Abstract
As a significant known risk factor for the development of ischemic stroke, carotid atherosclerosis is a potentially preventable and treatable disease process. The progression of improved diagnostic modalities, including magnetic resonance and computed tomography angiography, has provided enhanced plaque detection and characterization. The management of carotid artery stenosis has also continued to evolve from an aggressive, early surgical approach with the advent of the carotid endarterectomy to the initiation of progressive medical management options and the development of advanced percutaneous intervention. Carotid endarterectomy continues to be the clear treatment of choice in symptomatic patients with >70% carotid stenosis. However, strict risk factor modification, including improved antihypertensive therapy, lipid management, smoking cessation, and antiplatelet therapy, have led to less-compelling indications for immediate surgery in asymptomatic populations. In recent years, the evolution of improved percutaneous techniques and the development and approval of carotid stents have expanded the role of intervention. Several randomized trials have studied the efficacy of carotid artery stenting versus carotid endarterectomy in asymptomatic and symptomatic patients to help define the role of invasive therapy. The primary objective of this review is to summarize the current evidence and standards for the advanced diagnostic and management strategies used in asymptomatic and symptomatic patients with carotid artery stenosis.
Critical carotid artery atherosclerosis represents a multifactorial process with early degenerative plaque formation and subsequent development of flow-limiting stenosis, thrombosis, and embolization. Atherosclerosis typically begins when patients are young, with the first deposition of atheromatous plaque consisting of cholesterol, lipid, and inflammatory cells. As the plaque matures, it has the potential to produce a flow-limiting, thrombotically active matrix with infiltration of inflammatory cells. As a result, the patient is at considerable risk for acute ischemic events (1). The affected locations include the entire cerebrovascular circulation, but the extracranial portion of the carotid artery, with the proximal internal carotid and the carotid bifurcation, is most commonly affected.
The most pervasive complication of carotid atherosclerosis is the development of neurological symptoms and the potential for progression to a cerebrovascular accident (CVA). However, carotid atherosclerosis often goes undetected, and the presence of a carotid bruit is sometimes the only associated physical examination finding in patients with significant disease. Although considered a poor diagnostic marker for carotid stenosis, the detection of a carotid bruit was found to nearly double the expected stroke risk in asymptomatic patients (2). While these lesions are often asymptomatic, the initial clinical manifestations may include transient ischemic attacks (TIA), transient monocular blindness or amaurosis fugax, and focal, persistent neurological deficits related to a CVA.
Because of the potentially devastating consequences of carotid atherosclerotic disease, physicians need to be able to appropriately risk stratify patients to guide disease management. Advances in diagnostic modalities, medical treatment, surgery, and modern endovascular intervention have led to more effective management strategies for carotid atherosclerosis.
DIAGNOSIS
To complement the physical examination findings and clinical presentation of carotid atherosclerosis as noted above, various imaging modalities characterize the plaque and define the extent of disease. Carotid duplex ultrasonography is the diagnostic study of choice for screening and initial assessment of stenosis. Although not as accurate with stenoses of <50%, the study provides very accurate predictability for high-grade lesions (>70% stenosis) (3). Magnetic resonance angiography and computed tomography angiography are also useful and may be of considerable value in collaboration with ultrasound for further characterization of lesions producing >50% stenosis. The gold standard for carotid imaging, however, is cerebral angiography. Although conventional cerebral angiography provides comprehensive evaluation of the carotid distribution, including accurate characterization of plaque and collateral circulation (4), the risk of neurological complications (4%) and even death (1%) has led to its relatively uncommon use (5).
RISK FACTOR MODIFICATION
Once carotid atherosclerosis is diagnosed, the typical approach in asymptomatic patients, as with patients with coronary artery disease, is the identification and reduction of atherosclerotic risk factors. Hypertension, dyslipidemia, tobacco abuse, and poor glycemic control in diabetics are preventable causes of atherosclerosis.
As the single most prevalent cause of stroke, hypertension is a principal area of focus for the primary prevention of CVA and has been shown to be associated with an odds ratio of 2.11 for development of moderate carotid stenosis for every 20 mm Hg increase in systolic blood pressure (6). A recent meta-analysis of trials on blood pressure management in atherosclerotic disease revealed a 42% reduction in the incidence of stroke over a 2-to 5-year follow-up period after reduction of systolic blood pressure by an average of 5.8 mm Hg (7).
The effect of cholesterol on development of carotid stenosis has been well established and equates to an odds ratio of 1.10 for every increase of 10 mg/dL in cholesterol level (6). Another important factor is the direct relationship between low levels of high-density lipoprotein cholesterol and the development of atherosclerosis. A high ratio of total cholesterol to high-density lipoprotein cholesterol—an associated contributor to plaque development and progression—strongly justifies the aggressive treatment of dyslipidemia (8). A meta-analysis of trials studying the effects of lipid management found that statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) produced a relative risk reduction for stroke of approximately 26% over a mean follow-up period of 4.7 years (9). Further, in patients with a history of stroke or TIA, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial revealed a significant reduction of fatal or nonfatal stroke compared with placebo (11.2% vs 13.1%) with ≥50% low-density lipoprotein reduction with high-dose atorvastatin therapy (10). Another advantage of statins may be related to secondary effects that potentially contribute to carotid plaque regression. A study using high-resolution magnetic resonance imaging showed evidence of plaque regression at 6 and 12 months after lipid lowering by simvastatin, suggesting the possibility of vascular remodeling as a causative factor (11). Although the extent of benefit appreciated by the pleiotropic effects of statin therapy is still unclear, a clear connection between lower lipid levels and prevention of stroke is apparent.
Cigarette smoking has been associated with an increased risk of accelerated atherosclerosis, leading to a level IB indication for smoking cessation in primary stroke prevention. In a cohort of 4255 men and women from the Framingham Heart Study, cigarette smoking was shown to be directly associated with the risk of ischemic stroke (12). Furthermore, the risk of stroke decreased significantly 2 years after participants quit smoking and was equal to the risk for nonsmokers 5 years after cessation. Another analysis of 415 patients with carotid disease revealed an odds ratio of 1.08 per every 5 pack-years of smoking (5). Using ultrasound-measured carotid wall thickness in 5116 older patients, this remarkable dose-related relationship was further confirmed. Comparing current smokers, previous smokers, and patients who had never smoked, the prevalence of clinically significant (≥50%) internal carotid stenosis increased from 4.4% in those who never smoked to 7.3% in former smokers and 9.5% in current smokers (P <0.0001) (13). Furthermore, the carotid wall thickness of current smokers in comparison to nonsmokers was more pronounced than the 10-year age-related difference of nonsmokers (0.39 mm vs 0.31 mm).
Since diabetics have a significantly higher risk of ischemic stroke than the general population, the initiation of strict glycemic control in patients with diabetes mellitus has been suggested as another important measure in the prevention of carotid atherosclerosis. A randomized, single-blinded study of 175 type 2 diabetics between the ages of 35 and 40 who were assigned to receive either of two insulin secretagogues, repaglinide or glyburide, was recently completed with 1-year follow-up measurement of carotid intimal media thickness (14). In both groups, the strict management of postprandial hyperglycemia produced a significant reduction of carotid intimal media thickness (52% for repaglinide, 18% for glyburide; P <0.01), suggesting that reduction of postprandial hyperglycemia could potentially help reduce the incidence of stroke as well as help the regression of established plaque.
MEDICAL MANAGEMENT
Once risk factors have been identified and aggressive risk factor modification has been implemented, the principal treatment approach to primary asymptomatic disease is antiplatelet therapy. Aspirin therapy at various doses has been shown to reduce the risk of TIA, stroke, and death as monotherapy in high-risk patients (15). The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial further revealed that in symptomatic patients, the use of combination therapy with clopidogrel and aspirin can reduce the incidence of asymptomatic embolization compared with using aspirin alone (16).
Another useful agent for secondary prevention of stroke is the combination of extended-release dipyridamole and low-dose aspirin. The second iteration of the European Stroke Prevention Study (ESPS-2) was a large, randomized trial evaluating antiplatelet therapy with aspirin, dipyridamole, or combination therapy in 6602 patients with symptomatic carotid stenosis or previous ischemic stroke (17). Using the primary endpoints of stroke, death, or stroke/death combination, the trial revealed a stroke risk reduction of 18% and 16% in the aspirin and dipyridamole monotherapy groups, respectively, compared with a 37% reduction in the combination group (P < 0.001). The risk of death and stroke/death was also similarly reduced in the combination group. This trial provided the basis for the American College of Cardiology and the American Heart Association to provide a level IB indication for the use of aspirin and dipyrimidine combination therapy for secondary stroke prevention.
CAROTID ENDARTERECTOMY
In addition to risk factor modification and medical therapy, surgery has been a longstanding treatment option for carotid atherosclerosis. The first successful carotid endarterectomy (CEA) was reported by Eastcott, Pickering, and Rob in 1954 (18). In 1975, Michael DeBakey reported the first follow-up results, which revealed the durability of surgery over a 19-year period. The first published trials dealing with investigation of surgery as a viable alternative to medical therapy came via the Carotid Artery Stenosis with Asymptomatic Narrowing Operation Versus Aspirin (CASANOVA) trial and the Mayo Asymptomatic Carotid Endarterectomy (MACE) trial, which were both ultimately considered suboptimal due to poor study design.
The first true evidence for the efficacy of surgery in critical asymptomatic narrowing was revealed in the Veterans Affairs Cooperative Trial, which pooled a sample of 444 asymptomatic men with >50% carotid stenosis per arteriogram and randomly assigned them to aspirin monotherapy or aspirin plus CEA. The study measured the primary endpoints of TIA, amaurosis fugax, and CVA for 48 months. The results revealed a lower incidence of ipsilateral stroke and TIA in the combination group (8.0% vs 20.6%, P < 0.001) with no significant difference in mortality at 30 days and 48 months (19).
The Asymptomatic Carotid Atherosclerosis Study (ACAS) provided more substantial evidence for the role of surgery. This study assigned 1662 patients with >60% stenosis per ultrasound or arteriography to receive either aspirin alone or aspirin and CEA with primary endpoint measurement of cerebral infarction in the study artery or perioperative stroke or death for a median follow-up period of 2.7 years. The primary endpoint reduction in this study (5.1% vs 11%) also favored the surgery group (20). Although differences in gender were not statistically significant due to study design, preliminary evidence suggested that men appreciated a greater absolute risk reduction from surgery than women.
The largest multicenter trial studying the benefit of surgery for asymptomatic patients was the Asymptomatic Carotid Surgery Trial (ACST). This study enrolled 3120 patients with >60% stenosis per ultrasound and assigned groups to immediate CEA (88% by 1 year) versus deferred surgery with a mean follow-up of 3.4 years. The results again revealed a 5-year risk reduction for perioperative stroke or death in the immediate CEA group compared with the deferral group (6.4% vs 11.8%) (21). A similar reduction in fatal or disabling stroke was also apparent in the immediate CEA group. A notable finding in this study was a 2-year delayed benefit in the surgery group, with worse outcomes prior to this period.
With regard to symptomatic carotid occlusive disease, the standard of care has historically been immediate surgery. The seminal trial for the institution of this approach was the North American Symptomatic Carotid Endarterectomy Trial (NASCET), which established the efficacy of CEA in symptomatic patients. The study was a randomized, prospective, multicenter trial enrolling 659 patients with >70% stenosis who presented with hemispheric or retinal TIA or CVA from an isolated carotid stenosis over a 4-month period prior to enrollment. Results of this trial revealed clear surgical efficacy with a lower cumulative risk of any ipsilateral stroke at 2 years (9% vs 26%, P < 0.001) and a similarly significant decrease in the rate of major or fatal stroke during the same period of time (2.5% vs 13.1%) (22). These results led to the universal conclusion that in symptomatic patients with >70% stenosis, CEA was superior to medical therapy.
Results of this trial were subsequently corroborated by the European Carotid Surgery Trial (ECST). ECST randomly selected 2518 patients divided into two groups, with one group consisting of patients with <30% stenosis and the other group with >70% stenotic lesions (23). This study showed that patients with mild to moderate stenosis appreciated little benefit from CEA compared with the early risks of surgery, particularly in those with <30% stenosis. Similar to NASCET, patients with severe stenosis gained the most significant endpoint reductions and appreciated the greatest benefit.
The American Heart Association and American Stroke Association currently have a grade IIA recommendation for CEA in otherwise healthy, asymptomatic men between the ages of 40 and 75 years with >60% stenosis. Recognizing that the true benefit in these patients is not revealed until several years after surgery, they suggest that CEA be considered very carefully in patients with other complicating comorbidities. In women with asymptomatic lesions, there has never been conclusive evidence of benefit with CEA, and some data reveal a greater risk of harm in this patient population. This has prompted the recommendation for surgical therapy in only symptomatic women. In patients with mild stroke or TIA, the most advantageous time for intervention is within 2 weeks of the event. Furthermore, the pooled analysis of these studies has led to a grade IA indication for CEA in carotid lesions of >70% stenosis regardless of symptomatology (24).
In symptomatic men with >50% stenotic lesions, CEA also received a clear indication within the 2-week time frame; however, the data have not shown a benefit in women with similar lesions, and risk factor management should be pursued in this population. There is no evidence of benefit in lesions >30% and significant harm in those with <30% stenosis irrespective of symptomatology (25). In patients with near complete or complete occlusion, the data do not support any significant benefit. Moreover, although CEA has a clear advantage in patients with severe contralateral occlusive disease, increased perioperative risk is a substantial consideration. In patients who have experienced a moderate to severe cerebrovascular event with subsequent disabling symptoms, the benefit of surgery is clearly outweighed by associated comorbidities, and CEA has not been recommended.
Despite several studies that clearly demonstrate the efficacy of CEA in appropriate patient populations, the surgery has inherent risks. The benefit revealed in the ACAS, ECST, and NASCET trials was largely dependent on a perioperative morbidity and mortality rate of <3%. However, a multicenter study evaluating the complication rates of CEA in asymptomatic patients revealed a postoperative stroke, myocardial infarction, and death rate of 4.5% (26). Another national study of Medicare patients with both asymptomatic and symptomatic disease reported an overall mortality rate as a high as 1.9% (27). Other associated postoperative complications include wound hematomas, hypotension, cranial nerve injuries, seizures, hyperperfusion syndrome, and intracerebral hemorrhage (28). Taking these variables into consideration is imperative to patient outcomes and safety when determining the surgical benefit in patients with carotid stenosis.
CAROTID ANGIOPLASTY AND STENTING
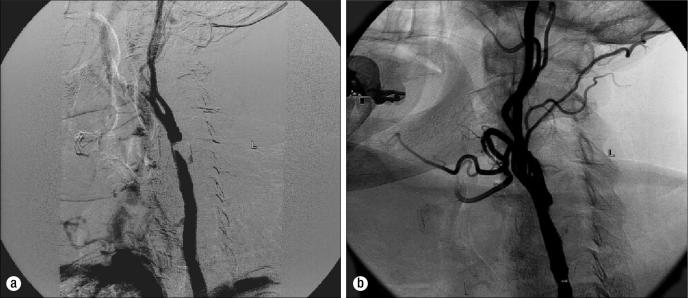
Given the potential advantages offered by percutaneous intervention of carotid atherosclerotic disease, carotid artery stenting (CAS) is progressively being seen as a viable alternative to surgery (Figure). The first successful carotid angioplasty for treatment of atherosclerotic carotid artery stenosis was performed by Klaus Mathias in 1980 (29). Since that time, however, the role of angioplasty in carotid atherosclerosis has largely remained a poorly understood and controversial topic in vascular medicine. Nevertheless, as percutaneous transluminal techniques and technology continue to advance for use in the already well established areas of coronary artery and peripheral vascular disease, the alternative of angioplasty as a means to counteract flow-limiting lesions of the carotid anatomy becomes increasingly attractive.
Figure.
Carotid angiograms (a) before and (b) after carotid artery stenting at Baylor University Medical Center. The patient had a history of previous carotid endarterectomy and had a high-grade distal common carotid artery restenosis that was successfully treated.
Despite a seemingly intuitive approach, angioplasty without stent placement has been plagued with poor results and multiple complications, leading to the relative reluctance to pursue balloon angioplasty as primary management of atherosclerotic carotid disease. The development of stent technology provided renewed enthusiasm to the arena of percutaneous carotid revascularization. Since the primary adverse event in carotid atherosclerosis is embolization of plaque material rather than blood flow impairment, it seemed intuitive that stenting would be able to provide an effective means of mechanical “plaque stabilization” by taking advantage of the scaffolding properties of these devices. Additionally, the minimally invasive nature of the procedure is attractive given the potentially decreased morbidity, reduced hospital stay, and lack of any residual scar when compared with CEA. The avoidance of general anesthesia represents another major advantage of the endovascular approach, particularly with monitoring of neurological status. The introduction of carotid intervention with stent placement is the single most influential factor in the resurgence of endovascular therapy as acceptable management of carotid atherosclerosis. As a result, carotid angioplasty with stenting readily replaced lone balloon angioplasty as a primary means for the percutaneous treatment of significant carotid stenosis.
Initial trials were relatively equivocal regarding the differences in outcome or major risks between CAS and CEA (30). However, two trials directly comparing these procedures have provided more data establishing the efficacy of CAS compared with CEA.
The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial was a randomized, multicenter European trial that compared CAS and CEA. A total of 1183 patents with severe symptomatic carotid stenosis, excluding patients at high surgical risk, were randomly assigned to CAS or CEA, with the primary endpoint of ipsilateral stoke and death. Despite the premature termination of the trial due to financial considerations, the 30-day results revealed no significant difference in primary endpoint outcomes (6.84% and 6.34%) between CAS and CEA (31). This trial, similar to other trials during the same period, was criticized for its optional use of embolic protection devices in the CAS group. These devices are designed to filter and trap debris that results from stent placement in the arteries. Although there is not sufficient evidence from prospective randomized trials on the effectiveness of embolic protection devices in preventing procedural complications, a recent review of outcomes revealed that such devices led to a lower incidence of minor (0.5% vs 3.7%) and major CVA (0.3% vs 1.1%) (32).
A more definitive trial, the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial, has now become the standard for the acceptance of endovascular intervention of the carotid arteries. SAPPHIRE randomly assigned 334 high-risk patients from 29 centers to either CAS or CEA. The study population consisted of asymptomatic patients with documented stenosis ≥80% and symptomatic patients with ≥50% stenosis. In contrast to the SPACE trial and other similar preceding trials, SAPPHIRE study researchers used a distal embolic protection device in each case and included high-risk patients—defined as those with age >80 years, congestive heart failure, chronic obstructive pulmonary disease, previous endarterectomy with restenosis, previous radiation or neck surgery, or distal or proximal lesions. The primary endpoint of the study was the cumulative incidence of a major cardiovascular event within 1 year, which included myocardial infarction within 30 days after the intervention and death or ipsilateral stroke between 31 days and 1 year after either procedure.
Despite some criticism for bias toward stenting since the study included restenosis and perioperative myocardial infarction and reported higher periprocedural complication rates than the previous standard of 3%, the study adequately demonstrated the equal efficacy of CAS and CEA in this population. This led to the US Food and Drug Administration's approval of carotid stent placement in patients with severe symptomatic stenosis. In terms of primary endpoint measurements, CAS with protection actually revealed a 39% reduced rate of events compared with CEA (12.1% vs 20.1%; P = 0.004) (33). The length of hospital stay and number of cranial nerve abnormalities after the procedure were also greater in the CEA group.
SAPPHIRE was truly a landmark study that more clearly defined the role of CAS in carotid disease. Currently, CAS has received a grade IIB indication from the American Heart Association/American Stroke Association for high-risk populations, including patients with difficult surgical anatomy, severe cardiopulmonary disease, recurrent stenosis after CEA, and radiation-induced stenosis (24). However, further definitive studies measuring long-term efficacy are currently under way and will provide greater insight into the potentially more prominent utility of CAS in a greater subset of patients.
Several trials studying the efficacy of CAS in lower-risk populations are currently ongoing, including the Carotid Revascularization Endarterectomy versus Stent Trial (CREST). CREST is a large, prospective, multicenter, randomized trial with two arms consisting of symptomatic patients with ≥50% stenosis and asymptomatic patients with ≥70% stenosis. A recent lead-in study from CREST enrolling 465 patients revealed a 5.6% stroke and death rate in symptomatic patients and a 2.4% rate in asymptomatic patients at 30 days—rates that are lower than those previously reported. Among other similar studies are the Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial, which is also a prospective, multicenter, randomized trial enrolling two arms of patients, including asymptomatic patients with >50% stenosis and symptomatic patients with >75% stenosis. Of the 397 patients enrolled in the study, 254 received CEA and the remaining 143 received CAS. The results of the study revealed no significant difference in 30-day (4.4% CEA vs 2.1% CAS) and 1-year (14.3% vs 10.9%) risk of mortality, stroke, or myocardial infarction.
Although carotid stenting seems to demonstrate efficacy equal to that of CEA, this technique clearly possesses its own complications. The most pervasive risk associated with percutaneous revascularization of the carotid arteries lies in the potential for distal embolization of established plaque with subsequent neurological events such as hemispheric TIA or stroke. Acute occlusion and in-stent thrombosis may also occur, with early follow-up data of interventional carotid cases reporting approximately 6% restenosis at 1 year (34). Typical catheter-associated complications also apply to the carotid arteries, with approximately 5% incidence of dissection reported (35).
A potentially devastating effect of carotid revascularization is a phenomenon known as cerebral hyperperfusion syndrome, which is considered to be the result of impaired autoregulation after restoration of cerebral blood flow (36). Due to the rapid change of pressure, a loss of momentary compensatory mechanisms is often manifested in the distribution of the stented artery, with symptoms presenting as motor seizures, transient focal deficits, and the possibility of an intracerebral hemorrhage. These complications are very difficult to manage due to their traditionally marginal response to surgical intervention, and most initial data revealed a significantly greater risk of stroke. Based on these potential complications, CAS has not yet received a recommendation for the primary treatment of carotid atherosclerotic disease.
Despite the potential for such procedural complications, more evidence supporting the utility of percutaneous carotid intervention in certain populations is removing previous misconceptions and encouraging further consideration.
CONCLUSION
The most definitive evidence for disease management in asymptomatic patients, regardless of the degree of disease, exists for medical therapy, with particular emphasis on statins, more effective antihypertensive medications, and antiplatelet therapy. Specifically, the advent of statins has provided a means for not only delaying plaque progression, but also possibly degenerating already formed lesions. These therapies have provided a practical alternative to the previously aggressive, early invasive approach to carotid occlusive disease.
Clearly, CEA has long been established as a safe and viable option in symptomatic carotid disease. The potential benefits of surgery must be weighed against the relative risks, considering the presence of a contralateral lesion, complications of surgery, gender, age, and time to surgery after a cerebrovascular event.
As the technology and operator experience in percutaneous techniques continue to advance, the role of carotid angioplasty and stenting will undoubtedly expand, as it has with other endovascular interventions. Nonetheless, the results of multiple ongoing trials are pending and will be critical to establishing the efficacy and safety of CAS as a routine and reliable approach to aggressive treatment of carotid occlusive disease in the future.
References
- 1.Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, Piepgras DG, Pistolese R, Ippoliti A, Holmes DR., Jr Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA. 2004;292(15):1845–1852. doi: 10.1001/jama.292.15.1845. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Kannel WB, Sorlie P, McNamara P. Asymptomatic carotid bruit and risk of stroke. The Framingham study. JAMA. 1981;245(14):1442–1445. [PubMed] [Google Scholar]
- 3.Tsuruda JS, Saloner D, Anderson C. Noninvasive evaluation of cerebral ischemia. Trends for the 1990s. Circulation. 1991;83(2 Suppl):I176–I189. [PubMed] [Google Scholar]
- 4.Wolpert SM, Caplan LR. Current role of cerebral angiography in the diagnosis of cerebrovascular diseases. AJR Am J Roentgenol. 1992;159(1):191–197. doi: 10.2214/ajr.159.1.1609697. [DOI] [PubMed] [Google Scholar]
- 5.Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990;21(2):209–222. doi: 10.1161/01.str.21.2.209. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, Hoeg JM, D'Agostino RB, Silbershatz H, Belanger AM, Poehlmann H, O'Leary D, Wolf PA. Cumulative effects of high cholesterol levels, high blood pressure, and cigarette smoking on carotid stenosis. N Engl J Med. 1997;337(8):516–522. doi: 10.1056/NEJM199708213370802. [DOI] [PubMed] [Google Scholar]
- 7.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693):827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 8.van Merode T, Hick P, Hoeks PG, Reneman RS. Serum HDL/total cholesterol ratio and blood pressure in asymptomatic atherosclerotic lesions of the cervical carotid arteries in men. Stroke. 1985;16(1):34–38. doi: 10.1161/01.str.16.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Corvol JC, Bouzamondo A, Sirol M, Hulot JS, Sanchez P, Lechat P. Differential effects of lipid-lowering therapies on stroke prevention: a meta-analysis of randomized trials. Arch Intern Med. 2003;163(6):669–676. doi: 10.1001/archinte.163.6.669. [DOI] [PubMed] [Google Scholar]
- 10.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA, Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) investigators High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 11.Corti R, Fayad ZA, Fuster V, Worthley SG, Helft G, Chesebro J, Mercuri M, Badimon JJ. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104(3):249–252. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The Framingham Study. JAMA. 1988;259(7):1025–1029. [PubMed] [Google Scholar]
- 13.Tell GS, Polak JF, Ward BJ, Kittner SJ, Savage PJ, Robbins J, Cardiovascular Health Study (CHS) Collaborative Research Group Relation of smoking with carotid artery wall thickness and stenosis in older adults. The Cardiovascular Health Study. Circulation. 1994;90(6):2905–2908. doi: 10.1161/01.cir.90.6.2905. [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Giugliano D, Nappo F, Marfella R, Campanian Postprandial Hyperglycemia Study Group Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110(2):214–219. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 15.Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, Ringelstein EB. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111(17):2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 17.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143(1–2):1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 18.Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;267(6846):994–996. doi: 10.1016/s0140-6736(54)90544-9. [DOI] [PubMed] [Google Scholar]
- 19.Hobson RW, 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB, The Veterans Affairs Cooperative Study Group Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med. 1993;328(4):221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 20.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421–1428. [PubMed] [Google Scholar]
- 21.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D, MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 22.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD, North American Symptomatic Carotid Endarterectomy Trial Collaborators Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339(20):1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 23.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial(ECST) Lancet. 1998;351(9113):1379–1387. [PubMed] [Google Scholar]
- 24.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Circulation. 2006;113(24):e873–e923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ, Carotid Endarterectomy Trialists' Collaboration Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361(9352):107–116. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LB, Samsa GP, Matchar DB, Oddone EZ. Multicenter review of preoperative risk factors for endarterectomy for asymptomatic carotid artery stenosis. Stroke. 1998;29(4):750–753. doi: 10.1161/01.str.29.4.750. [DOI] [PubMed] [Google Scholar]
- 27.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279(16):1278–1281. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 28.Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Harbaugh RE, Dempsey RJ, Caplan LR, Kresowik TF, Matchar DB, Toole JF, Easton JD, Adams HP, Jr, Brass LM, Hobson RW, 2nd, Brott TG, Sternau L. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 1998;97(5):501–509. doi: 10.1161/01.cir.97.5.501. [DOI] [PubMed] [Google Scholar]
- 29.Mathias K, Mittermayer Ch, Ensinger H, et al. Perkutane Katherdilatation von Karotisstenosen. ROFO. 1980;3:47–50. doi: 10.1055/s-2008-1056722. [DOI] [PubMed] [Google Scholar]
- 30.Coward LJ, Featherstone RL, Brown MM. Safety and efficacy of endovascular treatment of carotid artery stenosis compared with carotid endarterectomy: a Cochrane systematic review of the randomized evidence. Stroke. 2005;36(4):905–911. doi: 10.1161/01.STR.0000158921.51037.64. [DOI] [PubMed] [Google Scholar]
- 31.SPACE Collaborative Group. Ringleb PA, Allenberg J, Br¨ckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-pro-tected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368(9543):1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 32.Kastrup A, Gröschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34(3):813–819. doi: 10.1161/01.STR.0000058160.53040.5F. [DOI] [PubMed] [Google Scholar]
- 33.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 34.Gröschel K, Riecker A, Schulz JB, Ernemann U, Kastrup A. Systematic review of early recurrent stenosis after carotid angioplasty and stenting. Stroke. 2005;36(2):367–373. doi: 10.1161/01.STR.0000152357.82843.9f. [DOI] [PubMed] [Google Scholar]
- 35.Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology. 1996;201(3):627–636. doi: 10.1148/radiology.201.3.8939208. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder TV. The cerebral hyperperfusion syndrome after CEA and CAS: physiologic basis, prevention, and treatment Presented at the 31st Global: Vascular and Endovascular Issues, Techniques and Horizons, November 21, 2004. Available at http://www.veithsymposium.org/pdf2004/253.pdf; accessed January 10, 2008.