SUMMARY
Dorsoventral patterning is regulated by a system of interacting secreted proteins involving BMP, Chordin, Xolloid and Twisted gastrulation (Tsg). We have analyzed the molecular mechanism by which Tsg regulates BMP signaling. Overexpression of Tsg mRNA in Xenopus embryos has ventralizing effects similar to Xolloid, a metalloprotease that cleaves Chordin. In embryos dorsalized by LiCl treatment, microinjection of Xolloid or Tsg mRNA restores the formation of trunk-tail structures, indicating an increase in BMP signaling. Microinjection of Tsg mRNA leads to the degradation of endogenous Chordin fragments generated by Xolloid. The ventralizing activities of Tsg require an endogenous Xolloid-like activity, as they can be blocked by a dominant-negative Xolloid mutant. A BMP-receptor binding assay revealed that Tsg has two distinct and sequential activities on BMP signaling. First, Tsg makes Chordin a better BMP antagonist by forming a ternary complex that prevents binding of BMP to its cognate receptor. Second, after cleavage of Chordin by Xolloid, Tsg competes the residual anti-BMP activity of Chordin fragments and facilitates their degradation. This molecular pathway, in which Xolloid switches the activity of Tsg from a BMP antagonist to a pro-BMP signal once all endogenous full-length Chordin is degraded, may help explain how sharp borders between embryonic territories are generated.
Keywords: TGFβ, BMP, Chordin, Tolloid, Twisted gastrulation, Crossveinless, Xenopus
INTRODUCTION
Dorsoventral patterning in developing embryos is established in part by a gradient of BMP (Bone Morphogenetic Protein) signaling. This gradient is generated in the extracellular space by the BMP antagonist Chordin (Chd), the zinc metalloproteinase Xolloid (Xld), and Twisted gastrulation (De Robertis and Sasai, 1996; Holley and Ferguson, 1997; De Robertis et al., 2000; Harland, 2001; Ray and Wharton, 2001).
Chordin is a secreted protein containing four cysteine-rich domains (CRs) that mediate the direct binding of Chordin to BMP (Larraín et al., 2000). Binding of BMP to Chordin prevents binding of BMP to its cognate receptor (Piccolo et al., 1996), leading to dorsalization of Xenopus embryos in overexpression studies (Sasai et al., 1994; Sasai et al., 1995). In zebrafish, the strongest ventralized mutant, chordino, has been identified as a loss-of function mutation in the chordin gene (Schulte-Merker et al., 1997; Fisher and Halpern, 1999). In chordino mutants neural plate and dorsal mesoderm are reduced, and epidermis and ventral mesoderm are expanded at the gastrula stage (Hammerschmidt et al., 1996; Gonzalez et al., 2000). The opposite phenotype, dorsalization, is seen in bmp2b/swirl and bmp7/snailhouse loss-of-function mutants (Kishimoto et al., 1997; Schmid et al., 2000). In chordino:swirl double mutants, a swirl phenotype is seen, confirming that Chordin functions as a dedicated BMP antagonist (Hammerschmidt et al., 1996).
In Drosophila, short gastrulation (sog) is the chordin homolog (François et al., 1994; Holley et al., 1995), and decapentaplegic (dpp) and screw (scw) encode BMP homologs (Holley and Ferguson, 1997; De Robertis et al., 2000). Loss of function of sog reveals two very different functions. In the ventral side, it is required for the formation of neural tissue (Zusman et al., 1988; François et al., 1994; Jaźwińska et al., 1999), as expected for a BMP antagonist. However, in the dorsal side, Sog is required for the formation of the amnioserosa, the dorsalmost tissue of the fly embryo, which requires maximal BMP signaling (Ferguson and Anderson, 1992; Ross et al., 2001). The latter effect is paradoxical, as it means that Sog, a BMP antagonist expressed in the ventral neuroectoderm, is required to attain peak BMP signaling at a distance. It has been proposed that Sog/BMP complexes originating from ventral regions diffuse in the embryo and that BMP is released dorsally by the proteolytic activity of Tolloid (Holley et al., 1996; Ashe and Levine, 1999; De Robertis et al., 2000; Harland, 2001).
Tolloid (Tld) is a zinc metalloproteinase that plays a pivotal role in BMP metabolism in Drosophila (Ferguson and Anderson, 1992). Tld and its vertebrate homolog Xolloid (Xld) have been shown to cleave Sog/Chd at specific sites (Marqués et al., 1997; Piccolo et al., 1997; Goodman et al., 1998; Scott et al., 1999; Scott et al., 2001; Yu et al., 2000). Proteolytic cleavage of inactive Chordin/BMP complexes by Xolloid restores BMP signaling in Xenopus explants (Piccolo et al., 1997). The cleavage products of Chd contain functional CR modules that retain BMP binding activity (Larraín et al., 2000), raising the question of how the BMP signal is released and transferred to the receptor.
Twisted gastrulation (Tsg) has been recently identified as an additional player in the Chd/Sog, BMP/Dpp, Xld/Tld signaling pathway (Oelgeschläger et al., 2000; Scott et al., 2001; Ross et al., 2001; Chang et al., 2001). Tsg encodes a secreted protein that is required for the differentiation of amnioserosa cells in Drosophila (Mason et al., 1994). It acts as a permissive factor specifically required for peak Dpp signaling in the dorsal midline (Mason et al., 1997; Ross et al., 2001). The isolation of a vertebrate homolog of Tsg revealed the presence of two evolutionarily conserved domains. The N-terminal domain has some sequence similarity to the CR domains of Chd/Sog and has been shown to bind directly to BMP (Oelgeschläger et al., 2000). Tsg has also been shown to bind to Chd and Sog (Oelgeschläger et al., 2000; Yu et al., 2000; Scott et al., 2001; Chang et al., 2001) and to facilitate the binding of Chd/Sog to BMP/Dpp (Oelgeschläger et al., 2000; Ross et al., 2001). Both pro- and anti-BMP activities have been described for Tsg in overexpression studies. In Xenopus, ubiquitous expression of Tsg mRNA leads to reduction of dorsal anterior markers at the early neurula stage (Oelgeschläger et al., 2000; Chang et al., 2001). However, in zebrafish, Tsg overexpression leads to dorsalization and in particular to a dramatic expansion of the expression domain of the hindbrain marker krox20 (Ross et al., 2001). In co-injection studies, Tsg is able to compete the residual anti-BMP activity of proteolytic fragments of Chordin generated by Xolloid, acting as a permissive pro-BMP factor (Oelgeschläger et al., 2000). However, in co-injections with full-length Chordin, two distinct effects of Tsg are seen. At low Tsg/Chd ratios, Tsg increases the dorsalizing activity of Chd, whereas at high concentrations Tsg inhibits Chordin (Ross et al., 2001; Chang et al., 2001; Oelgeschläger et al., 2000). As Tsg facilitates the binding of Chordin to BMP and the formation of a ternary complex, the matter of why Tsg would inhibit the activity of full-length Chordin at any concentration in vivo remains unresolved.
We present studies on the mechanism of action of the various players in this biochemical pathway. We show that the inhibition of Chd activity by Tsg requires endogenous Xolloid activity and that microinjected Xenopus Tsg mRNA facilitates the degradation of endogenous Chordin protein in Xenopus embryos. Binding of Tsg to Chordin requires an intact C-terminal Xolloid cleavage site in Chd; once Chd is cleaved, Tsg/BMP complexes are released. Using binding to the BMP receptor as a biochemical assay, we show that Tsg has distinct and sequential activities on BMP metabolism. Initially, Tsg makes Chordin a better BMP antagonist by forming a ternary complex that prevents binding of BMP to its cognate receptor. After cleavage of Chordin by Xolloid, however, Tsg competes the residual inhibitory activity of Chordin fragments and promotes their degradation in vivo. We conclude that Xolloid acts as a proteolytic switch for the two functions of Tsg. The dual activities of Tsg, first antagonizing and then promoting BMP signaling, provides a novel molecular mechanism for the regulation of morphogenetic signals in the extracellular space.
MATERIALS AND METHODS
DNA constructs
Xenopus Chd-A, Chd-B and Chd-C constructs were prepared using the Tolloid cleavage sites of mouse Chordin (Scott et al., 1999), which are conserved in the frog protein. For Chd-B the DNA between Asp147 and Ser853 was PCR amplified, and for Chd-C, the region from Asp854 to the C terminus was used. Chordin PCR products, as well as the mouse Tsg open reading frame, were introduced in frame into a pCS2 expression vector that contains the Xenopus Chordin signal peptide and the N terminus until Ala41, followed by a Flag tag sequence. The Chd-A construct has been described previously (pCS2-CR1) (Oelgeschläger et al., 2000). To generate Chd-A+B and Chd-B+C, BamHI and SphI internal sites were used, respectively. All constructs were linearized with NotI and transcribed with SP6 polymerase to generate synthetic mRNA using the mMessage mMachine kit (Ambion).
Protein expression and purification
Proteins used in Fig. 4 were obtained by transient transfection of 293T cells. For affinity purification of Xenopus Tsg-HA, conditioned medium was harvested, concentrated and diafiltrated (Centricon 30,000) to exchange the conditioned medium for buffer A (200 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.2, 0.1 mM MgCl2, 0.1 mM EDTA, 0.5 M NaCl, 0.05% Tween 20 and a cocktail of protease inhibitors (Roche Molecular Biochemicals). This sample was subjected to affinity purification using an HA affinity matrix (Covance). After washing extensively in buffer A, the proteins were eluted with 1 ml buffer A containing 0.5 mg/ml HA peptide (Piccolo et al., 1997).
Fig. 4.
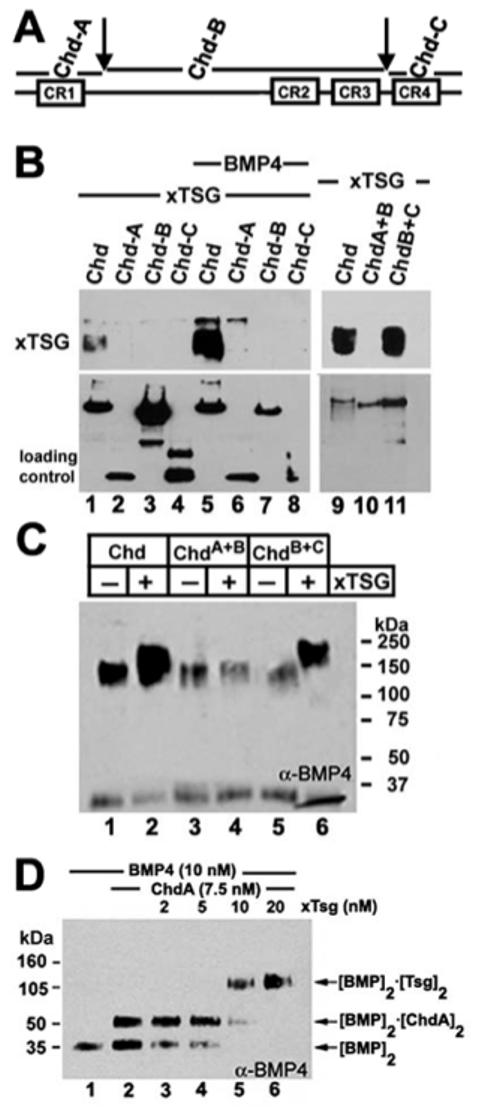
Binding of Xenopus Tsg to Chordin requires an uncleaved C-terminal Xolloid cleavage site. (A) Schematic representation of the cleavage sites of Xolloid in Chordin (arrows). Fragments of chordin mimicking the products of Xolloid digestion were prepared in 293T cells and designated as Chd-A, Chd-B, Chd-C, Chd-A+B and Chd-B+C. Each of these constructs contains the Xenopus Chordin signal peptide and an N-terminal Flag tag (except for Chd-A+B, which lacks the flag tag). (B) Western blot analysis of Xenopus Tsg-HA (5 nM) bound to the different Chordin fragments (5 nM each) after immunoprecipitation of Chordin in the absence (lanes 1 to 4) or presence (lanes 5 to 8) of 5 nM BMP4. As a loading control, membranes were stripped and probed with anti-Flag (lanes 1 to 8) or anti-I-Chd (lanes 9 to 11). (C) Anti-BMP4 immunoblot analysis of Xenopus Tsg-BMP-Chd ternary complexes after crosslinking with DSS (disuccinimidyl suberate). (D) BMP4 is dislodged from preformed Chd-A/BMP4 complexes by Xenopus Tsg. Chd-A and BMP4 were incubated for 1 hour at room temperature, followed by another hour of incubation in the presence of increasing amounts of Xenopus Tsg, and after that the crosslinker DSS was added. The complexes formed were analyzed by anti-BMP4 immunoblot. Note that Xenopus Tsg dislodges most the BMP4 from Chd-A at equimolar concentrations (lane 5) and that no Chd-A/BMP4/Xenopus Tsg ternary complexes were formed at any concentration.
Protein biochemistry
Assays for endogenous secreted Chordin were performed as described previously (Piccolo et al., 1996). In stage 10 dorsal lips incubated for 3 hours, only full-length secreted Chordin was detected. To visualize the cleavage products of endogenous Chordin, dorsal lips were explanted at stage 11 and the protein harvested after 12 hours of secretion at room temperature. In vitro digestions with the Xolloid protease were performed as described (Piccolo et al., 1997). For the BMP receptor (BMPR) binding assay, Chordin protein from baculovirus (Piccolo et al., 1996), affinity-purified Xenopus Tsg-HA and BMP4 (R&D Systems) were preincubated at room temperature for 1 hour. Then a BMPRIA-Fc protein (R&D Systems) was added for an additional hour. BMP bound to the BMPR was detected by anti-BMP4 western blot after protein A precipitation of the receptor (Larraín et al., 2000). Crosslinking and immunoprecipitation assays were performed as described (Larraín et al., 2000; Oelgeschläger et al., 2000).
Antibody purification
Antibodies for the N-terminal (anti-NChd) and for the inter-repeat (anti-I-Chd) region of Chordin were previously described (Piccolo et al., 1996; Piccolo et al., 1997). To analyze endogenous Chordin antisera were affinity-purified over nitrocellulose blots (Tang, 1993). Xenopus Chordin protein was separated by SDS-PAGE and transferred into nitrocellulose. Antibodies were bound to filter strips (for 16 hours at 4°C), washed five times (30 minutes each) with TBST, eluted on ice (for 3 minutes) with 2 ml of pH 2.8 buffer (0.1 M glycine, 0.5 M NaCl, 0.05% Tween-20) and immediately neutralized with 0.3 ml of 1 M Tris Buffer pH 8.0. For probing western blots, undiluted affinity-purified anti-NChd or a 1/3 dilution of anti-I-Chd were used.
Embryo manipulations and RT-PCR
Microinjections, in situ hybridization and mRNA synthesis were performed as described (Piccolo et al., 1997; Oelgeschläger et al., 2000; Sive et al., 2000). The probes for krox20 and otx2, gifts from Drs D. Wilkinson and E. Boncinelli, were linearized with EcoRI and NotI, respectively, and transcribed with T7 RNA polymerase (RT-PCR conditions and primers used are described at http://www.hhmi.ucla.edu/derobertis/index.html). For LiCl rescue experiments, embryos were microinjected ventrally at the 16-cell stage and treated with 120 mM LiCl in 0.1 × Barth’s medium (Sive et al., 2000) at the 32-64 cell stage for 25 minutes (Fainsod et al., 1994) and the dorsoanterior index (DAI) (Sive et al., 2000), estimated at stage 28. For lineage tracing of injected cells, 100 pg of lacZ mRNA was co-injected and visualized by Red-Gal staining.
RESULTS
Tsg and Xolloid ventralize Xenopus embryos
In zebrafish, microinjection of Tsg mRNA leads to dorsalization of the embryo, in particular to an expansion of the hindbrain marker krox20 in the neural plate (Ross et al., 2001). By contrast, in Xenopus, Tsg mRNA microinjection causes reductions of anterior structures (Oelgeschläger et al., 2000; Chang et al., 2001). To investigate this further, the effects of mouse or Xenopus Tsg mRNA were compared with the phenotype caused by injection of Xolloid mRNA, a known pro-BMP agent (Piccolo et al., 1997; Goodman et al., 1998; Ferguson and Anderson, 1992). Tsg caused a reduction of the head region (marked by krox20 and otx2) similar to that caused by Xolloid (Fig. 1A-C). In addition, co-injection of Tsg and Xolloid did not cause further reduction in head structures (Fig. 1D). As Xolloid cleaves Chordin (Piccolo et al., 1997), it seemed possible that the ventralizing activity of Tsg in Xenopus might be mediated through the Chordin pathway.
Fig. 1.
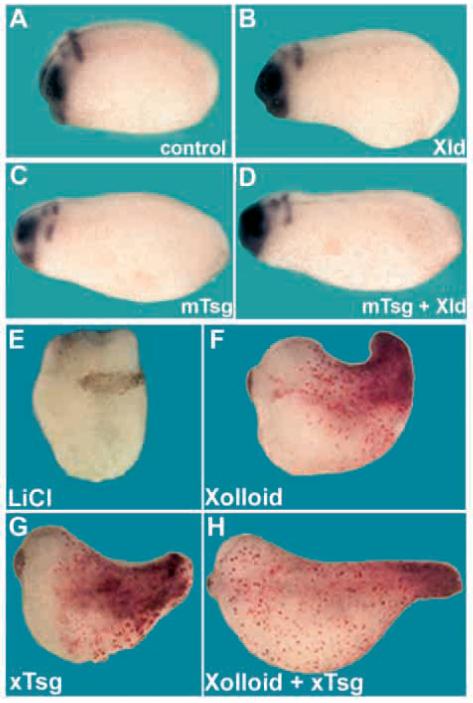
Tsg and Xolloid ventralize the Xenopus embryo. (A) Uninjected stage 18 control embryos stained for krox20 and otx2. (B) Embryos microinjected at the four-cell stage four times at the animal pole with 100 pg Xolloid, (C) 250 pg mouse Tsg or (D) both mRNAs. Same results were obtained using Xenopus Tsg mRNA (data not shown). For each mRNA combination at least 25 embryos were analyzed. (E-H) LiCl-treated embryos. (E) Radially dorsalized LiCl-treated embryo (n=40; dorsoanterior index, DAI=9.5); (F) embryo microinjected into a single blastomere of the marginal zone at the 16-cell stage with 200 pg Xolloid (26% with trunk/tail structures, n=23, DAI=8); (G) 500 pg Xenopus Tsg (32%, n=33, DAI=8.1); or (H) both mRNAs (51%, n=27, DAI=7). Lineage tracing with lacZ and Red-Gal shows that the cells injected with Xenopus Tsg or Xolloid mRNA contributed mostly to ventroposterior mesoderm in the rescued tail region.
LiCl is a dorsalizing agent that causes a large increase in the expression of Chordin and other organizer-specific genes (Sasai et al., 1994; Wessely et al., 2001). We asked whether the increase of Chordin expression is essential for the LiCl phenotype, in which case Xolloid mRNA would be expected to rescue the dorsoanteriorized phenotype. Microinjection of Xolloid mRNA into a single blastomere of the marginal zone at the 16-cell stage partially rescued trunk-tail structures in LiCl treated embryos (Fig. 1E,F). A similar rescue was caused by microinjection of Tsg mRNA (Fig. 1G). When Xolloid and Tsg mRNA were co-injected, an additive effect was observed (Fig. 1H). The injected cells, marked by lineage tracing with lacZ mRNA, contributed predominantly to ventral posterior mesoderm. The rescue of axial structures in dorsalized embryos demonstrates that Tsg is indeed a ventralizing (pro-BMP) agent in the context of the whole embryo, mimicking the rescue obtained by injection of Bmp4 mRNA (Fainsod et al., 1994). The rescue of LiCl treated embryos by the Xolloid metalloprotease also suggests an important role for Chordin or other Xolloid substrates in the dorsalization of the embryo. The similarity in phenotypes raised the possibility that Tsg, like Xolloid, may act by inactivating endogenous Chordin.
Tsg promotes the degradation of endogenous Chordin
We next tested the effect of Tsg mRNA on endogenous Chordin protein in the Xenopus gastrula. Dorsal marginal zone explants (DMZ) were prepared at the early gastrula stage, cells dissociated in Ca2+/Mg2+ free saline, incubated for 3 hours, and the secreted Chordin protein analyzed by western blot. Two affinity-purified antibodies were used, one raised against the central region of Chordin and one specific for the N terminus (Fig. 2A). Microinjection of Tsg mRNA caused a marked reduction in the amount of secreted endogenous full-length Chordin (Fig. 2B,C; lanes 1 and 2). In Xolloid-injected embryos, a stable Chordin degradation fragment (corresponding to the endogenous Xolloid cleavage product containing cysteine-rich domains CR2 and CR3; Fig. 2A) was detected, but was destabilized by co-injection of Xenopus Tsg mRNA (Fig. 2B, compare lanes 3 and 4). We conclude that Xenopus Tsg overexpression leads to the degradation of endogenous Chordin fragments in the embryo. This activity of Tsg helps explain why Tsg can block the induction of secondary axes by full-length Chordin mRNA (Oelgeschläger et al., 2000; Ross et al., 2001; Chang et al., 2001).
Fig. 2.
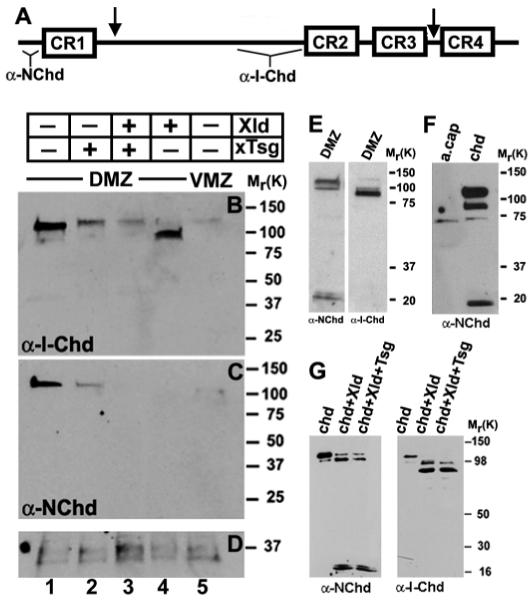
Tsg promotes the degradation of endogenous Chordin fragments. (A) Schematic representation of the cleavage sites of Xolloid in Chordin (arrows). The regions of Chordin protein used to generate the anti-N-Chd and anti-I-Chd antibodies are indicated. (B-D) Embryos were injected marginally into each blastomere at the four-cell stage with Xenopus Tsg (2 ng total, lane 2), Xolloid (0.8 ng total, lane 4) or both mRNAs (lane 3). Dorsal marginal zones (DMZ, lanes 1-4) or ventral marginal zones (VMZ, lane 5) were explanted at early gastrula (stage 10), cells were dissociated and the Chordin protein secreted during 3 hours analyzed in western blots using anti-I-Chd (B) or anti-N-Chd (C) antibodies. (D) Loading control showing a protein that crossreacts with the secondary antibody. (E) DMZs isolated at stage 11 and incubated for 12 hours at room temperature show the canonical Chordin degradation fragments but no additional products. (F) Proteins secreted by animal caps from uninjected (lane 1) and chordin-injected embryos (lane 2) were detected by anti-N-Chd immunoblot. Xenopus embryos were injected into the animal pole with 200 pg of chordin mRNA, ectodermal explants isolated at blastula stage, and dissociated cells incubated for 12 hours at room temperature. (G) Western blot analysis of Xenopus Chordin protein probed with anti-N-Chd or anti-I-Chd after digestion for 10 hours at room temperature with control medium (lane 1), Xolloid (lane 2), and Xolloid and Xenopus Tsg (lane 3). Note that the pattern obtained for Chordin digestion in vivo (E,F) is the same one as obtained after in vitro digestion of Chordin (G), and that additional proteolytic fragments were not observed in the embryo.
In Drosophila, Tsg changes the specificity of Tolloid cleavage causing the formation of a new fragment of the Chordin homolog Sog, called Supersog, which has been found both in embryo extracts and by in vitro digestion of Sog with Tolloid in the presence of Tsg (Yu et al., 2000). Supersog consists of the first CR repeat and additional amino acid sequence of the inter-repeat domain, and has novel inhibitory specificities (Yu et al., 2000). In Xenopus embryos, endogenous Chd proteolytic products could be detected in dissociated DMZ explants (Fig. 2E). In this experiment, DMZs were explanted at stage 11 and incubated for 12 hours; the longer incubation times were required to detect Chordin fragments. The fragments observed correspond to the two previously characterized cleavages of Chordin by Xolloid (Piccolo et al., 1997). We were unable to detect fragments of the Superchordin type in vivo (Fig. 2E), in animal caps injected with chd mRNA (Fig. 2F) or in biochemical assays (Fig. 2G). A third cleavage site within the inter-repeat region has been described in vitro for mouse Chordin in the presence of Tsg (Scott et al., 2001). We did not detect this cleavage in Xenopus, either in vivo (Fig. 2E) or in vitro (Fig. 2G), but we note that the sequence of this cleavage site is not conserved between mouse and Xenopus Chordin. Clearly, there are differences on how species regulate the Chordin pathway despite its evolutionary conservation.
Ventralization by Xenopus Tsg requires Xolloid activity
Embryos were microinjected with Tsg mRNA at the 32-cell stage into the animal pole and lineage traced with lacZ mRNA. As shown in Fig. 3A, the domain of krox20 expression in the Xenopus neurula was reduced in the injected site. When all animal blastomeres were injected at the four-cell stage with Xenopus Tsg mRNA, krox20 expression was reduced in width and intensity (Fig. 3B,C). When a dominant-negative Xolloid (dnXld) mRNA (Piccolo et al., 1997) was co-injected with Xenopus Tsg mRNA, the reduction of krox20 in Xenopus was blocked (Fig. 3E). This suggested that ventralization by Xenopus Tsg requires endogenous Xolloid activity. Similarly, secondary axes induced by either Xenopus or mouse full-length chordin mRNA were antagonized by co-injection of Xenopus Tsg or mouse Tsg mRNA (Fig. 3F,G and data not shown), but secondary axis formation was restored by co-injection of dominant negative Xld mRNA (Fig. 3I). In animal cap explants, Xenopus Tsg mRNA decreased neural induction by chordin, and this effect was blocked by co-injection of dominant negative Xld mRNA (Fig. 3P, lanes 4, 5 and 7). We conclude from these experiments that the ventralizing activity of Xenopus Tsg in Xenopus embryos requires an endogenous Xolloid-like activity. In other words, the pro-BMP activity of Tsg requires the cleavage of full-length Chordin by Xolloid metalloprotease.
Fig. 3.
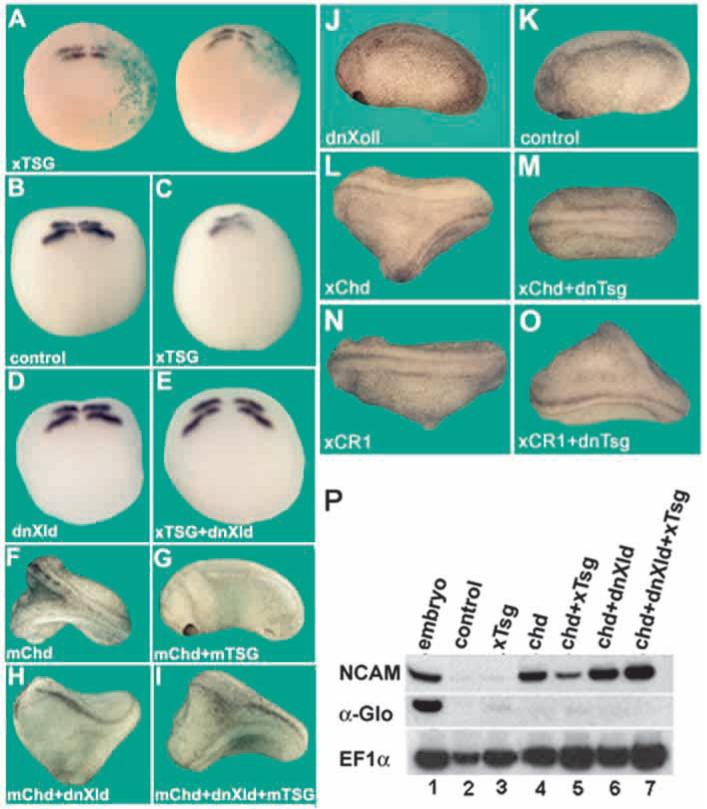
The ventralizing activity of Tsg is dependent on endogenous Xolloid. (A) Albino embryos were microinjected once into an animal cell with 500 pg Xenopus Tsg and lacZ mRNA at the 32-cell stage and krox20 in situ hybridization performed. Note that krox20 expression is reduced on the injected side. (B) krox20 in situ hybridization of uninjected embryos at neural plate stage. (C) Embryos injected with 250 pg Xenopus Tsg mRNA, (D) 250 pg dominant negative (dn) Xld mRNA or, (E) co-injected with Xenopus Tsg and dnXld mRNAs (n=25 or more for each mRNA combination). All embryos were injected 4 times in the animal pole at the four-cell stage. Similar results were obtained using mouse Tsg mRNA (data not shown). (F) Ventral injection of 5 pg mouse Chd mRNA induces secondary axes; (G) injection of 5 pg mouse Chd and 500 pg mouse Tsg mRNA; (H) injection of 5 pg mouse Chd and 500 pg dnXld mRNA, and (I) 5 pg mouse Chd, 500 pg mouse Tsg and 500 pg dnXld mRNAs. (J,K) Injection of 500 pg of dnXld mRNA(J) and uninjected controls (K). Similar results were obtained using Xenopus Tsg and Xenopus chd mRNA (data not shown). Injection of 5 pg of chd mRNA induced strong secondary axes in 47% of the cases (F); these axes were not seen after chd and Tsg co-injection (G), but in 14-50% of the embryos injected with chd, Tsg and dnXld mRNA, strong secondary axes were rescued (I). Note in J that dnXld was unable to induce secondary axes on its own. (L) Ventral injection of 5 pg Xenopus Chd mRNA induces secondary axes (44%). (M) Co-injection of 500 pg dnTsg mRNA reduced the axis-forming activity of Xenopus Chd (14%). (N) 20 pg Xenopus CR1 mRNA induced weak secondary axes. (O) Co-injection of dnTsg mRNA enhanced the secondary axis phenotype caused by Xenopus CR1. For all injections, at least 35 embryos were analyzed. (P) RT-PCR analysis of animal cap explants injected with the indicated combinations of mRNAs and analyzed at stage 25; total amounts of mRNA injected per embryo were 800 pg Xenopus Tsg, 40 pg Xenopus chd and 1 ng dnXld. NCAM is a pan-neural marker, α-Glo (α-globin), a ventral mesoderm marker, and EF1α was used as a loading control.
The critical importance of Chordin proteolytic cleavage was also seen in experiments using a dominant-negative Tsg (dnTsg) construct consisting of only the C-terminal domain (Oelgeschläger et al., 2000). Injected dnTsg has opposing activities on full-length Xenopus Chordin or on the CR1 (Chd-A) fragment. When co-injected with Chordin, dnTsg mRNA inhibited the formation of double axes (Fig. 3L,M). When co-injected with Xenopus CR1 mRNA, the opposite result (an increase in dorsalizing activity) was observed (Fig. 3N,O). These results further support the view that Tsg has dual function on BMP signaling. Decreasing the activity of endogenous Tsg reduces dorsalization by the BMP antagonist Chordin, presumably because lower amounts of ternary complex of Tsg, Chd and BMP are formed. However, reducing Tsg activity increases the BMP antagonist function of CR1, presumably by interfering with the ability of endogenous Tsg to dislodge BMP bound to the CR1 module.
In zebrafish, overexpression of Tsg has a dorsalizing phenotype (Ross et al., 2001), which is the opposite of what we observe in Xenopus. Zebrafish embryos may have low levels of endogenous Tolloid activity, as the loss-of-function of Tolloid has only a weak mini-fin phenotype (Connors et al., 1999) and injection of dn Tld mRNA results in only mild dorsalization (Blader et al., 1997). A possible explanation for the difference in phenotypes (Fig. 3) (Oelgeschläger et al., 2000; Ross et al., 2001) is that in zebrafish Tsg overexpression may favor primarily the formation of inhibitory ternary complexes of full-length Chordin, BMP and Tsg, which are more stable than those of Xenopus because of low Tolloid levels.
Binding of Tsg to Chd is regulated by Xolloid
The ventralizing activity of Tsg and its dependence on Xolloid activity suggests that the anti-BMP activity of ternary complexes should be inactivated by proteolytic cleavage of Chordin by Xolloid. To test this, we investigated the binding site of Xenopus Tsg in Chordin by subdividing the molecule into three fragments, designated Chd-A, Chd-B and Chd-C (Fig. 4A), which mimic the Xolloid cleavage products (Piccolo et al., 1997; Scott et al., 1999). Immunoprecipitation experiments showed that Xenopus Tsg binds to full-length Chordin but not to Chd-A, Chd-B or Chd-C in the absence or presence of BMP4 (Fig. 4B, lanes 1-8). After longer exposures, a trace amount of Xenopus Tsg bound to Xenopus Chd-A protein in the presence of BMP4 was observed (Fig. 4B, lane 6) (Scott et al., 2001), but this binding is not considered significant. A fragment consisting of Chd-A+B was unable to bind Xenopus Tsg, whereas a Chd-B+C construct bound Xenopus Tsg as efficiently as full-length Chd (Fig. 4B, lanes 10-11). Furthermore, in experiments using the chemical crosslinker DSS (disuccinimidyl suberate), full-length Chordin and the Chd-B+C fragment were able to form ternary complexes with Xenopus Tsg and BMP4, whereas Chd-A+B did not bind Tsg (Fig. 4C).
Chd-A, which contains the CR1 BMP-binding module does not form a ternary complex when incubated with Xenopus Tsg and BMP (Oelgeschläger et al., 2000). To determine whether Tsg is able to dislodge BMP pre-bound to Chd-A fragment, order-of-addition experiments were performed. After preincubation of Chd-A and BMP4 for 1 hour (Fig. 4D, lane 2), the addition of equimolar amounts of Xenopus Tsg was able to dislodge BMP4 from the Chd fragment, forming a binary complex consisting of Xenopus Tsg and BMP4 (Fig. 4D, lanes 5 and 6).
Taken together, the results suggest that formation of the ternary complex of full-length Chd, BMP and Xenopus Tsg requires an uncleaved C-terminal cleavage site. When Xolloid cleaves Chordin at this site, a binary complex of Tsg and BMP is released.
Xolloid as a proteolytic switch
To study the effects of Xenopus Tsg on BMP signaling, we used a direct assay measuring binding of BMP4 to a BMP-receptor-Fc fusion protein. In the presence of 0.5 or 5 nM full-length Chordin, 5 nM affinity-purified Xenopus Tsg protein potentiated the inhibition of receptor binding (Fig. 5A, lanes 3 and 5), whereas 5 nM of Xenopus Tsg alone had no effect on BMP binding (Fig. 5A, lane 6). This shows that the ternary complex of Xenopus Tsg, Chd and BMP is a more potent BMP antagonist than Chordin alone.
Fig. 5.
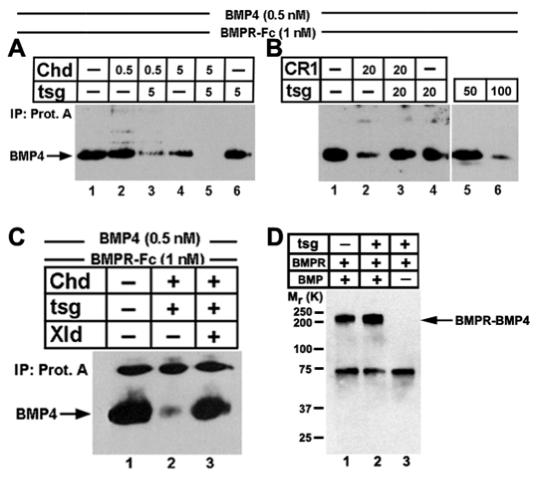
Xenopus Tsg functions as a BMP antagonist in the presence of full-length Chordin, but promotes binding of BMP to its receptor in the presence of Chordin fragments. BMP4 was incubated for 1 hour at room temperature with Chordin, CR1, and affinity purified Xenopus Tsg-HA. Subsequently, type I BMP-receptor-Fc fusion protein (R&D Systems) was added, precipitated using protein-A, and analyzed by anti-BMP immunoblot. (A) Xenopus Tsg makes Chordin a better BMP antagonist; the concentration of each component in nM is indicated. (B) Xenopus Tsg restores binding of BMP4 to its cognate receptor in the presence of 20 nM CR1 (lane 3). Note that at high concentrations (100 nM, lane 6) Xenopus Tsg by itself can function as a BMP antagonist in this biochemical assay. (C) The Chd/BMP/Xenopus Tsg ternary complex was digested for 10 hours at room temperature with Xolloid. Lane 3 shows that BMP4 is reactivated and binds to its receptor. (D) Xenopus Tsg does not bind to BMPR-IA. Xenopus Tsg, BMP4 and the BMPRIA-Fc were incubated 1 hour at room temperature before DSS crosslinker was added. The complexes formed were analyzed by anti-BMP4 western blot after protein A immunoprecipitation. The arrow indicates the BMPR-Fc-BMP4 complex. The band at 75 kDa is unspecific as it is also observed in the absence of BMP4 in the reaction (lane 3).
When CR1 protein (Chd-A fragment, Fig. 4A) was added, a concentration of 20 nM was required to inhibit BMP binding to its receptor (Larraín et al., 2000). Importantly, addition of an equimolar amount of Xenopus Tsg restored BMP binding (Fig. 5B, compare lanes 2 and 3). Xenopus Tsg therefore competes the residual anti-BMP activity of the CR1 fragment. Xenopus Tsg by itself at 20 or 50 nM did not interfere with BMP binding (Fig. 5B, lanes 4 and 5). We note, however, that at higher concentration (100 nM), Xenopus Tsg did inhibit receptor binding (Fig. 5B, lane 6). This helps explain results in which Xenopus Tsg alone acts as a BMP antagonist (Scott et al., 2001; Ross et al., 2001; Chang et al., 2001). The concentration of Tsg required to inhibit BMP binding to its receptor, however, is 20-fold higher than that required for the synergy with full-length Chordin and therefore presumably non-physiological. In addition, the Xenopus Tsg protein does not bind to the BMP receptor in immunoprecipitation and crosslinking assays, even in the presence of BMP4 (Fig. 5D). We conclude that Tsg displaces BMPs bound to CR fragments, providing a permissive signal that allows binding of BMP4 to its receptor.
The opposing activities of Tsg on full-length Chordin and on its proteolytic fragments suggested that the switch between the two activities of Tsg is controlled by the Xolloid metalloproteinase. This hypothesis was tested biochemically by digesting the inhibitory ternary complex with Xolloid and determining its effect on BMP binding to its receptor. We used conditions in which 0.5 nM BMP was quantitatively complexed with Chordin and Xenopus Tsg (5 nM each) for 1 hour. This blocked BMP binding to its receptor (Fig. 5C, lanes 1 and 2). After addition of Xolloid and efficient digestion of Chordin into its three fragments (Fig. 2G), binding to BMP receptor was restored (Fig. 5C, lane 3). This biochemical experiment, together with the findings that Tsg promotes the degradation of Chordin fragments and that formation of the ternary complex requires an intact C-terminal Xolloid cleavage site, argues against the possibility that Xenopus Tsg cooperates in BMP antagonism with Chordin fragments generated by Xolloid (Scott et al., 2001). We conclude that digestion of Chordin by Xolloid can efficiently reactivate latent BMPs complexed with Chordin and Xenopus Tsg, restoring receptor binding, even in the presence of Xenopus Tsg and Chordin proteolytic fragments.
DISCUSSION
We have examine the molecular mechanism by which Tsg regulates BMP signaling in the Xenopus embryo. Overexpression of vertebrate Tsg has a phenotypic effect comparable to that of Xolloid (Fig. 1). The rescue of LiCl dorsalization by Tsg and Xolloid suggests that both factors can increase BMP signaling in Xenopus. This ventralization seems to be mediated by the inactivation of endogenous Chordin. Analysis of endogenous Chordin secreted by the Spemann organizer using affinity-purified antibodies indicates that microinjection of Tsg mRNA causes the degradation of Chordin proteolytic fragments resulting from Xolloid cleavage (Fig. 2). Experiments using a dominant-negative Xld construct show that the ventralizing activity of Tsg, and its ability to inhibit microinjected chd mRNA, requires endogenous Xolloid activity (Fig. 3). Xolloid provides the key step regulating the formation of a ternary complex between Chordin, Tsg and BMP: once Chordin is cleaved at the C-terminal site, Tsg is unable to bind the Chordin fragments (Fig. 4).
A model for BMP signaling regulation
The opposing activities of Tsg on BMP binding to its receptor (Fig. 5) suggest a sequential molecular mechanism (Fig. 6) that may help reconcile disparate observations in the literature (Oelgeschläger et al., 2000; Scott et al., 2001; Ross et al., 2001; Chang et al., 2001). First, Tsg forms a ternary complex with Chordin and BMP, which is a potent inhibitor of BMP signaling (Fig. 5A). This antagonist function must be the predominant one in zebrafish, as loss-of-function of Tsg and Chordin using antisense morpholinos ventralizes the embryo (Ross et al., 2001). Second, after cleavage of Chordin by Xolloid, Tsg competes the residual activity of Chordin fragments, providing a permissive signal that promotes BMP binding to its cognate receptor. This function is consistent with injection experiments in Xenopus embryos, in which reduction of endogenous Xenopus Tsg activity enhances the anti-BMP activity of CR1 fragments (Oelgeschläger et al., 2000). Third, overexpression of Tsg facilitates the degradation of endogenous Chordin in Xenopus (Fig. 2B). This activity may help explain why Tsg can ventralize the embryo and inhibit axis duplication by Chordin (Oelgeschläger et al., 2000; Ross et al., 2001; Chang et al., 2001) in a Xolloid-dependent manner. We propose that in overexpression experiments, an excess of Tsg protein displaces the equilibrium in the reaction depicted in Fig. 6, so that after cleavage of Chordin by Xolloid Tsg dislodges BMP from the proteolytic products and facilitates their degradation in vivo. The Tsg/BMP binary complex acts as a permissive signal, because at physiological concentrations Tsg does not interfere with BMP binding to its receptor. Finally, at high concentrations Tsg can also act as a BMP antagonist in the absence of Chordin (Fig. 5B, lane 6) (Ross et al., 2001), inducing in animal cap explants the cement gland marker XAG-1, but not the neural marker NCAM, by partially inhibiting BMP activity (Chang et al., 2001; Wilson et al., 1997) (M.O. and E.M.D.R., unpublished).
Fig. 6.
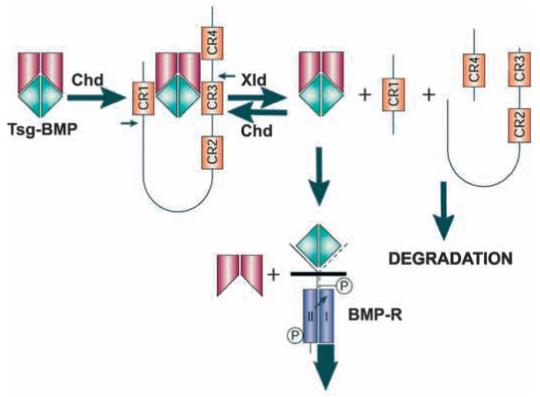
Model for a biochemical pathway that regulates BMP signaling in the extracellular space. Tsg-BMP complexes can be bound by full-length Chordin to form a ternary complex that is a potent BMP antagonist. Xolloid cleaves Chordin releasing Tsg-BMP binary complexes and Chordin fragments. In the presence of full-length Chordin, the binary complex will re-bind to Chordin, reforming the ternary complex. After all full-length Chordin is cleaved by Xolloid, however, Tsg is able to dislodge BMP from Chordin and to destabilize the Chordin proteolytic products, displacing the equilibrium. This model explains why Tsg has the dual ability to increase BMP antagonism by full-length Chordin and to promote BMP signaling after Xolloid cleavage.
Multiple Chordin-like proteins
A variety of extracellular proteins contain CR domains similar to those of Chordin. These include fibrillar procollagens (type I, II, III and V), members of the Nel-like family, CRIM-1, Kielin, Amnionless, Neuralin and Crossveinless 2 (Scriver et al., 1995; Watanabe et al., 1996; Kolle et al., 2000; Matsui et al., 2000; Kalantry et al., 2001; Coffinier et al., 2001; Conley et al., 2000). Several of these proteins, such as procollagen II, CRIM-1, Kielin, Neuralin and Crossveinless 2 (Cv-2), have been shown to modulate TGF-β/BMP signaling (Zhu et al., 1999; Larraín et al., 2000; Matsui et al., 2000; Coffinier et al., 2001; Nakayama et al., 2001; Conley et al., 2000).
The case of Cv-2 is particularly interesting. This Drosophila molecule contains five adjacent CR domains of the Chordin type. cv-2 mutants lack the crossveins of the fly wing, which require peak Dpp signaling (Conley et al., 2000). A similar phenotype is observed in the wing after overexpression of Sog and in partial loss-of-function dpp alleles (Yu et al., 1996). Thus, Cv2 is a CR-containing protein that increases Dpp signaling. Another Drosophila mutation with the same defects in wing vein patterning is crossveinless (cv), first identified many years ago (Bridges, 1920). The mutation has been recently mapped to a second Drosophila Tsg gene homolog (L. Marsh, communication to Fly Base: FBgn0000394). Drosophila Tsg is required for peak BMP signaling in the dorsal midline of the fly embryo, and Cv is required for maximal BMP signaling in the crossveins of the wing. The observation that a second Tsg works together with the Cv-2 CR-containing protein in promoting BMP signaling suggests that the Chd/BMP/Xld/Tsg pathway shown in Fig. 6 may provide a general paradigm for cell-cell signaling modulation.
Generating borders
The present results provide mechanistic insights into how sharp borders may be generated in embryos. In Drosophila, Tsg is required for the peak BMP signaling (Mason et al., 1994; Mason et al., 1997) that induces a sharp band of Mad phosphorylation in the dorsal-most tissue (Ross et al., 2001). The pathway depicted in Fig. 6 shows how in lateral regions of the embryo, in which free full-length Chordin is still present, Tsg/BMP binary complexes released by Xolloid will have a higher affinity for Chordin than for the BMP receptor (Oelgeschläger et al., 2000), promoting the re-formation of inhibitory ternary complexes that can diffuse further (Holley et al., 1996). However, once all Chd is proteolytically cleaved by Xolloid, the function of Tsg switches from an inhibitory to a permissive signal that increases binding of BMPs to their cognate receptors. This switch in activity would facilitate the formation of sharp boundary differences. In lateral regions, in which ternary complexes are constantly re-formed and re-cleaved as diffusion takes place, the situation is conceptually analogous to that occurring in an organic chemistry fractional distillation column. Although much remains to be learned about this interesting patterning system, the opposing functions of Tsg suggest a novel molecular mechanism for the establishment of cell differentiation territories in the embryo.
Acknowledgments
We thank Drs L. Zipursky, D. Schmucker and O. Wessely for comments on the manuscript, and S. Y. Li and A. Cuellar for technical assistance. M. O. and J. L. were HFSPO and Pew postdoctoral fellows, respectively. This work was supported by the NIH (R37 HD21502-15). E. M. D. R. is a Howard Hughes Medical Institute Investigator.
REFERENCES
- Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398:427–431. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- Blader P, Rastegar S, Fischer N, Strahle U. Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science. 1997;278:1937–1940. doi: 10.1126/science.278.5345.1937. [DOI] [PubMed] [Google Scholar]
- Bridges C. The mutant crossveinless in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1920;6:660–663. doi: 10.1073/pnas.6.11.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Holtzman DA, Chau S, Chickering T, Wolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Tran U, Larraín J, De Robertis EM. Neuralin is a novel Chordin-related molecule expressed in the mouse neural plate. Mech. Dev. 2001;100:119–122. doi: 10.1016/s0925-4773(00)00507-4. [DOI] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/minifin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Localized enhancement and repression of the activity of the TGF-β family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992;114:583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Fisher S, Halpern ME. Patterning the zebrafish axial skeleton requires early chordin function. Nat. Genet. 1999;23:442–446. doi: 10.1038/70557. [DOI] [PubMed] [Google Scholar]
- François V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Gonzalez EM, Fekany-Lee K, Carmany-Rampey A, Erter C, Topczewski J, Wright C, Solnica-Krezel L. Head and trunk in zebrafish arise via coinhibition of BMP signaling by bozozok and chordino. Genes Dev. 2000;14:3087–3092. doi: 10.1101/gad.852400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SA, Albano R, Wardle FC, Matthews G, Tannahill D, Dale L. BMP1-related metalloproteinases promote the development of ventral mesoderm in early Xenopus embryos. Dev. Biol. 1998;195:144–157. doi: 10.1006/dbio.1997.8840. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Harland RM. A twist on embryonic signalling. Nature. 2001;410:423–424. doi: 10.1038/35068657. [DOI] [PubMed] [Google Scholar]
- Holley SA, Ferguson EL. Fish are like flies are like frogs: conservation of dorsal-ventral patterning mechanisms. BioEssays. 1997;4:281–284. doi: 10.1002/bies.950190404. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffman FM, Ferguson EL. A conserved system for dorsal-ventral patterning in insects and vertebrates involving short gastrulation and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Jaźwińska A, Rushlow C, Roth S. The role of brinker in mediating the graded response to Dpp in early Drosophila embryos. Development. 1999;126:3323–3334. doi: 10.1242/dev.126.15.3323. [DOI] [PubMed] [Google Scholar]
- Kalantry S, Manning S, Haub O, Tomihara-Newberger C, Lee HG, Fangman J, Disteche CM, Manova K, Lacy E. The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nat. Genet. 2001;27:412–416. doi: 10.1038/86912. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee K-H, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Kolle G, Georgas K, Holmes GP, Little MH, Yamada T. CRIM1, a novel gene encoding a cysteine-rich repeat protein, is developmentally regulated and implicated in vertebrate CNS development and organogenesis. Mech. Dev. 2000;90:181–193. doi: 10.1016/s0925-4773(99)00248-8. [DOI] [PubMed] [Google Scholar]
- Larraín J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G, Musacchio M, Shimell MJ, Wünnenberg-Stapleton K, Cho KWY, O’Connor MB. Production of DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, Marsh JL. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8:1489–1501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- Mason ED, Williams S, Grotendorst GR, Marsh JL. Combinatorial signaling by Twisted Gastrulation and Decapentaplegic. Mech. Dev. 1997;64:61–75. doi: 10.1016/s0925-4773(97)00049-x. [DOI] [PubMed] [Google Scholar]
- Matsui M, Mizuseki K, Nakatani J, Nakanishi S, Sasai Y. Xenopus kielin: A dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline. Proc. Natl. Acad. Sci. USA. 2000;97:5291–5296. doi: 10.1073/pnas.090020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, Wen D. A novel Chordin-like protein inhibitor for Bone Morphogenetic Proteins expressed preferentially in mesenchymal cell lineages. Dev. Biol. 2001;232:372–387. doi: 10.1006/dbio.2001.0200. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RP, Wharton KA. Twisted perspective: new insights into extracellular modulation of BMP signalling during development. Cell. 2001;104:801–804. doi: 10.1016/s0092-8674(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Schmid B, Fürthauer M, Connors SA, Trout J, Thisse B, Thisse C, Mullins MC. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KWY, Greenspan DS. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev. Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KWY, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–478. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- Scriver C, Beaudet A, Sly W, Valle D. The Metabolic & Molecular Bases of Inherited Disease. III. McGraw-Hill; New York: 1995. pp. 4029–4077. [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. [Google Scholar]
- Tang WJ. Blot-affinity purification of antibodies. Methods Cell Biol. 1993;37:95–104. doi: 10.1016/s0091-679x(08)60245-9. [DOI] [PubMed] [Google Scholar]
- Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, Nakamura Y, Hirai Y, Maekawa H, Takahashi E. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschläger M, Pera EM, De Robertis EM. Neural induction in the absence of mesoderm: β-catenin dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev. Biol. 2001;234:161–173. doi: 10.1006/dbio.2001.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- Yu K, Sturtevant MA, Biehs B, François V, Padgett RW, Blackman RK, Bier E. The Drosophila decapentaplegic and short gastrulation genes function antagonistically during adult wing vein development. Development. 1996;122:4033–4044. doi: 10.1242/dev.122.12.4033. [DOI] [PubMed] [Google Scholar]
- Yu K, Srinivasan S, Shimmi O, Biehs B, Rashka KE, Kimelman D, O’Connor MB, Bier E. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-β1 and BMP-2. J. Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman SB, Sweeton D, Wieschaus EF. short-gastrulation, a mutation causing delays in stage-specific cell shape changes during gastrulation in Drosophila melanogaster. Dev. Biol. 1988;129:417–427. doi: 10.1016/0012-1606(88)90389-2. [DOI] [PubMed] [Google Scholar]


