2005). The neurotoxic effects are thought to be in part attributed to viral products including the transcriptional activator Tat. Tat, a regulatory protein of 86 to 104 amino acids, has been shown to be neurotoxic in vivo and in vitro (Sabatier et al., 1991; Nath et al., 1996; New et al., 1997; Brailoiu et al., 2006). Several Tat fragments including Tat37-72 (Philippon et al., 1994), Tat31-61 (Nath et al., 1996), Tat1-86 (Bonavia et al., 2001), Tat1-72 (Singh et al., 2004) and recombinant Tat1-72 (Aksenov et al., 2006) or recombinant Tat1-86 (Perez et al., 2001; Brailoiu et al., 2006) are neurotoxic. The fragment between residues 31-61 of Tat is required for producing neurotoxicity (Nath et al., 1996).
Neurons from cerebral cortex, hippocampus or basal ganglia appear to be highly susceptible to the neurotoxic effect of Tat proteins (Bonavia et al., 2001; Maragos et. al., 2003, Singh et al., 2004; Aksenov et al., 2006, Brailoiu et al., 2006). The issue whether or not Tat acts directly on central neurons or indirectly via a release of glutamate has not been clearly resolved. Results from several studies showed that the neurotoxic effect of Tat seems to be glutamate-mediated, as Tat potentiates glutamate-induced toxicity (Bonavia et al., 2001) and that glutamate receptor antagonists reduce or block Tat-induced neurotoxicity (Magnuson et al., 1995; Nath et al., 1996; Perez et al., 2001). On the other hand, the neurotoxic effect of Tat in cultured neurons has been attributed to a calcium overload, as it markedly increases intracellular calcium concentrations [Ca2+]i (Kruman et al., 1998; Haughey et al., 1999; Bonavia et al., 2001; Haughey and Mattson, 2002; Brailoiu et al., 2006). Both calcium influx and release from internal stores are thought to be involved in Tat-induced increase of [Ca2+]i (Bonavia et al., 2001; Haughey et al., 1999). The first objective of this study was to determine whether or not Tat30-86 acts directly on central neurons or indirectly via the release of glutamate.
Several studies report variable effects of Tat on synaptic response or release of putative transmitter. For example, Tat47-57 and the recombinant Tat1-86 inhibited long-term potentiation in hippocampal slices (Behnisch et al., 2004; Li et al., 2004). Tat1-72 enhanced glutamate transmission in suprachiasmatic nucleus (Clark et al., 2005), while not affecting the release of glutamate and other neurotransmitters from human and rat cortical nerve endings (Feligioni et al., 2003). Several Tat fragments evoked release of acetylcholine from human and rat cerebrocortical synaptosomes (Feligioni et al., 2003). The second aim was then to evaluate the effect of Tat on spontaneous quantal events in cultured rat cortical neurons, using the spontaneously occurring excitatory or inhibitory synaptic currents as an index.
Experimental procedures
Cerebrocortical neuronal culture
Cerebral cortical neurons were dissociated from neonatal (1-3-days old) Sprague Dawley rats as previously described (Brailoiu et al., 2006). Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee. After decapitation, brains were removed and quickly immersed in ice-cold Hank’s balanced salt solution without calcium and magnesium (HBSS, Mediatech, Herndon, VA). After removing meninges, cerebral cortices were minced in 1 mm3 blocks and subject to enzymatic digestion with papain for 20 min at 37°C followed by mechanical trituration. After centrifugation at 500x g, fractions enriched in neurons were collected and re-suspended in Neurobasal-A medium (Invitrogen, Carlsbad, CA) containing 2 mM glutamine, 100 units/ml penicillin G, 100 μg/ml streptomycin and 10% fetal bovine serum. Cells were plated on round glass coverslips in 24-well plates. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2. The mitotic inhibitor cytosine β-arabino furanoside (1 μM) (Sigma-Aldrich, St. Louis, MO) was added to the culture to inhibit glial cell proliferation (Schoniger et al., 2001). Cultures were maintained by changing the media every 2-3 days.
Electrophysiology
Visual patch-clamp recordings were made from cultured cortical neurons (5-10 days in culture). The whole-cell patch-clamp technique was similar to that described earlier (Brailoiu et al., 2002; Wu et al., 2003). One coverslip was transferred to the recording chamber (model RC-22C, Warner Instrument Inc., Hamden, CT) and superfused with Krebs solution oxygenated with 95% O2 and 5% CO2 at a rate of 1-2 ml/min using a valve control perfusion system (model BPS-4; ALA Scientific Instruments Inc., Westbury, NY). All experiments were carried out at room temperature (21 ± 1°C). The Krebs solution had the following composition (in mM): 127 NaCl, 1.9 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3 and 10 glucose. In experiments where Ca2+-free Krebs solution was used, Ca2+ was omitted and EGTA (2.5 mM) was added; the pH was adjusted with NaOH. Patch electrodes pulled from thin-walled borosilicate glass capillaries when filled with a solution containing (in mM) 130 K+ gluconate, 1 MgCl2, 2 CaCl2, 4 ATP, 0.3 GTP, 10 EGTA and 10 HEPES, had a resistance of 2-5 MΩ; the pH of the solution was adjusted to 7.2. After forming a gigaohm seal, the capacitance transients were compensated and the holding potential set to -60 mV. Short pulses of suction were applied to break the membrane patch and to establish the whole-cell configuration. Signals were recorded using an Axopatch 1C amplifier (Axon CNS/Molecular Devices, Sunnyvale, CA) in current-or voltage-clamp mode, filtered at 2 KHz, and recorded on a two-channel Gould chart recorder RS 3200 (Gould Instruments Systems, Inc., Valley View, OH). Experimental protocols were controlled and data acquired by a personal computer using the Clampex 8.0 software (Axon CNS/Molecular Devices). Steady-state I-V relationships of Tat-induced currents were investigated in cultured rat cortical neurons voltage-clamped to -60 mV, which is close to the resting membrane potential of these neurons. The I-V relationships were obtained by applying a series of 400 ms voltage command steps every 5 s from a holding potential of -60 mV to different potentials (-140 to 0 mV) with 10 mV increments, before and during the superfusion of Tat30-86. Currents elicited by each voltage command step in control media were subtracted from their counterparts in the presence of Tat to yield a steady-state I-V curve of Tat-induced currents.
Optical imaging using DiSBAC4(3)
DiSBAC4(3), bis-[1,3-dibutylbarbituric acid] trimethineoxonol, a slow-response voltage-sensitive fluorescent dye, was used to assess relative changes in membrane potential of single cells. Optical imaging using voltage sensor probes has proven to be a reliable approach in monitoring membrane potential changes in neurons (Ebner and Chen, 1995; Kunkler et al., 2005; Chen et al., 2006). Briefly, the cells were incubated for 30 min in HBSS containing 0.5 mM DiSBAC4(3). The fluorescence (excitation wavelength = 480 nm, emission wavelength = 540 nm) was continuously recorded at a rate of 10 points min-1. Background values (windows of identical area placed beside the cells) were always subtracted. The dye partition between the cell membrane and the cytosol is a function of membrane potential. Depolarization of the membrane leads to a sequestration of the dye into cytosol and is associated with an increase in the fluorescence intensity (Brauner et al., 1984), whereas during membrane hyperpolarization the dye concentrates in the cell membrane, leading to a decrease of fluorescence intensity.
Calibration of DiSBAC4(3) Fluorescence
Calibration of DiSBAC4(3) fluorescence was performed using the Na+-K+ ionophore gramicidin in Na+-free physiological solution (Brauner et al., 1984). The osmolarity was maintained constant by addition of N-methylglucamine. In the the presence of gramicidin (1 μM), the Na+ concentration gradient is zero, and the membrane potential is approximately equal to K+ equilibrium potential which is determined by the Nernst equation. The intracellular K+ and Na+ concentrations were assumed to be 130 mM and 10 mM, respectively. The addition of gramicidin with various concentrations of K+ to the cultured neurons alters the cell membrane potential, thereby, altering fluorescence. Extracellular K+ concentrations used in this study were 17, 25 and 80 mM; consequently, membrane potentials varied between -56, -45 and -14 mV, as calculated by the Nernst equation. According to the calibration measurements, changes in DiSBAC4(3) fluorescence intensity by 1.092 are equivalent to a change in membrane potential of 1 mV.
Ca2+ measurement
Cytosolic Ca2+concentrations [Ca2+]i were measured by the microfluorimetric technique, as previously described (Brailoiu et al., 2006). Cultured neurons were loaded with the fluorescent Ca2+ indicator Fura-2 AM (3 μM) by incubation of the cells in HBSS plus Fura-2 AM for 45 min and HBSS alone for an additional 15-60 min to allow de-esterification of the dye. Coverslips were mounted in a 500 μl bath on the stage of a TE2000U Eclipse Nikon inverted microscope equipped with a Photometrics CoolSnap HQ CCD camera (Roper Scientific, Tucson, AZ). Fura-2 fluorescence (excitation wavelength = 340 and 380 nm, emission wavelength = 520 nm) of single cells was acquired at a frequency of 0.3 Hz using a Lambda 10-2 filter shutter. Experiments were analyzed off-line using the MetaFluor software. The ratio of the fluorescence signals (340 nm/ 380 nm) was converted to Ca2+ concentrations (Grynkiewicz et al., 1985).
Statistics
Paired t-test followed by one way ANOVA was used to evaluate significant differences between controls and Tat-treated neurons; P<0.05 was considered statistically significant.
Chemicals
Bicuculline methobromide, 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), D-2-Amino-5-phosphonovaleric acid (AP-5) were from Sigma-Aldrich (St. Louis, MO), HIV-1 Tat30-86 was from Phoenix Pharmaceuticals, Inc. (Burlingame, CA).
Results
Tat30-86-induced depolarizations
Whole-cell patch clamp recordings in current- or voltage-clamped mode were made from cultured cortical neurons, which had a mean resting membrane potential of -54 ± 1.7 mV and mean input resistance of 1021 ± 82 MΩ (n=67). A subpopulation of cortical neurons discharged spontaneously (n=14). Similarly, the mean membrane potential of cortical neurons was -54.2 ± 2.41 mV (n=210), as measured by the optical imaging technique using DiSBAC4(3).
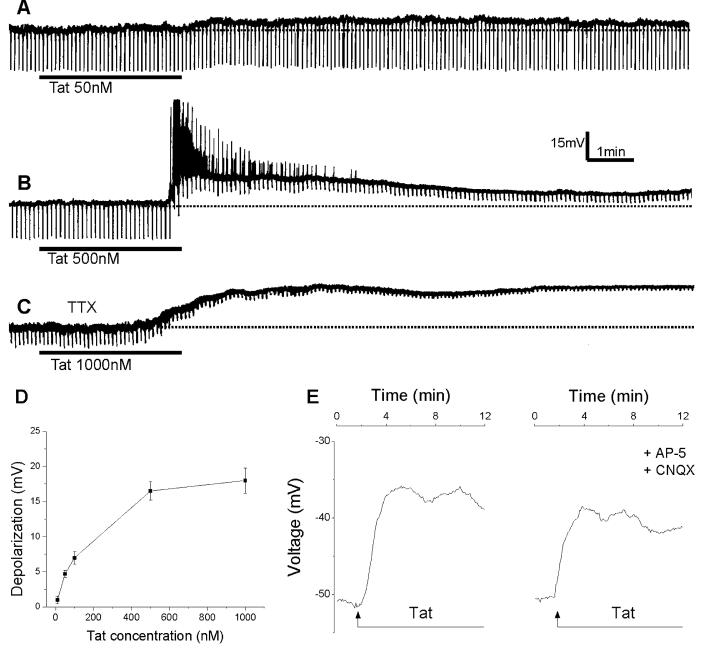
Tat30-86 (50-1000 nM) applied by superfusion induced a membrane depolarization in 80% (24/30) of the neurons tested. The membrane depolarization was relatively slow in onset and long-lasting (Fig.1A & B). At the highest concentration (1 μM) tested, Tat30-86 induced a long-lasting depolarization lasting for min (Fig. 1C). The depolarization was associated with a decrease in membrane resistance and persisted in tetrodotoxin (TTX, 0.5 μM)-treated neurons (Fig. 1C), indicating a direct excitatory effect of Tat30-86. The amplitude of Tat-induced depolarizations was concentration-dependent (Fig. 1D).
Figure 1.
Depolarizations of cultured rat cortical neurons by Tat30-86. A, Tat30-86 (50 nM) applied by superfusion produced a small membrane depolarization with no detectable changes in membrane resistance. The broken line denotes resting membrane potential. B, a higher concentration of Tat30-86 (500 nM) produced a long-lasting depolarization accompanied by intense discharge and a marked decrease in membrane resistance. C, Tat30-86 (1000 nM) induced a long-lasting depolarization in a neuron pretreated with tetrodotoxin (TTX; 0.5 μM). A, B and C represent actual recordings from three different neurons. Downward deflections superimposed on the membrane potential are hyperpolarizing electrotonic potentials induced by constant current pulses (30 pA, 300 ms) and are used to monitor membrane resistance changes. D, concentration-response relationship of Tat-induced depolarizations; data points represent mean ± S.E. of 3-6 neurons. E. Depolarizations of cerebral cortical neurons induced by Tat 30-86 as monitored by optical imaging using DiSBAC4(3); the depolarization is slightly reduced by pretreatment with AP-5 and CNQX; recordings are from two different neurons.
Pretreatement of neurons with the non NMDA glutamate receptor antagonist CNQX (10 μM) and the NMDA glutamate receptor antagonist AP--5 (50 μM) for 10 min reduced the amplitude of depolarizations induced by Tat30-86 (500 nM) from 16.48 ± 2.8 mV (n=64) to 12.08 ± 2.6 mV (n=82); the difference was statistically significant (P < 0.05); a representative example is shown in Fig. 1E.
Membrane excitability
While not causing a noticeable change in membrane potential, lower concentrations of Tat30-86 (10 nM) increased the number of action potentials in response to a step depolarizing current pulse, suggesting an increase in membrane excitability. A representative experiment is illustrated in Fig. 2A. The number of action potentials elicited at a given stimulus intensity was plotted against the stimulus intensity before and during Tat30-86 (10 nM) treatment; a leftward shift of the response-intensity curve supports the contention that membrane excitability is increased by Tat30-86 (Fig. 2B).
Figure 2.
Neuronal excitability increase by Tat30-86. A, a cortical neuron discharged 4 action potentials in response to a step depolarizing current pulse (30pA, 500ms, not shown). Tat30-86 (10 nM) increased the number of action potentials to 8 in response to the same depolarizing stimulus (lower panel). B, stimulus-response curves obtained before and after perfusion of 10 nM Tat (n=4); * denotes P<0.05.
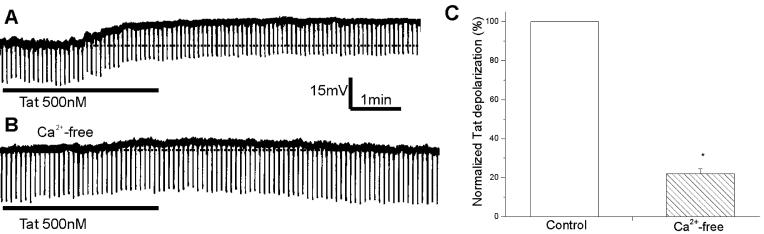
Next, the effect of Tat30-86 (500 nM) on membrane depolarizations was evaluated in neurons perfused with Ca2+-free Krebs solution (n=4) and compared with that obtained in neurons perfused with regular Krebs solution (n=4). The depolarization was markedly decreased in Ca2+-free Krebs (Fig. 3A & B); the average response was reduced to 20% of the control response (Fig. 3C); the difference was statistically significant (P<0.05).
Figure 3.
Comparison of Tat30-86-induced responses of rat cerebral cortical neurons in normal Krebs and Ca2+-free Krebs. A, superfusion of Tat (500 nM) produced a membrane depolarization accompanied by a small decrease in input resistance. B, this neuron was perfused with Ca2+-free Krebs solution for 5 min prior to administration of Tat30-86. C, normalized Tat-induced depolarizations in normal Krebs solution (control, n=4) and Ca2+-free Krebs solution (n=4). Asterisk indicates a statistically significant difference (P< 0.05).
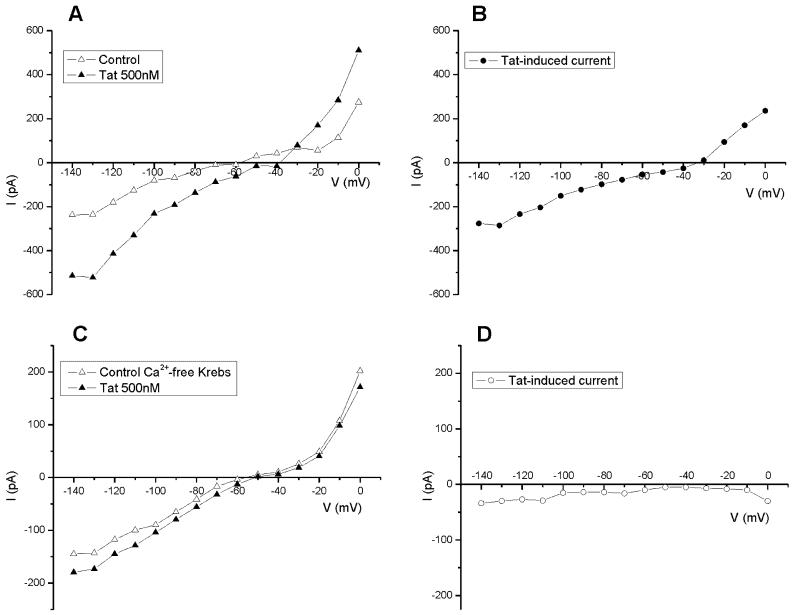
Steady state I-V relationship of Tat-induced currents
In normal Krebs solution, Tat30-86 (50-1000 nM) induced an inward current which was voltage-dependent and had a reversal between -30 and 0 mV (Fig. 4A & B). The I-V relationship was obtained for 4-6 cells at each concentration tested. In neurons perfused with Ca2+-free Krebs solution (n=4), the I-V relationship of Tat-induced responses shows a reduced inward current, and the responses were not reversed between -140 and 0 mV (Fig. 4C & D).
Figure 4.
I-V relationship of Tat30-86-induced currents in normal Krebs solution and Ca2+-free Krebs solution. A, steady-state I-V curve before (open triangle, control)) and after (filled triangle) application of Tat (500 nM). B, the reversal potential of Tat-induced current in this neuron was about -30 mV. C, steady-state I-V curve before (open triangle, control) and after (filled triangle) application of Tat30-86 (500 nM) in a neuron perfused with Ca2+-free Krebs solution for 10 min before application of Tat. D, Tat-induced current did not reverse between -140 and 0 mV. The neuron was held at -60mV.
Effects of Ta30-86t on spontaneous synaptic currents
Spontaneously occurring miniature inhibitory postsynaptic currents (mIPSCs) or miniature excitatory postsynaptic currents (mEPSCs) were recorded from a population of rat cultured cortical neurons for a period of 4 min before and 10 min after superfusion of Tat30-86.
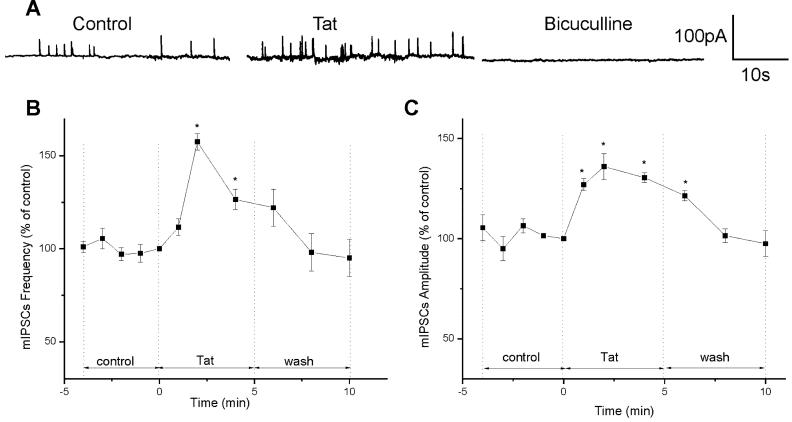
mIPSCs were recorded in the presence of CNQX (10 μM) and AP-5 (50 μM). At the end of the experiment, mIPSCs were blocked by the GABAA receptor antagonist bicuculline (20 μM), confirming that mIPSCs were mediated by GABA acting on GABAA receptors (Fig. 5A). The mean frequency of mIPSCs during the control period was 0.51 ± 0.05 s-1 and the mean amplitude was 43 ± 3.2 pA (n=4). Tat30-86 (10 nM), which did not cause a significant change in the holding current, increased the frequency of mIPSCs to 157.5 ± 4.5 % (0.80 ± 0.08 s-1), reaching the peak 2 min after administration (Fig. 5A & B). Tat30-86 enhanced the amplitude of mIPSCs to 127 ± 3% (54.6 ± 1.6 pA) in the first min of the treatment and reached the peak of 136 ± 6.5% (58.5 ± 3.8 pA) 2 min after treatment (Fig. 5A & C). The amplitude declined slowly, reaching the basal values in about 8 min. The effect of Tat on mIPSCs was reversible (Fig. 5 B & C).
Figure 5.
Effects of Tat30-86 on mIPSCs in cultured rat cortical neuorns. A, examples of actual recording of mIPSCs before and after administration of Tat30-86 (10 nM); mIPSCs were abolished by treatment with bicuculline (20 μM). B, the peak effect of Tat on mIPSC frequency occurred at 2 min, followed by a fast decay. C, the peak effect on mIPSCs amplitude occurred also at 2 min, but the time course of decay was slower as compared to that of the frequency. Data are expressed as percent of values at time zero and the results from 4 experiments are averaged. Asterisks denote statistically significant differences (P<0.05) from control.
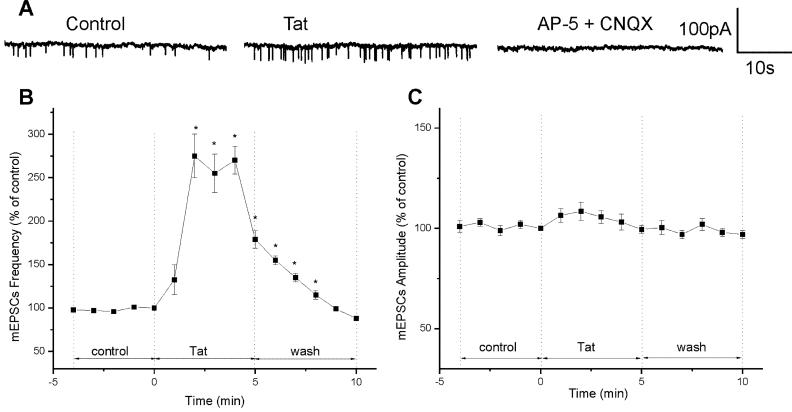
mEPSCs, which were recorded from a population of cultured cortical neurons in the presence of bicuculline (20 μM) and the glycine receptor antagonist strychnine (1μM), were abolished by the non-NMDA and NMDA glutamate receptor antagonists CNQX (10 μM ) and AP-5 (50 μM) (Fig. 6 A). The mean frequency and amplitude of mEPSCs recorded during control period was 0.35 ± 0.03 s-1 and 58.8 ± 3 pA (n=4). Two min after perfusing with Tat30-86 (10 nM), the frequency of mEPSCs increased to 275 ± 25% (0.96 ± 0.2 s-1) and returned to the basal level 10 min after withdrawing Tat30-86 from the perfusing solution (Fig. 6A & B). Tat30-86 increased the amplitude of mEPSCs to 108.5 ± 4.5% (63.79 ± 3.2 pA) (Fig. 6C), which was not statistically significant (P>0.05).
Figure 6.
Effect of Tat30-86 on mEPSCs. A, examples of recording of mEPSCs before and after administration of Tat30-86 (10 nM); mEPSCs were blocked with AP-5 (50 μM) and CNQX (10 μM). B, Tat30-86 increased the frequency of mEPSC by 275 ± 25% after 2 min; after a short plateau, the frequency returned to basal levels 10 min after administration. C, Tat30-86 increased the amplitude of mEPSCs to 108.5 ± 4.5%, which was not statistically significant (P>0.05). Data are expressed as percent of values at time zero and the results from 4 experiments are averaged. Asterisks denote statistically significant differences (P<0.05) from control.
Effect of Tat on cytosolic Ca2+
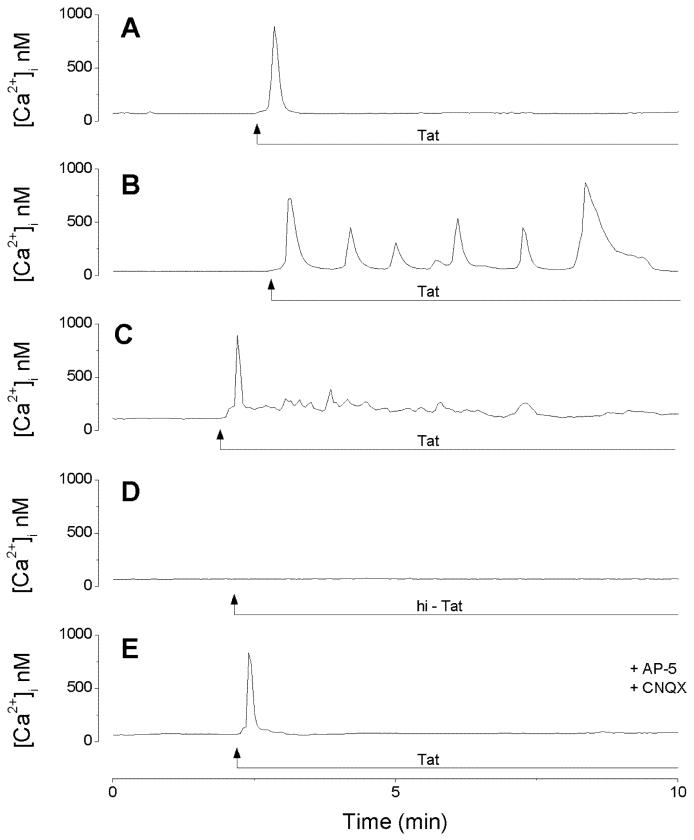
The mean basal [Ca2+]i value of rat dissociated cerebrocortical neurons was 84 ± 6.3nM (n=178); this is in agreement with the concentration (30-200 nM) reported in mammalian central neurons (Connor, 1986). Administration of Tat30-86 (500nM) induced three types of calcium responses. In 76 neurons (43%), Tat30-86 produced a fast and transitory increase in [Ca2+]i, resembling a single spike, with a mean amplitude of 772 ± 5.3 nM; an example is illustrated in Fig. 7A. In 37 neurons (21%), Tat30-86 induced [Ca2+]i oscillations consisting of an initial, transient spike followed by several spikes; the amplitude of the first spike was 678 ± 3.7 nM (Fig. 7B). Lastly, Tat30-86 produced a fast, transient spike of 785 ± 6.4 nM followed by a low amplitude plateau phase of 163 ± 1.8nM in 16 neurons (9%) (Fig. 7C). In 49 out of 179 neurons tested (27%), Tat30-86 did not increase [Ca2+]i. Heat inactivated Tat30-86 (60°C, 30 min) did not produce a change in [Ca2+]i in any of the 85 neurons tested (Fig. 7D). In neurons pretreated with CNQX (10 μM) and AP-5 (50 μM), Tat30-86 induced a fast and transitory increase in cytosolic Ca2+ of 752 ± 6.8 nM in 86 out of 129 neurons tested (67%), an example is shown in Fig. 7E.
Figure 7.
Calcium responses induced by Tat30-86 (500nM) in cerebral cortical neurons. A, Tat30-86 produced a fast and transitory increase in [Ca2+]i in this neuron; a similar response was produced in 43% of the cells tested. B, calcium oscillations produced by Tat30-86 in this neuron; this type of response was present in 21% of the cells tested. C, Tat30-86 produced a spike followed by a plateau in 9% of cells tested. D, Heat inactivated Tat30-86 (hi-Tat) did not increase [Ca2+]i in any of the 85 neurons tested. E, pretreatment with AP-5 (50 μM) and CNQX (10 μM) abolished the plateau, while preserving the fast, transient [Ca2+]i increase.
Discussion
Our studies show that Tat30-86 caused at least three, concentration-dependent responses in cultured cortical neurons: 1) increase of membrane excitability at lower concentrations (10 nM); 2) increase of spontaneously occurring synaptic currents; and 3) membrane depolarization and increase of cytosolic Ca2+ at higher concentrations (≥50 nM).
The observation that Tat30-86 depolarizes or excites cultured rat cortical neurons is in agreement with the excitatory effect of HIV-1 Tat on cultured human fetal neurons and neurons of rat hippocampal slices (Magnuson et al., 1995; Nath et al., 1996; Cheng et al., 1998). The concentration of Tat that induced a depolarization varied between micromolar (Magnuson et al., 1995) and femtomolar (Cheng et al., 1998). In our study, the minimal concentration effective in depolarizing cultured cortical neurons was about 50 nM. The differences could be explained by different fragments used in these studies; for example, Tat31-61 (Nath et al., 1996) or recombinant HIV-1Tat1-72 (Cheng et al., 1998) versus Tat30-86 in our experiments. The active site was found between residues 31 to 61, as Tat31-61, but not Tat48-85, depolarized neurons in rat hippocampal slices, increased cytosolic Ca2+ and induced neurotoxicity in cultured human fetal neurons (Nath et al., 1996). Moreover, different methodologies of applying HIV-1 Tat may result in different concentrations reaching the target neurons; for example, pressure ejection (Nath et al., 1996; Cheng et al., 1998) versus superfusion in our study.
It is noteworthy that membrane potential changes monitored by optical imagining techniques using a slow response, voltage-sensitive fluorescent dye DiSBAC4(3) are comparable to that recorded by direct electrophysiological recording techniques. This is in agreement with earlier reports indicating that optical imaging with voltage-sensitive dyes is a reliable approach for monitoring voltage changes in neurons (Ebner and Chen, 1995; Kunkler et al., 2005; Chen et al., 2006).
As previously reported (Magnuson et al., 1995; Cheng et al., 1998), depolarizations induced by Tat30-86 were insensitive to TTX, indicating a direct effect of Tat30-86 on cultured rat cortical neurons. The threshold concentration of Tat30-86 causing a detectable change in the membrane potential was approximately 50 nM. Tat30-86 at lower concentrations (10 nM), while not causing a noticeable change in the membrane potential, increased the excitability of the neurons, as demonstrated by the increased number of discharges in response to a step depolarizing current pulse.
Tat30-86-induced depolarizations were markedly reduced in Ca2+-free Krebs solution, indicating an influx of Ca2+ may account for a large portion of membrane currents induced by Tat30-86. This is in agreement with a reduction of Tat-depolarizations in the absence of extracellular Ca2+ reported in cultured human fetal neurons (Cheng et al., 1998). Ca2+ influx in neurons is realized primarily through voltage- and/or ligand-gated channels such as ionotropic glutamate receptors. In this respect, the steady-state I-V relationships in neurons voltage-clamped to -60 mV revealed that the reversal potential of Tat30-86 induced inward currents is between -30 and 0 mV. This value of reversal potential suggests the involvement of a non-selective cationic conductance. Activated ionotropic glutamate receptors (NMDA, AMPA or kainate) are permeable to Na+, Ca2+ and K+. The excitatory effect of Tat and Tat-induced increase in cytosolic Ca2+ is suggested to be mediated by a direct activation of NMDA receptors (Haughey et al., 2001; Song et al., 2003; Self et al., 2004,) or potentiation of NMDA receptors secondary to a release of glutamate (Clark et al., 2005). NMDA receptors are characterized by voltage-dependent Mg2+ block and the reversal potential is close to 0 mV (Mayer et al., 1984). However, our experiments indicate a quasi-linear I-V relationship with an inward rectification, suggesting that at the negative holding potentials where NMDA receptors are partially blocked, an inward rectifying K+ conductance may be active. This could also explain the reversal potential being more negative than that of NMDA receptors, since the reversal potential for K+ in our experimental conditions is close to -90 mV.
In addition to depolarizing the membrane, Tat30-86 facilitates the spontaneous release of excitatory and inhibitory transmitters in cultured rat cortical neurons. Several studies report that Tat increases cytosolic Ca2+ concentrations (Haughey et al., 1999, Bonavia et al., 2001, Brailoiu et al., 2006). A consequence of increase in cytosolic Ca2+ is facilitation of transmitter release in neurons (Augustine et al., 2003; Brailoiu et al., 2003). The observation that Tat30-86 increases the frequency of mEPSCs or mIPSCs in rat cortical neurons is indicative of a presynaptic site of action. Spontaneous miniatures, which have been recorded in cultured cortical neurons (Prange and Murphy, 1999), are produced by action potential-independent release of transmitter vesicles. Generally, the change in the frequency of miniatures indicates a presynaptic effect, while the change in the amplitude of miniatures could be due to presynaptic and postsynaptic effects.
Tat30-86 markedly increased the frequency of mEPSCs, while not significantly affecting their amplitude, indicating a stimulatory effect on glutamate release, but not on vesicles refilling. In our study, mEPSCs were abolished by CNQX and AP-5, indicating they are produced by glutamate acting on non-NMDA and NMDA receptors. It has been reported that Tat1-72 enhanced glutamate transmission in neurons of the mouse suprachiasmatic nucleus (Clark et al., 2005). On the other hand, Tat failed to affect the release of glutamate from human and rat cortical nerve endings (Feligioni et al., 2003). Recombinant Tat1-72 increased neuronal vesicular release by 50-75%, as measured by imaging methods (FM1-43 uptake assay) in primary cortical neurons (Perry et al., 2005). Our result provides direct evidence that Tat30-86 stimulates the spontaneous excitatory transmitter release in cultured rat cortical neurons.
Another important finding is that Tat30-86 increased the frequency and amplitude of mIPSCs by 57% and 36%, respectively. mIPSCs were abolished by treatment with bicuculline (20 μM), indicating that they are mediated by GABA acting on GABAA receptors. This is the first report that Tat30-86 modulates inhibitory transmitter release in central synapses. In human and rat synaptosomes, Tat is found not to affect the release of GABA, while stimulating the release of acetylcholine (Feligioni et al., 2003). We have previously reported that different calcium mobilizing second messengers such as IP3 and cyclic ADP ribose stimulate neurosecretion, but have a different effect on vesicles refilling: IP3 enhanced the amplitude of spontaneous miniatures; whereas cyclic ADP ribose had no effect (Brailoiu and Miyamoto, 2000). Tat stimulates IP3 production in cultured human fetal neurons (Hughey et al., 1999), but increases the level of cyclic ADP ribose in rat cortical nerve endings (Feligioni et al., 2003). It may be hypothesized that Tat, by acting on different signaling pathways, may differentially influence the amplitude of miniature synaptic currents in different cellular models.
With respect to the cytosolic Ca2+ concentration [Ca2+]i, Tat30-86 induced 3 types of Ca2+ responses: single spike (43%), oscillations (21%), and spike followed by plateau (9%). These patterns are similar to those produced by recombinant Tat1-72 in cultured fetal human brain cells (Haughey et al., 1999). Moreover, the transient increase in cytosolic Ca2+ persisted in neurons pretreated with NMDA and non NMDA receptor antagonists AP-5 and CNQX; whereas the plateau or oscillations were abolished, as previously reported in cultured fetal human brain cells (Haughey et al., 1999). Thus, it may be concluded that Tat30-86 directly depolarizes cultured rat cortical neurons, which may account for its excitatory action, and indirectly by releasing glutamate from adjacent neurons.
In summary, our results show that Tat30-86 is a biologically active fragment and that it excites mammalian neurons directly and indirectly via releasing glutamate from neurons adjacent to the neuron under investigation.
Acknowledgement
This study was supported by NIH Grants NS18710 and HL51314 from the Department of Health and Human Services.
Abbreviations
- AP-5
D-2-Amino-5-phosphonovaleric acid
- CNQX
6-Cyano-7-nitro-quinoxaline-2,3-dione
- DiSBAC4(3)
bis-[1,3-dibutylbarbituric acid] trimethineoxonol
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N,N-tetraacetic acid
- GABA
γ-aminobutyric acid
- HBSS
Hank’s balanced salt solution
- HEPES
N-(2-Hydroxyethyl) piperazine-N’-(2-ethanesulfonic acid)
- HIV-1
human immunodeficiency virus 1
- IP3
inositol-1,4,5-trisphosphate
- mEPSCs
miniature excitatory postsynaptic currents
- mIPSCs
miniature inhibitory postsynaptic currents
- NMDA
N-methyl-D-aspartate
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Editor
Cellular Neuroscience: Dr. Menahem Segal, Weizmann Institute of Science, Department of Neurobiology, Hertzl Street, Rehovot 76100, Israel
References
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: The role of oxidative stress and D1 dopamine receptor. Neurotoxic. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Brailoiu GC, Mameli G, Dolei A, Sawaya BE, Dun NJ. Acute exposure to ethanol potentiates human immunodeficiency virus type 1 Tat-induced Ca2+ overload and neuronal death in cultured rat cortical neurons. J Neurovirol. 2006;12:17–24. doi: 10.1080/13550280500516427. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Miyamoto MD. Inositol trisphosphate and cyclic adenosine diphosphate-ribose increase quantal transmitter release at frog motor nerve terminals: possible involvement of smooth endoplasmic reticulum. Neurosci. 2000;95:927–931. doi: 10.1016/s0306-4522(99)00509-6. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Patel S, Dun NJ. Modulation of spontaneous transmitter release from the frog neuromuscular junction by interacting intracellular Ca2+ Stores: Critical role for nicotinic acid adenine dinucleotide phosphate (NAADP) Biochem J. 2003;373:313–318. doi: 10.1042/BJ20030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu GC, Lai CC, Chen CT, Hwang LL, Lin HH, Dun NJ. Sympathoinhibitory action of nociceptin in the rat spinal cord. Clin Exp Pharmacol Physiol. 2002;29:233–237. doi: 10.1046/j.1440-1681.2002.03635.x. [DOI] [PubMed] [Google Scholar]
- Brauner T, Hulser DF, Strasse RJ. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochm Biophys Acta. 1984;771:208–216. doi: 10.1016/0005-2736(84)90535-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Geisler WS, Seidemann E. Optimal decoding of correlated neural population responses in the primate visual cortex. Nat Neurosci. 2006;9:1412–1420. doi: 10.1038/nn1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neurosci. 1998;82:97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Clark JP, 3rd, Sampair CS, Kofuji P, Nath A, Ding JM. HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am J Physiol Regul Integr Comp Physiol. 2005;289:R656–R662. doi: 10.1152/ajpregu.00179.2005. [DOI] [PubMed] [Google Scholar]
- Connor JA. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci USA. 1986;83:6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Prog Neurobiol. 1995;46:463–506. doi: 10.1016/0301-0082(95)00010-s. [DOI] [PubMed] [Google Scholar]
- Feligioni M, Raiteri L, Pattarini R, Grilli M, Bruzzone S, Cavazzani P, Raiteri M, Pittaluga A. The human immunodeficiency virus-1 protein Tat and its discrete fragments evoke selective release of acetylcholine from human and rat cerebrocortical terminals through species-specific mechanisms. J Neurosci. 2003;23:6810–6818. doi: 10.1523/JNEUROSCI.23-17-06810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein Tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J. Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31:S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, Matsushita M, Moriwaki A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H. HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning. Ann Neurol. 2004;55:362–371. doi: 10.1002/ana.10844. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 Tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neurosci. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 Tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7:1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Litzburg A, Zhang D, Dewhurst S, Gelbard HA. HIV-1 transactivator of transcription protein induces mitochondrial hyperpolarization and synaptic stress leading to apoptosis. J Immunol. 2005;174:4333–4344. doi: 10.4049/jimmunol.174.7.4333. [DOI] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, Filippi P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virol. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. J Neurosci. 1999;19:6427–6438. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoniger S, Wehming S, Gonzalez C, Schobitz K, Rodriguez E, Oksche A, Yulis CR, Nurnberger F. The dispersed cell culture as model for functional studies of the subcommissural organ: preparation and characterization of the culture system. J Neurosci Methods. 2001;107:47–61. doi: 10.1016/s0165-0270(01)00351-x. [DOI] [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Nath A, Harris BR, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;995:39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9:399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- Wu SY, Ohtubo Y, Brailoiu GC, Dun NJ. Effects of endomorphin on substantia gelatinosa neurons in rat spinal cord slices. Br J Pharmacol. 2003;140:1088–1096. doi: 10.1038/sj.bjp.0705534. [DOI] [PMC free article] [PubMed] [Google Scholar]