SUMMARY
The determination of the vertebrate dorsoventral body axis is regulated in the extracellular space by a system of interacting secreted molecules consisting of BMP, Chordin, Tolloid and Twisted Gastrulation (Tsg). Tsg is a BMP-binding protein that forms ternary complexes with BMP and Chordin. We investigated the function of Tsg in embryonic patterning by generating point mutations in its two conserved cysteine-rich domains. Surprisingly, Tsg proteins with mutations in the N-terminal domain were unable to bind BMP, yet ventralized the embryo very effectively, indicating strong pro-BMP activity. This hyperventralizing Tsg activity required an intact C-terminal domain and could block the anti-BMP activity of isolated BMP-binding modules of Chordin (CRs) in embryonic assays. This activity was specific for CR-containing proteins as it did not affect the dorsalizing effects of Noggin or dominant-negative BMP receptor. The ventralizing effects of the xTsg mutants were stronger than the effect of Chordin loss-of-function in Xenopus or zebrafish. The results suggest that xTsg interacts with additional CR-containing proteins that regulate dorsoventral development in embryos.
Keywords: Twisted-gastrulation, BMP, Chordin, Tolloid, Crossveinless, TGFβ, Cell-cell signaling, Xenopus, Zebrafish, CR modules
INTRODUCTION
The establishment of the vertebrate body plan requires inductive signals from the dorsal blastopore lip, also known as Spemann’s organizer, in amphibians (Spemann and Mangold, 2001; De Robertis and Aréchaga, 2001; Stern, 2001). Surprisingly, many of the secreted factors expressed specifically in the organizer were found to be antagonists of growth factor signaling, such as the BMP (Bone Morphogenetic Protein) antagonists Chordin, Noggin, Follistatin, Xnr3 and Cerberus (reviewed by De Robertis et al., 2000). Studies in vertebrates as well as invertebrates indicated that a BMP signaling gradient, which is established in the early embryo by the localized secretion of these organizer specific inhibitors, plays a central role in the determination of the dorsoventral body axis.
Chordin is a large secreted protein abundantly expressed in Spemann’s organizer, reaching extracellular concentrations in the 6-12 nanomolar range (Piccolo et al., 1996). Chordin binds directly to BMP and prevents BMP binding to its cognate receptor, causing dorsalization of the embryo in overexpression studies (Sasai et al., 1995; Piccolo et al., 1996). The binding of Chordin to BMP is mediated by four cysteine-rich domains (CRs) of about 70 amino acids each (Larraín et al., 2000). A large number of additional extracellular proteins containing CR domains have now been identified, and interactions with BMP or TGFβ have been documented for many of them (reviewed in García-Abreu et al., 2002).
In Drosophila, the chordin homolog short gastrulation (sog) is expressed in ventral neuroectoderm and is required for the formation of neural tissue (François et al., 1994). In addition, Sog is necessary for the formation of the dorsal-most tissue of the fly embryo, the amnioserosa, which requires maximal BMP activity (Ross et al., 2001; Eldar et al., 2002).
In zebrafish, the ventralized chordino phenotype is caused by a loss-of-function mutation in the zebrafish chordin gene (Schulte-Merker et al., 1997; Hammerschmidt and Mullins, 2002). The neural plate and dorsal mesoderm are reduced, and ventral mesoderm is expanded in chordino mutants (Hammerschmidt et al., 1996a; Gonzalez et al., 2000). The opposite phenotype, dorsalization, is observed in bmp2b/swirl, bmp7/snailhouse, Smad5/somitabun and Tolloid/mini-fin mutants (Mullins et al., 1996; Hammerschmidt and Mullins, 2002). In addition, chordino;swirl double mutants display the swirl phenotype, confirming that Chordin functions genetically as an anti-BMP (Hammerschmidt et al., 1996b). Recent studies on swirl/chordino genetic interactions indicate that in zebrafish, Chordin also functions in the formation of ventral tail fin tissue that requires maximal BMP signaling (Wagner and Mullins, 2002). This suggests that in vertebrates Chordin, like Sog in the amnioserosa of the fly, may promote BMP signaling as well.
In mouse, the targeted inactivation of chordin results in a ventralized gastrulation phenotype only in a low percentage of the homozygous mutant embryos. Most mutants lack anterior notochord and pharyngeal endoderm, causing a phenotype similar to human DiGeorge syndrome, but do not have gastrulation phenotypes (Bachiller et al., 2003). However, mice homozygous for mutations in chordin and noggin lack the forebrain, indicating that Noggin can partially compensate for the loss of Chordin (Bachiller et al., 2000).
In Xenopus, inhibition of Chordin production by morpholino oligonucleotides causes a phenotype similar to that of zebrafish chordino, with embryos developing with smaller heads and enlarged ventroposterior structures (Oelgeschläger et al., 2003). This relatively weak ventralization contrasts with the strong requirement for Chordin observed when the embryos are experimentally manipulated. Indeed, dorsalization of embryos by LiCl (Kao and Elinson, 1988), dorsal mesoderm induction by Activin (Green et al., 1992) and CNS induction by dorsal lip grafts, all show a complete dependence on the presence of Chordin (Oelgeschläger et al., 2003).
Tsg is a co-factor of Chordin that can bind both to BMP and to Chordin, generating trimolecular complexes that antagonize BMP signaling (Oelgeschläger et al., 2000; Chang et al., 2001; Scott et al., 2001; Larraín et al., 2001). The cleavage of Chordin by the Xolloid (Xld)/Tolloid (Tld) zinc-metalloproteinase generates protein fragments that include intact BMP-binding cysteine-rich modules (CRs) and retain anti-BMP activity (Larraín et al., 2000). Tsg facilitates the cleavage of Chordin by Tolloid (Scott et al., 2001; Larraín et al., 2001) and antagonizes the residual anti-BMP activity of the proteolytic cleavage products of Chordin (Oelgeschläger et al., 2000; Larraín et al., 2001). Thus, in this second aspect of its activity Tsg behaves as a pro-BMP. The cleavage of Chordin by Xld/Tld constitutes the molecular switch that controls the anti-BMP and pro-BMP activities of Tsg protein in this biochemical pathway (Larraín et al., 2001).
The multifunctional properties of Tsg hamper the analysis of its function in embryonic patterning. For example, in Xenopus embryos overexpression of xTsg has a pro-BMP effect, resulting in ventralization (Oelgeschläger et al., 2000). In zebrafish, overexpression of zTsg causes an anti-BMP, dorsalized phenotype (Ross et al., 2001). The difference in phenotype may be attributed to a lower endogenous activity of Tolloid during early zebrafish development (Connors et al., 1999; Larraín et al., 2001). Indeed, the ventralizing activity of xTsg in Xenopus could be reversed by overexpression of a dominant-negative Xolloid (Larraín et al., 2001).
In the present study, we dissected the anti-BMP and pro-BMP activities of xTsg by generating a series of point mutations that affected specifically one or the other activity of xTsg. The Tsg protein contains two evolutionary conserved cysteine-rich domains. The C-terminal cysteine-rich region does not have any significant homology to known protein motifs and its role in the biochemical activities of Tsg is unknown. The N-terminal cysteine-rich domain of Tsg has some homology to the BMP-binding modules of Chordin (CRs) and is necessary and sufficient for the direct interaction of xTsg with BMP (Oelgeschläger et al., 2000). As shown here, mutations in the N-terminal domain generated mutant xTsg proteins that no longer bound BMP and had greatly enhanced ventralizing activities, preventing the formation of CNS and dorsal mesoderm in Xenopus embryos. This hyperventralizing activity of xTsg mutants required an intact C-terminal domain, and phenotypes were much stronger than those caused by Chordin loss-of-function, both in Xenopus and zebrafish embryos. Hyperventralizing xTsg mutations specifically antagonized proteins containing BMP-binding modules of the Chordin type. Our data indicate that xTsg inactivates CR-modules through its C-terminal conserved domain and that xTsg may interact, in addition to Chordin, with other CR-containing proteins required for the regulation of early dorsoventral patterning.
MATERIALS AND METHODS
DNA constructs and morpholinos
Site-directed mutagenesis was performed with the QuickChange mutagenesis kit (Stratagene). Point mutations were introduced into Xenopus and mouse Tsg constructs lacking the endogenous signal peptide and fused to the signal peptide of Xenopus Chordin followed by a FLAG-tag (pCS2-ChdN-Tsg) (Oelgeschläger et al., 2000; Larraín et al., 2001). For the mutations in S36, S54, C59, C180 and C198 in xTsg, and C185 and C203 in mouse Tsg, the respective amino acids were mutated to Alaninc; W67 in Xenopus Tsg and the corresponding W66 in mouse Tsg were mutated into Glycine.
A combination of two anti-chordin morpholinos was used as described (Oelgeschläger et al., 2003).
Embryo manipulations and chordino genotyping
Microinjections, in situ hybridization and RNA synthesis were performed as described (Piccolo et al., 1997; Oelgeschläger et al., 2000; Sive et al., 2000). For LiCl rescue experiments, embryos were microinjected at the two to four cell stage four times with a total of 2 ng TsgW67G or 8 ng of a 1:1 mixture of the two Chordin morpholinos. Embryos at the 32-64 cell stage were treated for 29 minutes with 120 mM LiCl in 0.1×MBS saline (Larraín et al., 2000; Sive et al., 2000). The dorsoanterior index (DAI) was determined at stage 30 (Kao and Elinson, 1988). Treatment of animal cap explants with human recombinant Activin protein (R&D Systems) was as described (Piccolo et al., 1999). RNA was isolated at stage 25. RT-PCR conditions and PCR primers used have been described elsewhere (Sasai et al., 1995) (http://www.hhmi.ucla.edu/derobertis/index.html). To genotype chordinott250 mutant embryos two primers were used for PCR: chd-2 GCA GAA ACG TCT ACG TTT CC and chd-3 CGT TTT AGT TGG TGC TCT TGA CG. Following digestion with MspI, the wild-type allele was digested whereas the mutant allele was not.
Protein biochemistry
Xenopus Tsg and the mutant Tsg proteins were affinity purified using the anti-FLAG M2 affinity gel column (Sigma). The anti-FLAG column (0.5 ml) was washed once with 5 ml 0.1 M glycine, pH 3.5 and three times with 5 ml aliquots of TBS (50 mM Tris HCl, 150 mM NaCl, pH 7.4). Conditioned medium from human 293 cells transfected with pCS2-ChdN-xTsg or Tsg point mutants was harvested and loaded directly onto the column. After washing three times with 10 ml TBS, the bound protein was eluted with five aliquots of 1 ml TBS containing 100 μg/ml FLAG peptide (Sigma). Protein concentrations were estimated by comparison with BSA standards after Coomassie Blue staining. Analyses of protein secreted by animal cap explants were performed as described (Oelgeschläger et al., 2000). The supernatant was used for western blots probed with an antibody specific for the inter-repeat region of Chordin (α-I-Chd) (Piccolo et al., 1997) that had been blot-affinity purified (Larraín et al., 2001). For the expression of proteins in co-cultures, 293T cells were independently transfected with mouse Chordin, Bmp4, Tsg and TsgW66A expression plasmids using FuGENE (Roche), and chemical crosslinking and immunoprecipitation experiments carried out as described (Oelgeschläger et al., 2000; Larraín et al., 2001).
RESULTS
Tsg mutations reveal distinct activities for the N and C terminus
The N-terminal cysteine-rich domain of xTsg shares homology with the CRs of Chordin (Fig. 1A) (Oelgeschläger et al., 2000). Chordin CRs mediate the direct binding of Chordin to BMPs and are characterized by 10 cysteines spaced in a characteristic manner (Larraín et al., 2000). The conserved cysteines, as well as a conserved tryptophan, and potential glycosylation sites, were mutated (Fig. 1A,B) and tested by microinjection into Xenopus embryos.
Fig. 1.
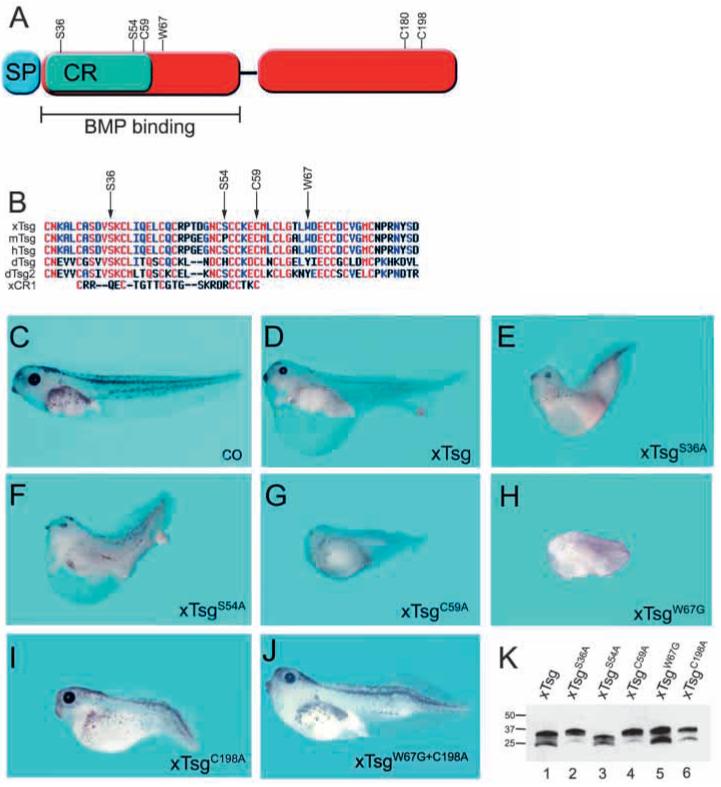
The ventralizing activity of Tsg resides in its C-terminal cysteine-rich domain. (A) The Tsg mutations presented here. (B) Alignment of the N-terminal cysteine rich domain of Tsg proteins from Xenopus (xTsg), mouse (mTsg), human (hTsg), Drosophila (dTsg, dTsg2) and Chordin CR1. (C-J) Stage 42 wild-type control embryo (C) and embryos radially microinjected at the four-cell stage with a total of 2 ng xTsg mRNA (D), xTsgS36A (E), xTsgS54A (F), xTsgC59A (G), xTsgW67G (H), xTsgC198A (I), xTsgW67G+C198A (J). Each construct was analyzed in at least three independent experiments with n>30. (K) xTsg and mutant xTsg proteins secreted by dissociated animal cap cells after microinjection of 2 ng of the indicated mRNAs.
Embryos overexpressing wild-type xTsg mRNA developed with reduced head structures, distended trunk endoderm and a posteriorized anus that detached from the main endodermal mass later in development (Fig. 1D). Three of the point mutants examined caused a hyperventralized phenotype when compared with xTsg. Embryos microinjected with mRNA coding for xTsgS36A, xTsgC59A and xTsgW67G lost head and dorsal structures and had an enlargement of ventral blood islands (Fig. 1E,G,H and data not shown). These phenotypes were indistinguishable from those of Xenopus embryos overexpressing mRNAs encoding BMP4 or BMP7 (not shown).
The C-terminal cysteine-rich domain of Tsg did not show any significant homology to known protein motifs in the databases but is conserved across Tsgs from different species. Mutations in several of its conserved cysteine residues were tested, two of which (xTsgC180A, xTsgC198A) had a phenotypic effect causing dorsalization, with reduced trunk and enlarged head structures (Fig. 1I and data not shown).
When W67G, the strongest ventralizing (pro-BMP) mutation, was combined with one of the dorsalizing mutations (C198A), the resulting double mutant (xTsgW67G+C198A) had almost no ventralizing activity in overexpression experiments (Fig. 1J, compare with 1H). However, in a subset of embryos injected with this double mutant weak phenotypes, including reduced head structures and defects in tail development could be observed. Mutation of a potential glycosylation site (xTsgS54A) resulted in a typical xTsg phenotype, with reduced head structures and a posteriorized anus, although the phenotype was stronger than its wild-type counterpart (compare Fig. 1D,F). S54 seems to be a site of glycosylation in vivo, as the mutant protein had faster electrophoretic mobility (Fig. 1K, compare lanes 1 and 3). Perhaps the enhanced activity of this glycosylation site mutant is due to increased diffusibility in the embryo.
We conclude from these data that mutations in the N-terminal cysteine-rich domain enhanced the ventralizing (pro-BMP) activity of Tsg, whereas those in the C-terminal domain caused the opposite effect, dorsalization. The phenotype of the double mutant further demonstrated that the hyperventralizing activity of xTsg mutants requires the function of the C-terminal cysteine-rich domain.
The hyperventralizing xTsgW67G mutant
To compare the ventralizing effects of wild-type xTsg and TsgW67G on CNS patterning, we analyzed the neural plate marker Sox2 by in situ hybridization. At early neurula stages, microinjection of 1 ng of xTsg mRNA led to reduction of the neural plate and the formation of a ring of Sox2 positive cells surrounding the slit-shaped blastopore (Fig. 2A,B). This ring may indicate a posteriorization of the embryo, and correlated with the posteriorized anus phenotype at tadpole stages. The mass of posteriorized and detached endoderm expressed Sox2, which marks the proctodeum (Fig. 2K,L) (Chalmers et al., 2000). At higher concentrations (4 ng), xTsg mRNA reduced the neural plate further, indicating that the wild-type protein has pro-BMP effects (Fig. 2C) (Oelgeschläger et al., 2000). Results were different when the hyperventralizing mutant TsgW67G was microinjected: the reduction of the neural plate was much more severe and the ring of Sox2-positive cells as well as the posteriorized anus were not seen (Fig. 2D,I).
Fig. 2.
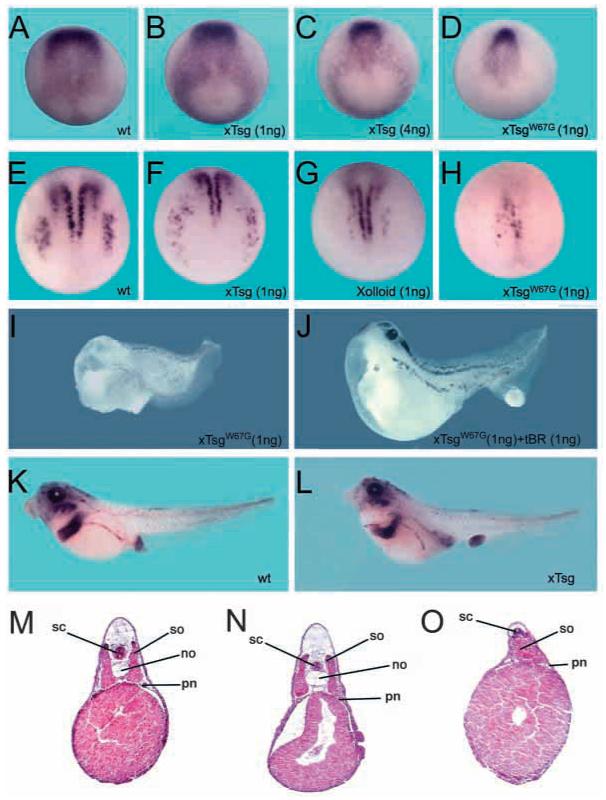
Effects of xTsg and xTsgW67G on neural patterning and dorsal development. (A-D) Expression of Sox-2 at stage 12 in embryos either uninjected (A), or microinjected in the animal pole at the four-cell stage with a total of 1 ng xTsg mRNA (B), 4 ng xTsg mRNA (C) or 1 ng xTsgW67G mRNA (D). (E,F) Expression of N-tubulin at stage 14 in wild-type embryos (E), or embryos microinjected at the two to four cell stage with 1 ng of xTsg (F), Xolloid (G) or xTsgW67G (H) mRNA. At least 10 embryos per sample were stained in each of two independent experiments. (I) Embryo injected four times into the marginal region at the two to four cell stage with 250 pg xTsgW67G mRNA. (J) Embryo co-injected with 250 pg TsgW67G and 250 pg dominant-negative BMP receptor (tBR) mRNA. (K,L) Sox-2 in situ hybridization of stage 42 embryos. (M-O) Histological sections of control, xTsg and xTsgW67G injected embryos. sc, spinal cord; so, somites; no, notochord; pn, pronephros.
Staining for N-tubulin, a marker for differentiated neurons, confirmed the decrease in anterior neural plate caused by the microinjection of xTsg mRNA. In these embryos, the lateral sensory (Rohon-Beard) neurons formed a ring surrounding the blastopore slit (Fig. 2E,F). Microinjection of xTsgW67G led to an almost complete inhibition of early neurogenesis (Fig. 2H) similar to, although more severe than, microinjection of Xolloid mRNA (Fig. 2G). The anti-neural effect of TsgW67G was mediated by increased BMP signaling, as co-injection of mRNA encoding dominant-negative BMP receptor (tBR) rescued the formation of dorsoanterior CNS structures such as forebrain and eyes (Fig. 2J). Most of the rescued embryos had a posteriorized anus that detached from the trunk (Fig. 2J). This phenotype was also observed in embryos injected into the animal pole with 1 ng of constitutively active BMP receptor or Smad5 mRNA (data not shown) (Beck et al., 2001). Thus, at this point it is not possible to establish whether the detachment of the proctodeum represents a pro-BMP or anti-BMP activity.
In histological sections, embryos overexpressing wild-type xTsg showed a mild reduction of the spinal cord and somites (Fig. 2M,N) and in some cases a hypoplastic notochord (data not shown) (Oelgeschläger et al., 2003). By contrast, embryos injected with TsgW67G showed considerable reduction of the brain, spinal cord, notochord and somites (Fig. 2O) (data not shown).
We conclude that xTsg mRNA inhibited development of the anterior neural plate and posteriorized the embryo. In the hyperventralizing mutant TsgW67G, the pro-BMP activities of wild-type xTsg were exacerbated.
Tsg and TsgW67G antagonize dorsalization of mesoderm by Activin
In Xenopus, formation of dorsal mesoderm is mediated by a horizontal signal secreted by Spemann’s organizer (Dale and Slack, 1987). This signal can be mimicked by treatment of ectodermal explants with Activin protein (Green et al., 1992; Dyson and Gurdon, 1998). Activin induces the expression of chordin in ectodermal explants, and we have recently shown that this expression of endogenous Chordin is required for dorsal mesoderm induction (Oelgeschläger et al., 2003). Treatment of ectodermal explants with 2 ng/ml Activin triggered the convergence-extension movements that accompany dorsal mesoderm formation (Fig. 3B). In explants microinjected with xTsg or TsgW67G, animal cap elongation was blocked (Fig. 3C,D). RT-PCR analysis confirmed that the induction by Activin of the dorsal mesodermal markers MyoD and α-Actin and of the pan-neural marker NCAM was inhibited by wild-type xTsg or TsgW67G mRNAs (Fig. 3F). Overexpressed xTsg is known to induce degradation of Chordin protein secreted by Spemann’s organizer (Larraín et al., 2001). As expected, microinjection of xTsg mRNA led to a decrease of full-length Chordin protein secreted by Activin-treated animal caps (Fig. 3E, lanes 2, 3). By contrast, TsgW67G increased the amount of Chordin protein secreted by Activin-treated animal caps (Fig. 3E, lane 4). Thus, although full-length Chordin protein was abundantly produced in animal caps in the presence of TsgW67G (Fig. 3E, lane 4), this Chordin protein was inactive in mesoderm dorsalization (Fig. 3F, lane 5).
Fig. 3.
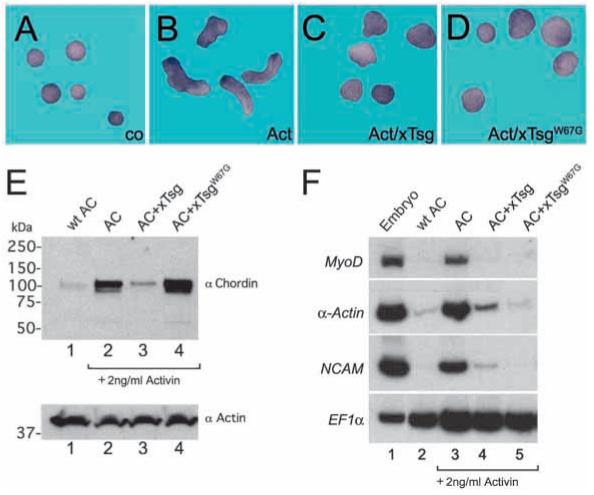
Wild-type xTsg and the hyperventralizing mutant xTsgW67G antagonize the dorsalization of mesoderm by Activin. (A-D) Animal cap explants were isolated at stage 8 and treated with 2 ng/ml Activin protein. Activin induces elongation (B). Microinjection of a total of 2 ng xTsg (C) or xTsgW67G (D) mRNA at the eight-cell stage into the animal blastomeres prevented elongation. (E) The accumulation of Activin-induced endogenous Chordin protein (lane 2) was reduced by wild-type xTsg mRNA injection (lane 3) and increased by xTsgW67G (lane 4). (F) RT-PCR analysis of animal cap explants treated with Activin after microinjection of 2 ng xTsg or xTsgW67G mRNA; both mRNAs inhibited the induction of dorsal mesodermal (MyoD, alpha-Actin) or neural (NCAM) marker genes by Activin. EF1α served as a loading control.
We conclude that xTsg and TsgW67G antagonize Activin dorsalization by different molecular mechanisms. The ventralizing activity of wild-type xTsg may be explained by the increased degradation of Chordin, but TsgW67G caused the accumulation of full-length Chordin protein that had lost its dorsalizing (anti-BMP) activity.
Hyperventralizing Tsg proteins do not bind BMP
To investigate the molecular mechanisms that underlie the ventralizing activities of xTsg and TsgW67G, we carried out a series of functional and biochemical experiments. As reported previously, xTsg RNA induced expression of the cement gland marker XAG-1 in animal cap explants (Fig. 4A, lane 3) (Chang et al., 2001; Larraín et al., 2001). However, this anti-BMP effect was not sufficient to induce the expression of the pan-neural marker NCAM, which requires even lower levels of BMP signaling (Fig. 4A). We tested the various xTsg point mutants in animal cap explants and found that, like wild-type xTsg, the dorsalizing mutant xTsgC198A induced XAG-1 but not NCAM expression (Fig. 4A, lane 5). The hyperventralizing mutations xTsgS36A, xTsgC59A and xTsgW67G did not induce XAG-1 expression in animal cap explants (Fig. 4A, lane 4 and data not shown). One possible explanation for this difference was that the N-terminal mutations might prevent binding of xTsg to BMP.
Fig. 4.
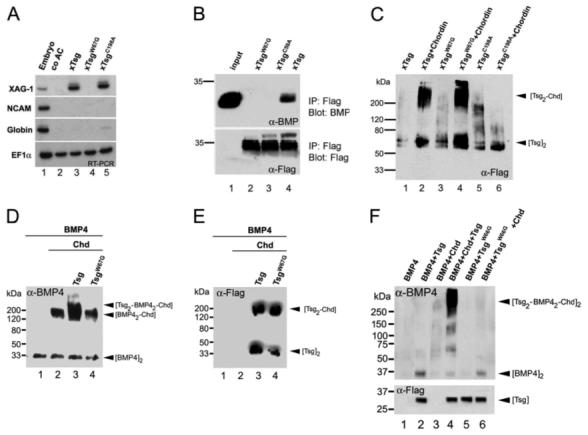
Biochemical analyses of xTsg mutant proteins. (A) RT-PCR of animal cap explants injected with 2 ng xTsg, xTsgW67G or xTsgC198A mRNA. (B) Immunoprecipitation of BMP4 bound to xTsg. Recombinant BMP4 (5 nM) was preincubated with either 20 nM xTsgW67G, xTsgC59A or wild-type xTsg protein (lanes 2, 3, 4). Immunoprecipitation was performed with a polyclonal antibody recognizing an N-terminal peptide present in the Tsg expression vector (Piccolo et al., 1999) and the immunoblot probed with anti-BMP4 or anti-Flag antibodies. (C) Tsg-Chordin complexes formed after crosslinking of 20 nM of the indicated affinity-purified xTsg proteins to 5 nM xChordin protein with DSS (disuccinimidyl suberate). xTsg proteins were visualized via the FLAG epitope. Note that xTsgW67G binds to Chordin (lane 4) and that xTsgC198A does not (lane 6). (D) Chemical crosslinking of BMP-Chordin-Tsg ternary complexes after incubation of BMP4 (5 nM, lane 1) with Chordin (5 nM, lane 2) and xTsg (20 nM, lane 3) or xTsgW67G (20 nM, lane 4) proteins. The western blot was stained with anti-BMP4 monoclonal antibody. (E) Anti-FLAG immunoblot of the same experiment shown in D, after stripping of the BMP4 antibody and incubation with an anti-FLAG antibody. (F) Crosslinking with DSS of 293T supernatants from cell cultures transfected separately with the indicated murine expression vectors and then co-cultured. Formation of a ternary BMP4-Chordin-Tsg complex led to a significant increase in BMP avidity by the monoclonal antibody (lane 4). The amounts of BMP4 in the supernatants vary, due to binding to the extracellular matrix of the cultured cells.
To test this biochemically, we prepared affinity-purified xTsg proteins and incubated them with recombinant BMP4 protein. BMP4/xTsg complexes were immunoprecipitated via the N-terminal FLAG-tag of the Tsg proteins, and immunoprecipitated proteins visualized with antibodies specific for BMP4. Wild-type xTsg bound BMP4 protein (Fig. 4B, lane 4) (Oelgeschläger et al., 2000). By contrast, two N-terminal mutations that were unable to induce XAG-1 expression in animal caps, TsgC59A and TsgW67G, did not bind BMP4 at detectable levels (Fig. 4B, lanes 2 and 3). Thus, the induction of XAG-1 in animal caps by overexpressed xTsg correlated with a mild anti-BMP activity of xTsg mediated by direct binding to BMP. Importantly, TsgC59A and TsgW67G, which were devoid of BMP-binding activity, had strong ventralizing (pro-BMP) activity in biological assays.
TsgW67G binds to Chordin
To test whether binding to Chordin was affected by the mutations, affinity-purified mutant xTsg proteins were incubated with full-length Xenopus recombinant Chordin protein, and complexes chemically crosslinked with disuccinimidyl suberate (DSS). Wild-type xTsg protein formed a high molecular weight complex in the presence of Chordin, corresponding to a dimer of xTsg bound to Chordin (Fig. 4C, lane 2). A similar complex was observed in the presence of TsgW67G and Chordin protein (Fig. 4C, lane 4). By contrast, Chordin-Tsg complexes were not detectable using the C-terminal mutant TsgC198A (Fig. 4C, lane 6).
We conclude from these biochemical experiments that the hyperventralizing mutant TsgW67G is not affected in its ability to bind to Chordin, and that dorsalizing mutations in the C-terminal domain prevent binding of xTsg to Chordin.
TsgW67G does not form ternary complexes with Chordin and BMP
We next asked whether the lack of BMP binding activity in hyperventralizing xTsg mutants affected the formation of ternary complexes with BMP and Chordin. Affinity-purified xTsg proteins (at a concentration of 20 nM) were incubated with 5 nM BMP4 and 2 nM Chordin protein, chemically crosslinked with DSS, and BMP4 visualized with a monoclonal antibody specific for BMP4 (Masuhara et al., 1995). Incubation of BMP4 and Chordin protein led to the formation of a high molecular weight complex with a molecular mass corresponding to a BMP4 dimer bound to a Chordin monomer (Fig. 4D, lane 2). Incubation of wild-type Tsg protein with Chordin and BMP4 generated a ternary complex corresponding to a Tsg dimer bound to a BMP4 dimer and Chordin (Fig. 4D, lane 3).
By contrast, when TsgW67G protein was used, only the Chordin-BMP4 complex was detectable (Fig. 4D, lane 4). When the same blot was incubated with a Flag-Tsg specific antibody, complexes of Chordin and TsgW67G were detectable (Fig. 4E, lane 4). As ternary complexes were not formed, this indicates that the full-length Chordin protein bound to TsgW67G lost its BMP4 binding ability. This result was surprising, as Chordin is a large protein containing four BMP-binding domains of the CR type. Tsg is a rather small molecule, five times smaller than Chordin, with only one BMP interaction domain. These results raise the possibility that TsgW67G might inactivate the BMP-binding activity of Chordin in an active way (see below).
The inability of TsgW67G to form ternary complexes with Chordin and BMP was confirmed using a co-culture system of human 293T kidney cells. Cell cultures separately transfected with expression constructs for mouse Chordin, BMP4, mTsg or mTsgW66G were mixed, cultured together for 24 hours, and proteins secreted into the culture medium analyzed after chemical crosslinking. These conditions greatly increased the avidity of the monoclonal antibody for BMP4 in trimolecular complexes. (Fig. 4F, lanes 2-4 and data not shown). Even under these conditions, which greatly facilitate the detection of trimolecular Tsg-Chd-BMP complexes, the TsgW66G mutation precluded the binding of BMP4 to Chordin complexes (Fig. 4F, lane 6).
We conclude from these biochemical experiments that the hyperventralizing mutations prevent binding of Tsg to BMP4 but allow binding to full-length Chordin. Binding of xTsgW67G to Chordin prevents binding of BMP to either protein, and this inhibition correlates with the enhanced ventralizing activity of this Tsg mutant.
TsgW67G inhibits CR-modules
When Chordin is digested by the Tolloid/Xolloid metalloprotease the molecule is cleaved twice, releasing intact CR modules that can still bind and inhibit BMP. Unlike full-length Chordin, individual CR modules do not form stable complexes with xTsg. However, wild-type xTsg has the ability to antagonize the residual anti-BMP activity of the proteolytic fragments (Oelgeschläger et al., 2000; Larraín et al., 2001). We tested the effects of TsgW67G on Xenopus CR1, a protein that mimics the N-terminal cleavage product. Microinjection of mRNA encoding xCR1 induced the expression of the anterior neural marker genes Rx2a and Six3, the pan-neural marker gene NCAM, and the cement gland marker XAG-I in animal cap explants (Fig. 5A, lane 3). Co-injection of wild-type xTsg mRNA inhibited the expression of anterior neural marker genes but expression of XAG-1 was still observed (Fig. 5A, lane 4). Co-injection of TsgW67G mRNA completely blocked the induction of XAG1 and of neural markers by xCR1 (Fig. 5A, lane 5). In addition, expression of the epidermal BMP target gene Msx1, which was inhibited by xCR1, was restored by TsgW67G mRNA (Fig. 5A).
Fig. 5.
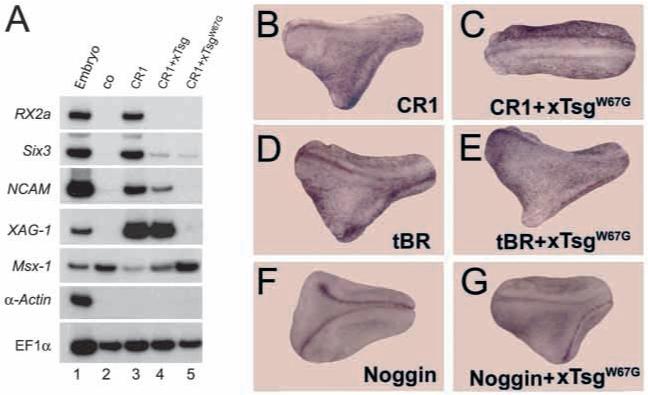
xTsgW67G inactivates an isolated CR module but not Noggin or a dominant-negative BMP receptor. (A) RT-PCR analysis of animal caps injected with the indicated mRNAs. Xenopus CR1 mRNA (80 pg total) was injected alone or together with 1 ng xTsg or xTsgW67G mRNA. (B) Microinjection of 20 pg CR1 mRNA induces secondary axes at high frequency (79%, n=79). (C) Formation of secondary axes was completely blocked by co-injection of 250 pg xTsgW67G mRNA (0%, n=39). (D) Secondary axes were efficiently induced by microinjection of 1 ng mRNA encoding a dominant-negative BMP receptor (tBR; 67%, n=49). (E) Axis-inducing activity of tBR was not affected by co-injection of 250 pg xTsgW67G (65%, n=57). (F) Injection of 0.1 pg Noggin mRNA induced secondary axes (68%, n=40). (G) The activity of Noggin was not inhibited by co-injection of 250 pg xTsgW67G mRNA (67%, n=42).
Thus, TsgW67G, like wild-type Tsg, can inactivate the anti-BMP activity of a CR-module. This was surprising, as we had previously assumed from biochemical experiments that xTsg inhibited CRs through mutual competition for BMP binding (Larraín et al., 2001). Because the hyperventralizing TsgW67G mutant does not bind BMP4, its effects on CR1 activity must be through a different, non-competitive, molecular mechanism.
TsgW67G is specific for CR-containing proteins
We next tested the effect of TsgW67G on other inhibitors of BMP signaling that lack CR-modules. Ventral injection of tBR mRNA resulted in the formation of partial secondary axes that were not affected by co-injection of TsgW67G mRNA (Fig. 5D,E). This shows that TsgW67G acts on the BMP signaling pathway, upstream of the BMP receptor. Microinjection of noggin mRNA induced secondary axes that were not affected by co-injection of TsgW67G mRNA (Fig. 5F,G). By contrast, axes induced by injection of xCR1 mRNA were abolished by co-injection of TsgW67G (Fig. 5B,C). As TsgW67G does not affect dorsalization by tBR or Noggin, we conclude that the effects of TsgW67G in the embryo are specific for CR modules.
Hyperventralizing Tsgs compared with Chordin loss of function
Chordin downregulation after microinjection of morpholino oligonucleotides results in a moderate ventralization of Xenopus embryos comparable with that of chordino mutant zebrafish (Oelgeschläger et al., 2003). The phenotype of the hyperventralizing mutants xTsgW67G and xTsgC59A is much more severe, causing extensive loss of the CNS, particularly in the anterior (Fig. 1G,H). Striking pro-BMP effects of xTsgW67G were observed in LiCl-treated Xenopus embryos. Embryos treated at the 32-cell stage with 120 nM LiCl expressed organizer genes such as Chordin throughout the marginal zone at gastrula and cause the development of embryos with radial head structures lacking trunk-tail structures (Fig. 6A,B; Dorsoanterior index, DAI=9.25, n=16). The effect of LiCl can be blocked by microinjection of Chordin morpholinos (Oelgeschläger et al., 2003). Even in the presence of LiCl, Chordin morpholinos produced a chordino-like weakly ventralized phenotype (Fig. 6D). Overexpression of TsgW67G had a strong effect on LiCl treated embryos. Microinjection of TsgW67G mRNA into each of the four blastomeres resulted in embryos that were strongly ventralized and lacked CNS and axial structures, despite having been treated with LiCl (Fig. 6C, DAI=1.1, n=11). Thus, the ventralizing effect of TsgW67G is much stronger than that of Chordin loss of function, and suppressed most dorsal development even in what should have been dorsalized LiCl-treated embryos.
Fig. 6.
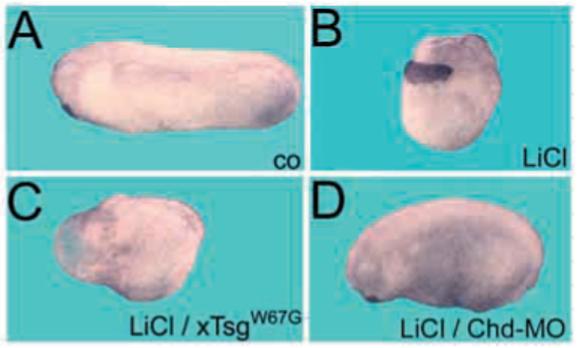
xTsgW67G overexpression prevents dorsalization by LiCl. (A) Untreated stage 30 embryo. (B) Radially dorsalized embryo obtained after LiCl treatment at the 32-cell stage (DAI=9.25, n=16). (C) Embryo microinjected into the marginal zone of each blastomere at the four cell stage with 500 pg xTsgW67G mRNA (DAI=1.1, n=11) or (D) microinjected with a total of 8 ng anti-Chordin morpholino oligos at the two-cell stage (DAI=6.4, n=36) prior to LiCl treatment at 32-cell stage. Note that xTsgW67G causes complete ventralization, which cannot be reversed by LiCl treatment.
In zebrafish, the complete loss-of-function phenotype of Chordin is well characterized (Hammerschmidt, 1996a; Wagner and Mullins, 2002). As xTsg binds to Chordin and can promote Chordin degradation in overexpression studies (Oelgeschläger et al., 2000; Scott et al., 2001; Larraín et al., 2001), it was important to determine whether the effects of xTsg hyperventralizing mutants should be explained solely by antagonism of Chordin. We overexpressed wild-type xTsg and two xTsg hyperventralizing variants in zebrafish embryos. Wild-type xTsg mRNA dorsalized these embryos (Fig. 7A-D), as reported previously for zebrafish Tsg (Ross et al., 2001). The opposite effect, ventralization, is observed in Xenopus (Fig. 2B,C) (Oelgeschläger et al., 2000). This difference has been proposed to be due to lower levels of Tolloid activity during early zebrafish development (Connors et al., 1999), which would cause accumulation of inhibitory Chordin/Tsg/BMP ternary complexes (Larraín et al., 2001).
Fig. 7.
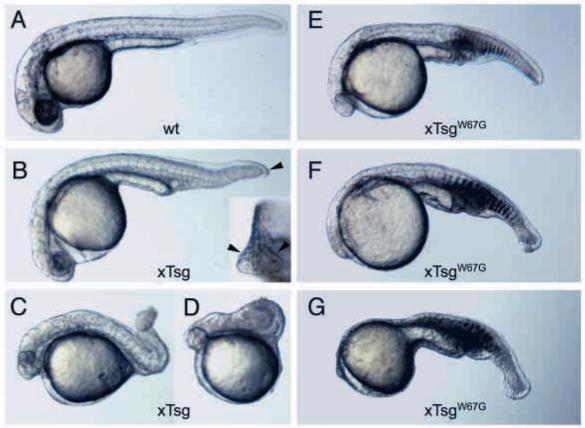
Overexpression of hyperventralizing xTsg constructs in zebrafish embryos inhibits dorsoanterior development. (A) Uninjected sibling. Wild-type zebrafish embryos were injected at the one-cell stage with 400 pg wild-type xTsg mRNA (B-D) or with 330 pg xTsgW67G mRNA (E-G). Embryos injected with wild-type xTsg mRNA in two independent experiments (n=144) displayed a range of dorsalization (as classified by Mullins et al., 1996): 13% Class 1 (not shown), 44% Class 2 (B), 17% Class 3 (C) and 24% Class 4 (D). Of the embryos displaying Class 1 or 2 dorsalizations, 51% also exhibited duplication of the terminal ventral fin (inset in B, indicated by arrowheads), suggestive of a tail ventralization in zebrafish. Of 199 embryos injected with 330 pg xTsgW67G mRNA in two independent experiments, 73% were moderately ventralized to levels comparable with chordino (E). 14% showed a phenotype more severe than chordino (F) and 3% displayed an even more ventralized phenotype (G). At higher doses (800 pg), xTsgW67G mRNA caused phenotypes stronger than chordino in 86.8% of embryos (n=91), with 18.7% of the type shown in G and 67% of the type shown in F. Note the almost complete absence of brain and trunk somites in G. Injection of 200 pg xTsgC59A mRNA into 60 embryos (not shown) also caused ventralization, with 82% appearing similar to E, and 5% resembling F.
However, we now report that overexpression of wild-type xTsg in zebrafish also caused a pro-BMP phenotype in the tail region. About half of the weakly dorsalized embryos had a duplication of the ventral fin tip, visible in posterior views (see inset in Fig. 7B). These duplications are typically seen in a weak allele of chordino, m52, and in mercedes/ogon ventralized mutants (Hammerschmidt et al., 1996a; Wagner and Mullins, 2002). Perhaps at later stages of development, when the tail is patterned, higher Tolloid levels favor the pro-BMP activity of xTsg mRNA. A duplicated ventral fin has been recently reported for Smad5 mutant fish (Kramer et al., 2002), making less clear whether duplication of the ventral fin always reflects a pro-BMP effect.
Whereas wild-type xTsg overexpression may cause both dorsalization and ventralization in zebrafish, hyperventralizing xTsgW67G and xTsgC59A constructs had only pro-BMP effects. As seen in Fig. 7E-G, the anterior CNS was hypoplastic, lacking brain and eyes in the most severe cases, and trunk somites were defective or absent. The degree of ventralization caused by xTsgW67G and xTsgC59A was more severe (Fig. 7) than the ventralization caused by complete loss-of-function of Chordin in zebrafish (Hammerschmidt et al., 1996a; Ross et al., 2001; Wagner and Mullins, 2002) or by knock-down using Chordin morpholinos in Xenopus (Oelgeschläger et al., 2003). When 800 pg xTsg W67G mRNA was injected into embryos resulting from din/+ x din/+ crosses (n=147), we recovered 14 embryos that had a din/din genotype by PCR but a ventralized phenotype stronger than that in chordino mutants. We used the tt250 allele of chordino, which results in a null mutation (Hammerschmidt et al., 1996a; Schulte-Merker et al., 1997; Wagner and Mullins, 2002). Therefore, these experiments demonstrate that xTsgW67G can exert ventralizing effects even in the absence of Chordin protein.
As the hyperventralizing Tsg activities are specific for proteins containing CR modules (Fig. 5), these data indicate that other CR-containing proteins, in addition to Chordin, must be required for the establishment of dorsal cell fate in Xenopus and zebrafish development.
DISCUSSION
The multiple activities of Tsg
In an effort to separate the multiple activities of Tsg, we introduced point mutations in xTsg, concentrating on the evolutionarily conserved cysteine-rich domains located at the N and C termini. Three mutations in the N terminus at positions 36, 59 and 67 generated a series of mutants with increasing ventralizing (pro-BMP) activities. The strongest one, xTsgW67G, resulted in phenotypes corresponding to ventral belly pieces (’Bauchstücke’) lacking CNS and dorsal mesoderm. Such embryos are typically seen after UV-treatment and microinjection of BMP4 or BMP7 mRNA into Xenopus embryos. These N-terminal mutations exacerbate the ventralizing activity of overexpressed wild-type xTsg in Xenopus (Oelgeschläger et al., 2000).
Mutations in conserved cysteines of the C-terminal domain at positions 180 and 198 resulted in the opposite phenotype, a mild dorsalization, with enlarged head structures and shortened trunks. This phenotype indicated anti-BMP activity, which could be explained by their maintaining BMP-binding ability in combination with a loss of the ventralizing activity contained in the C-terminal domain. When the strongest ventralizing and dorsalizing mutations were combined in the same molecule, the resulting molecule had almost no activity in overexpression assays. Thus, the two conserved domains of Tsg appear to have opposing biological activities.
The hyperventralizing Tsg mutants
Biochemical studies showed that xTsgC59A and xTsgW67G mutants were unable to bind BMP4 but were still able to bind to full-length Chordin. The C-terminal mutations, however, were unable to bind Chordin, suggesting that their dorsalizing activity is caused exclusively by BMP binding. The strongest ventralizing mutant, xTsgW67G, bound to full-length Chordin and prevented the recruitment of BMP into a ternary complex (Fig. 4D,F).
The hyperventralizing mutants of xTsg described here are not a neomorphic activity resulting from the point mutations. This is because wild-type xTsg also has ventralizing effects in Xenopus embryos (Fig. 2B,C) (Oelgeschläger et al., 2000). Wild-type xTsg mRNA has very potent ventralizing effects (inhibition of CNS, notochord or muscle) in Xenopus embryos in which the levels of Chordin have been reduced by microinjection of chordin antisense morpholino oligos (Oelgeschläger et al., 2003).
xTsgW67G was not only able to antagonize the activity of full-length Chordin, but also that of an isolated CR module (Fig. 5). We had previously shown that CR1 was able to bind and antagonize BMP and that this binding could be competed by increasing amounts of xTsg protein without formation of ternary complexes (Larraín et al., 2001). It was assumed that xTsg acted via competition for BMP binding. However, as xTsgW67G did not bind BMP, the inactivation of the anti-BMP activity of this CR module must be achieved through a different molecular mechanism. This raises the possibility that the C-terminal domain of Tsg contains a biochemical activity able to inactivate CR domains. Although the C-terminal domain of Tsg presented some homology to bacterial nitrate reductases (narH) in blast searches, these similarities were weak and the catalytic residues not conserved. It will be important to compare this domain of Tsg to other enzymes once its three-dimensional structure is available.
The N-terminal domain could have an autoinhibitory effect on the biochemical activity of the C terminus. Autoinhibitory mechanisms by intramolecular interactions have been described for a variety of enzymes including the Src tyrosine kinase, GSK3β and ubiquitin ligases (Nguyen and Lim, 1997; Harwood, 2001; Du et al., 2002), and are commonly described as the jack-knife model. A requirement of the N-terminal domain for autoinhibition of Tsg might explain why multiple mutations in this domain have similar phenotypic effects on an intriguing activity that resides in the C terminus of the molecule.
xTsg must regulate multiple CR-containing proteins
The ventralizing (pro-BMP) activity of xTsgW67G was specific for proteins containing CR modules, as it was able to block Chordin and an isolated CR-domain, but did not inhibit the structurally unrelated BMP antagonist Noggin or a dominant-negative BMP receptor (tBR). Biochemical analyses have so far failed to detect stable binding of xTsg or xTsgW67G to isolated CR domains (Oelgeschläger et al., 2000; Larraín et al., 2001) (M.O. and E.M.D.R., unpublished). The ventralizing activity of xTsgW67G mRNA is very potent, for it can almost completely eliminate formation of CNS and dorsal mesoderm when overexpressed in Xenopus or zebrafish embryos. In addition, xTsgW67G completely blocked the dorsalizing effects of LiCl and Activin protein.
The effects of xTsgW67G cannot be solely due to inactivation of endogenous Chordin protein. In zebrafish embryos, overexpression of hyperventralizing Tsg mutants (Fig. 7) resulted in embryos that were much more ventralized than a chordino null allele (Hammerschmidt et al., 1996a; Schulte-Merker et al., 1997; Wagner and Mullins, 2002). Experiments using chordino mutant zebrafish embryos confirmed that xTsgW67G can exert ventralizing effects that are independent of the presence of Chordin. Taken together, these results indicate that hyperventralizing xTsg mutants must act on other CR-containing proteins in addition to Chordin.
Chordin is probably only the tip of the iceberg. CR-containing proteins are part of a growing family of secreted proteins (Garcia-Abreu et al., 2002), many of which have been shown to bind BMP. For example, CTGF and isoforms of procollagens contain single CR modules and have been shown to bind both BMPs and TGFβs (Abreu et al., 2002; Zhu et al., 1999; Larraín et al., 2000). Kielin, CRIM1 (cysteine-rich motor neuron 1), Crossveinless 2, Neuralin 1/Ventroptin and Neuralin 2 contain multiple CR repeats and have been implicated in the regulation of BMP and TGFβs (Matsui et al., 2000; Kolle et al., 2000; Larraín et al., 2000; Coffinier et al., 2001; Nakayama et al., 2001; Sakuta et al., 2001; Coffinier et al., 2002). Any of these CR-containing proteins, or as yet undiscovered ones, may contribute to dorsoventral patterning in the course of normal development.
Acknowledgments
We thank Drs K. Matsuhara, N. Ueno and I. Dawid for materials; C. Coffinier, I. Haas, E. Pera, A. Tomilin and O. Wessely for comments on the manuscript; and U. Tran, D. Geissert and A. Cuellar for technical assistance. M.O. was a HFSPO postdoctoral fellow and J.L. a Pew Latin Fellow. This work was supported by the NIH (HD21502-17 to E.M.D.R. and GM56326 to M.C.M.) and a Developmental Grant Award to M.C.M. from The Center for Research in FOP and Related Disorders. E.M.D.R. is a Howard Hughes Medical Institute Investigator.
REFERENCES
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland R, Rossant J, De Robertis EM. The organizer secreted factors Chordin and Noggin are required for forebrain development in the mouse. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Schneyder N, Tran U, Anderson R, Rossant J, De Robertis EM. The role of Chordin/BMP signals in mammalian pharyngeal development and DiGeorge phenotype in mice. Development. 2003;130:3567–3578. doi: 10.1242/dev.00581. [DOI] [PubMed] [Google Scholar]
- Beck CW, Whitman M, Slack JM. The role of BMP signaling in outgrowth and patterning of the Xenopus tail bud. Dev. Biol. 2001;238:303–314. doi: 10.1006/dbio.2001.0407. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Slack JM, Beck CW. Regional gene expression in the epithelia of the Xenopus tadpole gut. Mech. Dev. 2000;96:125–128. doi: 10.1016/s0925-4773(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of Tolloid/mini-fin in dorsoventral formation in the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Tran U, Larraín J, De Robertis EM. Neuralin is a novel Chordin-related molecule expressed in the mouse neural plate. Mech. Dev. 2001;100:119–122. doi: 10.1016/s0925-4773(00)00507-4. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Ketpura N, Tran U, Geissert D, De Robertis EM. Mouse Crossveinless-2 as the vertebrate homolog of a Drosophila extracellular receptor of BMP signaling. Gene Exp. Patt. Mech. Dev. 2002;2:189–194. [Google Scholar]
- Dale L, Slack JM. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987;100:279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The establishment of Spemann’s Organizer and patterning of the vertebrate embryo. Nat. Rev. Gen. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Aréchaga J, editors. International Journal of Developmental Biology. Vol. 45. The University of the Basque Country Press; Bilbao, Spain: 2001. The Spemann Organizer 75 years on. [Google Scholar]
- Du F, Navarro-Garcia F, Xia Z, Tasaki T, Varshavsky A. Pairs of dipeptides synergistically activate the binding of substrate by ubiquitin ligase through dissociation of its autoinhibitory domain. Proc. Natl. Acad. Sci. USA. 2002;99:14110–14115. doi: 10.1073/pnas.172527399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson S, Gurdon JB. The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell. 1998;93:557–568. doi: 10.1016/s0092-8674(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. doi: 10.1038/nature01061. [DOI] [PubMed] [Google Scholar]
- François V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- García-Abreu J, Coffinier C, Larraín J, Oelgeschläger M, De Robertis EM. Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene. 2002;287:39–47. doi: 10.1016/s0378-1119(01)00827-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez EM, Fekany-Lee K, Carmany-Rampey A, Erter C, Topczewski J, Wright CV, Solnica-Krezel L. Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes Dev. 2000;14:3087–3092. doi: 10.1101/gad.852400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JB, New HV, Smith JC. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996a;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzjia GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996b;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Mullins MC. Dorsoventral patterning in the zebrafish: bone morphogenetic proteins and beyond. In: Solnica-Krezel L, editor. Pattern Formation in Zebrafish. Springer-Verlag; Heidelberg, Germany: 2002. pp. 72–95. [DOI] [PubMed] [Google Scholar]
- Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev. Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kolle G, Georgas K, Holmes GP, Little MH, Yamada T. CRIM1, a novel gene encoding a cysteine-rich repeat protein, is developmentally regulated and implicated in vertebrate CNS development and organogenesis. Mech. Dev. 2000;90:181–193. doi: 10.1016/s0925-4773(99)00248-8. [DOI] [PubMed] [Google Scholar]
- Kramer C, Mayr T, Nowak M, Schumacher J, Runke G, Bauer H, Wagner DS, Schmid B, Imai Y, Talbot WS, Mullins MC, Hammerschmidt M. Maternally supplied Smad5 is required for ventral specification in zebrafish embryos prior to zygotic Bmp signaling. Dev. Biol. 2002;250:263–279. [PubMed] [Google Scholar]
- Larraín J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraín J, Oelgeschläger M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Mizuseki K, Nakatani J, Nakanishi S, Sasai Y. Xenopus kielin: A dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline. Proc. Natl. Acad. Sci. USA. 2000;97:5291–5296. doi: 10.1073/pnas.090020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuhara K, Nakase T, Suzuki S, Takaoka K, Matsui M, Anderson HC. Use of monoclonal antibody to detect bone morphogenetic protein-4 (BMP-4) Bone. 1995;16:91–96. doi: 10.1016/s8756-3282(94)00014-x. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJM, Furutani-Seiki M, Granato M, Haffter P, Heisenberg C-P, Jiang Y-J, Kelsh RN, Nüsslein-Volhard C. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, Wen D. A novel Chordin-like protein inhibitor for Bone Morphogenetic Proteins expressed preferentially in mesenchymal cell lineages. Dev. Biol. 2001;232:372–387. doi: 10.1006/dbio.2001.0200. [DOI] [PubMed] [Google Scholar]
- Nguyen JT, Lim WA. How Src exercises self-restraint. Nat. Struct. Biol. 1997;4:256–260. doi: 10.1038/nsb0497-256. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signaling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev. Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;9:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Guadenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular antagonist. Nature. 2001;410:423–424. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura Si., Yamamoto TS, Ueno N, Noda M. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science. 2001;293:111–115. doi: 10.1126/science.1058379. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KWY, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–478. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. Int. J. Dev. Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- Stern CD. Initial patterning of the central nervous system: how many organizers? Nat. Rev. Neurosci. 2001;2:92–98. doi: 10.1038/35053563. [DOI] [PubMed] [Google Scholar]
- Wagner DS, Mullins MC. Modulation of BMP activity in dorsal-ventral pattern formation by the chordin and ogon antagonists. Dev. Biol. 2002;245:109–123. doi: 10.1006/dbio.2002.0614. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J. Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]


