Abstract
Recently, there has been renewed interest in the organization and neurobiology of remote memory, and the pace of work in this area has accelerated. Yet the recent literature does not suggest that a consensus is developing, and there is disagreement about both facts and their interpretation. This article undertakes a comprehensive review of the three kinds of evidence that have been most prominent in recent discussion: studies of retrograde amnesia in memory-impaired patients who have well-characterized lesions, neuroimaging of healthy volunteers, and work with experimental animals including lesion studies, imaging and mouse genetics. The available evidence tells a coherent story and leads to some straightforward conclusions about the neuroscience of remote memory.
Introduction
Remote memory and remote memory impairment have fascinated clinicians and experimentalists for more than 100 years [1,2], and it is widely recognized that these topics have important implications for understanding the structure and organization of normal memory. This review considers three kinds of evidence that have been prominent in recent work: studies of retrograde amnesia in memory-impaired patients, studies of healthy volunteers using neuroimaging, and studies of experimental animals.
Retrograde amnesia in memory-impaired patients
Retrograde amnesia refers to loss of information that was acquired before the onset of memory impairment. Quantitative studies of retrograde amnesia using objective tests began in the early 1970s [3,4], and additional studies have been carried out since that time. It would seem straightforward enough to determine the status of remote memory in memory-impaired patients. Is memory impaired or intact? If impaired, what time periods are affected? What is the status of memory for facts (e.g. past news items, or famous names and faces), and what is the status of memory for autobiographical events that occurred at a particular time and place? Yet the literature does not reflect an easy consensus on these matters. There are four reasons for this state of affairs: the lack of thorough, neuroanatomical information about many of the patients under study; a tendency to treat ‘amnesia’ as a homogenous condition rather than a disorder that varies both in severity and in its qualitative features, depending on what structures are damaged; the preponderance of single-case studies; and the ascendance of theory over data.
Our review draws heavily on anatomical information about patients as a first step in understanding their phenotype. Work involving groups of patients given the same tests has proven especially illuminating in this regard. These data tell a consistent and compelling story and enable some simple conclusions to be made about the nature of retrograde amnesia.
Memory for facts
Retrograde amnesia for factual information can be either temporally-limited, covering only a few years, or prolonged, depending on the locus and extent of damage. In six patients with damage limited to the hippocampus (hippocampus proper plus dentate gyrus and subiculum), retrograde amnesia for past news events was temporally graded and extended no more than a few years into the premorbid time period (Figure 1a) [5] (for two additional patients, see [6]). Two other patients with neurohistological confirmation of limited hippocampal lesions plus cell loss in the entorhinal cortex had more extended, but still temporally limited, retrograde amnesia that covered one or two decades (LM and WH in [7]). Note though that when remote memory tests are coarsely constructed (e.g. measuring past memory decade by decade, rather than year by year), retrograde amnesia might not be detected after hippocampal lesions (three patients in [8•]; patient RB in [9]).
Figure 1.
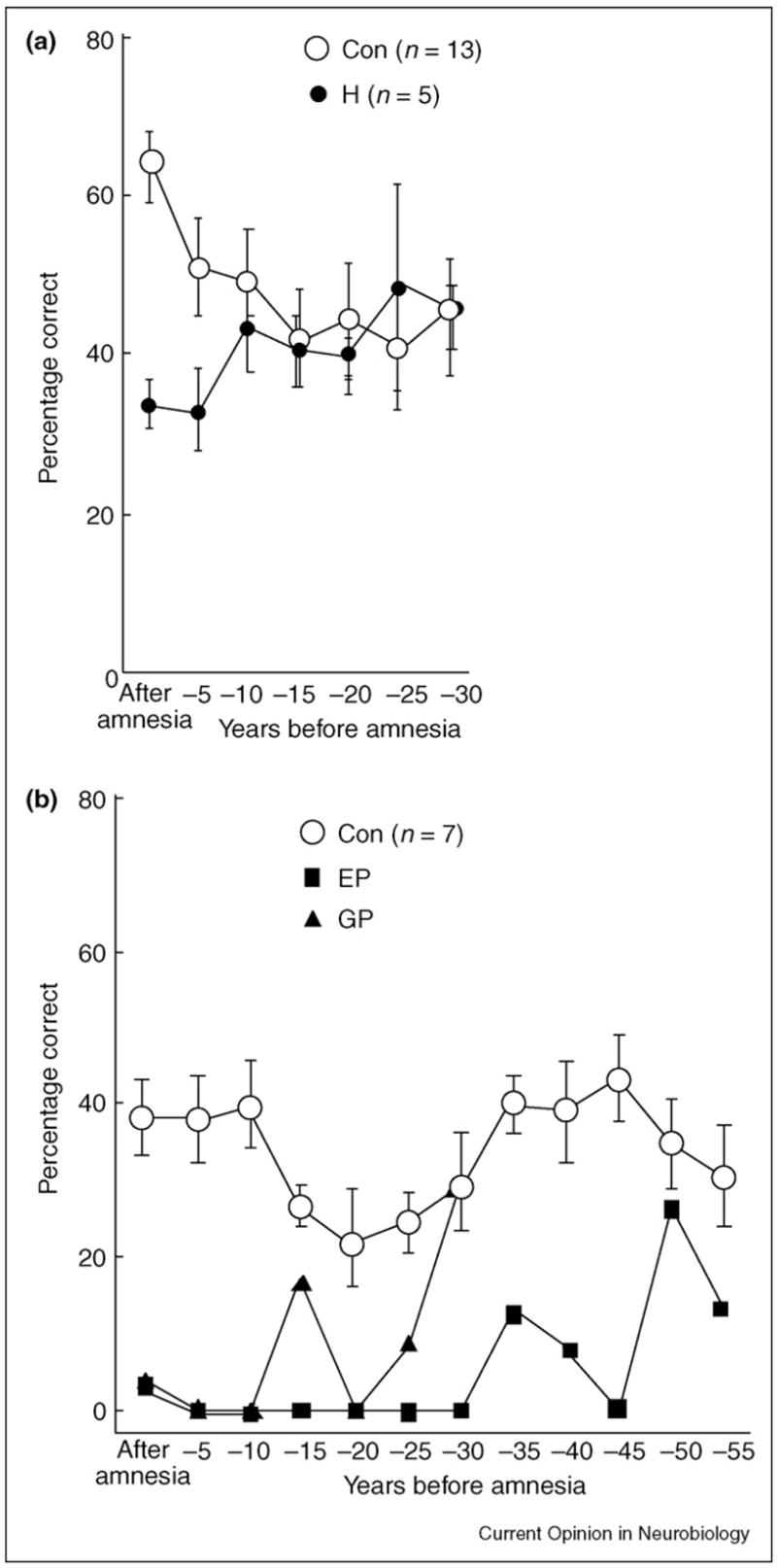
Recall performance on a test of news events. The scores of the patients have been aligned relative to the onset of their amnesia, so that performance can be shown for the time period after the onset of amnesia and for five-year intervals preceding the onset of amnesia (retrograde amnesia). The data point at –5 represents 1–5 years before amnesia, the point at –10 represents 6–10 years before amnesia, and so on. Brackets show standard error of the mean. (a) The scores for six patients with damage limited primarily to the hippocampal region (H) and 13 controls (Con) on 279 questions about news events that had occurred 1951–2005. (b) The scores for two patients (EP and GP) with large medial temporal lobe lesions and seven controls (Con) on questions about 279 news events that had occurred 1951–2005 (GP) or 300 news events that had occurred 1938–2005 (EP and the controls). GP became amnesic at the age of 41, and EP became amnesic at the age of 70. Adapted from [10•].
In contrast to the limited retrograde amnesia reported in all group studies of patients with circumscribed hippocampal lesions, large lesions of the medial temporal lobe produce extended retrograde amnesia that can cover many decades, with sparing only of memories from early life (patients EP and GP in Figure 1b) [10•]. Two other patients with large lesions of the medial temporal lobe (atrophy of both the hippocampus and the parahippocampal gyrus) exhibited retrograde amnesia covering at least two or three decades depending on the test, and they performed consistently more poorly than the three patients who had limited hippocampal damage [8•]. Memory was not tested for earlier time periods. Finally, the lowest scores of all were obtained by seven patients who had damage that extended bilaterally into the lateral temporal lobe.
These findings help to explain reports of patients whose retrograde amnesia is extensive and ungraded (i.e. unrelated to how long ago the memory was formed); for example, they can account for an early, influential report of five patients with mixed etiology [3]. One of these patients (NT) had a left circumscribed hippocampal lesion and a right temporal lobectomy that removed most of the medial and lateral temporal lobe [11]. (Unaccountably, NT’s retrograde amnesia has been attributed to selective hippocampal damage [12,13].) Patients such as NT (and also DRB [14] and GT [15]) might exhibit ungraded retrograde amnesia because their damage extends laterally into the temporal lobe. It is also possible that the retrograde amnesia might not be as ungraded as it appears to be in some cases. The difficulty is that testing seldom covers early adulthood and adolescence. Consider that EP exhibited three to five decades of retrograde amnesia but still obtained good scores on questions that covered the period before he was 25 years old (Figure 1b) [10•]. With this in mind, one must allow for the possibility that other patients who are reported to have extended, ungraded retrograde amnesia (e.g. NT or VC) [16] might also have exhibited intact very remote memory if testing had included items from early life.
To summarize, the evidence describes three different profiles of retrograde amnesia for factual information. After limited hippocampal lesions, retrograde amnesia is temporally-limited, typically covering only a few years. After circumscribed medial temporal lobe lesions, retrograde amnesia is extended and ungraded, often across decades, but performance remains good for information acquired early in life. After lesions that extend into the lateral temporal lobe, retrograde amnesia is even more severe. Unless testing includes items from early childhood and adolescence, it remains possible that memories from early life are intact in many patients who have been described as having severe and extended retrograde amnesia (e.g. [13,16]).
Memory for spatial layouts
The ability to navigate environments learned long ago has been assessed in four single-case studies of memory-impaired patients: EP [17], KC [18], SB [19] and TT [20]. EP was asked to recall the spatial layout of the region where he grew up, and from which he moved away as a young adult >50 years earlier. He performed as well as or better than control subjects who had grown up in the same region and also moved away. Working from questions covering an area of 50 square miles, he described how to travel from his home to other locations in the neighborhood and how to travel from one location to another, and he constructed alternative routes to travel between locations when his preferred route was blocked. He was also able to imagine himself in a particular orientation at a given location and then to point towards a specific landmark. By contrast, EP could demonstrate no knowledge of the neighborhood to which he moved after he became amnesic.
Studies of patients KC and SB also led to the conclusion that the hippocampus is not necessary for mental navigation of remotely learned environments [21•]. Nevertheless, Moscovitch et al. suggest that the spatial information available to these patients might yet be found to be coarser, or to contain fewer details, than the spatial information available to controls. Patient TT, a retired London taxi driver, was tested using a virtual reality simulation of central London [20]. Despite being unable to learn his way around a new neighborhood, TT had detailed knowledge of landmarks he had learned long ago as a taxi driver. He also had considerable knowledge of the spatial arrangement of streets and he successfully navigated several routes. Yet he performed poorly on 5 of the 13 routes tested — routes that tended to involve traveling off the main arterial roads. Note that the findings for TT are not in direct conflict with the findings for EP: TT was tested for information he had acquired during nearly 40 years beginning at about age 26, whereas EP was tested for information he had acquired while growing up, all before the age of 28.
In any case, conclusions about the neural basis of findings exhibited by KC and TT must remain somewhat tentative. KC developed amnesia following a head injury that caused widespread neuropathology, along with bilateral hippocampal damage. As those who have studied him suggest [22], this case is far from ideal for drawing conclusions about the function of any particular brain structure. TT’s condition (limbic encephalitis associated with antibodies to voltage-gated K+ channels) can cause damage beyond the hippocampus, and he did exhibit some generalized atrophy in addition to abnormalities in the anterior temporal lobes (case 8 in [23]). Particularly notable was his zero score on the childhood portion of the autobiographical memory interview [24], a standardized test instrument that has been used in several settings. It is of interest that patients EP and GP, who have large lesions of the medial temporal lobe, and also six patients who have circumscribed hippocampal damage [10•], all obtained scores of eight or nine (the maximum score) on this simple, standardized test of remote autobiographical memory (see also patient BE [6] and patients MR and PD [25]). Accordingly, for a patient such as TT who scored zero on this same test, one must allow for the possibility that the neuropsychological findings have been influenced by damage beyond the medial temporal lobe (e.g. anterior temporal lobes).
It will always be possible to suppose that more detailed, more sensitive tests will bring out impairments that were not previously detected. Even so, the available cases demonstrate a striking contrast between the ability to acquire new spatial memory after medial temporal lobe damage and the ability to access remote spatial memory. Accordingly, spatial information that initially requires the integrity of medial temporal lobe structures must be reorganized as time passes so as to depend much less (or not at all) on these same structures.
Memory for personal episodes
Remote autobiographical memory has been assessed in five recent studies that involved groups of patients who have well-characterized lesions [8•,10•,25,26,27•]. Not included here are group studies that did not describe the lesions using structural magnetic resonance imaging (MRI) or did not distinguish between medial and lateral temporal lobe tissue [28–30].
One approach has been to carry out a detailed analysis of narrative content [26,27•]. Six patients with limited hippocampal lesions and two patients with large medial temporal lobe lesions (EP and GP) successfully recalled detailed memories from their early lives. Their recollections and the recollections of 25 matched controls contained the same number of details (±5%) and were also similar in several other measures. By contrast, three other patients (HC, PH and GT) had medial temporal lobe damage plus significant additional damage to the neocortex, including the frontal, lateral temporal or occipital lobes. These patients were markedly impaired at producing well-formed, detailed autobiographical memories specific to time and place.
Another study included three patients with limited hippocampal damage, two with large medial temporal lobe lesions, and seven with lesions that included both the medial and lateral temporal lobes [8•]. The patients were given a modified version of the autobiographical memory interview [24], and recollections were assessed from the recent premorbid period, early adulthood and childhood. The patients with lesions limited to the medial temporal lobe were significantly impaired only at the most recent time period. By contrast, the patients with lateral temporal lobe lesions performed significantly below controls at all time periods.
Two other studies [10•,25] investigated memory for personal episodes using the autobiographical memory interview. Although this test is simple, and potentially not as sensitive as detailed assessments of narrative content, it has the advantage that results can be compared across laboratories. Accordingly, discordant results recorded in the literature that are based on this test cannot be due to test differences, subjective methods or obscure scoring techniques. In the first study [25], two patients with lesions limited bilaterally to the medial temporal lobe (MR and PD) scored well on the childhood portion of the autobiographical memory interview (6/9 and 7/9, respectively), but a patient with lesions that extended substantially beyond the medial temporal lobe (EK), especially on the left, performed more poorly (4/9). In the second study [10•], six patients with limited hippocampal damage and two patients with large medial temporal lobe lesions (EP and GP) all scored 8/9 or 9/9 on the childhood portion of the interview. Three patients with additional damage in the neocortex (PH, GT and HC) scored more poorly on the same test (0/9, 0/9 and 7/9) (Figure 2) [10•].
Figure 2.
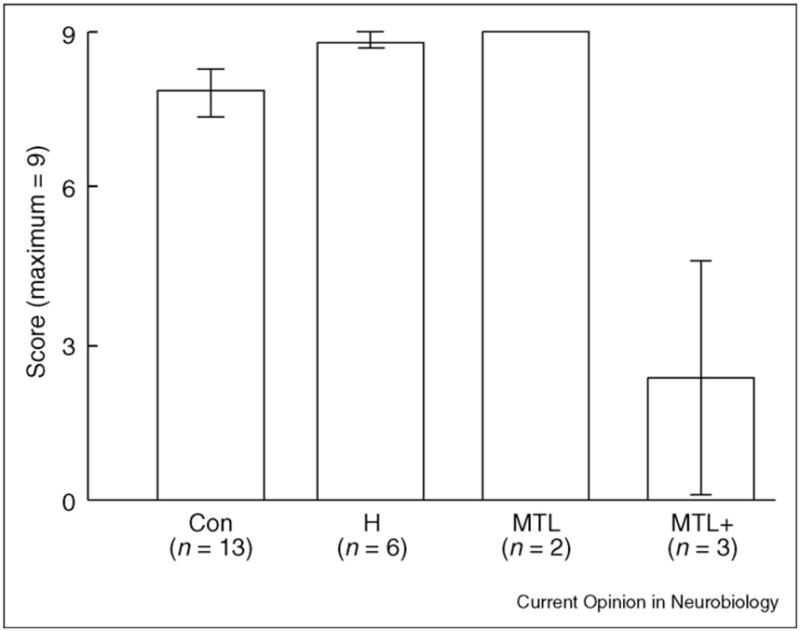
Performance on the childhood portion of the autobiographical memory interview (maximum score = 9). Abbreviations: Con, controls; H, patients who have circumscribed hippocampal lesions; MTL, patients EP and GP, who have large medial temporal lobe lesions; MTL+, patients with medial temporal lobe lesions and additional damage in the neocortex. Brackets show standard error of the mean. Adapted from [10•,27•].
The data from these group studies all point in the same direction. First, remote autobiographical memory is intact when damage is limited to the hippocampus or adjacent medial temporal lobe structures (see also [7,31]). Second, the findings emphasize the importance of lateral temporal and frontal lobe pathology for understanding impaired remote autobiographical memory [8•,25,27•] (see also [28,32]).
Given this consensus from group studies, it is interesting that several single-case studies report impaired remote autobiographical memory (with scores of 0–2 out of 9 on the childhood portion of the autobiographical memory interview). In some instances, the impairment has been attributed to hippocampal damage. Yet, when one examines these studies, one finds that investigators have usually described damage that extends beyond the medial temporal lobe: KC was a case of head trauma with significant damage in frontal, parietal and occipital cortices [22]; VC [16] has evidence of hypometabolism in the right thalamus and right parietal cortex [33]; RS has damage to the basal forebrain and medial frontal lobe [34]; TT [20] has evidence of abnormality in the anterior temporal lobes (case 8 in [23]); LD has basal forebrain and right lateral temporal lobe damage [35]; SS [36] has damage to the lateral temporal lobe bilaterally [37]; and NT [11,13] sustained a resection of most of the right temporal lobe.
Of these single cases, perhaps VC has been the most carefully documented [16]. Although his brain was not fully described using quantitative MRI, the volume of the lateral temporal lobes was reported to be normal. It is striking that VC’s 1/9 score on the childhood portion of the autobiographical memory interview differs sharply from the good scores (and sometimes maximum scores of 9) that have been obtained on the same test by as many as 12 patients with MRI documentation of limited medial temporal lobe damage (n = 8 [10•], n = 2 [25], n = 1 [6] and n = 1 [38]). With the possible exception of VC, we are unaware of memory-impaired patients who have damage limited to the medial temporal lobe (as documented by neurohistology or thorough MRI) and who do so poorly at recollecting remote autobiographical memories.
Lastly, it is notable that the well-known patient HM (who had bilateral medial temporal lobe resection for epilepsy at age 27) was long considered to have intact remote autobiographical memory from before the age of 17 [39,40]. More recently, however, HM’s remote autobiographical memory was reported to be impaired [41]. HM was asked to provide details for just a single event from each of five time periods. Although he was unable to recall a memory from early childhood (0–11 years old), he produced nearly 100 details for a more recent time period (12–17 years old) and scored above the control mean. The data thus provide an ambiguous picture. Yet even if HM is considered deficient, there is some question as to whether his deficiency can be attributed to his medial temporal lobe lesions. First, extended retrograde amnesia for autobiographical events has been reported in patients with temporal lobe epilepsy who have not had surgery [42]. HM’s seizures began at age 10, and young age at onset is associated with especially severe retrograde amnesia [43]. Second, recent MRI scans document several abnormalities outside HM’s medial temporal lobe, including cortical and subcortical atrophy, large amounts of abnormal white matter, and subcortical infarcts [44•]. These findings are thought to have appeared during the past decade, and they complicate the interpretation of HM’s autobiographic memory abilities, which were assessed during the same time period.
A second patient in the same report [41], who became amnesic at age 58, also deserves mention. WR had damage to the right superior temporal gyrus in addition to medial temporal lobe structures bilaterally. Even so, autobiographical memory was described as borderline for the teenage years and normal for ages 18–35 years. Thus, WR’s data are more consistent with a temporally limited retrograde amnesia than with a pervasive impairment in remote autobiographical memory.
It has sometimes been proposed that remote autobiographical recollections should not be described as episodic memories in the same sense as the rich and unique recollections that healthy individuals readily recall from their very recent past. For example, it has been proposed that remote memory in amnesia consists mostly of often-told stories that have become disconnected from their original temporal context and are now part of personal semantic knowledge [45]. If this proposal has merit with respect to patients who have medial temporal lobe damage, we suggest that the idea also applies to the remote memory of healthy individuals. In any case, the empirical question at issue here is whether remote autobiographical memory in patients who have restricted medial temporal lobe lesions is or is not deficient. The available data make a compelling case that these remote memories are intact and accessible.
Neuroimaging
Neuroimaging studies of recent and remote memory present at least three important challenges. First, as has been noted by others, neuroimaging does not identify structures that are essential for performance of the task under study, and activity can reflect cognitive processes that are incidental to task performance. For example, studies of retrieval from the remote past are complicated by the fact that participants are not only retrieving but are also incidentally encoding the test material for possible later use. After all, individuals can usually remember what they did while they were in the scanner. There is now direct evidence that activity recorded during a memory retrieval test can be related to how well the test items will be remembered later [46,47].
Second, many of the baseline conditions (e.g. rest) commonly used in neuroimaging studies are associated with significant brain activity [48], including activity in the medial temporal lobe [49]. Accordingly, when such a baseline condition is contrasted with a memory task of interest, baseline activity will work against finding an effect in the medial temporal lobe that is related to the task. And, if mental activity during the baseline condition varies across experimental conditions (e.g. how much participants reminisce during the baseline condition after retrieving a remote memory versus how much they reminisce after retrieving a recent memory), then the results could be misleading. Third, comparisons of recent and remote memory must take into account that memories from different time periods typically differ not only in age but also in accessibility and vividness. Given these challenges, it is perhaps not surprising that the results from neuroimaging studies are rather mixed. Our review focuses primarily on studies that have tried to compare recent and remote memory.
Memory for facts
Three studies assessed the ability to recognize remotely learned and more recently learned famous faces or famous names, for example from the 1940s through to the 1990s [47,50,51]. Two of these studies reported greater activity in the right hippocampus [51] or right entorhinal cortex [50] for recently learned material than for more remotely learned material. The third study, which compared famous faces from the 1960s and 1970s to faces from the 1990s, found no effect of time period, but activity within the hippocampus did predict how well non-famous faces presented during scanning were remembered later [47]. Thus, activity related to new learning might have masked an effect of time period. An additional study of memory for past public events found no effect of time period within the medial temporal lobe [52]. Further work can be expected to illuminate the aforementioned baseline issue, as well as issues related to item selection (e.g. whether items from remote time periods were in fact learned remotely; for one useful approach, see [51]) and whether findings should be based on all test items or only on items answered correctly.
Memory for spatial layouts
Studies of remote spatial memory have often found activity in the parahippocampal cortex — for example, when long-time residents mentally navigated downtown Toronto [53] and when experienced taxi drivers recalled complex routes around London [54]. A broader region of the posterior medial temporal lobe, including the hippocampus, was activated when students mentally navigated familiar streets in London [55]. Importantly, activity in the parahippocampal cortex, and sometimes also in the hippocampus, occurred when volunteers learned new routes [56,57]. Accordingly, it is possible that the findings obtained when individuals mentally navigate familiar environments are related to encoding of the experience in the scanner and less to retrieval from remote memory. It is also true that studies of remote spatial memory to date have focused mainly on comparisons between spatial and nonspatial memory, not on comparisons between recent and remote spatial memory. Further work will be needed to establish what such comparisons reveal and how the findings compare to lesion data.
Memory for personal episodes
The available studies of recent and remote autobiographical memory provide mixed results. Three studies obtained findings in correspondence with the findings from lesion studies. Thus, when volunteers recalled recent memories, activity was greater in the hippocampal region bilaterally [58], in the right hippocampus [59] or in the left parahippocampal gyrus [60] than when the volunteers recalled remote memories. In one of these studies, the same findings were obtained even when the recent and remote memories were matched for whether they contained many details or only a few details [60]. Two other studies obtained ostensibly opposite results [61,62]. Activity in the hippocampus was greater during retrieval of remote events than during retrieval of recent events, although in these studies the remote events (mean = 5–10 years old) were not as remote as in most other studies of this issue.
Another finding has been that recent and remote autobiographical remembering activates medial temporal lobe structures to a similar extent [63,64]. In perhaps the most thorough study [65], the authors avoided a prescanning interview for selecting test items, because the interview complicates the determination of whether a memory retrieved later is recent or remote [66]. This study compared reminiscences in response to family photographs, which were on average either two years old or 33 years old. Although the left anterior hippocampus was more active during recall of recent memories than during recall of remote memories, this difference was not apparent when the greater vividness of recent memories was accounted for. As already discussed, one of the principal challenges in neuroimaging studies of recent and remote memory is that activity related to the age of a memory might be masked by activity related to encoding the test material into memory. In their study, Gilboa et al. [65] tried to address this possibility by employing a baseline task (constructing an imagined event for an unfamiliar photograph) that would also be expected to promote encoding activity. This procedure requires the untested assumption that generating imagined events and reminiscing about real events promote encoding and successful memory storage to the same extent.
In any case, the findings from this study [65], as well as those from other neuroimaging studies, do not correspond to the findings from lesion studies — namely, that remote autobiographical memories are spared following damage to the medial temporal lobe. Yet, given the number of important factors that can influence the extent of activity in the hippocampus and related structures (e.g. the encoding of novel material for later use, the vividness of the material being recalled, and perhaps also accuracy and effort), one should consider the possibility that activity related to the age of a memory might often be masked or over-ridden. One is also struck by the fact that each neuroimaging study is virtually unique and different from every other study with respect to test materials and design. Work on this problem would benefit from developing some standard test materials and procedures.
One promising direction is the possibility of prospective studies in which the time and strength of learning can be precisely known. In one study [67•], volunteers studied 320 scenes and then took recognition memory tests later the same day and also one day, one month and three months later (80 scenes tested at each interval). As time passed after learning, there was a reduction in hippocampal activity bilaterally during memory testing and a parallel increase in activity in ventral medial frontal cortex. Some unique and useful innovations in the experimental design and data analysis helped to reduce the influence of nonspecific factors that could have operated across the retention interval, and these innovations might explain why other prospective studies did not obtain the same result [68,69]. The findings are unlikely to be related to differences in the vividness of memory across time, because the largest decrease in hippocampal activity occurred between the first and second day, even though memory performance was stable across this interval. These results describe a gradual process of reorganization of memory, whereby the hippocampus becomes less important and areas of neocortex become more important. The suggestion that this process sometimes occurs within a month in humans, rather than during many years, connects this tradition of work to similar studies in experimental animals.
Studies of experimental animals
Studies that have compared recent and remote memory in experimental animals have been especially useful for two reasons. First, recent and remote memory can be studied prospectively by introducing manipulations at particular times after a scheduled training event. Second, studies in animals can provide insight into what occurs as recent memories gradually become remote memories (see [70•] for a comprehensive review). Three kinds of evidence from experimental animals are relevant: the nature of retrograde amnesia following hippocampal lesions, findings from imaging, and mouse genetic studies.
Lesion studies
Prospective studies of retrograde amnesia began only in the 1990s, but >20 studies have already been reported. The large majority found recent memory to be impaired and remote memory to be intact after hippocampal lesions (i.e. temporally graded retrograde amnesia) [71]. In these studies, recent memory is typically tested one day after training, and remote memory is typically tested after ~30 days. The work has involved five species (cats, monkeys, rabbits, rats and mice) and a variety of spatial and nonspatial memory tasks, including contextual fear conditioning, trace eyeblink conditioning, object discrimination learning, social transmission of food preference, and place learning in the water maze.
In one informative study [72], mice given trace eyeblink conditioning received hippocampal lesions, cerebellar lesions or medial prefrontal cortex lesions either one day or four weeks after conditioning. Cerebellar lesions severely impaired both the recently and the remotely established conditioned response (CR). Hippocampal lesions impaired the recently acquired CR but not the remotely acquired CR. Medial prefrontal lesions severely impaired the remotely acquired CR but had only a small effect on the recently acquired CR. These findings describe a process by which memory gradually becomes independent of the hippocampus as time passes after learning. The increasing importance of medial frontal cortex is consistent with the idea that memory gradually becomes embedded in a distributed cortical network, and that the frontal cortex is a node in that network.
Although temporally graded retrograde amnesia has been described for both spatial tasks and nonspatial tasks (for spatial tasks, see [73–75]), it is interesting that virtually all the cases where remote memory was found to be impaired, rather than spared, following hippocampal lesions have involved tasks such as the water maze, where an animal must navigate to a particular point in space (e.g. [76•,77]). Indeed, remote spatial memory in the water maze was impaired even when rats were trained daily from 21–90 days of age and the hippocampal lesion was introduced 100 days after the completion of training [78].
It is puzzling that remote spatial memory in the water maze is impaired by hippocampal lesions, especially considering that patients with even more extensive lesions can mentally navigate their childhood neighborhoods (see earlier in this review). One possibility is that hippocampal lesions deprive animals of detailed information they need to perform the water maze task, while leaving intact coarser information sufficient to perform other spatial tasks, such as discriminating among the arms of a maze (as in [73,74]). A second possibility is that successful performance in the water maze requires new learning (e.g. the rat must continually update its position in space) and new learning requires the hippocampus [79]. With permanent hippocampal lesions, it will be difficult to explore the idea that some performance requirement of the water maze task is important for understanding why rats cannot express remote memory. Reversible lesions, which make it possible to test memory using a functional hippocampus, should be useful in this regard [80].
Some recent findings are consistent with the idea that remote memory for the water maze is independent of at least some aspects of hippocampal function. A lesion of the temporoammonic projection from entorhinal cortex to the CA1 field of the hippocampus disrupted spatial memory in the water maze when the lesion was made one day after training but not 21 days after training [81]. In this case, the lesion presumably disrupted hippocampal–neocortical interactions needed to develop long-term, stable memory but left intact sufficient hippocampal circuitry to perform the task. Indeed, rats with this lesion could learn the task and retain it one day later, but memory was impaired 28 days later.
Together, the available data provide strong evidence for the temporary role of the hippocampus in the formation and maintenance of memory. Less information is available about the relative importance for recent and remote memory of the cortex adjacent to the hippocampus (in the rat, the perirhinal and postrhinal cortices), and studies of retrograde amnesia involving this cortex would be illuminating.
Imaging and mouse genetics
A different strategy is to track changes in brain metabolic activity (e.g. using [14C]2-deoxyglucose) or changes in the expression of activity-related genes, such as c-Fos or Zif268, after retrieval of recent or remote memory. In the first work of this kind, mice learned a spatial discrimination task and were then tested after five days or 25–30 days [75,82]. The hippocampus and entorhinal cortex were active after retrieval of a recent memory but not after retrieval of a remote memory. Conversely, several cortical regions, including the prefrontal, temporal and anterior cingulate cortex, were more active after retrieval of remote memory.
A different study documented the same pattern of changes for contextual fear conditioning [83]. Activity in the CA1 field of the hippocampus was substantially diminished after retrieval of a 36-day-old memory compared with activity after retrieval of a one-day-old memory, but multiple, distributed cortical regions (e.g. prefrontal, temporal and anterior cingulate cortex) were more active after the remote memory test (Figure 3). (For a detailed assessment of changes within hippocampal subregions after recent and remote tests in the water maze, see [84].) As might be expected, the specific cortical regions that are recruited to support remote memory depend on the task. Thus, for a task that relies on olfaction (social transmission of food preference), remote memory testing was associated with increased activity in piriform, entorhinal and orbitofrontal cortex, but not anterior cingulate cortex [85].
Figure 3.
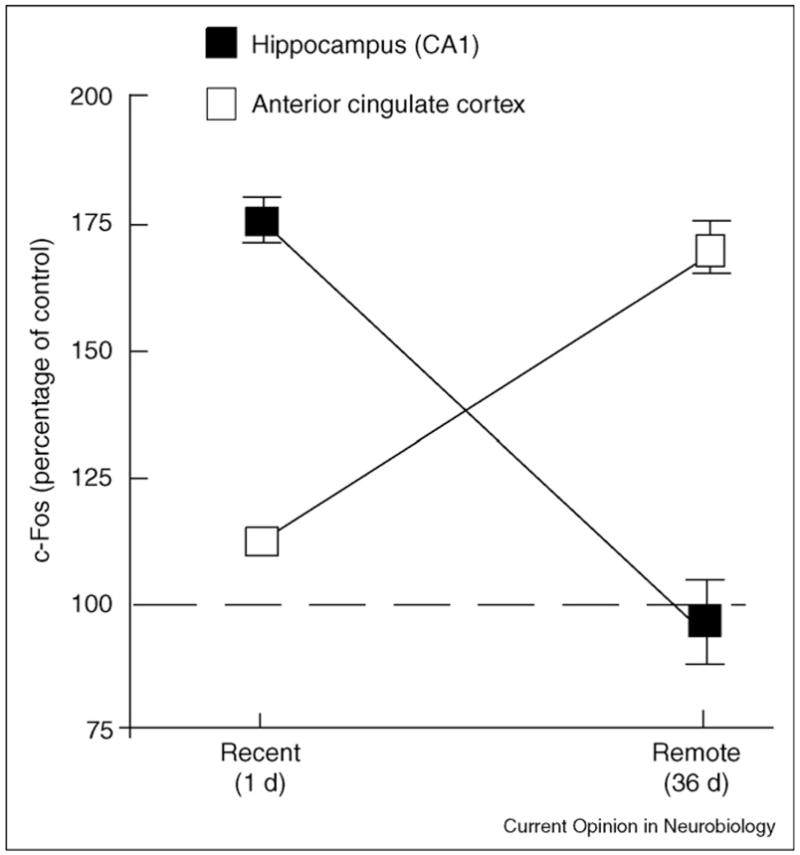
Expression of the activity-dependent gene c-Fos after recent and remote memory tests for contextual fear conditioning in mice. c-Fos expression is shown in two different brain regions as a percentage relative to controls that were not given footshock. The anterior cingulate cortex was one of several cortical regions that were more active during the remote test than during the recent test. Adapted, with permission, from [83].
These findings reflect the increasing importance of distributed cortical regions for the representation and expression of memory as time passes after learning. The idea is not that memory is literally transferred from the hippocampus to the neocortex but that gradual changes in the neocortex (e.g. synaptogenesis [75]) increase the complexity, distribution and connectivity among multiple cortical regions. The fact that remote memory, but not recent memory, was nevertheless disrupted by focal inactivation of prefrontal or anterior cingulate cortex [75,83] could indicate a specific role of these structures in effortful retrieval or executive function [86] or a role in memory storage per se. Other regions, including the temporal cortex, are also likely sites of memory storage. Neurophysiological studies in rats have provided evidence for a dialogue between the hippocampus and the neocortex that occurs after training and that might be part of the process by which recent memories become remote memories [87].
The process by which recent memories become remote memories has also been studied in genetically modified mice. In one study [88], induction of a mutant, constitutively active form of alpha Ca2+/calmodulin-dependent protein kinase II (α-CaMKII) in the entorhinal and subicular cortex beginning immediately after training disrupted water maze memory, but activation beginning three weeks after training had no effect. The results suggest that these regions have a time-limited role in memory formation similar to the hippocampus.
Other work has focused on the role of neocortex in supporting the development of remote memory. In one study, mice heterozygous for a null mutation of the gene encoding α-CaMKII had pervasive abnormalities in cortical plasticity but intact hippocampal plasticity (as measured by tests of long-term potentiation [LTP]) [89]. These mice acquired two different tasks successfully (contextual fear conditioning and the water maze) and exhibited good retention for 1–3 days. However, they forgot abnormally rapidly and were impaired at 10–50 days after training. A second study of the same two tasks involved mice with cortical abnormalities due to overexpression of a dominant-negative form of p21-activated kinase [90]. In this case as well, hippocampal plasticity was normal (i.e. LTP and late-LTP) but synaptic plasticity was impaired in the temporal cortex (enhanced LTP and reduced long-term depression) and there were also abnormalities in dendritic spine density and morphology. Again, memory was intact at short intervals (many hours or a few days) and impaired at longer intervals. These findings demonstrate a requirement for cortical plasticity that is revealed as the role of the hippocampus decreases, and in this respect the results are consistent with imaging studies in experimental animals that describe the emerging importance of the neocortex as time passes after training.
Perspective
A wide range of findings from both humans and experimental animals demonstrate that the hippocampus has a temporary role in the formation and maintenance of memory. The process by which a recent memory becomes gradually independent of the hippocampus, and cortical regions become increasingly engaged, is termed memory consolidation. New imaging work using experimental animals and improved studies of memory-impaired patients provide strong support for these ideas. Nonetheless, discussion has continued about the possible special status of autobiographical memory and spatial memory, especially in the context of multiple trace theory (MTT) [21•]. MTT posits that autobiographical memories, as well as detailed spatial memories, do not consolidate in the sense just described but instead depend on medial temporal lobe structures for the lifetime of the memories.
Yet, as more data have been collected, and with increased attention to neuroanatomy, the need for special treatment of certain kinds of memories is dwindling. All the group studies of patients who have well-characterized lesions find remote memory, including autobiographical memory, to be intact after damage limited to the medial temporal lobe. Single-case studies that report impaired remote memory almost invariably describe damage outside the medial temporal lobe or describe patients who have incompletely characterized lesions. There is the sense, of course, in which MTT is not refutable, because more penetrating assessments of remote memory might some day reveal impairments that were not previously detected. Yet it is questionable whether such a line of inquiry would improve on what has already been learned from the detailed analysis of narrative content [26].
The more interesting point is that MTT could hold to its tenet that detailed memory is special and hippocampus-dependent, and also accommodate all the data, by the simple suggestion that all memories lose richness of detail and become fact-like as time passes. In other words, the suggestion would be that remote memory contains no detailed, episodic memories — neither in patients nor in the rest of us. With this suggestion, MTT is neither useful nor necessary for understanding the status of remote memory because it makes no unique predictions. What is left are issues of definition around how to distinguish a detailed, personal memory from a fact-like memory about the same event, and questions about whether similar or different neural mechanisms within the medial temporal lobe are used for representing memory for detail and memory for fact.
There are two promising developments. First, it is now possible to carry out detailed, volumetric analyses of the brains of memory-impaired study patients, and reports involving groups of patients who have well-characterized lesions have been very instructive. Single-case reports where the anatomy is described only qualitatively from MRI scans no longer meet the standards of evidence needed in contemporary neuropsychological work. Second, study of experimental animals is benefiting from several novel approaches, including imaging using immediate-early genes and behavioral analysis of genetically modified mice. Not too long ago, remote memory and remote memory impairment were studied only descriptively. Now the first steps are being taken towards understanding their mechanism.
Acknowledgments
Our work is supported by the Medical Research Service of the Department of Veterans Affairs, NIMH grant 24600, and the Metropolitan Life Foundation. We thank Brock Kirwan, Hilde Lechner, Yael Shrager and Christine Smith for helpful comments.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
- 1.Ribot T. Les Maladies de la Memoire [English translation: Diseases of Memory] New York: Appleton-Century-Crofts; 1881. [Google Scholar]
- 2.Russell WR, Nathan PW. Traumatic amnesia. Brain. 1946;69:280–300. doi: 10.1093/brain/69.4.280. [DOI] [PubMed] [Google Scholar]
- 3.Sanders HI, Warrington DK. Memory for remote events in amnesic patients. Brain. 1971;94:661–668. doi: 10.1093/brain/94.4.661. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR, Slater PC, Chace PM. Retrograde amnesia: temporal gradient in very long-term memory following electroconvulsive therapy. Science. 1975;187:77–79. doi: 10.1126/science.1109228. [DOI] [PubMed] [Google Scholar]
- 5.Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38:127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 6.Kapur N, Brooks DJ. Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus. 1999;9:247–254. doi: 10.1002/(SICI)1098-1063(1999)9:3<247::AID-HIPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment following bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Bright P, Buckman JR, Fradera A, Yoshimasu H, Colchester ACF, Kopelman MD. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learn Mem. 2006;13:545–557. doi: 10.1101/lm.265906. This study of four groups of patients assessed fact memory and autobiographical memory with several tests. Patients who had lateral temporal damage exhibited impaired remote autobiographical memory for all time periods, including childhood. Patients who had medial temporal lobe damage had impaired autobiographical memory for only the most recent time period. See also [27•]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Bayley PJ, Hopkins RO, Squire LR. The fate of old memories following medial temporal lobe damage. J Neurosci. 2006;26:13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. This study describes two phenotypes of retrograde memory impairment: temporally limited retrograde amnesia that covers only a few years, and extended retrograde amnesia that covers decades but spares early memories. The first profile is associated with limited hippocampal lesions and the second with large medial temporal lobe lesions. The findings suggest that the cortex adjacent to the hippocampus has a role in memory storage that extends well beyond the temporary role of the hippocampus itself. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warrington EK, Duchen LW. A re-appraisal of a case of persistent global amnesia following right temporal lobectomy: a clinico-pathological study. Neuropsychologia. 1992;30:437–450. doi: 10.1016/0028-3932(92)90091-y. [DOI] [PubMed] [Google Scholar]
- 12.Chan D, Revesz T, Rudge P. Hippocampal, but not parahippocampal, damage in a case of dense retrograde amnesia: a pathological study. Neurosci Lett. 2002;329:61–64. doi: 10.1016/j.neulet.2002.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Cipolotti L, Moscovitch M. The hippocampus and remote autobiographical memory. Lancet Neurol. 2005;4:792–793. doi: 10.1016/S1474-4422(05)70232-5. [DOI] [PubMed] [Google Scholar]
- 14.Damasio AR, Eslinger PJ, Damasio H, Van Hoesen GW, Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Arch Neurol. 1985;42:252–259. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- 15.Reed JM, Squire LR. Retrograde amnesia for facts and events: findings from four new cases. J Neurosci. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cipolotti L, Shallice T, Chan D, Fox N, Scahill R, Harrison G, Stevens J, Rudge P. Long-term retrograde amnesia…the crucial role of the hippocampus. Neuropsychologia. 2001;39:151–172. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 17.Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum RS, Priselac S, Kohler S, Black S, Gao F, Nadel L, Moscovitch M. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat Neurosci. 2000;3:1044–1048. doi: 10.1038/79867. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum RS, Gao F, Richards B, Black S, Moscovitch M. ‘Where to? ’ Remote memory for spatial relations and landmark identity in former taxi drivers with Alzheimer’s disease and encephalitis. J Cogn Neurosci. 2005;17:446–462. doi: 10.1162/0898929053279496. [DOI] [PubMed] [Google Scholar]
- 20.Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- 21•.Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. A summary of remote memory studies in humans that considers the relative merits of three different views of recent and remote memory. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum RS, Kohler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, Gao F, Tulving E. The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, Clover L, Parkinson A, Bien CG, Omer S, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–712. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 24.Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- 25.Eslinger PJ. Autobiographical memory after temporal lobe lesions. Neurocase. 1998;4:481–495. [Google Scholar]
- 26.Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;37:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 27•.Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005;46:799–810. doi: 10.1016/j.neuron.2005.04.034. A detailed volumetric analysis of structural brain images is presented for five patients who had limited medial temporal lobe damage and intact remote autobiographical memory. Three other patients with impaired remote autobiographical memory had medial temporal lobe damage plus additional damage to the neocortex. Together with [26], the data suggest that damage must extend beyond the medial temporal lobe to impair remote autobiographical memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopelman MD, Stanhope N, Kingsley D. Retrograde amnesia in patients with diencephalic, temporal lobe or frontal lesions. Neuropsychologia. 1999;37:939–958. doi: 10.1016/s0028-3932(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 29.Moscovitch M, Yaschyshyn T, Ziegler M, Nadel L. Remote episodic memory and retrograde amnesia: was Endel Tulving right all along? In: Tulving E, editor. Memory, Consciousness and the Brain: The Tallinn Conference. Psychology Press/Taylor & Francis; 2000. pp. 331–345. [Google Scholar]
- 30.Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKinnon DF, Squire LR. Autobiographical memory and amnesia. Psychobiology. 1989;17:247–256. [Google Scholar]
- 32.Kapur N. Syndromes of retrograde amnesia: a conceptual and empirical synthesis. Psychol Bull. 1999;125:800–825. doi: 10.1037/0033-2909.125.6.800. [DOI] [PubMed] [Google Scholar]
- 33.Kapur N, Thompson P, Kartsounis LD, Abbott P. Retrograde amnesia: clinical and methodological caveats. Neuropsychologia. 1999;37:27–30. doi: 10.1016/s0028-3932(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 34.Kitchener EG, Hodges JR, McCarthy R. Acquisition of post-morbid vocabulary and semantic facts in the absence of episodic memory. Brain. 1998;121:1313–1327. doi: 10.1093/brain/121.7.1313. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor M, Butters N, Miliotis P, Eslinger P, Cermak LS. The dissociation of anterograde and retrograde amnesia in a patient with herpes encephalitis. J Clin Exp Neuropsychol. 1992;14:159–178. doi: 10.1080/01688639208402821. [DOI] [PubMed] [Google Scholar]
- 36.Cermak LS, O’Connor M. The anterograde and retrograde retrieval ability of a patient with amnesia due to encephalitis. Neuropsychologia. 1983;39:213–224. doi: 10.1016/0028-3932(83)90039-8. [DOI] [PubMed] [Google Scholar]
- 37.Verfaellie M, Koseff P, Alexander MP. Acquisition of novel semantic information in amnesia: effects of lesion location. Neuropsychologia. 2000;38:484–492. doi: 10.1016/s0028-3932(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 38.Schnider A, Bassetti C, Schnider A, Gutbrod K, Ozdoba C. Very severe amnesia with acute onset after isolated hippocampal damage due to systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 1995;59:644–646. doi: 10.1136/jnnp.59.6.644-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. Semin Neurol. 1984;4:249–259. [Google Scholar]
- 40.Sagar HH, Cohen NJ, Corkin S, Growdon JM. Dissociations among processes in remote memory. In: Olton DS, Gamzu E, Corkin S, editors. Memory Dysfunctions. 444. Annals of the New York Academy of Sciences; 1985. pp. 533–535. [DOI] [PubMed] [Google Scholar]
- 41.Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Kapur N. Autobiographical amnesia and temporal lobe pathology. In: Parkin AJ, editor. Studies in the Neuropsychology of Memory. Psychology Press; 1997. pp. 37–62. [Google Scholar]
- 43.Lah S, Lee T, Grayson S, Miller L. Effects of temporal lobe epilepsy on retrograde memory. Epilepsia. 2006;47:615–625. doi: 10.1111/j.1528-1167.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 44•.Salat DH, van der Kouwe AJW, Tuch DS, Quinn BT, Fischl B, Dale AM, Corkin S. Neuroimaging H.M.: a 10-year follow-up examination. Hippocampus. 2006;16:936–945. doi: 10.1002/hipo.20222. New MRI of findings for patient HM document significant abnormalities outside the medial temporal lobe, thought to have appeared during the past decade. These findings raise concerns as to whether cognitive impairment identified during this same time period, such as possible difficulty with remote autobiographical memory [41], can be attributed to HM’s medial temporal lobe resection. [DOI] [PubMed] [Google Scholar]
- 45.Cermak LS. The episodic–semantic distinction in amnesia. In: Squire LR, Butters N, editors. Neuropsychology of Memory. Guilford Press; 1984. pp. 55–62. [Google Scholar]
- 46.Stark CEL, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernard FA, Bullmore E, Graham K, Thompson S, Hodges J, Fletcher PC. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage. 2004;22:1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 48.Gusnard DA, Akbudak E, Shulman GL, Raichel ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain activation. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark CEL, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haist F, Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- 51.Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao S. Medial temporal lobe activity for recognition of recent and remote famous names: an event related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Maguire EA, Henson RN, Mummery C, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- 53.Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. ‘I have often walked down this street before’: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- 54.Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumaran D, Maguire EA. The human hippocampus: cognitive maps or relational memory? J Neurosci. 2005;25:7254–7259. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 57.Shelton AL, Gabrieli JDE. Neural correlates of encoding space from route and survey perspectives. J Neurosci. 2002;22:2711–2717. doi: 10.1523/JNEUROSCI.22-07-02711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piefke M, Weiss P, Zilles K, Markowitsch H, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- 59.Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–5307. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niki K, Luo J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J Cogn Neurosci. 2002;14:500–507. doi: 10.1162/089892902317362010. [DOI] [PubMed] [Google Scholar]
- 61.Rekkas PV, Constable RT. Evidence that autobiographic memory retrieval does not become independent of the hippocampus: an fMRI study contrasting very recent with remote events. J Cogn Neurosci. 2005;17:1950–1961. doi: 10.1162/089892905775008652. [DOI] [PubMed] [Google Scholar]
- 62.Piolino P, Giffard-Quillon G, Desgranges B, Chetelat G, Baron J, Eustache F. Re-experiencing old memories via hippocampus: a PET study of autobiographical memory. Neuroimage. 2004;22:1371–1383. doi: 10.1016/j.neuroimage.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Ryan L, Nadel L, Keil D, Putnam K, Schuyner D, Trouard T, Moscovitch M. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- 64.Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- 66.Rekkas PV, Constable RT. Hemodynamic retrieval intensity in hippocampus is decreased by pre-exposure to autobiographic test items. Brain Res Bull. 2006;70:467–473. doi: 10.1016/j.brainresbull.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 67•.Takashima A, Petersson K, Rutters F, Tendolkar I, Jensen O, Zwarts M, McNaughton BL. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. During the three months after learning, the authors report a decrease in hippocampal activity during memory retrieval and a parallel increase in activity within the ventral medial prefrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stark CEL, Squire LR. fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 69.Bosshardt S, Degonda N, Schmidt CF, Goesiger P, Nitsch RM, Hock C, Henke K. One month of human memory consolidation enhances retrieval-related hippocampal activity. Hippocampus. 2005;15:1026–1040. doi: 10.1002/hipo.20105. [DOI] [PubMed] [Google Scholar]
- 70•.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. A comprehensive review of the neurobiology of recent and remote memory, focusing especially on studies that used experimental animals. [DOI] [PubMed] [Google Scholar]
- 71.Squire LR, Clark RE, Bayley PJ. Medial temporal lobe function and memory. In: Gazzaniga M, editor. The Cognitive Neurosciences III. MIT Press; 2004. pp. 691–708. [Google Scholar]
- 72.Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho YH, Kesner RP, Brodale S. Retrograde and anterograde amnesia for spatial discrimination in rats: Role of hippocampus, entorhinal cortex and parietal cortex. Psychobiology. 1995;23:185–194. [Google Scholar]
- 74.Ramos JM. Retrograde amnesia for spatial information: dissociation between intra and extramaze cues following hippocampus lesions in rats. Eur J Neurosci. 1998;10:3295–3301. doi: 10.1046/j.1460-9568.1998.00388.x. [DOI] [PubMed] [Google Scholar]
- 75.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 76•.Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. This study demonstrates impaired remote memory in three different spatial tasks — the standard water maze, a dry-land version of the water maze and an annular water maze — even with training-to-surgery intervals as long as 14 weeks. The findings highlight the differences between the water maze and other spatial and nonspatial tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin SJ, de Hoz L, Morris RGM. Retrograde amnesia: neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding. Neuropsychologia. 2005;43:609–624. doi: 10.1016/j.neuropsychologia.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Clark RE, Broadbent NJ, Squire LR. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus. 2005;15:340–346. doi: 10.1002/hipo.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knowlton BJ, Fanselow MS. The hippocampus, consolidation and on-line memory. Curr Opin Neurobiol. 1998;8:293–296. doi: 10.1016/s0959-4388(98)80154-2. [DOI] [PubMed] [Google Scholar]
- 80.Riedel G, Micheau J, Lam AGM, Roloff EvL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RGM. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 81.Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- 82.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 83.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 84.Gusev PA, Cui C, Alkon DL, Gubin AN. Topography of Arc/Arg3.1 mRNA expression in the dorsal and ventral hippocampus induced by recent and remote spatial memory recall: Dissociation of CA3 and CA1activation. J Neurosci. 2005;25:9384–9397. doi: 10.1523/JNEUROSCI.0832-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rudy JW, Biedenkapp JC, O’Reilly RC. Prefrontal cortex and the organization of recent and remote memories: an alternative view. Learn Mem. 2005;12:445–446. doi: 10.1101/lm.97905. [DOI] [PubMed] [Google Scholar]
- 87.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 88.Yasuda M, Mayford MR. CaMKII activation in the entorhinal cortex disrupts previously encoded spatial memory. Neuron. 2006;50:309–318. doi: 10.1016/j.neuron.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 89.Frankland PW, O’Brien C, Ohno M, Kirkwood A, Silva AJ. α-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 90.Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron. 2004;42:773–787. doi: 10.1016/j.neuron.2004.05.003. [DOI] [PubMed] [Google Scholar]


