Abstract
The purpose of this work was to study the effect of pH on the liposomal encapsulation of a model camptothecin anti-tumor agent, DB-67, by considering the state of ionization and bilayer membrane/water partitioning of the drug as a function of pH. A novel fluorescence method was developed to monitor intravesicular pH in liposomal formulations containing entrapped DB-67 by using the drug itself as a pH indicator. Fluorescence spectra were recorded in aqueous buffers and liposomes and used to estimate the ionization constant of the A-ring phenol of DB-67 (pKa2) and shifts in ionization constants (pKa1 and pKa2) due to membrane binding. Bilayer/water partitioning studies by equilibrium dialysis were employed to show that DB-67 is highly membrane bound over the entire pH range examined though binding decreases with an increase in pH. The observed ionization constants of membrane-bound DB-67 obtained from the equilibrium dialysis experiments were consistent with observations from fluorescence measurements and previous permeability results. The pH dependence of DB-67 loading using a passive loading technique was found to reflect the pH dependence of membrane binding of the drug. This results in poor encapsulation efficiency of DB-67 at high pH, necessitating further development of formulation strategies to improve loading efficiency.
Keywords: Liposomes, nanoparticles, intraliposomal pH, membrane binding, lipid bilayers, camptothecins, tumor drug delivery, fluorescence, drug encapsulation
1. INTRODUCTION
Liposomal encapsulation of anti-tumor agents may enhance anti-tumor efficacy by exploiting the passive tumor accumulation of liposomal nanoparticles (Gabizon, 2001). Efficient techniques for loading drug into liposomes are required to achieve therapeutic drug concentrations in vivo and prolonged liposomal drug retention is necessary to avoid potential side-effects due to premature leakage. Drug efflux from the liposomes must occur following the accumulation of liposomes in solid tumors. Since liposomes do not permeate deep into tumor tissue, the drug must diffuse through the tumor interstitium to reach distant tumor cells (Yuan et al., 1994). Thus, the release rate of drug from liposomes should be optimized such that most of the liposomally entrapped drug is retained in circulating liposomes but released at the tumor site at a controllable rate following tumor accumulation. The development of such liposomal delivery systems requires an understanding of the lipid bilayer permeability and partitioning behavior of the encapsulated drug.
Silatecan 7-t-butyldimethylsilyl-10-hydroxycamptothecin (DB-67, Scheme I) is a novel, highly potent, lipophilic camptothecin with enhanced blood stability and promising anti-tumor activity both in vitro and in vivo (Bom et al., 2000; Lopez-Barcons et al., 2004) that has been recently approved by the FDA for phase I clinical studies. Camptothecins, including DB-67, undergo a pH dependent hydrolysis in solution (Fassberg and Stella, 1992; Xiang and Anderson, 2002). Four different DB-67 species may exist in solution with the fraction of each species depending on solution pH. Scheme I shows the equilibria for ring opening of DB-67 lactone (I) and its subsequent ionization. At physiological pH, the ring-opened ionized carboxylate form (III) predominates.
Scheme 1.
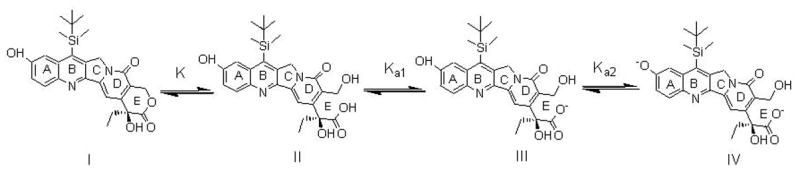
Equilibria between DB-67 lactone (I), DB-67 carboxylic acid (II) and the DB-67 carboxylate monoanion (III) and dianion (IV).
Previously, liposomal formulation strategies for neutral camptothecins have focused on the benefits of membrane binding of the lactone to improve the stability of camptothecins toward hydrolysis (Burke et al., 1993; Peikov et al., 2005; Zhang et al., 2004). We have recently investigated the potential of a novel pH gradient strategy for improving the liposomal retention of neutral camptothecins such as DB-67 by encapsulating the drug in rigid gel phase liposomes at high pH (Joguparthi and Anderson, 2007; Joguparthi et al., 2007). This strategy relies on entrapping the ring-opened, ionized carboxylate in liposomes to prolong retention while the species released from liposomes is still the neutral lactone. Since membrane binding facilitates drug loading into liposomes, and because drug ionization was expected to reduce membrane binding, we investigated the liposomal drug loading efficiency of DB-67 as a function of pH.
The success of the pH gradient strategy for prolonging drug retention was previously found to be partially diminished by the loss of trans-membrane pH gradients under physiological conditions, resulting in a faster drug release from liposomes than desired (Joguparthi and Anderson, 2007). This loss of pH gradient was attributed to the rapid equilibration of CO2/H2CO3 between the intravesicular and extravesicular compartments due to the high concentration of carbonate in physiological fluids (Joguparthi and Anderson, 2007). In order to directly confirm the loss of pH gradients and to monitor the pH in liposomes, we developed a fluorimetric method to monitor the intraliposomal pH in DB-67 liposomal formulations above pH 7.4. Commercially available fluorescence-based pH probes that are often used to monitor intracellular and intraliposomal pH (e.g., fluorescein (pKa = 6.4), SNARF-1 (pKa = 7.5), HPTS (pKa = 7.3), BCECF (pKa = 6.5), etc.) exhibit spectral properties that are highly sensitive to pH and local microenvironment (i.e., aqueous vs. interfacial regions) in the physiological pH range (pH 7–8) (Valeur, 2002). However, none of these are useful probes for liposomes containing entrapped DB-67 at a higher pH. Additionally, the chosen probe should be stable under liposomal formulation conditions and should not interfere with liposome loading or permeation of DB-67. To avoid the additional complexities involved in finding such an optimal probe, we chose to investigate the potential of DB-67 as a self-probe to monitor intraliposomal pH. The pKa of the A-ring phenol group of DB-67 in aqueous solution is 8.67 ± 0.20 (Xiang and Anderson, 2002), within the pH region of interest (7.4–10) for studies of liposomal formulations of DB-67 involving anionic entrapment and release. The pH dependence of the fluorescence emission and excitation spectra of DB-67 in aqueous solution or at lipid bilayer membrane interfaces has not been previously characterized.
2. MATERIALS AND METHODS
2.1. Materials
DB-67 was provided by the Novartis Pharmaceuticals Corporation (East Hanover, NJ). Pre-cut dialysis membrane discs (MWCO: 12000–14000) were obtained from Spectrum Laboratories (Rancho Dominguez, CA). Phospholipids 1, 2-distearoyl-sn-glycero-3-phosphatidylcholine (DSPC, >99% purity) and 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[polyethylene glycol 2000] (m-PEG DSPE, MW = 2806, >99% purity) were purchased as powders from Avanti Polar Lipids (Alabaster, AL). Sephadex® G-25M pre-packed size exclusion columns were obtained from GE Healthcare Biosciences Corporation (Piscataway, NJ). All other reagents were purchased from Fisher Scientific (Florence, KY).
2.2. Fluorescence Measurements of DB-67 in Aqueous Buffers
Aqueous drug solutions for fluorescence measurements were prepared by diluting an ethanolic stock solution of DB-67 (3 mM) into various buffers (70 mM Na phosphate (pH=7.3–7.7); 100 mM Na borate (pH=8.4–9.5); and 80 mM Na carbonate (pH=10.1)) to obtain a target concentration of 1 μM. Following drug addition, the solutions were incubated overnight at 37ºC to allow the reversible lactone ring-opening reaction to reach equilibrium. For concentration quenching studies, DB-67 solutions at various concentrations (0.0002–20 mM) were prepared in 80 mM Na carbonate buffer (pH=10.1). DB-67 solutions at 37°C and at varying pH were scanned (FluoroMax-3, Jobin Yvon Inc, Edison, NJ) by fixing the excitation and emission wavelengths at 380 nm and 560 nm to obtain fluorescence emission and excitation spectra. Potential photobleaching and photodegradation of DB-67 during fluorescence measurements were tested by continuous measurement of the excitation and emission spectra of DB-67 solutions.
2.3. Preparation of Liposomes for Fluorimetric, Equilibrium Dialysis and Drug Loading Measurements
DB-67 solutions were prepared in 85 mM Na acetate (pH 4), 80 mM Na citrate (pH 6.1–6.8), 70 mM Na phosphate (pH 7.3–7.7), 100 mM Na borate (pH 8.4–9.5) and 70–80 mM Na carbonate (pH 9.0–10.1) buffers at 1 μM (pH 4–7.3), 5 μM (pH 7.7) and 10 μM (pH 8.4–10.1) as described earlier. Pre-weighed DSPC and m-PEG DSPE (95:5 mol%) were dissolved in chloroform, evaporated under N2 and dried in a vacuum oven overnight. The dried lipid films were hydrated with 2 ml of drug solution in a 60°C water bath and extruded 10 times through two stacked 200 nm pore size polycarbonate membranes (Nuclepore, Pleasanton, CA) using an extrusion device (LiposoFast, Avestin, Ottawa, Canada) to prepare drug loaded unilamellar vesicles. Blank liposomes for equilibrium dialysis measurements were prepared similarly to the drug loaded liposomes at various pH values using the same buffers used for preparing drug loaded vesicles. Following their preparation both blank and drug loaded vesicles were allowed to cool to room temperature for 3 hours and stored at 5°C prior to their use in fluorescence and equilibrium dialysis measurements. Particle size of the vesicles was determined by dynamic light scattering (DLS) using a Malvern Zetasizer 3000 (Malvern Instruments Ltd, Malvern, UK). The pH, particle size and lipid (DSPC) concentration of all liposome suspensions were monitored to ensure no aggregation or lipid degradation during storage.
2.4. Fluorescence Measurements in Liposomes
Liposome entrapped drug was separated from unentrapped drug by loading 0.5 ml of the liposome suspensions prepared at varying pH (7.7–10.1) on a Sephadex® G-25 size exclusion column and eluting with 5 ml of the corresponding buffer. The first 4 ml of the eluent suspension was collected and its fluorescence excitation (250–400 nm) and emission (400–600 nm) spectra were immediately recorded. Light scattering from liposome particles was reduced by a long pass filter (400 nm cut off) in the emission pathway. Excitation and emission spectra were further corrected for scattering effects by subtracting the excitation and emission spectra of corresponding blank vesicles. Following the fluorescence measurements, 100 μl of each liposome suspension was immediately diluted into 100 μl of acidified methanol for drug analysis and another 100 μl of the suspension was transferred into a test tube, dried under a stream of N2 and stored at −25°C for later lipid analysis.
Liposomes prepared with 70 mM Na carbonate buffer (pH=9.0) were used to measure the change in intraliposomal pH of vesicles when the extravesicular buffer was exchanged with phosphate buffered saline (PBS, pH=7.4) or “carbonated” PBS. Phosphate buffer was “carbonated” by adding sodium bicarbonate to obtain a physiological (Laiken and Fanestil, 1989) concentration of carbonate (24 mM) with the pH and total solute concentration maintained at physiologically relevant levels. The extravesicular carbonate buffer was exchanged for PBS or C-PBS by passing vesicles (0.5 ml) through Sephadex® columns. The eluent suspensions (5 ml) were collected and fluorescence emission spectra were immediately recorded as detailed earlier.
2.5. Determination of DB-67 Loading Efficiency
Aliquots (0.25 ml) of drug loaded liposome suspension at varying pH (4–9.5) were loaded onto a Sephadex® G-25 size exclusion column pre-equilibrated with the same buffer used in preparing the liposomes and eluted with 5 ml of additional buffer. The eluent suspension from 2 to 5 ml was collected, and 100 μl of the collected suspension was immediately transferred to an HPLC autosampler vial for DB-67 analysis. The pipette tip used for the transfer was washed with 100 μl of acidified methanol to recover adsorbed drug and this solution was transferred into the same vial. A 100 μl aliquot of the Sephadex® column eluent liposome suspension was transferred into a test tube and dried under a stream of N2 for lipid analysis. A 100 μl aliquot of the original drug liposome suspension was also collected for lipid and drug analysis and treated similarly to the Sephadex® column eluent suspension. Samples collected for drug and lipid analysis were stored at −25°C prior to their analysis by HPLC.
2.6. Determination of Bilayer Membrane/Water Partition Coefficients
Bilayer membrane/water partition coefficients for DB-67 were determined by equilibrium dialysis (1 ml Teflon® cells, Equilibrium Dialyzer, Spectrum Laboratories). One ml of blank liposome suspension (0.08–16 mg/ml) prepared in buffers at varying pH (4–9.5) were loaded into the receiver compartment and dialyzed at 37°C against 1 ml of DB-67 (0.05–5 μM) solution in the same buffer in the donor compartment. Equilibration times varied from 12 to 120 hours depending on pH. From pH 4 to 8, equilibrium was attained with respect to both the inner and outer leaflets of the liposome bilayer. Previous studies of liposome release kinetics of DB-67 at varying pH suggested that the half-life for liposome release was on the order of several days above pH 8.5 (Joguparthi and Anderson, 2007). Therefore, above pH 8.5, liposomes were allowed to equilibrate only for 12 hours and the drug was assumed to be at equilibrium only with respect to the outer bilayer leaflet. After equilibrium was established, 100 μl of liposome suspension and drug solution from the donor and receiver compartments were withdrawn using a 1 ml syringe and transferred to an HPLC auto sampler vial followed by a wash of the syringe with 200 μl of acidified methanol to recover adsorbed drug and samples for drug analysis were stored at −25°C prior to their analysis by HPLC. A 100 μl sample was collected from the receiver compartment (liposomes), dried under a stream of N2 gas, and stored at −25ºC for later lipid analysis.
2.7. HPLC Analyses
Samples collected in equilibrium dialysis, drug loading and fluorescence experiments were analyzed for DB-67 lactone and carboxylate concentrations using a previously developed isocratic HPLC method with fluorescence detection (Joguparthi et al., 2007). Lipid (DSPC) analysis was performed by HPLC coupled to an evaporative light scattering detector (ELSD) as described previously (Joguparthi et al., 2007).
3. Data Analyses
3.1. Analysis of Membrane Partitioning Data
Four different DB-67 species may exist in solution depending on solution pH as shown in Scheme 1. The fraction of the ring opened unionized species (II) has been shown to be negligible in solution for various camptothecins, including DB-67 (Fassberg and Stella, 1992; Xiang and Anderson, 2002), and has been assumed to be negligible in the current treatment. The various partitioning and ionization equilibria in the liposome membrane and in the aqueous core are depicted in Scheme 2. Membrane partitioning of DB-67 at each pH was determined by equilibrium dialysis. At equilibrium the total drug concentration in the receiver chamber can be related to the concentration in the bilayer membrane and accessible aqueous phase as follows:
| (1a) |
where Cr and Vr are the drug concentration and total volume in the receiver compartment, Cm is the molar solute concentration in the liposome membrane, Cw is the molar solute concentration in the aqueous phase and, Vm and Vw as the respective volumes of the membrane and aqueous phases. The apparent volume partition coefficient, Kpapp at each pH (4–9.5) was determined using the following relation:
| (1b) |
where Cd is the donor solution concentration of DB-67. The apparent partition coefficient obtained at each pH value can be related to the concentration of species I, III and IV in the membrane and aqueous phases as follows:
| (1c) |
or
Scheme 2.
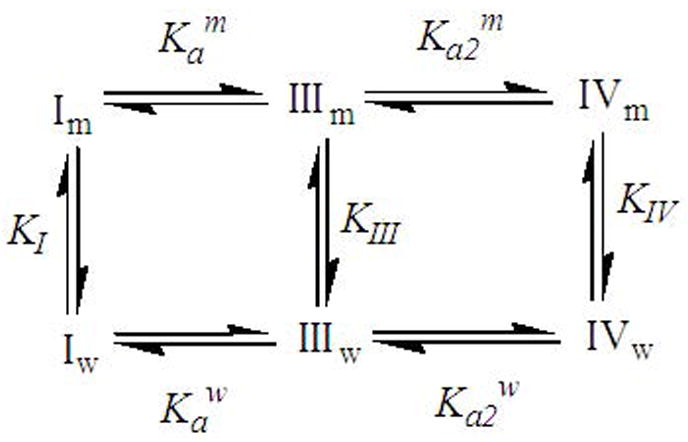
The ionization and binding equilibra considered in the data analyses. I, II and III refer to the DB-67 lactone, DB-67 carboxylate monoanion and dianion, respectively. Their corresponding membrane/water partition coefficients are represented by KI, KIII and KIV. The equilibrium constants Ka and Ka2 refer to the ionization of the E-ring lactone and A-ring hydroxyl groups, respectively, and the subscripts and superscripts m and w refer to the intravesicular and extravesicular compartments.
| (1d) |
where the superscripts and superscripts m and w refer to the membrane and aqueous phase, Ka is the effective ionization constant for lactone ring opening and ionization of the E-ring carboxylic acid group, and Ka2 is the ionization constant for the 10-hydroxyl group on the A ring. The intrinsic partition coefficients of the three species are:
| (1g) |
where KI, KIII and KIV are the intrinsic partition coefficients of DB-67 lactone (I), monoanion (III) and dianion (IV). The pH at the interface was assumed to be equal to that of the aqueous phase in the current treatment. Further, due to the low concentration of drug used in these studies (10−8–10−6 M), deviations in activity coefficients due to possible self-association could be neglected. The values of Ka and pKa2 in solution have been previously found to be (3.4 ± 0.3) × 10−7 and 8.67 ± 0.20 (Xiang and Anderson, 2002). The apparent partition coefficients obtained at each pH (4–9.5) were fit to Eqn. 1d using the non-linear least squares regression software Scientist® ((Micromath Scientific Software, St Louis, MO) and solving for KI, Kam and Ka2m
3.2. Analysis of Fluorescence Spectra in Aqueous Solutions
Fluorescence excitation spectra of DB-67 solutions at varying pH (7.7–10) were used to estimate the value of pKa2 in aqueous buffers. In this pH region, DB-67 exists predominantly as the monoanion (III) and dianion (IV) (Xiang and Anderson, 2002). Assuming that the intensity at each wavelength results from the independent contributions of each species, the total intensity normalized by total concentration, St’(λ), can be expressed as:
| (2a) |
| (2b) |
| (2c) |
where C is the total concentration of DB-67 in solution, λ is the wavelength, SIII(λ) and SIV(λ) are the intrinsic intensities of species III and IV as a function of wavelength (λ) and f IIIW and f IVW are the fractions of species III and IV, respectively, in aqueous buffers.
The fluorescence excitation spectra of DB-67 in aqueous solution at each pH (7.7–10.1) normalized by the concentration of DB-67 also determined at each pH were fit by least-squares regression analysis (see Appendix I) to Eqn. 2a to obtain an estimate of K a2w and the intrinsic excitation spectra of species III and IV.
3.3. Analysis of Fluorescence Spectra in Liposomes
Fluorescence spectra for liposome suspensions were obtained immediately after unentrapped drug was separated from the liposomes containing entrapped drug by size exclusion chromatography. Previous studies have shown that the half-life for lipid bilayer transport of DB-67 ranged from approximately 3 hours at pH 4 to 70 hours at pH 9.5 (Joguparthi and Anderson, 2007). Since size-exclusion chromatography and fluorescence measurement for each sample was typically completed within 5 minutes, the entire fluorescence signal was assumed to reflect the intraliposomal microenvironment at any pH studied. The fluorescence emission spectra of DB-67 at various pH values (7.7–10.1) were used to estimate the value of pKa2′ for entrapped DB-67. Membrane partitioning studies suggested that in this pH region (7.7–10.1), the membrane binding of DB-67 decreases with increasing pH. Therefore, pKa2′ obtained here is assumed be the effective ionization constant of DB-67 in the intraliposomal microenvironment. Assuming that the drug exists predominantly as two species (mono- and di-anion) in this pH region, the total intensity from the liposome suspensions corrected for the effects of liposome light scattering and normalized for drug concentration can be expressed as:
| (3a) |
| (3b) |
| (3c) |
| (3d) |
where [L] is the lipid concentration in the suspension, Ci is the intraliposomal concentration of DB-67, St(λ) is the total intensity of DB-67 in the vesicles, SIII(λ) and SIV(λ) are the intrinsic intensities of species III and IV in the intraliposomal microenvironment, SL(λ) is the scattering intensity of the blank liposome particles, and f IIIL and f IIVL are the fractions of species III and IV in the intraliposomal microenvironment. C is the total drug concentration in the liposome suspension, Vi and Vo are the total volumes of the intra- and extravesicular compartments in the liposome suspension, and × is the ratio of the extravesicular to intravesicular volume. is the intravesicular proton concentration and Ka2′ is the effective intraliposomal ionization constant of the A-ring phenol.
The effective ionization constant can be expressed as:
| (3e) |
| (3f) |
| (3g) |
where and are the intraliposomal concentrations of species III and IV, and are the concentrations of species III in the vesicle aqueous core and inner monolayer, and are the concentrations of species IV in the vesicle aqueous core and inner monolayer, and a and b are the ratios of total entrapped volume to the volumes of the entrapped aqueous core ( ) and inner monolayer ( ), respectively. Eqns. 3e–g can be simplified to relate the effective ionization constant to the ionization constants of DB-67 in the aqueous and membrane phases as follows:
| (3h) |
The fluorescence emission spectra of the liposome suspensions at each pH (7.7–10.1) were fit to Eqn. 3a by least-squares regression analysis (Appendix I) to obtain a value of Ka2′ and the intrinsic emission spectra of species III and IV in the liposome microenvironment. The ionization constant (Ka2m) of the A-ring phenol group on the bilayer membrane was estimated from the effective ionization constant in the intraliposomal microenvironment using Eqn. 3h and compared to the value obtained by equilibrium dialysis.
The intraliposomal pH in liposomes prepared with pH 9 Na carbonate buffer was measured after exchanging the extravesicular carbonate buffer with PBS or C-PBS and measuring the corresponding fluorescence emission spectra. These spectra were used to estimate (Appendix I) the fractions of species III and IV in the intraliposomal microenvironment. The intraliposomal pH was then calculated from the fractions of the two species and the effective intraliposomal ionization constant (Ka2′) using the Henderson-Hasselbalch equation.
4. RESULTS
4.1. Validation of Fluorescence Methods
One of the objectives of the present work was to develop and validate a fluorescence method for monitoring intraliposomal pH in the region between pH 7–0. The fluorescence method was first validated in aqueous solution by estimating the ionization constant of the A-ring phenol group (pKa2) of DB-67 in aqueous buffers. The concentration quenching of DB-67 was investigated to select a concentration region devoid of quenching effects for fluorescence measurements in solution. Above a concentration of 20 μM, the fluorescence intensity of DB-67 was found to decrease with increasing drug concentration. A 1 μM concentration of DB-67 was therefore employed for fluorescence measurements. During fluorescence studies of liposomal suspensions, the vesicles were diluted (by size exclusion chromatography on Sephadex® columns) such that the suspension drug concentration was ~ 1 μM. The photostability of DB-67 during spectral measurements was investigated by intermittent excitation of the drug in aqueous buffers or unilamellar vesicles, every 10 seconds. There was no evidence of photodegradation as confirmed by the reproducible emission intensity, indicating that DB-67 was photostable during the measurement of excitation and emission spectra. Further, each spectral measurement was completed within a minute by the use of a short integration time (0.1 second) and a wavelength step size of 1 nm.
The particle sizes of liposome suspensions employed in the experiments here were typically 195 ± 50 nm. The lipid concentration of the vesicle suspensions (after size exclusion chromatography) was typically 0.5–2 mg/ml. Fluorescence spectra of blank vesicles at a similar lipid concentration were used to correct the overall fluorescence signal from liposomes containing drug for the light scattering contribution.
4.2. Membrane Partitioning of DB-67
The DSPC/m-PEG DSPE bilayer/water partitioning of DB-67 was investigated over a lipid concentration range of 0.08–16 mg/ml and a drug concentration range of 0.05–5 μM. At pH< 8, the drug concentration range was only varied from 0.05–0.5 μM due to the low solubility of DB-67 (Xiang and Anderson, 2002). Figure 1 shows a representative lipid concentration versus time profile observed during dialysis measurements at pH 8 where the equilibration time was the longest. The lipid concentration was found to be stable throughout the equilibrium dialysis measurements at all the pH values (4–9.5) explored. All the buffers employed in the dialysis measurements were anionic and the buffer concentrations were typically at least 1000-fold greater than the drug concentration. Therefore it was assumed that no pH gradients were generated due to drug transport across the bilayer during equilibration.
Figure 1.
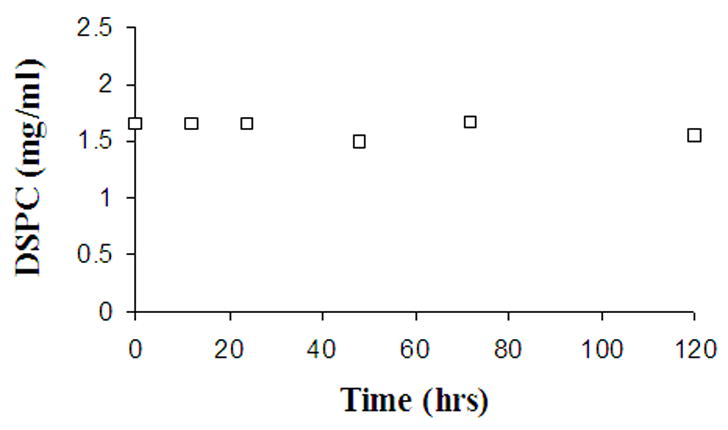
Concentration of DSPC measured as a function of time during equilibrium membrane/water partitioning studies at pH 8.
Shown in Figure 2 are representative partition coefficient (Kp) values observed as a function of lipid concentration at three different pH settings (4.11, 6.76, 8.95). Within the concentration range employed in these studies, the partition coefficient of DB-67 was found to be independent of drug and lipid concentration over the entire pH range examined (4–9.5). The partition coefficients obtained using different lipid and drug concentrations at each pH were fit to Eqn. 1d to obtain values of Kam and Ka2m Displayed in Figure 3 are the observed (symbols) and model fitted (solid line) values of Kp for DB-67 as a function of pH. The Kp values ranged from 2443 ± 230 at pH of 4.1 to 57 ± 5 at pH 9.5. These values correspond to a percentage of total drug that is membrane bound inside the vesicles of approximately 99% and 76% at pH 4.1 and 9.5, respectively. The ionization constants Kam and Ka2m were found to be 1.95 ± 0.32 × 10−8 and 1.1 ± 0.6 × 10−9, respectively, which are similar to the values determined previously from permeability measurements (Kam = 1.08 ± 0.17 × 10−8, Ka2m = 1.53 ± 0.64 × 10−9). Table I summarizes the ionization constants on the membrane measured by various methods. These pKa values correspond to shifts of the pKa values in aqueous buffers by 1.2 and 0.3 units upon membrane binding. Ignoring the membrane binding of the carboxylate dianion (IV) resulted in a poor fit of the partition coefficients in the pH region 8.5–9.5. The dashed line in Figure 3 shows the fitted pH-partitioning profile if the binding constant for species IV was considered to be negligible. The ionization constants for DB-67 in the membrane and aqueous phases were used to estimate the intrinsic partition coefficients of the various species depicted in Scheme II. The intrinsic partition coefficients of the neutral lactone (KI), monoanion (KIII) and dianion (KIV) were found to be 2443 ± 230 (Joguparthi et al., 2007), 141 ± 23 and 73 ± 37, respectively.
Figure 2.

Values of Kp, the membrane/water partition coefficients for DB-67, observed as a function of lipid concentration at pH 4.11 (Δ), 6.76 (□) and 8.95 (○). The error bars shown are the standard deviations of the experimental measurements.
Figure 3.
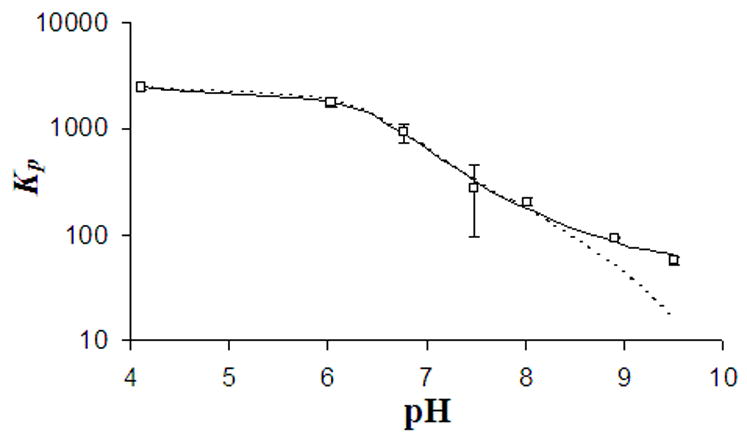
Plot of Kp versus pH for DB-67 obtained by the fitting the observed partition coefficients (□) at varying pH (4–9.5) to Eqn. 1d by considering (solid line) or ignoring (dashed line) partitioning of the dianion species.
Table I.
| Experimental Method | Kam (pKam) | Ka2m pKa2m) |
|---|---|---|
| Membrane Permeability Studies | 1.08 ± 0.17 × 10−8 (7.97 ± 0.16) | 1.53 ± 0.64 × 10−9 (8.81 ± 0.42) |
| Membrane Partitioning Studies | 1.95 ± 0.32 × 10−8 (7.71 ± 0.16) | 1.1 ± 0.6 × 10−9 (8.95 ± 0.54) |
| Fluorescence Spectroscopic Studies | N/A | 5.8 ± 1.1 × 10−10 (9.23 ± 0.19) |
Ionization constants (mean ± std) of DB-67 when bound to the gel phase (DSPC) bilayer, measured using various methods
4.3. Fluorescence Measurements of DB-67 in Aqueous Buffers
Shown in Figure 4 are the excitation (panel A) and emission (panel B) spectra of DB-67 at 37°C in aqueous buffers varying in pH but having the same drug concentration (1 μM). The emission spectrum exhibited an intensity maximum at 560 nm. The emission intensity was observed to increase with an increase in pH but no shifts were observed in the emission maxima. The excitation spectrum showed pH dependent shifts in intensity as well as in the excitation maximum. At pH<8.5, the excitation maximum was 380 nm. At pH>8.5, the excitation maximum was found undergo a red shift to 420 nm. The excitation spectra were fit to Eqn. 2a–c and solved for Ka2w and the intrinsic spectra for species III and IV by least-squares regression analysis (Appendix I). The deconvoluted individual spectra of species III and IV are shown in Figure 5. The value of pKa2w was found to be 8.57, not significantly different from the pKa2 of 8.67 ± 0.20 observed in these laboratories previously from solubility studies of DB-67 (Xiang and Anderson, 2002).
Figure 4.
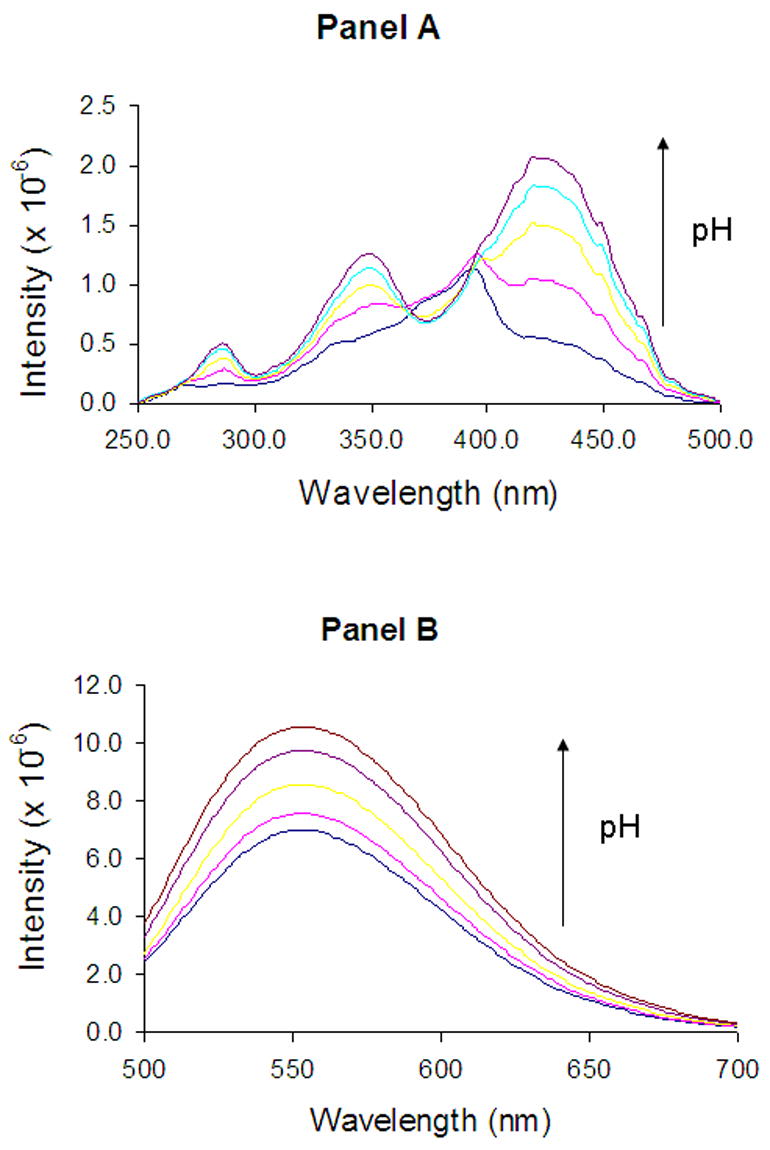
Fluorescence excitation (panel A) and emission (panel B) spectra of DB-67 aqueous buffers of varying pH (7.7–10.1). The arrows show the direction of increasing pH. pH values of the solutions for which the spectra are displayed are 8, 8.5, 9, 9.6 and 10.1, respectively.
Figure 5.
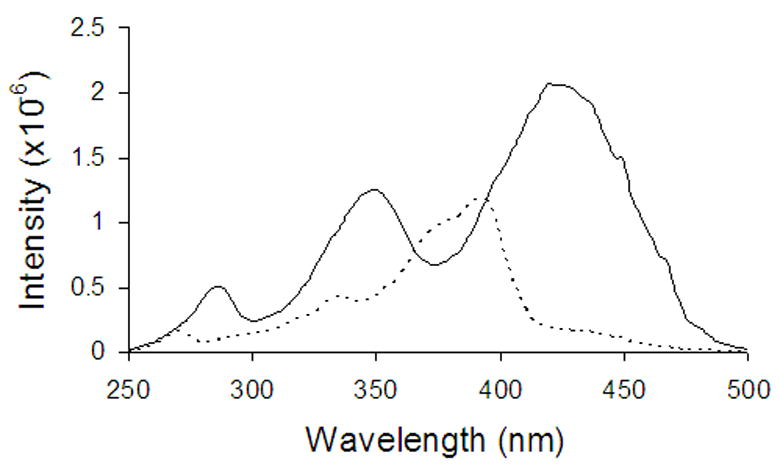
Deconvoluted (using Eqn. 2a) excitation spectra of the DB-67 monoanion (_ _ _ _) and dianion ( _____ ) in aqueous buffers.
4.4. Fluorescence Measurements of DB-67 in Unilamellar Vesicles
Fluorescence excitation and emission spectra were measured in liposomes containing entrapped DB-67 as a function of pH. Since the entrapment efficiency of the drug was pH dependent (see results on drug loading), the concentration of DB-67 in each liposome suspension was measured and the spectra were normalized for any differences in drug concentration. Blank liposome suspensions prepared at the same lipid concentration (16 mg/ml) as that in the drug loaded vesicles were treated the same as the drug loaded vesicles and used as a control to correct for scattering.
Shown in Figure 6 are the excitation (panel A) and emission (panel B) spectra of DB-67 entrapped in vesicles at varying pH. The excitation maximum was 397 nm and pH independent. The total fluorescence intensity decreased with increasing intraliposomal pH. The emission spectra showed a pH dependent red-shift in the emission maximum. In addition to the emission peak at 560 nm observed in aqueous solution, an additional peak was observed at 445 nm. The emission intensity at 445 nm decreased with increasing pH, while the intensity at 560 nm was relatively insensitive to a change in pH. The emission spectra were used to estimate the effective ionization constant of liposomally entrapped DB-67 (Ka2′). The emission spectra were fit to Eqns. 3a–c (after correcting for light scattering and normalizing for differences in intraliposomal drug concentration) and solved (by least-squares regression analysis, Appendix I) for Ka2′ and the individual spectra of species III and IV in the intraliposomal microenvironment. The deconvoluted spectra are shown in Figure 7. The value of Ka2′ was estimated to be 7.2 ± 0.4 × 10−10. The value of Ka2′ was estimated from the value of Ka2′ (using Eqn. 3h) to be 5.8 ± 1.1 × 10−10, close to that observed in our previous permeability studies as well as membrane partitioning studies here.
Figure 6.
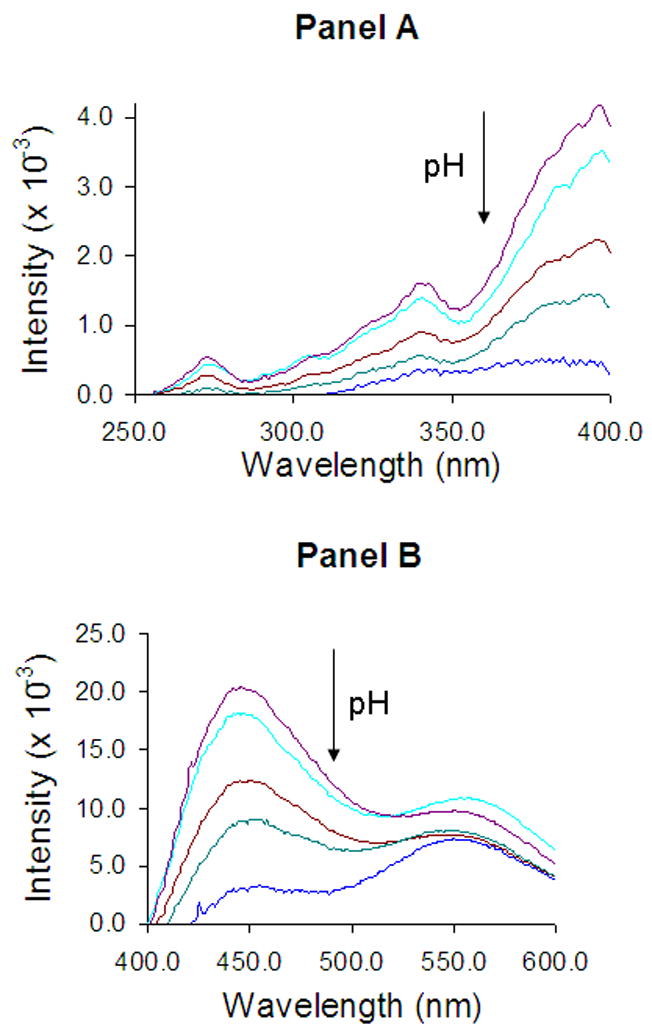
Normalized fluorescence excitation (panel A) and emission (panel B) spectra of DB-67 in liposomes of varying pH (7.8–10.1). The arrows show the direction of increasing pH. Values for pH in the vesicles for which the spectra are displayed are 7.8, 8.4, 9, 9.5 and 10.1, respectively.
Figure 7.
Deconvoluted emission spectra (using Eqn. 3a)of the DB-67 monoanion (_ _ _) and dianion ( _____ ) in liposomes.
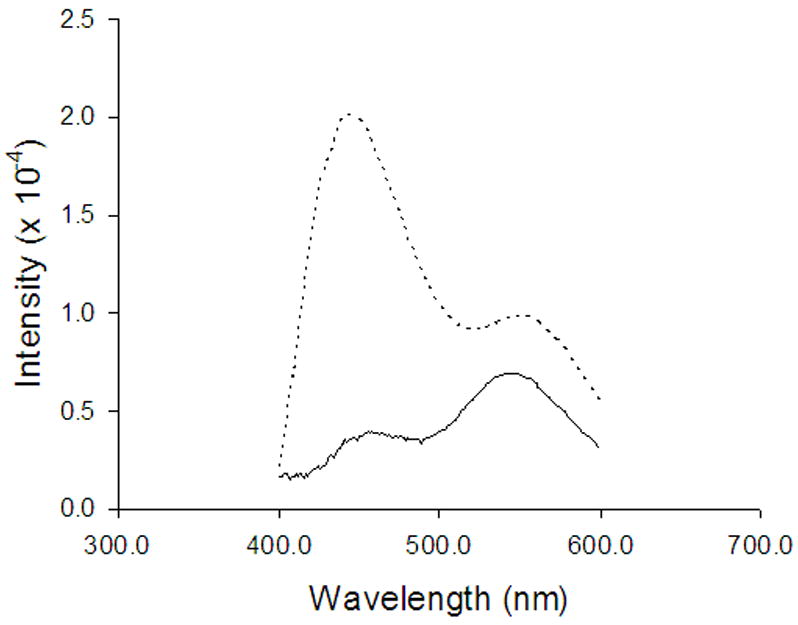
Previous liposomal release studies suggested that the faster than desired release rate under physiological conditions from liposomes encapsulated with DB-67 carboxylate is due to the instantaneous loss of trans-bilayer pH gradients due to the rapid equilibration of CO2 (Joguparthi and Anderson, 2007). A mathematical model to predict changes in intravesicular pH due to flux of CO2 was also developed (Joguparthi and Anderson, 2007). To confirm the experimental observations and model predictions in the release studies, the fluorescence emission spectra of liposomes containing entrapped DB-67 were obtained after exchanging (by passing through a Sephadex® gel column) the extravesicular buffer with PBS or C-PBS. The fluorescence emission spectra were used to calculate (Appendix I) the pH inside the vesicles. Figure 8 shows the observed fluorescence emission spectra in PBS or C-PBS buffer. The pH inside the vesicles was measured to be 7.4 ± 0.2 in C-PBS buffer and 10.3 ± 0.2 in PBS buffer. Previous model calculations (Joguparthi and Anderson, 2007) estimated an intravesicular pH decline to 7.8 in C-PBS and an increase to 10.2 in PBS buffer. The experimental values here are close to the calculated values confirming the model predictions.
Figure 8.
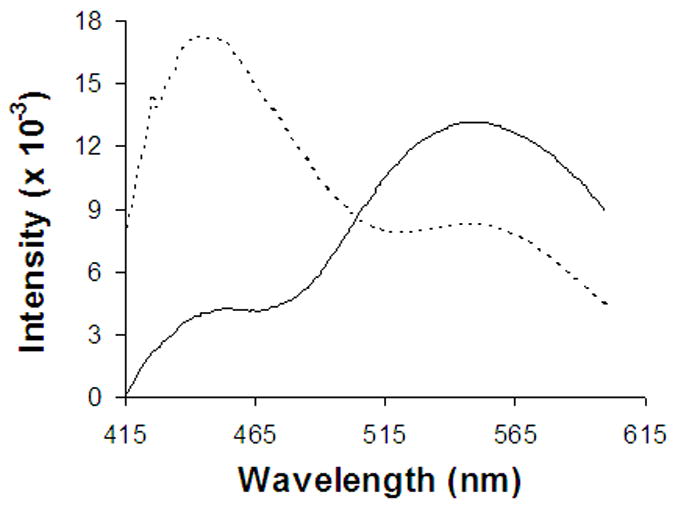
Normalized fluorescence emission spectra of DB-67 in pH 9 Na carbonate liposomes measured immediately after exchanging the extravesicular buffer with PBS ( _____ ) or C-PBS(- - - -).
4.5. Determination of Drug Loading Efficiency
The loading efficiency of DB-67 into vesicles was investigated as a function of intravesicular pH. Encapsulation efficiency was calculated as the percent of total drug retained in the liposome fraction after size exclusion. Shown in Figure 9 is the percent of DB-67 encapsulated in vesicles as a function of pH. Encapsulation efficiency varied from 75% at pH 4 to about 15% at pH 9.5. The drug loading profile as a function of pH in Figure 9 follows the same pattern as the pH-partitioning profile shown in Figure 3. The high entrapment efficiency of DB-67 at pH<8 is likely due to the greater membrane binding in this pH region.
Figure 9.
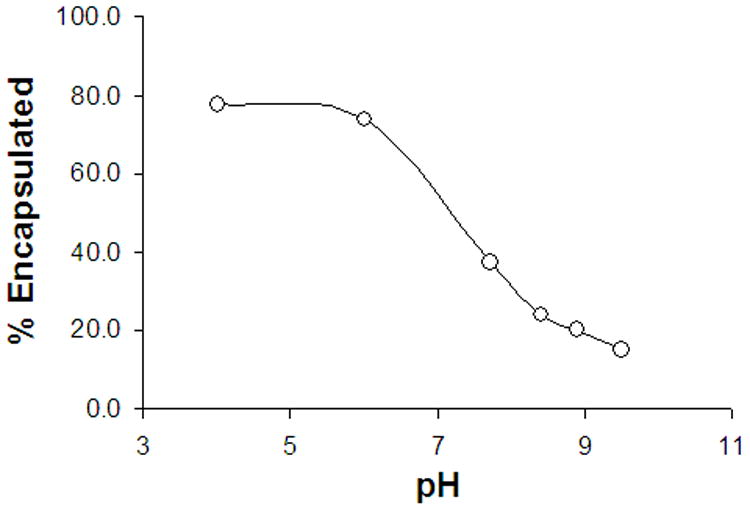
Percent of initial DB-67 encapsulated in vesicles when liposomes were prepared by hydration-extrusion procedure as a function of varying pH.
5. DISCUSSION
5.1. Fluorescence Method for Measurement of Intraliposomal pH using DB-67
Liposomal formulations of drug candidates requiring prolonged blood circulation time in vivo rely on trans-membrane pH gradients to retain the drug inside the vesicles during circulation (Maurer-Spurej et al., 1999). Trans-membrane pH gradients are also used for the purpose of drug loading into vesicles during their formulation (Dos Santos et al., 2004; Madden et al., 1990). The maintenance of established pH gradients both in vitro and in vivo may be critical to ensure efficient drug loading and retention.
Recent formulation studies in these laboratories have been aimed at developing a sustained release liposomal formulation of a novel camptothecin, DB-67 by various methods, including the use of a trans-bilayer pH gradient (Joguparthi and Anderson, 2007; Joguparthi et al., 2007). A more rapid rate of release of DB-67 under physiological conditions (i.e., in the presence of CO2 at physiological concentrations) than in buffers at the same pH but lacking CO2 was attributed to a drop in intravesicular pH caused by the influx of CO2. In the present work, the use of entrapped DB-67 as a self-probe of intraliposomal pH has been explored as a means of monitoring the pH during release studies. To assess the feasibility of using DB-67 to monitor intraliposomal pH, the pH dependent fluorescence properties of DB-67 were examined. Initial method development and validation studies were conducted using 1 μM DB-67 solutions at varying pH (7.7–10.1). The pH dependent changes in the excitation spectra (panel A in Figure 4) were used to estimate a value for the pKa2 of DB-67 of 8.57, similar to that reported previously for DB-67 (Xiang and Anderson, 2002) and similar to the value reported for other 10-hydroxy containing camptothecins (Fassberg and Stella, 1992).
Spectral measurements were also performed in liposomes containing entrapped DB-67 at varying intravesicular pH. In contrast to the studies in aqueous buffers, the excitation spectra (panel A in Figure 6) exhibited no change in excitation maximum with pH, but the total fluorescence intensity was found to decrease with increasing pH. A new peak at 445 nm was observed in the emission spectra in addition to the peak at 560 nm observed in solution (panel B in Figure 6). In addition to the blue shift in the emission maximum to 445 nm from the 560 nm observed in solution, the ratio of the intensities at 445 and 560nm ( ) decreased with increasing pH.
Spectral properties of fluorescent dyes are sensitive to both the probe environment as well as the prototropic equilibria. Therefore the nature of the probe environment has to be carefully considered in the interpretation of pH effects on the observed spectra (Klonis et al., 1998; Klonis and Sawyer, 2000). Recent fluorescence studies of DB-67 in organic solvents of varying polarity revealed that the emission maximum lies in the wavelength region of 400–450 nm and increases with increasing solvent polarity (Xiang et al., 2006). In water, the emission maximum has been observed to undergo a large red shift to 560 nm compared to that in organic solvents. In spectral measurements in liposomes, two peaks were observed at 440 nm and 560 nm, respectively, with the ratio of the two peaks dependent on pH, indicating either a change in the probe microenvironment, a change in the fraction of the various species, or both. To confirm the effect of solvent polarity, the emission spectra of DB-67 were measured in water-ethanol mixtures as depicted in Figure 10. In 100% ethanol, an emission maximum was observed at 430 nm, shifting to 560 nm in 90% ethanol. Increasing the percentage of water beyond 10% in the water-ethanol mixtures produced further decreases in the intensity of the emission at 430 nm confirming that the ratio of the two peaks was strongly dependent on solvent polarity. Thus, the change in the ratio of the two peaks at 445 and 560 nm observed in liposomes as a function of pH clearly suggests a potential change in the probe microenvironment (i.e. change in drug location).
Figure 10.
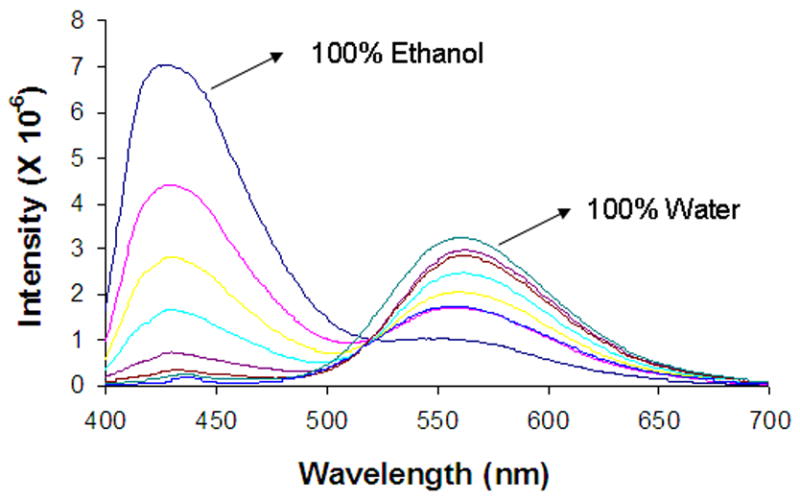
Fluorescence emission spectra of DB-67 in ethanol-water mixtures starting from 100% ethanol as the solvent and changing to 100% water as the solvent. The % (v/v) ethanol in ethanol/water mixtures used to generate the displayed spectra in the figure are 100%, 97.5%, 95%, 90%, 50%, 25% and 0%, respectively. The decrease in the percent of ethanol is characterized by the decrease in the emission intensity at 430 nm and increase in the intensity at 560nm.
Membrane partitioning studies indicated that both the monoanion and dianion are highly membrane bound in the intraliposomal microenvironment. The estimated partition coefficients for these two species correspond to percentages of membrane bound intravesicular drug of 90% and 82% for the monoanion and the dianion, respectively. Since the fractions of species bound do not differ dramatically at the high lipid/water volume ratios found inside vesicles, the increase in the emission maximum of DB-67 in the vesicles with increasing pH may be due predominantly to changes in the fractions of monoanion and dianion rather than to differences in the microenvironment in which drug is located. One of the pH values (pH 7.8) employed in the liposome experiments was close to the effective pKa (=7.7) for the E-ring lactone ring-opening/ionization in membrane bound DB-67. However, ignoring the spectrum at this pH did not significantly change the estimated value of the effective pKa2 of DB-67 in the intraliposomal microenvironment. This is likely due to the fact that the E-ring is far away from the quinoline nucleus that is responsible for the major fluorescence signal. This is also corroborated by the observation that emission spectra in liposomes were pH independent in the pH 4–7 region (data not shown). The pH dependence of the emission spectra of DB-67 in liposomes in the pH region 7.8–10.1 was used to estimate the effective pKa2 of DB-67 in the intraliposomal microenvironment. The value of the ionization constant on the membrane (Ka2m) was estimated from the effective pKa2 and found to be similar (see Table I) to the value obtained from previous permeability measurements and the membrane partitioning studies described herein. These results confirm that the pH dependent spectral properties of DB-67 can be exploited to probe the intravesicular pH.
The fluorescence method developed was used to confirm the previous experimental observations and model predictions (Joguparthi and Anderson, 2007) that when starting with a high intravesicular pH, the established pH gradients are instantaneously lost due to the rapid trans-bilayer CO2 equilibration. When liposomes having an initial internal pH of 9.0 were dialyzed against C-PBS, a strong blue shift in the spectra was seen in the case of C-PBS suggesting that the predominant species has changed from that of the dianion to the monoanion. The measured pH (7.4 ± 0.2) in the case of C-PBS was close to that predicted (7.8) confirming the loss of pH gradients. The small difference between the predicted and observed is likely due to the relative insensitivity of the method in this pH region, which is ~1.5 pKa units away from the apparent pKa2 (9.14 ± 0.23) in the intraliposomal microenvironment. When liposomes having an initial internal pH of 9.0 were dialyzed against PBS, the pH was predicted to rise due to the efflux of CO2 from the intraliposomal carbonate buffer. This was confirmed by the pH measurements. The measured pH in PBS (10.3 ± 0.2) was not different from that predicted (10.2) since this pH region is close to the apparent pKa2 in the intraliposomal microenvironment.
5.2. Membrane Partitioning and Liposomal Loading of DB-67
The membrane partitioning of DB-67 was investigated to obtain independent estimates of the ionization constants for the E-ring lactone ring-opening/ionization and A-ring phenol group on the bilayer membrane. The ionization constants Kam and Ka2m were found to be 1.95 ± 0.32 × 10−8 (pKam = 7.71) and 1.1 ± 0.6 × 10−9 (pKa2m = 8.96), respectively. The intrinsic partition coefficients of species I, III and IV were found to be 2443 ± 230, 141 ± 23 and 73 ± 37, respectively. These values suggest that DB-67 is highly membrane bound even at pH 9.5. The values of the ionization constants obtained in this study are consistent with previously estimated values from permeability measurements.
The shifts in the ionization constants of DB-67 upon binding to the inner leaflet of the liposomal bilayer are consistent with a microenvironment having reduced water exposure. Recent molecular dynamics studies in these laboratories have shown that DB-67 lactone resides at the membrane-water interface with both the E-ring lactone and Aring phenol group residing in regions of reduced water exposure (Xiang et al., 2006). These simulations also suggested that the 10-hydroxyl group on the A-ring is farther away from the lipid/water interface (and closer to the chain region) than the E-ring lactone probably due to the presence of two hydrogen bonding groups in the lactone ring. We have ignored the neutral ring-opened carboxylic acid (species II) in the treatment of our data here based on previous (Fassberg and Stella, 1992; Xiang and Anderson, 2002) observations that this species is negligible in solution at any pH. This assumption should also be valid for membrane bound DB-67 because membrane binding should further favor the lactone over the ring-opened species (II). The observed shift in the ionization constants also indicates that, unlike in solution, the carboxylate/lactone equilibrium is shifted towards DB-67 lactone at physiological pH in the intraliposomal microenvironment. A consequence of this is that a higher intraliposomal pH is necessary than would be expected in the absence of membrane binding to significantly prolong the release of DB-67 from liposomes.
Liposomal loading of DB-67 was investigated as a function of pH to explore the potential of passive liposomal loading to prepare liposomes at high intravesicular pH. The liposomal loading profile shown in Figure 9 appears to be very similar to the pH partitioning profile of DB-67. The high encapsulation efficiency in the pH region 4–7 is likely due to DB-67 lactone being the predominant species in this region. The decrease in encapsulation efficiency with increasing pH suggests that the loading of DB-67 occurs primarily through membrane binding as opposed to aqueous core loading which would be expected to the pH independent. This indicates that a high drug concentration is required for formulation of liposomes (when using a high intravesicular pH) by passive loading approaches in order to achieve a high entrapped concentration. Alternative strategies are therefore needed to improve the encapsulation efficiency of DB-67 at a high intraliposomal pH.
6. CONCLUSIONS
The pH dependent fluorescence properties of DB-67 have been examined to explore its use as a probe of intravesicular pH. The emission spectra of DB-67 in the intraliposomal microenvironment exhibit a pH dependent shift in the emission maxima in the pH region of interest (7–10) that enables the use of DB-67 as an intravesicular pH probe. Previous predictions for the changes in intravesicular pH under physiological conditions (Joguparthi and Anderson, 2007) were confirmed using this method. The membrane partitioning of DB-67 as a function of pH was consistent with observations from fluorescence measurements. DB-67 was found to be highly membrane bound in the intraliposomal microenvironment which results in a decrease in the ionization constants of the E-ring lactone and A-ring phenol. The observed ionization constants of DB-67 in its membrane bound state are consistent with observations from fluorescence measurements here and previous permeability measurements. The pH dependence of DB-67 loading using a passive loading technique was found to reflect the pH dependence of membrane binding of the drug. This results in poor encapsulation efficiency of DB-67 at high pH, necessitating further development of formulation strategies to improve loading efficiency.
Acknowledgments
This work was financially supported by a grant from the NIH (NCI RO1 CA87061-03).
The authors also thank Dr. Paul Bummer for use of his ELSD instrument.
APPENDIX I
Spectral Deconvolution by Least-Squares Regression Analysis
The total fluorescence intensity at each wavelength in experiments in aqueous buffers can be expressed as a sum of intrinsic intensities of species III and IV as:
| (1) |
Since spectra were obtained at varying pH, the total intensity can be expressed as:
| (2) |
where i is the pH value and n is the total number of pH values where the spectra were measured. The best fit value for the ionization constant (Ka2w) was obtained by minimization of the following objective function defined to represent the difference between the sum of squares of deviations of the observed from the predicted intensity values.
| (3) |
where the subscripts expr and calc represent the experimentally observed and calculated spectra. At the minima of the above objective function, the first derivative with respect to SIII (λ) or SIV (λ) is zero:
| (4a) |
| (4b) |
Eqns. 4a & 4b are a system of linear equations with two unknowns SIII (λ) and SIV (λ); having the following solutions:
| (5a) |
| (5b) |
The solutions 5a & 5b can be substituted into the objective function (Eqn. 3) to find the optimal value of Ka2w at which the difference between the experimental and calculated spectra is minimized. The spectra obtained from liposomes was similarly solved (after correcting for liposome scattering and normalizing for drug concentration) to find the value of Ka2w for ionization of the A-ring phenol in the intraliposomal microenvironment. The measured Ka2′ was used to predict the pH values in vesicles by least squares analysis. In order to predict the pH, the objective function (Eqn. 3) was derivatized with respect to fIII,i and fIV,i to obtain two linear equations (similar to Eqns. 4a & 4b shown above) with fIII,i and fIV,i as the unknowns. These equations can be solved to obtain the fractions of the two species. From the values of the fraction of various species (fIII,i & fIV,i ) and the apparent ionization constant in the vesicle (Ka2′), the pH inside the vesicles can be calculated using the well known Henderson-Hasselbalch equation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bom D, et al. The novel silatecan 7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high lipophilicity, improved human blood stability, and potent anticancer activity. J Med Chem. 2000;43:3970–3980. doi: 10.1021/jm000144o. [DOI] [PubMed] [Google Scholar]
- Burke TG, et al. Lipid bilayer partitioning and stability of camptothecin drugs. Biochemistry. 1993;32:5352–5364. doi: 10.1021/bi00071a010. [DOI] [PubMed] [Google Scholar]
- Dos Santos N, et al. pH gradient loading of anthracyclines into cholesterol-free liposomes: enhancing drug loading rates through use of ethanol. Biochim Biophys Acta. 2004;1661:47–60. doi: 10.1016/j.bbamem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 1992;81:676–684. doi: 10.1002/jps.2600810718. [DOI] [PubMed] [Google Scholar]
- Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7:223–225. [PubMed] [Google Scholar]
- Joguparthi V, Anderson BD. Liposomal delivery of hydrophobic weak acids: Enhancement of drug retention using a high intraliposomal pH. 2007J Pharm Sci doi: 10.1002/jps.21135. in press. [DOI] [PubMed] [Google Scholar]
- Joguparthi V, et al. Liposome transport of hydrophobic drugs: Gel phase lipid bilayer permeability and partitioning of the lactone form a hydrophobic camptothecin, DB-67. J Pharm Sci. 2007 doi: 10.1002/jps.21125. in press. [DOI] [PubMed] [Google Scholar]
- Klonis N, et al. Spectral properties of fluorescein in solvent-water mixtures: applications as a probe of hydrogen bonding environments in biological systems. Photochem Photobiol. 1998;67:500–510. [PubMed] [Google Scholar]
- Klonis N, Sawyer WH. Effect of solvent-water mixtures on the prototropic equilibria of fluorescein and on the spectral properties of the monoanion. Photochem Photobiol. 2000;72:179–185. doi: 10.1562/0031-8655(2000)072<0179:eoswmo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Laiken ND, Fanestil DD. Body Fluids and Renal Function: Physiology of body fluids. In: West JB, editor. Best and Taylor’s physiological basis of medical practice. 12. Baltimore: William & Wilkins Publishing Co; 1989. pp. 406–418. [Google Scholar]
- Lopez-Barcons LA, et al. The novel highly lipophilic topoisomerase I inhibitor DB67 is effective in the treatment of liver metastases of murine CT-26 colon carcinoma. Neoplasia. 2004;6:457–467. doi: 10.1593/neo.04139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden TD, et al. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: a survey. Chem Phys Lipids. 1990;53:37–46. doi: 10.1016/0009-3084(90)90131-a. [DOI] [PubMed] [Google Scholar]
- Maurer-Spurej E, et al. Factors influencing uptake and retention of amino-containing drugs in large unilamellar vesicles exhibiting transmembrane pH gradients. Biochim Biophys Acta. 1999;1416:1–10. doi: 10.1016/s0005-2736(98)00204-1. [DOI] [PubMed] [Google Scholar]
- Peikov V, et al. pH-dependent association of SN-38 with lipid bilayers of a novel liposomal formulation. Int J Pharm. 2005;299:92–99. doi: 10.1016/j.ijpharm.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Valeur B, editor. Molecular Fluorescence. Wiley VCH; Germany: 2002. [Google Scholar]
- Xiang TX, Anderson BD. Stable supersaturated aqueous solutions of silatecan 7-t-butyldimethylsilyl-10-hydroxycamptothecin via chemical conversion in the presence of a chemically modified beta-cyclodextrin. Pharm Res. 2002;19:1215–1222. doi: 10.1023/a:1019862629357. [DOI] [PubMed] [Google Scholar]
- Xiang TX, et al. Molecular dynamics simulations and experimental studies of binding and mobility of 7-tert-butyldimethylsilyl-10-hydroxycamptothecin and its 20(S)-4-aminobutyrate ester in DMPC membranes. Mol Pharm. 2006;3:589–600. doi: 10.1021/mp0600081. [DOI] [PubMed] [Google Scholar]
- Yuan F, et al. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3360. [PubMed] [Google Scholar]
- Zhang JA, et al. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 2004;270:93–107. doi: 10.1016/j.ijpharm.2003.10.015. [DOI] [PubMed] [Google Scholar]


