Summary
A critical step in the SOS response of E. coli is the specific proteolytic cleavage of the LexA repressor. This reaction is catalyzed by an activated form of RecA, acting as a co-protease to stimulate the self-cleavage activity of LexA. This process has been re-examined in light of evidence that LexA is dimeric at physiological concentrations. We found that RecA-dependent cleavage was robust under conditions in which LexA is largely dimeric, and conclude that LexA dimers are cleavable. We also found that LexA dimers dissociate slowly. Furthermore, our evidence suggests that interactions between the two subunits of a LexA dimer can influence the rate of cleavage. Finally, our evidence suggests that RecA stimulates the transition of LexA from its non-cleavable to its cleavable conformation, and therefore operates, at least in part, by an allosteric mechanism.
Keywords: LexA, RecA, Dimer, SOS, specific cleavage
Introduction
The LexA repressor of E. coli regulates the cellular SOS response to DNA damage. SOS-inducing treatments activate another regulatory protein, RecA, which in turn inactivates LexA by a specific cleavage reaction, leading to de-repression of the SOS regulon.1 In vitro, RecA-mediated cleavage can be observed if RecA is activated by binding to single-stranded DNA and ATP.2 However, the action of RecA is indirect; LexA has a latent self-cleavage activity that can occur rapidly at high pH,3 but requires activated RecA to stimulate it under physiological conditions. Since the role of RecA in LexA cleavage is indirect, we call it a “co-protease”.4
LexA acts as a repressor in its dimeric form, but two studies of dimer stability have given very different results. Using analytical ultracentrifugation, Schnarr et al.5 measured a dimer dissociation constant of 50 μM, while a more recent study by Mohana-Borges et al.6 found Kdimer <1 nM by assaying pressure-dependent changes in LexA fluorescence. We do not understand the basis for this disparity. The concentration of LexA in vivo is ∼2 μM in cells without DNA damage, and it drops to ∼0.2 μM in cells with activated RecA.7 Therefore these contrasting estimates of Kdimer make very different predictions about the assembly state of cellular LexA.
The domain structure of LexA accounts for its loss of repressor function upon cleavage. LexA is made up of two domains, an N-terminal DNA binding domain and a C-terminal domain that provides both LexA dimerization and cleavage activity (Figure 1(c)). LexA cleavage occurs between Ala84 and Gly85,8 which lie in the cleavage site region (residues 79-95) within the catalytic C-terminal domain (CTD).9 Although cleavage does not destroy the DNA-binding domain, it separates it from the CTD, weakening DNA binding.
Figure 1.

(a) and (b) Conformations of LexA. The LexA crystal structures solved by Luo et al.9 of (a) full-length S119A mutant protein (PDB 1JHH), and (b) a truncated quadruple mutant (L89P, Q92W, E152A, and K156A) (PDB 1JHE). Residues beginning at aa 75 are shown. Figures were drawn using SwissPDB Viewer.53 Each dimer has one grey and one green monomer with its Cleavage Site Region (79-95) in orange and the cleavage site (84/85) in black. In (a) the green monomer shows the non-cleavable conformation (the other side is in the cleavable conformation). In (b) both monomers are in the cleavable conformation. The alpha carbons of selected residues are shown, and labeled with the wild-type amino acid; the active site in magenta (S119 and K156), location of IndS mutations in blue (L89, Q92, E152), and G124 at the dimer interface in cyan. (c) Schematic of LexA. The functional domains of LexA are shown: DNA-Binding Domain (residues 1-69), Cleavage Site Region (79-95), and the Catalytic Core (111-202).9 The LexA cleavage site, shown with a dotted line, is between Ala84 and Gly85. (d) Schematic of the LexA truncations used in this study, WT/ΔN and WT/PKA, contain residues 65-202 of LexA. The His6 tag used for purification, and the PKA tag for radiolabeling, are at the N termini. The phosphorylated serine is marked (*). A tryptic fragment similar to WT/ΔN undergoes self-cleavage and RecA-mediated cleavage at rates similar to those of full-length LexA.3 The truncation prevented LexA from binding the DNA used to activate RecA, simplifying interpretation of data.
Structures of three LexA mutant proteins revealed that the cleavage site region can adopt two conformations, while the rest of the CTD is essentially unchanged.9 As shown in Figure 1 (a) and (b), in the non-cleavable (NC) conformation the cleavage site is distant from the active site, whereas in the cleavable (C) conformation the cleavage site sits in the active site, positioned adjacent to the catalytic residues Ser119 and Lys156.10 In the C conformation, Lys156 is buried and in an environment where a positive charge is not likely to be stable.9 De-protonation of Lys156 appears to be required for cleavage,11 since autocleavage is efficient only at high pH.3 Roland et al.12 proposed that, in addition to de-protonation, LexA also undergoes a disfavored conformational change (L to L*). According to this model, only ∼0.01% of LexA molecules are in a cleavable, de-protonated L* state at pH 7, while the rest are in a non-cleavable conformation. The two conformations of LexA observed crystallographically, C and NC, appear to correspond with these biochemically detected states. Although it remains formally possible that L and L* are not identical to NC and C, respectively, there are no data to suggest they are different. Therefore in this paper we will treat these states as being the same.
It is not clear how RecA accelerates cleavage of LexA. RecA binding may have an allosteric effect on LexA that alters the relative stabilities of the NC and C forms, or that reduces the pKa of Lys156. However, the crystal structures of the LexA CTD in the NC and C forms are very similar except for the movement of the cleavage site region; Luo et al.9 suggest that this argues against an allosteric effect by RecA. Instead the authors postulate that RecA binds preferentially to the C conformation of LexA, stabilizing this form and promoting cleavage.9; 12 Identifying the RecA-binding site on LexA would likely provide insight into this mechanism, but its location is not known. Mutations that specifically impair RecA-dependent cleavage have not been identified in LexA; mutations of LexA that slow or block RecA-dependent cleavage have similar effects on autocleavage, suggesting that they are altering catalysis and are independent of the action of RecA.11; 13
The difficulty in isolating such mutants contrasts notably with the related λ CI repressor, where RecA-specific mutations have been identified.14; 15 Despite the fact that the catalytic mechanisms and structures of RecA substrates are similar,3; 9; 16-20 RecA may stimulate the cleavage of these proteins through different interactions. For example, some RecA mutants have distinct effects on the cleavage of LexA, CI, and another related protein, UmuD.21; 22 Because of these differences, it may not be possible to determine the RecA binding site of LexA with RecA-specific mutations, even though this was effective with CI. For this reason, we have taken a biochemical approach to these questions. We find that LexA dimers are efficiently cleaved. In addition, our evidence suggests that RecA can bind to the NC form of LexA, a finding that implies that RecA acts, at least in part, by an allosteric mechanism.
Results
Kinetic constants for RecA-mediated LexA cleavage
We developed an improved assay for RecA-mediated cleavage of LexA. We used a truncated form of LexA, termed WT/ΔN, which lacks most of the N-terminal DNA-binding domain, and another variant, WT/PKA, that can be radiolabeled by phosphorylation at a site for Protein Kinase A (PKA) (Figure 1(d)). These truncations cleaved at rates similar to that of full-length LexA; RecA-mediated cleavage was about twice as fast for the truncations compared to full-length LexA, and autocleavage at high pH was the same for all LexA proteins, as assayed using Coomassie-stained gels (Supplemental Figure 1). Phosphorylation of WT/PKA had no further effect on these activities. RecA was activated with a GT-rich oligo known to bind preferentially to RecA23 (see Methods and Materials). Using this assay, we determined cleavage rates by measuring the fraction of added protein cleaved with time, and found that the rate of cleavage was constant for more than 2 h at ≥ 20 nM RecA (Figure 2(a)).
Figure 2.
Kinetic analysis of RecA-dependent cleavage of WT/PKA. Radiolabeled WT/PKA was incubated with 20 nM activated RecA at 30°C, and sampled at appropriate intervals. Reactions were run on Tris-Tricine gels and analyzed as described in the Materials and Methods. (a) Representative data showing the fraction of WT/PKA cleaved with time for (from left to right) 4 (closed squares), 8 (open circles), 12 (diamonds), 16 (triangles), 24 (open squares), and 32 μM WT/PKA (closed circles). (b) Initial rates were determined by linear fits of time-points (typically four) within the first 30% of cleavage. Each data point shows the mean of a minimum of three independent samples. Error bars show the standard deviation of the data. The data fit a Michaelis-Menten curve of Vmax = 2.2 ± 0.1 LexA/RecA/min, and KM = 0.9 ± 0.1 μM WT/PKA. The right-hand panel shows an expansion of the data for low protein concentrations.
We first used this assay to measure kinetic constants for RecA-mediated LexA cleavage. Though it acts indirectly, RecA does acts as a catalyst, and hence this reaction can be considered an enzymatic reaction with RecA and LexA as enzyme and substrate, respectively. Cleavage rates of WT/PKA at 0.2 to 32 μM were measured in the presence of 20 nM activated RecA. At this low concentration of RecA, the data could be analyzed by Michaelis-Menten kinetics. The data were fit to a curve with Vmax = 2.2 ± 0.1 LexA/RecA/min and KM = 0.9 ± 0.1 μM (Figure 2(b)). The Vmax, measured at 30°C, was faster than the rate of 1.3 LexA/RecA/min determined for full-length LexA at 37°C.24 This difference was probably due to changes in the procedure for RecA activation as well as the difference in activities between full-length LexA and WT/PKA (Supplemental Figure 1(a)). The KM of the truncation for RecA, 0.9 μM, was comparable to the value of 0.5 μM determined for full-length LexA in the presence of 0.2 μM RecA,24 indicating that the truncation of LexA does not greatly alter the protein’s interaction with RecA.
Rapid cleavage of WT/PKA by excess RecA
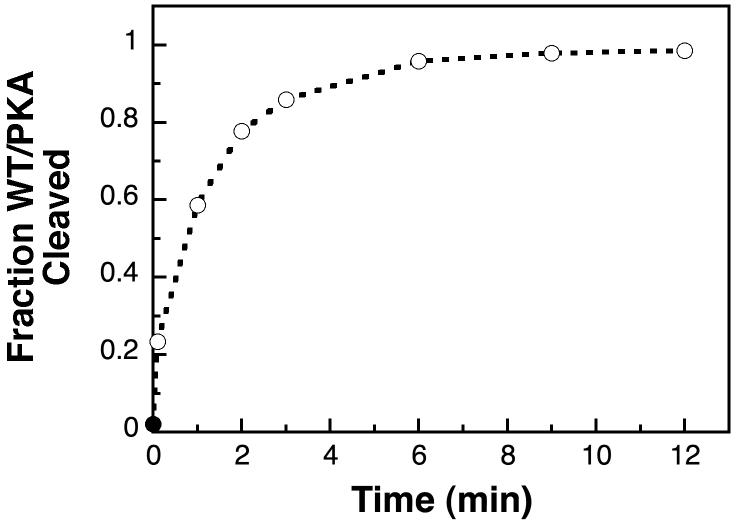
As described above, it is possible that LexA only binds RecA when it is in the C conformation. In order to test if, at any given time, there is a large fraction of LexA that cannot bind RecA, we measured the cleavage of WT/PKA in the presence of a two-fold molar excess of RecA. Addition of 1 μM RecA to 0.5 μM WT/PKA resulted in rapid cleavage of a significant fraction of the substrate (Figure 3); ∼20% of the WT/PKA was cleaved in the 6 seconds it took to start and stop the reaction. Within 3 min, 85% of the substrate was cleaved, and the reaction neared completion by 9 min. Very similar results were obtained when only 0.5 μM RecA was used (data not shown), indicating that at both concentrations there was sufficient RecA to bind the available LexA molecules. These data appear to contradict the model that RecA binds only the C conformation of LexA, but an alternative interpretation is presented in the Discussion.
Figure 3.
Rapid cleavage of WT/PKA with excess RecA. A representative time-course shows the cleavage of 0.5 μM WT/PKA in the presence of 1 μM activated RecA (open circles) at 30°C. The first time-point was stopped 6 s after adding RecA, and was 23% cleaved. Prior to adding RecA, the WT/PKA was 2% cleaved (solid circle).
Dimeric stability of LexA and truncations
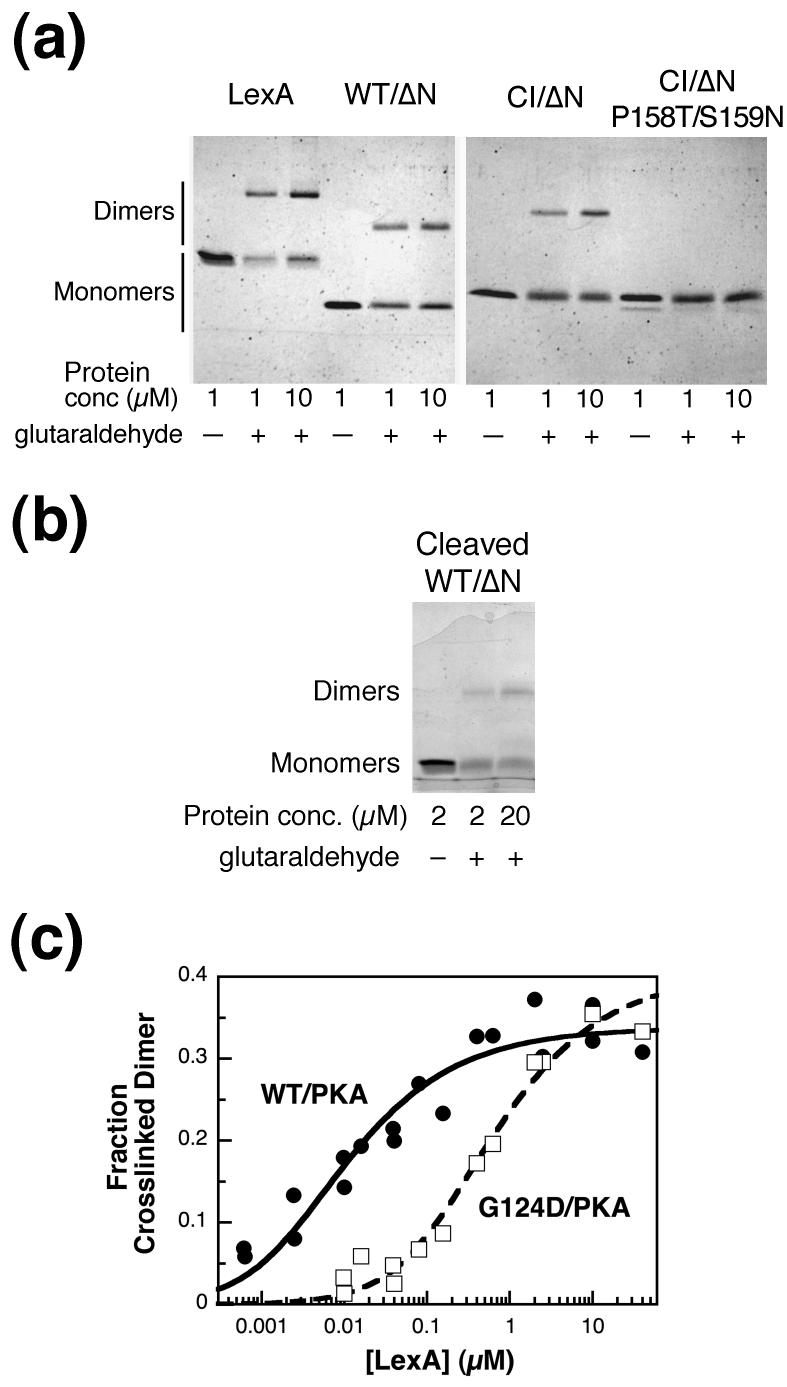
Recent data suggest that LexA is dimeric at lower concentrations than previously believed.6 To assess LexA dimerization under our cleavage reaction conditions, we developed a glutaraldehyde cross-linking assay and applied it to full-length and truncated LexA. Although the RecA-dependent cleavage buffer contained 20 mM Tris, which can also react with glutaraldehyde, Coomassie-stained gels demonstrated that cross-linked dimers of either full-length LexA or WT/ΔN formed at 1 and 10 μM (Figure 4(a)). To facilitate visual comparison, equal amounts of protein were loaded in each lane for a given protein.
Figure 4.
Cross-linking of LexA and truncations. (a) LexA, WT/ΔN, CI/ΔN, and CI/ΔN P158T/S159N at 1 and 10 μM were incubated at 30°C in the presence or absence of 8 mM glutaraldehyde for 10 min, as indicated. Equal molar amounts of each sample were run on 15% Laemmli gels and stained with Coomassie blue. The faint band under CI/ΔN P158T/S159N is cleaved protein, presumably resulting from autodigestion, that was present after the protein purification. (b) An experiment done as in part (a) was carried out with cleaved LexA WT/ΔN at 2 or 20 μM. A higher concentration was used than in part (a) because the C-terminal fragment, like full-length LexA, did not resolve well after cross-linking at lower concentrations. (c) Radiolabeled WT/PKA (closed circles) and G124D/PKA (open squares) were cross-linked as above at the concentrations indicated, and the amount of protein in the dimer band was quantified as described in Materials and Methods. Each data point represents one sample, and the data from multiple experiments have been combined. Data were fit to curves for a monomer-dimer equilibrium to calculate the KD and the maximum cross-linked dimer (max), where L is the concentration of the LexA truncation. Fraction Cross-linked Dimer Observed=(max)*(4L+ KD- (KD2+ 8L* KD)1/2)/4L, where L is the concentration of the LexA truncation. KD= 10 ± 3 nM, max= 0.34 ± 0.01 for WT/PKA and KD= 0.6 ± 0.1 μM, max= 0.40 ± 0.02 for G124D/PKA.
We verified that this assay is responsive to the oligomeric state of the protein using the closely related λ CI repressor, for which dimerization mutants are available.25 We used a truncated form of CI that lacks the N-terminal DNA-binding domain. While full-length CI has a Kdimer of ∼10 nM,26 this truncated form is expected to have weaker dimerization because it lacks an α-helix, helix 5 of the N-terminal domain, which contributes to the stability of CI dimers.25; 27 CI/ΔN formed cross-linked dimers, but the proportion was smaller at 1 μM than at 10 μM (Figure 4(a)) indicating the concentration-dependence of dimerization in this range. Furthermore, a CI/ΔN variant carrying two mutations (P158T and S159N) that further decrease the dimeric stability of CI25; 28 was not cross-linked as dimers at either 1 or 10 μM. Based on these results we conclude that the glutaraldehyde cross-linking assay is responsive to the dimerization state of the protein.
In contrast, the fraction of cross-linked dimers of WT/ΔN appeared the same at 1 and 10 μM (Figure 4(a)). With full-length LexA, some protein was lost at the lower concentration during the cross-linking, but the ratio of the monomeric and dimeric bands was also constant at 1 and 10 μM. The constant fraction of dimer observed at these and higher concentrations (data not shown), suggests that both proteins were nearly completely dimeric at and above 1 μM (also see below). Thus the large amount of monomer observed after cross-linking does not appear to indicate that there is a large population of free monomers in solution, but rather to reflect a limit in the extent to which cross-links between monomers within a dimer can occur (see Materials and Methods).
To quantify dimerization, cross-linking was performed on radiolabeled WT/PKA over a range of concentrations from 0.6 nM to 40 μM. At and above 0.5 μM WT/PKA, one-third of the protein cross-linked as dimers (Figure 4(c)). At lower concentrations the fraction of protein in the dimer band decreased, further affirming the ability of the assay to detect concentration-dependent changes in assembly. Assuming that WT/PKA was fully dimeric when the maximum fraction of dimers was observed, Kdimer was estimated to be about 10 nM. However, this estimate is only approximate, since cross-linking is not an equilibrium method. In any case, as predicted from the data of Mohana-Borges et al.,6 WT/PKA appears to be predominantly dimeric at ≥ 0.2 μM.
To further verify that this assay is responsive to the dimerization state of LexA, we created a dimerization mutant, using the structure as a guide. Gly124, a residue at the LexA dimer interface,9 was changed to charged Asp, making G124D/PKA. We expected dimerization to be weakened, since the change places negative charges opposite one another in the dimer interface (Figure 1(a)). Dimerization of radiolabeled G124D/PKA was assessed by cross-linking over a range of concentrations (Figure 4(c)). These data indicated that the mutation significantly impaired dimeric stability. Although the data with the mutant protein did not reach a plateau at the highest concentration tested, a fit to the monomer-dimer equation gave a Kdimer of ∼0.6 μM, roughly 50 times weaker than WT. The behavior of the mutation supports our conclusion that WT/PKA is largely dimeric at ≥ 0.1 μM.
Effect of dimerization on RecA-dependent cleavage
Since LexA is largely dimeric at cellular concentrations (0.2 to 2 μM),7 we asked whether dimerization affects RecA-mediated cleavage. It is known that dimerization has different effects on RecA-mediated cleavage of various substrates. While λ CI can only cleave when it is monomeric,25; 29; 30 cleavage of the related 434 repressor is more efficient for dimers than monomers.31 UmuD dimers also undergo cleavage, and this cleavage can be intermolecular—that is, one subunit of UmuD can be cleaved by the active site of the other subunit.32
Two lines of evidence, already shown, strongly suggest that LexA dimers are efficient substrates for RecA-mediated cleavage. At 0.5 μM WT/PKA, where we estimate that the protein was ∼90% dimeric (Figure 4(c)), RecA-dependent cleavage was rapid (Figure 3). This probably did not result from rapid dissociation to monomers, since we find that equilibration of dimers is a slow process (see below). Second, no inhibition of cleavage was seen at higher concentrations of WT/PKA (Figure 2(b)). We conclude that RecA stimulates the cleavage of LexA dimers.
In order to test whether LexA monomers can also be cleaved, we compared RecA-dependent cleavage rates of the WT/PKA and G124D/PKA labeled proteins at concentrations where the fraction of dimers was different (data not shown). At and above 1 μM, G124D/PKA was cleaved 25% more slowly than WT/PKA, suggesting that the mutation had a small effect on cleavage that was independent of its effect on dimerization.
If LexA monomers cannot cleave, then we would predict that the G124D/PKA mutant protein would be cleaved at far slower rates at low concentrations. We found that it was cleaved about 3-fold more slowly than WT/PKA at a concentration of 40 nM. These data suggest the possibility that monomers can undergo RecA-mediated cleavage, although perhaps at a slower rate than dimers. However, enough uncertainty exists in the estimates of Kdimer that these data cannot be interpreted unambiguously, and the issue of monomer cleavage requires further study.
Slow equilibration of LexA dimers
Two lines of evidence strongly suggest that equilibration among LexA monomers and dimers is a slow process. Both are based on experiments in which cross-linking was allowed to occur for only 30 sec., and using forms of LexA with different gel mobilities, so that heterodimers could be resolved from homodimers. First, heterodimers between cleaved and intact LexA formed in 5-10 min. When cross-linking was performed on a mixture of 5 μM of each full-length LexA and autodigested WT/ΔN, the heterodimer (L-X in Figure 5(a)) was detected, but it took more than 5 min at 30°C for the proportion of heterodimers to reach a constant value. Similar results were obtained for heterodimers of WT/ΔN and the cleaved protein (Figure 5(b)), suggesting that LexA and WT/ΔN interact with the cleavage fragment similarly.
Figure 5.
Slow formation of LexA heterodimers. (a) LexA and the cleavage fragment (X) from WT/ΔN; (b) WT/ΔN and the cleavage fragment (X) from WT/ΔN; (c) LexA and WT/ΔN. In each case, proteins were mixed together (at final concentrations 5 μM each) and incubated at 30°C. At the indicated times (in min) aliquots were mixed with glutaraldehyde to 16 mM. After 30 s, reactions were stopped with glycine to 160 mM. Samples were run on 15% Laemmli gels and stained with Coomassie blue. Bands are labeled: L, full-length LexA monomer; X, cleaved WT/ΔN monomer; and Δ, WT/ΔN monomer. Dimers are labeled by combinations of these labels; e.g., L-Δ, LexA-WT/ΔN heterodimer. In (d), a representative experiment is shown when 0.5 μM LexA and 0.5 μM labeled WT/PKA were cross-linked as above. The counts in the heterodimer band were compared to the sum of the counts in the homodimer and heterodimer bands. Data varied by 10-20% between experiments. Within 2 h, 50 ± 8% of the labeled protein was in the heterodimer.
Second, intact LexA heterodimerized even more slowly than the cleavage fragment. When 5 μM full-length LexA and WT/ΔN were mixed, the formation of the heterodimer (L-Δ in Figure 5(c)) took more than 10 min to reach equilibrium. We quantified the rate of this interaction using 0.5 μM each full-length LexA and labeled WT/PKA (Figure 5(d)). Within the first 30 s, nearly 10% of the protein rapidly formed heterodimers. This was followed by slower association, with a half-time of 10-15 min. At equilibrium, 50% of the labeled WT/PKA that was in dimers was heterodimerized with LexA. Because the full-length LexA and WT/PKA appear to behave similarly in dimer and cross-linking studies (Figures 4 and 5), these data suggest that dimers of full-length LexA dissociate and re-associate slowly, on a time scale of tens of minutes.
As just described, mixtures containing the C-terminal cleavage fragment (X) appeared to equilibrate more rapidly than the mixture of WT/PKA and full-length LexA (Figure 5(a-c)). This suggests that the C-terminal fragments might dimerize less well than its uncleaved precursor—that is, they might dissociate faster, making monomers more readily available for heterodimer formation. In cross-linking experiments with the C-terminal fragment, a higher proportion of cross-linked dimer was observed when cross-linking occurred at 20 μM than at 2 μM (Figure 4(b)), consistent with weak dimerization by this cleavage product.
Stoichiometry of LexA: RecA interaction
We used the quantitative cleavage assay to address another important question about RecA-mediated LexA cleavage. Activated RecA is not a single molecule, but a ternary complex of RecA, ssDNA and ATP (or ATP-γ-S) in a helical filament.33 Analysis of electron microscope images suggests that LexA binds in a deep helical groove in this filament, and that the stoichiometry of this interaction is substantially less than 1 LexA per RecA.34 Although the interpretation of these data is complicated by our finding that LexA dimers cleave, implying that LexA binds to RecA as a dimer (see Discussion), they strongly suggest that the simple picture of one LexA binding site per RecA subunit is not correct.
As an independent approach to measuring the stoichiometry, we used the cleavage assay, together with a non-cleavable mutant form of LexA. Non-cleavable LexA mutants can competitively inhibit RecA-dependent cleavage of WT LexA,35 providing an indirect assay for RecA binding. We reasoned that we could calculate the number of LexA-binding sites on RecA by measuring the stoichiometry of inhibition by an especially effective inhibitor. K156A is a mutation of the catalytic residue Lys156 which blocks cleavage, and the full-length mutant protein inhibits WT LexA cleavage strongly even at low concentrations.10; 11
We found that the truncated form of K156A, K156A/ΔN, was also an excellent inhibitor of RecA-dependent cleavage. The cleavage of 1 μM WT/PKA in the presence of 20 nM RecA and 20 nM K156A/ΔN was reduced to 25 ± 9% of the uninhibited rate (data not shown). Such strong inhibition at this low concentration demonstrates that K156A/ΔN binds RecA much more tightly than WT/PKA, which has a KM of ∼1 μM (Figure 2(b)). We have interpreted this enhanced affinity to mean that K156A is in a state, perhaps the C conformation, that interacts well with RecA. The C conformation could be favored in K156A if energy does not have to be expended to bury the charged side-chain of Lys156.9
The number of LexA binding sites in RecA was estimated by determining the inhibition of WT/PKA cleavage with increasing concentrations of K156A/ΔN. A similar approach has been used for CI.20 We were concerned that only one monomer in a K156A/ΔN dimer might be able to bind RecA at one time (see Discussion), which would cause an over-estimate of the number of binding sites. To obviate this concern, we used an excess of WT/PKA to ensure that nearly all the K156A/ΔN subunits were present in heterodimers. K156A/ΔN at 10 - 60 nM was pre-incubated with 5 μM WT/PKA for 1 h at 30°C before adding 0.1 μM RecA. At 10 nM K156A/ΔN, the lowest concentration tested, the rate of cleavage of the labeled substrate dropped by 17 ± 1% relative to an uninhibited reaction (Supplemental Figure 2). These data suggest that under our conditions 100 nM RecA provided no more than 60 ± 10 nM LexA binding sites, and probably significantly fewer (see Discussion).
Poor inhibition by a quadruple mutant of LexA
Although Figure 3 appears to support a model that RecA can interact with LexA in the NC as well as the C conformation, it remains possible that RecA binds more tightly to the C conformation. This model would predict that mutations of LexA that stabilize the C form should enhance RecA binding, and is consistent with the strong inhibition by K156A/ΔN.
Of the three mutant LexA proteins whose structures were solved, only the so-called quadruple mutant (QM) exclusively occupied the C conformation.9 QM contains K156A and three additional mutations, L89P, Q92W, and E152A. These additional mutations, called IndS, each stimulate autocleavage of LexA in the absence of K156A,12; 36 an effect proposed to result from stabilizing the C conformation of the flexible cleavage site region.9 Thus one might predict that the QM mutant would be an even more efficient inhibitor than K156A. Unexpectedly, QM is much less inhibitory than is K156A (Y. Luo and N. C. J. Strynadka, personal communication). This observation suggested that at least one of the IndS mutations disrupts the RecA binding site on LexA, which would be the first such mutation identified in LexA.
We quantified the ability of K156A/ΔN and QM/ΔN, a truncation carrying the four mutations, to inhibit RecA-dependent cleavage of WT/PKA. The cleavage of 1 μM WT/PKA with 20 nM RecA was inhibited by 93% in the presence of 0.1 μM K156A/ΔN, but only 15% by the same amount of QM/ΔN (Table I). Again, the truncated forms of LexA provided effects comparable to those of the full-length proteins.
Table I.
Inhibition of WT/PKA cleavage by QM/ΔN and other variants
| Inhibitor | Mutations | Inhibition (%)a |
|---|---|---|
| K156A/ΔN | K156A | 93 ± 1 |
| QM/ΔN | L89P, Q92W, E152A, K156A | 15 ± 1 |
| QM-89/ΔN | Q92W, E152A, K156A | 36 ± 1 |
| QM-92/ΔN | L89P, E152A, K156A | 83 ± 1 |
| QM-152/ΔN | L89P, Q92W, K156A | 43 ± 3 |
| K156A/Q92W/ΔN | Q92W, K156A | 79 ± 2 |
| S119A/ΔN | S119A | 41 ± 4 |
| S119A/Q92W/ΔN | Q92W, S119A | 42 ± 1 |
The cleavage rate of 1 μM WT/PKA at 30°C was measured in the presence and absence of 0.1 μM of the indicated non-cleavable truncations. Heterodimers of LexA truncations were allowed to form for 1 h at 30°C before initiating the reactions with 20 nM RecA. The percent inhibition of WT/PKA was calculated from the initial rates of samples without (U) and with (I) inhibitor: 100*(U-I)/U. The averages of two independent experiments with standard deviations are provided.
To test if one of three IndS mutations in QM impairs its ability to bind RecA, we created versions of QM/ΔN in which L89P, Q92W, or E152A was restored to its wild-type counterpart. These proteins are named for the reverted residue; for instance, QM-89/ΔN is wild type at residue 89. Reversion of residue 89 or 152 had relatively small effects on the inhibition of QM (Table I); both QM-89/ΔN and QM-152/ΔN inhibited WT/PKA by roughly 40%. QM-92/ΔN, in which residue 92 was the WT Gln, inhibited by 83%, an effect more comparable to K156A/ΔN than the other triple mutants.
Although this finding appears to indicate that Gln92 is an essential contact for RecA, we found that the double mutant K156A/Q92W/ΔN protein was a relatively strong inhibitor (Table I). This pattern was also seen with truncations bearing S119A, which also affects a catalytic residue and blocks LexA cleavage, in place of K156A. S119A protein is not as strong an inhibitor as K156A,10 and both S119A/Q92W/ΔN and S119A/ΔN provided ∼40% inhibiti on (Table I). Therefore we conclude that Q92W, like the other IndS mutations in QM, does not directly block RecA binding. Instead, it appears that the combination of Q92W with either or both L89P and E152A had a negatively synergistic effect on the ability of LexA to bind RecA. In the context of QM, each of the IndS mutations had at least a small effect on inhibition, which could indicate that they alter a RecA binding site. Nevertheless it is clear that RecA can bind to LexA proteins that carry any one of these IndS mutations.
A second mechanism for K156A inhibition of RecA-dependent cleavage
In some inhibition experiments with K156A/ΔN, we observed that substrate cleavage became slower over time. This non-linearity was not an issue in the data described above, because progressively slower cleavage was not observed when WT/PKA and K156A/ΔN were pre-incubated. As described below, the increasing degree of inhibition appears to result from a slow interaction between WT/PKA and K156A/ΔN that is likely to be the formation of heterodimers.
The change in inhibition over time is clearly seen in an order-of-addition experiment (Figure 6). Using equimolar amounts of WT/PKA, K156A/ΔN, and RecA (0.5 μM), two of the three proteins were pre-incubated together for 60 min at 30°C before the final protein, either WT/PKA or RecA, was added to initiate cleavage. In Reaction 1 (open circles) the WT and K156A LexA truncations were pre-incubated to allow heterodimer formation. An early burst of substrate cleavage occurred, which was complete within ∼2 min; we infer that some RecA molecules initially bound to and catalyzed the cleavage of WT substrate before they bound K156A/ΔN. After the burst, cleavage occurred at a constant rate of 0.008 LexA/RecA/min. The simple expectation would be that in Reaction 2, in which WT/PKA was added to pre-incubated RecA and K156A/ΔN, there would be no burst, since RecA would be saturated with K156A/ΔN, and that cleavage would proceed at 0.008 LexA/RecA/min from the start (see solid line in Figure 6). Instead, the initial cleavage rate of Reaction 2 (closed squares) was 0.022 LexA/RecA/min, nearly three times faster than the constant rate observed in Reaction 1. After 15 min the rate of substrate cleavage in Reaction 2 gradually decreased from its initial rate to ∼0.01 LexA/RecA/min. This decrease occurred well before 30% of the substrate had been cleaved, and so was not due to the progressively decreasing concentration of substrate. In contrast, the rate of Reaction 1 remained constant beyond 25 min, when > 30% of the substrate was cleaved.
Figure 6.
LexA-modifying inhibition of WT/PKA cleavage. Representative cleavage reactions of 0.5 μM WT/PKA, K156A, and RecA are shown. Samples were pre-incubated at 30°C for 1 h before starting reactions by adding RecA (Reaction #1, open circles) or WT/PKA (Reaction #2, closed squares). Selected portions of the data (4.5-25 min for Reaction 1, and 0-6 min for Reaction 2) were fit to straight lines that correspond to rates of 0.008 and 0.022 LexA/RecA/min for Reactions 1 and 2 respectively. The solid line represents the expected kinetics of Reaction 2, based on the cleavage rate of Reaction 1 (see text).
These data demonstrate that the ability of K156A/ΔN to inhibit the cleavage of WT/PKA was affected by differences in the pre-incubation conditions. After pre-incubating WT/PKA and K156A/ΔN together, which allowed heterodimers to form, steady-state cleavage in Reaction 1 was inhibited by an additional ∼3-fold compared to the initial rate of cleavage in Reaction 2. In Reaction 2, inhibition gradually increased after adding WT/PKA. The rate of cleavage in Reaction 2 approached that of Reaction 1, although it remained slightly faster throughout the 30 min reaction. Because this biphasic behavior was not observed in the absence of K156A/ΔN, the data indicate that a slow interaction between WT/PKA and K156A/ΔN caused the three-fold difference in rates. Because the time-scale of this effect resembles that of heterodimer formation, we hypothesize that the rate change in Reaction 2 was the result of heterodimer formation between WT/PKA and K156A/ΔN, and call this effect “LexA-modifying inhibition”.
We cannot use the cross-linking assay to measure heterodimer formation between the WT and K156A truncations used in Figure 6, because these heterodimers do not resolve differently from homodimers on Laemmli gels. We have compared the rates and extents of heterodimer formation by the WT and K156A truncations with full-length LexA by Coomassie staining of cross-linked products, and have not detected any differences (data not shown). Therefore it is unlikely that the K156A mutation has a significant effect on dimer stability. Furthermore, there do not seem to be differences in heterodimer formation by full-length and truncated LexA, since heterodimer formation between cleaved LexA and either full-length LexA or WT/ΔN occurred at similar rates (Figure 5(a) and (b)). Heterodimer formation between 0.5 μM full-length LexA and WT/PKA was approximately half complete at 10 min (Figure 5(d)), a time scale similar to that on which cleavage slowed in Reaction 2 (Figure 6). Thus the formation of heterodimers between WT/PKA and K156A/ΔN probably coincided with the increase in LexA-modifying inhibition.
If heterodimer formation is inhibitory to WT/PKA cleavage, then the difference in the rates between reactions with fully equilibrated heterodimers and those initiated by adding WT/PKA should be affected by the fraction of substrate in heterodimers. Half of the WT/PKA is expected to be in heterodimers at equilibrium when equal amounts of WT/PKA and K156A/ΔN are present, based on the cross-linking of full-length and truncated LexA under similar conditions (Figure 5(d)). While keeping K156A/ΔN at 0.5 μM, the concentration of WT/PKA was increased in order to lower the fraction of the labeled substrate in heterodimers. Consistent with an inhibitory effect by heterodimers, the rates of the reactions initiated with either RecA or WT/PKA were more similar to each other under these conditions than in Figure 6 (data not shown). However, when substrate dimerization with K156A/ΔN was maximized by using low ratios of WT/PKA:K156A/ΔN, we did not observe a significant increase above the three-fold difference shown in Figure 6 (data not shown). Thus the LexA-modifying inhibition observed in Figure 6 appears to have been nearly maximal at equal amounts of WT/PKA and K156A/ΔN.
Discussion
We have re-examined RecA dependent cleavage of LexA in light of new evidence that LexA is a dimer at physiologically relevant concentrations. We discuss the cleavage of LexA dimers and the stoichiometry of the LexA: RecA interaction, then turn to possible mechanisms for LexA-modifying inhibition, and finally consider the form of LexA to which RecA binds.
Cleavage of LexA Dimers
Our evidence clearly indicates that dimers of LexA can undergo efficient RecA-mediated cleavage. We have shown that under cleavage conditions WT/PKA has a Kdimer on the order of 10 nM (Figure 4(b)). RecA-dependent cleavage of WT/PKA occurred well at > 1 μM, where the protein was almost entirely dimeric, and without any indication of inhibition at high concentrations (Figure 2(b)). Furthermore, in the presence of excess RecA, 0.5 μM WT/PKA cleaved rapidly (Figure 3) when we estimate that it was 90% dimeric (Figure 4(c)). Rapid dissociation of dimers cannot account for this rapid rate, because the formation of heterodimers between intact and cleaved WT/ΔN was relatively slow (Figure 5(b)). This experiment did not directly measure dissociation, but, given the constraints of the dissociation constant, the observed rate of heterodimer formation is not consistent with such rapid dissociation (data not shown). We conclude that LexA dimers undergo RecA-dependent cleavage efficiently, unlike dimers of λ CI.
The structures of the catalytic domains of LexA and CI9; 20 do not provide obvious clues to account for the different effects of dimerization on RecA-mediated cleavage, but the data reinforce previous conclusions that RecA interacts differently with its various substrates. Furthermore, unlike CI, LexA also undergoes autocleavage as dimers. At pH 10.4, where LexA autocleavage is efficient, non-cleavable variants S119A/ΔN and K156A/ΔN cross-linked equally well at 1 and 64 μM (data not shown), strongly suggesting that the dimer dissociation constant is < 1 μM at high pH. Since the rate constant for WT LexA autodigestion is invariant over the concentration range of 1 nM to 10 μM,16 this finding implies that autodigestion occurs efficiently in LexA dimers. In contrast, autodigestion of CI is inhibited by dimerization.16
Our data provide insight into the oligomeric state of LexA in vivo. The cellular concentration of LexA is ∼2 μM, dropping to ∼0.2 μM after DNA damage.7 At 2 μM, almost all the LexA would be dimeric. In the absence of DNA damage, only ∼20% of the LexA is free in solution, as judged by minicell experiments;7 presumably the bulk of it is bound non-specifically to DNA. While the effect of non-specific DNA binding on LexA cleavage is not known, there will still be sufficient LexA in solution for that protein to be dimeric. Moreover, our evidence (Figure 5) that dimers dissociate slowly indicates that pre-existing dimers will not be converted rapidly to monomers in vivo as cleavage progresses. Newly-made LexA, a source of monomers, is rapidly degraded in vivo.37 Although our data do not indicate conclusively whether monomers can be cleaved, the in vivo data suggest that the cell does not contain a large cleavage-resistant pool.37 In sum, we do not believe that our findings significantly alter our view of the SOS regulatory circuit.
The finding that LexA dimers are substrates for RecA-mediated cleavage does, however, complicate our view of the interaction between these two proteins because it is not clear if both subunits of a LexA dimer can interact with RecA simultaneously. Activated RecA is a helical filament polymerized on ssDNA, with six monomers per turn.38 Thus the 18-nucleotide ssDNA used to activate RecA in this work should bind six molecules of RecA,39 forming a RecA hexamer comprising about one turn of the filament. LexA, by contrast, has two-fold rotational symmetry about an axis through its dimerization interface.9 For both subunits of a LexA dimer to make the same interactions with two binding sites on RecA, the latter sites would have to be related by two-fold rotational symmetry as well, which is not compatible with the symmetry relationships in the RecA. Hence, it is very unlikely that both subunits of a LexA dimer can interact with two binding sites on the same RecA hexamer. Although it is in principle possible that, at high concentrations of RecA, a second RecA hexamer could contact the other LexA subunit in a LexA dimer, it seems unlikely for steric reasons that most LexA dimers could bind two RecA complexes simultaneously. The consequence of these mismatches in symmetry is that when LexA is predominantly dimeric the concentration of LexA that can interact with RecA may be as little as half of the total concentration of LexA.
Stoichiometry of LexA: RecA interaction
In the design of our experiment to measure the stoichiometry of the LexA: RecA interaction, we used a large ratio of substrate: inhibitor so that nearly all the tightly-binding inhibitor, K156A/ΔN, would be in the form of heterodimers with WT/PKA (Supplemental Figure 2). From the data, we estimate that at most there were 0.6 LexA binding sites per RecA molecule. However, uncertainties about the actual amount of K156A/ΔN and RecA present, either due to errors in measuring the concentrations, or the presence of a significant fraction of inactive protein in our preparations, make the precision of this estimate uncertain. Furthermore, competition for RecA binding due to the large excess of WT/PKA over K156A/ΔN is likely to have caused an over-estimate of the number of binding sites on RecA, despite the relatively high affinity with which K156A/ΔN binds compared to WT/PKA.10
The stoichiometry of the LexA: RecA interaction has been measured using different approaches. Yu and Egelman34 used electron microscopy to examine RecA filaments with and without LexA, and concluded that the ratio is about 0.4 LexA: RecA. In their analysis the authors assumed that LexA was binding as a monomer, as was believed at the time.5 If LexA is binding as a dimer, this ratio would be reduced. However, this effect might be counteracted to some extent by the flexibility between the two domains of LexA, and between the two monomers; flexibility might weaken the electron density contributed by LexA to the reconstructed image, making it is difficult to re-evaluate these data in light of our work. The stoichiometry between λ CI and RecA was determined using the same approach as used here,20 but this analysis was free of the complications caused by dimerization, since mutant CI proteins that dimerize poorly were used. The authors determined that the stoichiometry is ∼0.5 CI binding sites per RecA.
If, as all these data imply, the stoichiometry is in the range of ∼0.5 LexA: RecA, why might this be the case? A reasonable possibility, suggested by Yu and Egelman,34 is that LexA cannot bind to a site on RecA adjacent to another site that is occupied by a LexA molecule. This could reflect steric blockage or allosteric effects on RecA subunits adjacent to a bound LexA. Clearly, the molecular basis of the sub-molar stoichiometry requires further investigation.
The number of RecA binding sites affects our interpretation of the LexA cleavage rate. We measured a Vmax of 2.2 LexA/RecA/min (Figure 2(b)), but if there are only ∼0.5 active sites per molecule of RecA, the actual turnover number is probably at least 4/min or ∼0.07/s. This is in good agreement with the estimate of the maximum cleavage rate of 0.06/s by Roland et al.12 that was determined from the autocleavage of full-length LexA carrying the IndS mutation Q92W. The authors suggested that RecA acts to bring LexA into its cleavable state, which involves both a conformational change and de-protonation of Lys156. However that experiment was performed at 37°C, as opposed to the 30°C used here; perhaps deletion of the N-terminal domain has slightly altered one of these events and so increased the rate of cleavage.
LexA-modifying inhibition
In addition to competitive inhibition caused by K156A/ΔN bound to RecA, we observed a second form of inhibition, which we suggest depends on heterodimer formation and occurs through effects of the K156A/ΔN subunit on the WT/PKA subunit. LexA-modifying inhibition had an additional three-fold effect on the inhibition of RecA-dependent cleavage of WT/PKA. We discuss two possible mechanisms for this effect. In the first, a mechanism that we shall term “trans-binding”, the cleavage site from one subunit of a dimer occupies the active site of the other subunit. As noted by Luo et al.,9 relatively modest rearrangements of the loop region between Gln99 and Asp110 might allow an exchange of the cleavage site regions across the dimer. Some forms of trans-binding would lead to cleavage in trans, which we would be able to detect. We tested for RecA-dependent trans-cleavage of LexA in various ways (Supplemental Figure 3) and found no evidence that it occurs.
A second possibility is that LexA-modifying inhibition results from allosteric effects by K156A/ΔN on WT/PKA. In this view, there could be communication between the two subunits in a LexA dimer, so that when one subunit is in the C conformation the other subunit is driven somewhat towards the NC conformation. Because K156A is expected to stabilize the C conformation, cleavage of a WT molecule in a heterodimer might be inhibited by this mechanism. In support of this model, in the crystal structure of the S119A dimer, one subunit is in each conformation.9 In the structure of the QM variant, both subunits are in the C form,9 but the three IndS mutations in QM might favor the C form enough to overcome the modest effect we are proposing. We looked for effects of IndS mutations on the LexA-modifying inhibition of K156A (Supplemental Figure 4), to test if mutations that stabilize the C conformation of LexA increased the effect of WT/PKA in a heterodimer. The data were inconsistent with this model.
Other allosteric mechanisms remain possible. For example, in the heterodimer the catalytic efficiency of the WT active site may be three-fold less active. While the mechanism underlying LexA-modifying inhibition is puzzling, these findings may indicate that interactions between LexA monomers in the dimer affect function in a way that has not been recognized previously.
Form of LexA that binds to RecA
Luo et al.9 suggested that RecA may preferentially bind to and stabilize the C form. Based on the calculations of Roland et al.,12 fewer than one in 4000 LexA molecules is in the cleavable conformation at the pH 7.4 used for RecA-dependent cleavage. Thus, if RecA only recognizes LexA when it is in the C form, it should only bind and stimulate the cleavage of a tiny fraction of LexA. We found that, in the presence of excess RecA, 20% of the WT/PKA was cleaved in 6 s (Figure 3). This was ∼1000-times more LexA than was predicted to be in the cleavable conformation, and suggested that RecA did not bind the C conformation exclusively. Given the estimate of ∼0.5 RecA binding sites/molecule, we calculate that the rate of association would have to have been at least 3 × 108 M-1 s-1 if only the C conformation binds RecA. This calculation assumes that interconversion between the NC and C forms occurs on a time scale of msec or faster, so as to replenish the C form as it binds to RecA; if interconversion is slower, the rate of association would have to be even faster. As the calculated rate is about at the diffusion-controlled limit,40 we suggest that RecA does not bind exclusively to the C conformation of LexA. Instead, we propose that it can bind to both the NC and C forms. It is also possible that other LexA conformations exist in solution to which RecA can bind.
If RecA can bind to the NC form, we further suggest that RecA stimulates cleavage at least in part via an allosteric effect on the conformation of LexA. Although the structures of the C-terminal core domain (residues 109-202) of LexA in the two conformations are similar,9 including the active site, subtle changes might not be detected by X-ray crystallography. RecA could also cause additional changes not present in the crystal structure of the C form. The RecA binding site on LexA has not yet been identified; it may be close to, or distant from, the active site. If the LexA cleavage site region can move from the NC to C conformation after RecA binds, RecA binding must not block its access to the active site. This issue is made more complicated by recent findings that LexA and CI may have more than one binding site on RecA (C. E. Bell and E. H. Egelman, personal communication); this raises the possibility that the form of LexA that binds to one site on RecA is not the same as the form that binds the second.
How does the observation that the QM/ΔN protein, which is in the C conformation,9; 41 binds RecA poorly bear on this question? The K156A mutant protein is the strongest inhibitor of LexA cleavage known, but the effects of several IndS mutations on the affinity of K156A/ΔN were complex (Table I). We suggest that their effects on RecA binding may result from one or more of the following differences between these mutant proteins and K156A/ΔN alone. First, each of the IndS mutations increases the rate of autodigestion,36 an effect suggested, at least for Q92W,12 to result from stabilizing the C form. Perhaps increasing the stability of the C form in solution decreases the affinity for RecA. This model assumes that the K156A mutant protein is not itself predominantly in the C form. Second, as these mutations change the surface of LexA in a localized region,9 this region may be part of the RecA-binding site on LexA, and the mutations could weaken this interaction directly. Finally, it is possible that a segment of the cleavage site region, residues 87-94, is flexible in K156A (and in WT LexA) and less so in QM, and that this flexibility is somehow important for RecA-binding. This region was fully resolved in the QM structure (Figure 1(b)), while in the S119A structure, with one subunit each in NC and C conformations, the electron density for residues 88-93 was missing in the C form.9 Perhaps the IndS mutations inhibit flexibility of this region. The proline in L89P should restrict flexibility; Q92W creates an interaction, not possible with WT,9 that might decrease flexibility by anchoring this region at that point. It is unclear, however, how changing flexibility would affect RecA binding. More work is necessary to resolve this question.
In conclusion, the complexity of RecA has been appreciated for many years. Our data suggest that the LexA partner in the LexA: RecA interaction is also substantially more complex than has been assumed. Taken together with recent evidence for multiple binding sites on RecA for CI and LexA (C. E. Bell and E. H. Egelman, personal communication), our findings also imply that the role of RecA may also be even more complicated than currently thought. Our data suggest that RecA can bind to LexA in the non-cleavable conformation. In turn, this implies that RecA has an allosteric effect on the conformation of LexA, perhaps through either direct or indirect interactions of RecA with the cleavage site region. Finally, our data suggest that communication between LexA subunits in a dimer can affect the rate of cleavage.
Materials and Methods
Plasmid construction
PCR-based mutagenesis was performed as follows. For point mutations, pairs of mutagenic oligos were designed so that they annealed to opposite strands of a plasmid template, with 5′ ends at adjacent nucleotides. To cause deletions the oligos annealed just outside the deleted segment of the gene. Insertions were created with extra bases on the oligos, 5′ of their annealing sequences. After phosphorylation with polynucleotide kinase, oligos were used in PCR with Pfu Turbo (Stratagene), resulting in linear molecules that had incorporated the oligo-based mutations. These were then circularized by ligation of their blunt ends with T4 DNA ligase (NEB), followed by transformation into E. coli strain DH5α.
Plasmids for overproduction of all proteins used in this study carried operon fusions of the T7 promoter to the protein of interest. The plasmid pJWL920 was used for expression of WT/ΔN, a His6-tagged truncation of LexA that lacked the N-terminal domain (residues 2-64). It was made by PCR on pJWL228 (pET-11a (Novagen) containing lexA42), using oligos that substituted six His codons for residues 2-64. Other expression plasmids were made similarly.
The N-terminal His6 tag used for purifying the protein could be removed by digesting with trypsin, which cleaves after Arg67 and leaves a catalytically active protein.3 Since the rate of cleavage of the tagged protein was the same before and after trypsin-treatment (data not shown), experiments were performed with His-tagged proteins.
A substrate site for Protein Kinase A (PKA), Arg-Arg-Ala-Ser-Gln,43 was added between the His6 tag and the lexA coding sequence of WT/ΔN, using oligos designed similarly to those described above. The resulting plasmid, pJWL944, was used for expression of WT/PKA, which could be radiolabeled. A similar approach has been used with 434 repressor.31 pJWL966 was created from this plasmid, adding the G124D mutation, to express G124D/PKA.
The CI truncation plasmid was made by cloning a PCR product of a truncation of the cI gene from λ JL16344 into pET21a (Novagen) between the NdeI and XhoI sites. This construct expresses a CI truncation that starts at Met93, and has a His-tag (VPRGSLEHHHHH) added to the C-terminus. The P158T and S159N mutations were added by PCR, as described above.
Protein Purification
RecA was purified from an over-expression strain as described by Lusetti et al..45 LexA was made from pJWL228, and purified as described.46
To purify truncated LexA derivatives, BL21/pLysS cells containing T7p::lexA truncation plasmids were grown in 2 L LB at 37°C. IPTG was added to 1 mM when the cells reached OD590 of ∼0.5. Cells were grown 2 h more before they were pelleted. Cell pellets were resuspended in 60 ml Lysis Buffer (25 mM Tris, pH 7.5; 500 mM NaCl; 10 mM imidazole, pH 7.5) and lysed by sonication. The solution was centrifuged 40 min at 14,000g, and the supernatant was loaded onto a 1.5 ml Ni-NTA column (Qiagen). Column was washed with Lysis Buffer with 50 mM imidazole followed by a 25 ml gradient of 50 to 500 mM imidazole in lysis buffer. LexA truncations typically came off during the second half of the gradient, and were at least 95% pure. Proteins were dialyzed into LexA Storage Buffer (10 mM PIPES-NaOH, pH 7.0; 0.1 mM EDTA; 200 mM NaCl; 10% glycerol), and stored at -70°C. The concentration of WT/ΔN (16.3 kDa) was measured using an extinction coefficient of E280 = 6990 M-1 cm-1, calculated by the method of Pace47 for a protein with one Trp, one Tyr, and no Cys residues. The concentrations of other LexA truncations were measured by the assay of Bradford,48 using WT/ΔN as the standard.
The quadruple mutant (QM) has four mutations: L89P, Q92W, E152A, and K156A.9 QM-89/ΔN carries Q92W, E152A, and K156A, QM-92/ΔN has L89P, E152A, and K156A, and QM-152/ΔN has L89P, Q92W, and K156A. The crystallized QM protein also contained an inadvertent fifth mutation, D150H, which did not affect its behavior in the assay described below (data not shown); this mutation was not present in the proteins used in this study.
Labeling of WT/PKA
Kinase reactions were performed for 1 h at 30°C, and contained 100 μM WT/PKA or G124D/PKA in 50 mM Tris, pH 7.5; 70 mM NaCl; 10 mM MgCl2; 0.2 mM ATP (1 Ci [γ-32P] ATP/mmol ATP) with 9 U/μl catalytic subunit of PKA (Calbiochem). Unincorporated label was removed by extensive buffer exchange with LexA Storage Buffer in a Microcon YM-10 spin column (Millipore). Typically more than 50% of the protein was phosphorylated in this reaction.
The activity of WT/PKA, both before and after phosphorylation, was comparable with that of WT/ΔN (see Results). In contrast, when the PKA tag was added to the C terminus of WT/ΔN, autocleavage was ∼2-fold slower than that of WT/ΔN, and was further impaired by phosphorylation (data not shown).
LexA Cleavage
LexA autodigestion was performed in 100 mM CAPS-NaOH, pH 10.4; 200 mM NaCl at 37°C. RecA dependent cleavage of LexA was performed as described,46 with the following modifications. RecA was activated with the oligo SKBT25 (GCG TGT GTG GTG GTG TGC), which was selected for preferential binding to RecA.23 RecA activation reactions typically contained 10 μM RecA; 2.2 μM oligo (or 40 μM nucleotides); 20 mM Tris, pH 7.4; 2 mM MgCl2; 1 mM ATP-γ-S; and 1 mM DTT, and were incubated overnight on ice. RecA activity was constant between 3-5 nucleotides/RecA molecule (data not shown); a 1:4 ratio was used. The long activation time produced higher activity than incubations of 2 h or less (data not shown), possibly due to slow repacking of RecA to the oligo to eliminate gaps between RecA molecules. Cleavage reactions were performed in 20 mM Tris, pH 7.4; 5 mM MgCl2; 1 mM ATP-γ-S; and 1 mM DTT; 2 mM PIPES; 40 mM NaCl; 0.02 mM EDTA; and 2% glycerol. The last four components came from the addition of LexA and LexA Storage Buffer to make up 20% of the volume of all reactions. This afforded constant ionic conditions, even when large volumes of LexA were used. Unless otherwise indicated, cleavage was initiated by adding RecA to pre-warmed samples. Aliquots were stopped at various times by adding Sample Buffer to a final concentration of 30 mM Tris, pH 6.8; 5% glycerol; 2% SDS; 0.01% bromphenol blue; 2.5% ß-mercaptoethanol. Samples were run on 15% Laemmli gels49 and stained with Coomassie blue.
RecA-dependent cleavage of radiolabeled WT/PKA and G124D/PKA for quantification was performed as described above, except that reactions contained 0.1 mg/ml BSA, and cleavage was performed at 30°C, in order to measure the early part of reactions with low concentrations of substrate better than could be achieved at 37°C.
Reactions were run on Tris-Tricine gels (with 16.5 % resolving, 10% spacer, and 4% stacking gels).50 Gels were dried immediately after running, and exposed overnight to a phosphorimager screen. Cleavage was quantified by measuring the relative amounts of signal from the intact protein and the N-terminal fragment using a Typhoon 9410 Scanner. Linear fits of data with 30% or less cleavage were used to determine initial cleavage rates. The quantitative assay for cleavage depended upon reproducibly measuring the fraction of cleaved protein over time. We validated our conditions by taking samples over time of a RecA-dependent cleavage reaction in which the radiolabeled WT/PKA was allowed to approach complete cleavage. The counts in the bands from intact WT/PKA and the N-terminal cleavage product were measured; the sum of these two bands was essentially constant as the protein went from 1% to 90% cleaved (data not shown). In contrast, analysis of samples on 15% Laemmli gels detected a drop in total counts as cleavage progressed, due to poor resolution of the small N-terminal fragment.
Cleavage rates were linear with the amount of activated RecA at or above 20 nM RecA (data not shown); at 10 nM the rate dropped by ∼30%, suggesting that the RecA activation complex was unstable below 20 nM. The activity of 20 nM RecA was constant for more than 2 h (Figure 2(a)), allowing us to measure a wide range of rates. Previous studies used much higher concentrations of RecA10; 11 because the cleavage activity of RecA was impaired at and below 100 nM.24 The stable cleavage activity we observed at≥20 nM may be due to changes in the RecA activation protocol.
Glutaraldehyde cross-linking of LexA
The procedure was based on the method described by Craig,51 using buffer conditions similar to those of RecA-dependent cleavage (20 mM Tris-HCl, pH 7.4; 5 mM MgCl2; 2 mM PIPES; 40 mM NaCl; 0.02 mM EDTA; 2% glycerol). Omission of DTT should have no effect on LexA, which has no cysteines. Although the Tris-HCl in this buffer can react with glutaraldehyde, cross-linking was only a little less efficient than in a non-reactive buffer (data not shown). For equilibrium studies, protein solutions were equilibrated at 30°C before adding glutaraldehyde to a final concentration of 8 mM. Cross-linking was allowed to occur for 10 min, then quenched with 0.1 volume of 0.8 M glycine. For kinetic studies, proteins were mixed at time zero; at the indicated times aliquots were mixed with 16 mM glutaraldehyde for 30 s before adding glycine to 16 mM. Samples were analyzed by 15% Laemmli gels.
The limited amount of cross-linked dimer observed at high concentrations of LexA was not increased by increasing the cross-linking time or the concentration of glutaraldehyde, and similar results were obtained in Tris-free buffers (data not shown). We presume that this limitation exists because side reactions compete with formation of inter-monomer bonds. In particular, the lysine residue(s) that can form inter-monomer cross-links might also form intra-molecular cross-links, even when LexA is dimeric. We also carried out cross-linking of full-length λ CI at 5 μM. This protein forms dimers26 and higher order oligomers52 at this concentration; cross-linking resulted in about 60% cross-linked dimer and higher-order species (data not shown). A mutant protein (Y210H) that is dimeric at this concentration52 gave about 60% cross-linked dimer (data not shown). These findings indicate again that a limit in the extent to which cross-linking can occur does not necessarily reflect a comparable limit in the fraction of dimers.
The cleaved WT/ΔN used in some experiments was made by autocleavage for 3 h at 37°C of concentrated WT/ΔN. The reaction was then diluted fifty-fold into LexA Storage Buffer to lower the pH. Both cleavage products remained, although the N-terminal product did not resolve well in the 15% Laemmli gels.
Cross-linking was quantified using radiolabeled protein in the presence of 0.1 mg/ml BSA under the conditions described above. Samples were run on 15% Laemmli gels that were dried and then exposed overnight to a phosphorimager screen. The fraction cross-linked as dimers was calculated by comparing the counts in the dimer band to the counts in the entire lane. Calculating the total counts from the entire lane provided better reproducibility than the sum of the monomer and dimer bands, presumably because approximately one-quarter of the protein ran in a smear above these bands.
Supplementary Material
Acknowledgements
We are grateful Bill Montfort, John Osterhout, Matt Cordes, and to Sharotka Simon for helpful discussions; to Shelly Lusetti and Mike Cox for the gift of the RecA expression strain, and helpful suggestions regarding purification; to Yu Luo and Natalie Strynadka, and to Chuck Bell and Ed Egelman, for communicating unpublished results; and to Matt Cordes and Carol Dieckmann for reading the manuscript. This work was supported by NIH grant GM65180.
Abbreviations used
- CTD
C-terminal domain
- C
cleavable conformation of LexA
- NC
non-cleavable conformation of LexA
- WT
Wild-type
- PKA
Protein Kinase A
- IndS
super-inducible mutations of LexA
- QM
quadruple mutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Little JW, Mount DW. The SOS regulatory system of Escherichia coli. Cell. 1982;29:11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- 2.Craig NL, Roberts JW. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980;283:26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- 3.Little JW. Autodigestion of lexA and phage lambda repressors. Proc. Natl. Acad. Sci. USA. 1984;81:1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little JW. Mechanism of specific LexA cleavage: autodigestion and the role of RecA coprotease. Biochimie. 1991;73:411–421. doi: 10.1016/0300-9084(91)90108-d. [DOI] [PubMed] [Google Scholar]
- 5.Schnarr M, Pouyet J, Granger-Schnarr M, Daune M. Large-scale purification, oligomerization equilibria, and specific interaction of the LexA repressor of Escherichia coli. Biochemistry. 1985;24:2812–2818. doi: 10.1021/bi00332a032. [DOI] [PubMed] [Google Scholar]
- 6.Mohana-Borges R, Pacheco AB, Sousa FJ, Foguel D, Almeida DF, Silva JL. LexA repressor forms stable dimers in solution. The role of specific DNA in tightening protein-protein interactions. J. Biol. Chem. 2000;275:4708–4712. doi: 10.1074/jbc.275.7.4708. [DOI] [PubMed] [Google Scholar]
- 7.Sassanfar M, Roberts JW. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J. Mol. Biol. 1990;212:79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- 8.Horii T, Ogawa T, Nakatani T, Hase T, Matsubara H, Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell. 1981;27:515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Pfuetzner RA, Mosimann S, Paetzel M, Frey EA, Cherney M, Kim B, Little JW, Strynadka NC. Crystal structure of LexA: a conformational switch for regulation of self-cleavage. Cell. 2001;106:585–594. doi: 10.1016/s0092-8674(01)00479-2. [DOI] [PubMed] [Google Scholar]
- 10.Slilaty SN, Little JW. Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc. Natl. Acad. Sci. USA. 1987;84:3987–3991. doi: 10.1073/pnas.84.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin LL, Little JW. Autodigestion and RecA-dependent cleavage of Ind-mutant LexA proteins. J. Mol. Biol. 1989;210:439–452. doi: 10.1016/0022-2836(89)90121-6. [DOI] [PubMed] [Google Scholar]
- 12.Roland KL, Smith MH, Rupley JA, Little JW. In vitro analysis of mutant LexA proteins with an increased rate of specific cleavage. J. Mol. Biol. 1992;228:395–408. doi: 10.1016/0022-2836(92)90829-9. [DOI] [PubMed] [Google Scholar]
- 13.Lin LL, Little JW. Isolation and characterization of noncleavable (Ind-) mutants of the LexA repressor of Escherichia coli K-12. J. Bacteriol. 1988;170:2163–2173. doi: 10.1128/jb.170.5.2163-2173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimble FS, Sauer RT. Mutations in bacteriophage lambda repressor that prevent RecA-mediated cleavage. J. Bacteriol. 1985;162:147–154. doi: 10.1128/jb.162.1.147-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimble FS, Sauer RT. Lambda repressor inactivation: properties of purified ind-proteins in the autodigestion and RecA-mediated cleavage reactions. J. Mol. Biol. 1986;192:39–47. doi: 10.1016/0022-2836(86)90462-6. [DOI] [PubMed] [Google Scholar]
- 16.Slilaty SN, Rupley JA, Little JW. Intramolecular cleavage of LexA and phage lambda repressors: dependence of kinetics on repressor concentration, pH, temperature, and solvent. Biochemistry. 1986;25:6866–6875. doi: 10.1021/bi00370a020. [DOI] [PubMed] [Google Scholar]
- 17.Burckhardt SE, Woodgate R, Scheuermann RH, Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc. Natl. Acad. Sci. USA. 1988;85:1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peat TS, Frank EG, McDonald JP, Levine AS, Woodgate R, Hendrickson WA. Structure of the UmuD’ protein and its regulation in response to DNA damage. Nature. 1996;380:727–730. doi: 10.1038/380727a0. [DOI] [PubMed] [Google Scholar]
- 19.Bell CE, Frescura P, Hochschild A, Lewis M. Crystal structure of the lambda repressor C-terminal domain provides a model for cooperative operator binding. Cell. 2000;101:801–811. doi: 10.1016/s0092-8674(00)80891-0. [DOI] [PubMed] [Google Scholar]
- 20.Ndjonka D, Bell CE. Structure of a hyper-cleavable monomeric fragment of phage lambda repressor containing the cleavage site region. J. Mol. Biol. 2006;362:479–489. doi: 10.1016/j.jmb.2006.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutreix M, Moreau PL, Bailone A, Galibert F, Battista JR, Walker GC, Devoret R. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J. Bacteriol. 1989;171:2415–2423. doi: 10.1128/jb.171.5.2415-2423.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konola JT, Guzzo A, Gow JB, Walker GC, Knight KL. Differential cleavage of LexA and UmuD mediated by recA Pro67 mutants: implications for common LexA and UmuD binding sites on RecA. J. Mol. Biol. 1998;276:405–415. doi: 10.1006/jmbi.1997.1531. [DOI] [PubMed] [Google Scholar]
- 23.Tracy RB, Kowalczykowski SC. In vitro selection of preferred DNA pairing sequences by the Escherichia coli RecA protein. Genes Dev. 1996;10:1890–1903. doi: 10.1101/gad.10.15.1890. [DOI] [PubMed] [Google Scholar]
- 24.Lin L-L. Ph. D. Thesis. University of Arizona; 1988. A Genetic and biochemical analysis of LexA repressor cleavage in Escherichia coli K-12. [Google Scholar]
- 25.Gimble FS, Sauer RT. Lambda repressor mutants that are better substrates for RecA-mediated cleavage. J. Mol. Biol. 1989;206:29–39. doi: 10.1016/0022-2836(89)90521-4. [DOI] [PubMed] [Google Scholar]
- 26.Koblan KS, Ackers GK. Energetics of subunit dimerization in bacteriophage lambda cI repressor: linkage to protons, temperature, and KCl. Biochemistry. 1991;30:7817–7821. doi: 10.1021/bi00245a022. [DOI] [PubMed] [Google Scholar]
- 27.Beamer LJ, Pabo CO. Refined 1.8 A crystal structure of the lambda repressor-operator complex. J. Mol. Biol. 1992;227:177–196. doi: 10.1016/0022-2836(92)90690-l. [DOI] [PubMed] [Google Scholar]
- 28.Whipple FW, Kuldell NH, Cheatham LA, Hochschild A. Specificity determinants for the interaction of lambda repressor and P22 repressor dimers. Genes Dev. 1994;8:1212–1223. doi: 10.1101/gad.8.10.1212. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Knoll BJ, Little JW, Mount DW. Preferential cleavage of phage lambda repressor monomers by recA protease. Nature. 1981;294:182–184. doi: 10.1038/294182a0. [DOI] [PubMed] [Google Scholar]
- 30.Crowl RM, Boyce RP, Echols H. Repressor cleavage as a prophage induction mechanism: hypersensitivity of a mutant lambda cI protein to recA-mediated proteolysis. J. Mol. Biol. 1981;152:815–819. doi: 10.1016/0022-2836(81)90128-5. [DOI] [PubMed] [Google Scholar]
- 31.Pawlowski DR, Koudelka GB. The preferred substrate for RecA-mediated cleavage of bacteriophage 434 repressor is the DNA-bound dimer. J. Bacteriol. 2004;186:1–7. doi: 10.1128/JB.186.1.1-7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald JP, Peat TS, Levine AS, Woodgate R. Intermolecular cleavage by UmuD-like enzymes: identification of residues required for cleavage and substrate specificity. J. Mol. Biol. 1999;285:2199–2209. doi: 10.1006/jmbi.1998.2433. [DOI] [PubMed] [Google Scholar]
- 33.McEntee K, Weinstock GM, Lehman IR. Binding of the recA protein of Escherichia coli to single- and double-stranded DNA. J. Biol. Chem. 1981;256:8835–8844. [PubMed] [Google Scholar]
- 34.Yu X, Egelman EH. The LexA repressor binds within the deep helical groove of the activated RecA filament. J. Mol. Biol. 1993;231:29–40. doi: 10.1006/jmbi.1993.1254. [DOI] [PubMed] [Google Scholar]
- 35.Harmon FG, Rehrauer WM, Kowalczykowski SC. Interaction of Escherichia coli RecA protein with LexA repressor. II. Inhibition of DNA strand exchange by the uncleavable LexA S119A repressor argues that recombination and SOS induction are competitive processes. J. Biol. Chem. 1996;271:23874–23883. [PubMed] [Google Scholar]
- 36.Smith MH, Cavenagh MM, Little JW. Mutant LexA proteins with an increased rate of in vivo cleavage. Proc. Natl. Acad. Sci. USA. 1991;88:7356–7360. doi: 10.1073/pnas.88.16.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little JW. The SOS regulatory system: control of its state by the level of RecA protease. J. Mol. Biol. 1983;167:791–808. doi: 10.1016/s0022-2836(83)80111-9. [DOI] [PubMed] [Google Scholar]
- 38.VanLoock MS, Yu X, Yang S, Galkin VE, Huang H, Rajan SS, Anderson WF, Stohl EA, Seifert HS, Egelman EH. Complexes of RecA with LexA and RecX differentiate between active and inactive RecA nucleoprotein filaments. J. Mol. Biol. 2003;333:345–354. doi: 10.1016/j.jmb.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 39.Di Capua E, Engel A, Stasiak A, Koller T. Characterization of complexes between recA protein and duplex DNA by electron microscopy. J. Mol. Biol. 1982;157:87–103. doi: 10.1016/0022-2836(82)90514-9. [DOI] [PubMed] [Google Scholar]
- 40.Fersht A. Enzyme Structure and Mechanism. Second ed. W. H. Freeman and Co.; New York: 1985. [Google Scholar]
- 41.Okon M, Pfuetzner RA, Vuckovic M, Little JW, Strynadka NC, McIntosh LP. Backbone chemical shift assignments of the LexA catalytic domain in its active conformation. J. Biomol. NMR. 2005;31:371–372. doi: 10.1007/s10858-005-0944-8. [DOI] [PubMed] [Google Scholar]
- 42.Shepley DP, Little JW. Mutant LexA proteins with specific defects in autodigestion. Proc. Natl. Acad. Sci. USA. 1996;93:11528–11533. doi: 10.1073/pnas.93.21.11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark WA, Izotova L, Philipova D, Wu W, Lin L, Pestka S. Site-specific 32P-labeling of cytokines, monoclonal antibodies, and other protein substrates for quantitative assays and therapeutic application. BioTechniques. 2002;33:S76–S87. [PubMed] [Google Scholar]
- 44.Little JW, Shepley DP, Wert DW. Robustness of a gene regulatory circuit. EMBO J. 1999;18:4299–4307. doi: 10.1093/emboj/18.15.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lusetti SL, Wood EA, Fleming CD, Modica MJ, Korth J, Abbott L, Dwyer DW, Roca AI, Inman RB, Cox MM. C-terminal deletions of the Escherichia coli RecA protein. Characterization of in vivo and in vitro effects. J. Biol. Chem. 2003;278:16372–16380. doi: 10.1074/jbc.M212917200. [DOI] [PubMed] [Google Scholar]
- 46.Little JW, Kim B, Roland KL, Smith MH, Lin LL, Slilaty SN. Cleavage of LexA repressor. Methods Enzymol. 1994;244:266–284. doi: 10.1016/0076-6879(94)44022-0. [DOI] [PubMed] [Google Scholar]
- 47.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 49.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 50.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Analytical Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 51.Craig WS. Determination of quaternary structure of an active enzyme using chemical cross-linking with glutaraldehyde. In: Fleischer S, Fleischer B, editors. Methods Enzymol. Vol. 156. Academic Press; New York: 1988. pp. 333–345. [DOI] [PubMed] [Google Scholar]
- 52.Burz DS, Ackers GK. Cooperativity mutants of bacteriophage λ cI repressor: Temperature dependence of self-assembly. Biochemistry. 1996;35:3341–3350. doi: 10.1021/bi952055x. [DOI] [PubMed] [Google Scholar]
- 53.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.