Abstract
Ectopic production of adrenocorticotropic hormone by carcinoid tumors is relatively uncommon and may not be recognized by physicians. This report describes a woman who had Cushing syndrome from the ectopic secretion of adrenocorticotropic hormone by a carcinoid tumor. Her cause of death was a pneumonia that may have been secondary to her untreated hypercortisolism. There are threeinstructive elements of this case: 1) the recognition of Cushing syndrome, 2) the association of Cushing syndrome with low-grade (carcinoid tumors) as well as with high-grade (small cell carcinoma) neuroendocrine tumors, and 3) the need to treat the hypercortisolism as well as the tumor.
A 46-year-old white woman presented to Baylor University Medical Center (BUMC) in March 2007 for evaluation and treatment of refractory hypertension, hypokalemia, hyperglycemia, and weakness. In 2004, a previous workup at an outside hospital revealed a 9-cm pancreatic mass that was diagnosed as a low-grade neuroendocrine tumor consistent with a carcinoid tumor on biopsy. Four months after the initial presentation, new-onset neck pain led to the discovery of a right-sided supraclavicular node. This node was resected and showed a low-grade neuroendocrine tumor. Extensive liver and mediastinal metastases were discovered during the same period of time.
In December 2006, the patient was hospitalized at an outside hospital with new-onset facial swelling and weakness. A computed tomography (CT) scan of the chest revealed large paratracheal nodes. The diagnosis of presumed superior vena cava syndrome was made. The patient received one course of streptozocin and daunorubicin and was started on mediastinal radiation therapy, which she completed approximately 1 month prior to presenting at BUMC. During this hospitalization, the patient developed elevated blood pressure, new-onset hyperglycemia, and profound hypokalemia. On her initial presentation at BUMC, the patient complained of severe generalized weakness, left-sided flank pain with radiation to the left lower back, and urinary incontinence. She had a 5-pack-year history of smoking but had stopped 18 years previously. She admitted to only a social alcohol habit and denied any drug use.
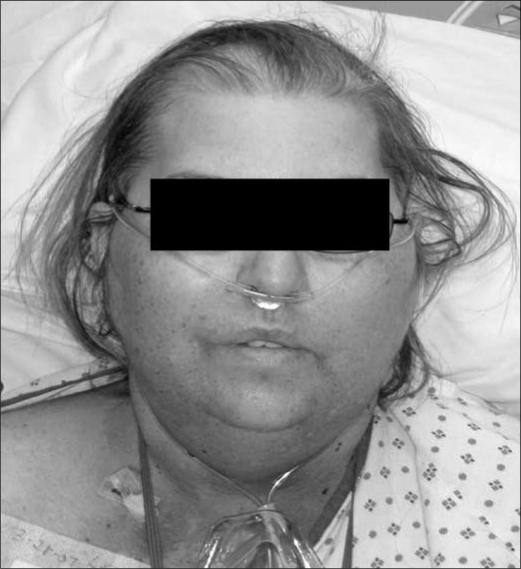
Her physical examination revealed a mildly ill-appearing, obese white woman in no acute distress (Figure 1). Her vital signs showed a blood pressure of 141/103 mm Hg but otherwise were within normal limits. She had moderate facial swelling. She had neither jugular venous pressure distention nor collateral circulation over her chest. She had left-upper-quadrant tenderness to palpation and 1+ bilateral lower extremity edema. She had a regular heart rate and rhythm. She had no murmurs, wheezes, rhonchi, crackles, abdominal distension, or any other significant findings.
Figure 1.
Our patient with moon-shaped facies and alopecia on admission.
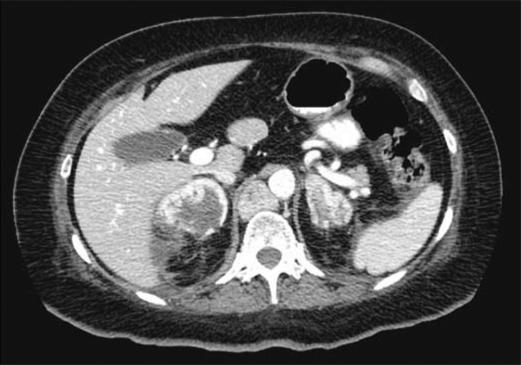
Routine laboratory results revealed modest hypokalemia (2.7 mmol/L; reference range, 3.5–5.3 mmol/L), hyperglycemia (145 mg/dL; reference range, <120 mg/dL), and anemia (hematocrit = 33.5%; reference range, 35%–46%). A random serum cortisol level showed significant elevation (135 mcg/dL; reference range, 6–28 mcg/dL) and an overnight, low-dose dexamethasone suppression test revealed a failure to suppress cortisol (129.4 mcg/dL; reference range, 3–16 mcg/dL). The serum adrenocorticotropic hormone (ACTH) level was elevated (743 pg/mL; reference range, 10–60 pg/mL). Her 5-hydroxyin-doleacetic acid level (4.3 mg/24 h; reference range, 3–15 mg/24 h) was within normal limits. A CT scan of her chest, abdomen, and pelvis revealed multiple enlarged lymph nodes in the mediastinum, retrocrural area, peripancreatic area, paraaortic area, aortocaval area, and aortic bifurcation area. She also had a large enhancing mass in the uncinate process of the pancreas suspicious for the primary tumor. Bilateral adrenal enlargement was also noted, with hemorrhage and a subcapsular hematoma in the right adrenal gland (Figure 2). A bone scan was negative for any osseous metastases.
Figure 2.
CT shows a left adrenal mass measuring 4.3 × 2.9 cm and a right adrenal mass measuring 4.1 × 4.9 cm. The right adrenal mass is hemorrhagic with resultant right-sided subcapsular hematoma.
Since ACTH secretion is more prevalent in high-grade neuroendocrine tumors than in carcinoid tumors, we treated our patient with carboplatinum and etoposide, as if she had small cell carcinoma. The hypertension was controlled with multiple antihypertensives, the hypokalemia was treated with potassium, and the hyperglycemia was managed with insulin. She developed dyspnea on exertion during the initial days of her hospitalization, but the dyspnea was appropriately responsive to nebulizer treatments and oxygen. A CT scan with a pulmonary embolism protocol was negative. Cardiac enzyme levels were normal, as were the findings of the electrocardiogram and two-dimensional echocardiogram. The ejection fraction was 70%. Blood cultures grew Klebsiella pneumoniae in two out of two sets. This was treated with imipenem/cilastatin and vancomycin. She also received empiric fungal coverage with fluconazole. However, her respiratory status continued to deteriorate, and she developed acute respiratory distress. The patient subsequently died after electing not to pursue ventilator support.
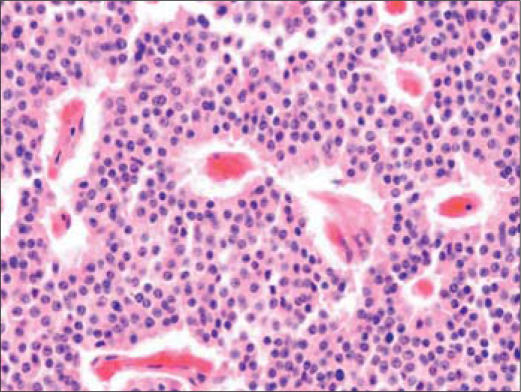
On autopsy, her cause of death was an overwhelming pneumonia due to Stenotrophomonas maltophilia. The original pancreatic mass was consistent with carcinoid (Figure 3). There was marked adenopathy, which was involved with her low-grade neuroendocrine tumor. There was no evidence of high-grade neuroendocrine tumor involvement. The adrenal glands showed extensive necrosis but no involvement with tumor.
Figure 3.
Section of pancreatic tumor showing an organoid pattern of polygonal cells with abundant eosinophilic cytoplasm, salt and pepper chromatin, and occasional mitotic figures, consistent with carcinoid tumor.
DISCUSSION
The decision to approach the treatment of this tumor with the presumption of either dedifferentiation to or simultaneous presentation with a high-grade neuroendocrine tumor was influenced by the overall picture and history of the patient. This patient had a biopsy-proven carcinoid tumor. The subsequent development of profound hypercortisolism led to the assumption that the patient's carcinoid tumor had dedifferentiated into a high-grade neuroendocrine tumor (i.e., small cell carcinoma).
Based on a population study, the annual incidence of endogenous Cushing syndrome is estimated to be approximately 1.2 million to 1.7 million cases per year. Of these cases, approximately 60% are due to Cushing disease, i.e., a pituitary ACTH-producing tumor; 22.3% are due to a benign adrenocortical adenoma; 6.6%, to an adrenal carcinoma; 6.0%, to a nonadrenal carcinoma; 3.6%, to a carcinoid tumor; and the remaining 1.5%, to miscellaneous diseases (1). The preponderance of ectopic ACTH-producing tumors originate from four different histological categories: small cell carcinomas of the lung (50%), pheochromocytomas (3%), pancreatic islet cell tumors (10%), and carcinoid tumors (15%) (2).
The carcinoid tumors that produce ectopic ACTH are overwhelmingly bronchial or thymic in origin and are very rarely gastrointestinal in origin. A thorough search of the literature showed 78 cases of thymic or bronchial carcinoids that produced ACTH as opposed to only one case of abdominal carcinoid that produced ACTH. These tumors, either through direct release or through production of precursors, preferentially express the proopiomelanocortin gene, which leads to the release of markedly higher ACTH concentrations in comparison to typical Cushing disease.
Ectopic ACTH production from small cell carcinomas of the lung has been well described in the literature. Small cell carcinoma of the lung comprises approximately 20% to 25% of all bronchogenic lung carcinomas (3). Of the patients with small cell carcinoma of the lung, there is a 4.5% incidence of associated paraneoplastic ACTH production (4). Extrapulmonary small cell carcinoma is a comparatively rare disease, representing approximately 4% of all small cell cancers (5).
Carcinoid tumors occur less frequently than small cell carcinoma of the lung. Approximately 55% of carcinoid tumors present in the gastrointestinal tract and 30% in the bronchopulmonary system (6). The general classification of these tumors is based on the embryological origin (foregut, midgut, hindgut). The metastatic potential of the tumor correlates with primary tumor size and origin. Clinical manifestations also differ based on tumor location. Carcinoid of the lung represents approximately 2% of all primary lung tumors, but only 5% of these primary pulmonary carcinoid tumors are associated with ACTH production. Although this makes ACTH production the second most common paraneoplastic manifestation in carcinoid tumors, behind the carcinoid syndrome, this subset of patients comprises only approximately 1% of all Cushing syndrome (7).
Pancreatic carcinoid tumors are one of the four foregut carcinoid tumors (lung, thymus, stomach, and pancreas). The pancreatic carcinoid tumors usually occur in the tail of the pancreas and metastasize to the liver and regional lymph nodes. These tumors are exceedingly rare, comprising approximately 0.55% of all carcinoid tumors, making pancreatic carcinoid the least common gastrointestinal carcinoid tumor (8). Our patient had intraabdominal carcinoid that originated in the pancreas and spread to the mediastinum. This should have alerted us that this carcinoid tumor could produce ACTH.
The morbidity and mortality associated with Cushing syndrome is related to the production of excess cortisol. Hypercortisolism results in multiple medical problems, including hypertension, obesity, osteoporosis, fractures, impaired wound healing, infectious diseases, glucose intolerance, and psychosis. There are various methods of treating Cushing syndrome depending upon the etiology. In Cushing disease, transsphenoidal resection of the pituitary adenoma offers the best chance of cure (9). Unilateral or bilateral resection may be performed for functioning adrenal adenomas. External beam radiotherapy is used as an adjunct to surgery when complete resection is not possible. Ectopic ACTH secretion secondary to an underlying malignancy is optimally managed by treating the tumor. However, in our patient, it was unlikely that the low-grade neuroendocrine tumor would respond to conventional chemotherapy.
The logic that led us to treat this woman as if she had a small cell carcinoma is based on the following facts. First, ectopic ACTH production is much more common in a high-grade neuroendocrine tumor (e.g., small cell carcinoma) than in a low-grade neuroendocrine tumor (e.g., a carcinoid tumor). Second, high-grade neuroendocrine tumors (e.g., small cell carcinomas) are much more responsive to chemotherapy than low-grade neuroendocrine tumors (e.g., carcinoid tumors). Third, the overwhelming preponderance of reported cases of ACTH-pro-ducing low-grade neuroendocrine tumors has been thoracic and not abdominal carcinoid tumors. Therefore, our hope was that this woman's low-grade neuroendocrine tumor (carcinoid) had dedifferentiated into a high-grade neuroendocrine tumor (e.g., small cell carcinoma) which caused her new-onset Cushing syndrome and which would have a much better chance of responding to chemotherapy. Unfortunately, the patient did not respond to the chemotherapy and possibly died as a consequence of immunosuppression from her Cushing syndrome.
Medical therapy for Cushing syndrome is often necessary in patients whose ACTH-producing tumor has not responded to therapy, who have had unsuccessful pituitary surgery, or who have had a recurrence of their disease. Medical therapy is also used in patients who are not fit to undergo surgery. It would have been reasonable for our patient to receive medical treatment.
Drugs used to reduce cortisol can be categorized into three groups based on the mechanism of action: steroidogenesis inhibitors, neuromodulators of ACTH release, and glucocorticoid receptor-blocking agents (10). Mitotane, trilostane, ketoconazole, aminoglutethimide, and metyrapone directly inhibit steroidogenesis in the adrenal glands at one or more enzymatic steps. These drugs must be used two to three times a day, and the doses need to be titrated to maintain a eucortisolemic state. They are useful in all forms of Cushing syndrome and are effective in about 70% of patients (11). Common side effects of these agents include nausea and diarrhea. Cyproheptadine, bromocriptine, somatostatin, and valproic acid are neuromodulators that affect corticotropin-releasing hormone or ACTH synthesis. These have been used as single therapeutic agents, but response rates are poor (12). Side effects include postural hypotension, sedation, weight gain, and hepatic toxicity. Mifepristone (RU-486) is a steroid that binds competitively to the glucocorticoid receptor and inhibits the action of the endogenous ligands. It has been used in only a few investigational studies and is not available commercially in the United States (12). Etomidate is a short-acting anesthetic that can immediately decrease adrenal steroid production. It is given parenterally and can be used for acute control of severe hypercortisolism (13).
When the primary tumor cannot be treated successfully with surgery and/or chemotherapy, then one of the above-mentioned drugs can be used to control the cortisol production. Bilateral adrenalectomy is often the next step when medical therapy fails. We considered this in our patient, but it became clear that she would not tolerate any type of surgery. Our patient died of pneumonia, which was likely related to the hypercortisolemic state.
References
- 1.Lindholm J, Juul S, J⊘rgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, J⊘rgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. Incidence and late prognosis of Cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117–123. doi: 10.1210/jcem.86.1.7093. [DOI] [PubMed] [Google Scholar]
- 2.Liddle GW, Nicholson WE, Island DP, Orth DN, Abe K, Lowder SC. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283–314. doi: 10.1016/b978-0-12-571125-8.50009-0. [DOI] [PubMed] [Google Scholar]
- 3.Morgan LC, Grayson D, Peters HE, Clarke CW, Peters MJ. Lung cancer in New South Wales: current trends and the influence of age and sex. Med J Aust. 2000;172(12):578–582. doi: 10.5694/j.1326-5377.2000.tb124122.x. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Laskey J, Evans WK, Goss PE, Johansen E, Khamsi F. Cush-ing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol. 1992;10(1):21–27. doi: 10.1200/JCO.1992.10.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Levenson RM, Jr, Ihde DC, Matthews MJ, Cohen MH, Gazdar AF, Bunn PA, Jr, Minna JD. Small cell carcinoma presenting as an extrapulmonary neoplasm: sites of origin and response to chemotherapy. J Natl Cancer Inst. 1981;67(3):607–612. [PubMed] [Google Scholar]
- 6.Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240(1):117–122. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penezi? Z, Savi? S, Vujovi? S, Tati? S, Ercegovac M, Drezgi?c M. The ectopic ACTH syndrome. Srp Arh Celok Lek. 2004;132(1–2):28–32. doi: 10.2298/sarh0402028p. [DOI] [PubMed] [Google Scholar]
- 8.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79(4):813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Nieman LK, Ilias I. Evaluation and treatment of Cushing's syndrome. Am J Med. 2005;118(12):1340–1346. doi: 10.1016/j.amjmed.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 10.Miller JW, Crapo L. The medical treatment of Cushing's syndrome. Endocr Rev. 1993;14(4):443–458. doi: 10.1210/edrv-14-4-443. [DOI] [PubMed] [Google Scholar]
- 11.Díez JJ, Iglesias P. Pharmacological therapy of Cushing's syndrome: drugs and indications. Mini Rev Med Chem. 2007;7(5):467–480. doi: 10.2174/138955707780619653. [DOI] [PubMed] [Google Scholar]
- 12.Nieman LK. Medical therapy of Cushing's disease. Pituitary. 2002;5(2):77–82. doi: 10.1023/a:1022308429992. [DOI] [PubMed] [Google Scholar]
- 13.Findling JW, Raff H. Cushing's syndrome: important issues in diagnosis and management. J Clin Endocrinol Metab. 2006;91(10):3746–3753. doi: 10.1210/jc.2006-0997. [DOI] [PubMed] [Google Scholar]