Abstract
In hippocampal neurons, neurotransmitter release can be regulated by protein kinase A (PKA) through a direct action on the secretory machinery. To identify the site of PKA modulation, we have taken advantage of the ability of the neurotoxin Botulinum A to cleave the synaptic protein SNAP-25. Cleavage of this protein decreases the Ca2+ responsiveness of the secretory machinery by partially uncoupling Ca2+-sensing from fusion per se. This is expressed as a shift toward higher Ca2+ levels of the Ca2+ to neurotransmitter release relationship and as a perturbation of synaptic delay under conditions where secretion induced by the Ca2+-independent secretagogue ruthenium red is unimpaired. We find that SNAP-25 cleavage also perturbs PKA-dependent modulation of secretion; facilitation of ruthenium red-evoked neurotransmitter release by the adenylyl cyclase activator forskolin is blocked completely after Botulinum toxin A action. Together with our observation that forskolin modifies the Ca2+ to neurotransmitter release relationship, our results suggest that SNAP-25 acts as a functional linker between Ca2+ detection and fusion and that PKA modulates an early step in the secretory machinery related to calcium sensing to facilitate synaptic transmission.
Synaptic transmission can be modulated through a number of mechanisms, both pre- and postsynaptic, some of which are implicated in memory formation (1–4). In a number of preparations, presynaptic activation of protein kinase A (PKA) can enhance the amount of neurotransmitter released per action potential (5–9). In rat hippocampal neurons, this increase in release occurs, at least in part, through an up-regulation of the efficacy of the secretory process at a step downstream from Ca2+ influx (10). The mechanism underlying this enhanced secretory response is not defined. However, we have determined that it is not caused by an increase in the number of functional synaptic boutons or by an increase in the number of morphologically docked vesicles within individual synaptic terminals (10). Two other prominent mechanisms might mediate this PKA-dependent facilitation of synaptic transmission: Either the Ca2+ sensitivity of the secretory machinery is increased, or the number of vesicles functionally primed for exocytosis could be enhanced by PKA. To distinguish between these possibilities, we have used the clostridial neurotoxin Botulinum A (BontA), which selectively cleaves the synaptic protein SNAP-25 in intact neurons (11, 12). We first report modifications to synaptic transmission that result from SNAP-25 cleavage and then go on to identify consequences for the PKA-dependent modulation of synaptic transmission.
MATERIALS AND METHODS
Cell Culture.
Mixed neuron–astrocyte cultures were prepared from neonatal rat pups as recently described (13). Coverslips were coated with a mixture of poly-l-lysine (0.1 mg/ml) and collagen (0.1 mg/ml). Culture media contained 5% fetal calf serum and Mito+ serum additive (Collaborative Biomedical Products, Bedford MA). A mitotic inhibitor (5 μM 5-fluoro-2-deoxyuridine and uridine) was added after the fourth day in culture to halt glial proliferation. One-third of the culture media was replaced with fresh medium every third day. Cells were used after ≥9 days in culture. Cultures treated with BontA (Wako Bioproducts, Richmond, VA) were exposed to the toxin overnight at a concentration of 2 μg/ml.
Electrophysiological Recordings.
For dual cell recordings, standard whole-cell patch clamp recordings were obtained from the neurons by using two Axopatch 1-D amplifiers (Axon Instruments, Foster City, CA). The normal internal solution was 140 mM KGluconate, 10 mM EGTA, 4mM Mg-ATP, 0.2 mM Tris-GTP, and 10 mM Hepes (pH 7.35). The standard external saline contained 135.5 mM NaCl, 7 mM MgCl2, 3 mM CaCl2, 5 mM KCl, 10 mM Hepes, and 8 mM glucose (pH 7.35). The concentration of MgCl2 was elevated from the usual 2 mM to 7 mM to decrease spontaneous synaptic activity in the cultures and to decrease the likelihood of recording polysynaptic responses. In experiments where synaptic delays were measured, the observation that high extracellular Ca2+ in addition to the high concentration of MgCl2 also failed to block synaptic responses provided an additional test of the monosynaptic nature of the synaptic currents because such treatments are well known to decrease cellular excitability. The reversal potential of the inhibitory synaptic currents (IPSCs) was monitored periodically during the course of the experiments. It was found to stabilize at ≈−75 mV within a few minutes after obtaining a whole-cell recording. Data were analyzed by using pclamp software (Axon Instruments). For the recording of miniature IPSCs (minis), the internal recording solution consisted of 117.5 mM CsGluconate, 10 mM NaCl, 4 mM MgCl2, 5 mM EGTA, 2 mM Mg-ATP, 0.2 mM Tris-GTP, and 15 mM Hepes (pH 7.3). The reversal potential for the miniature IPSCs stabilized at ≈−35 mV within a few minutes after obtaining whole-cell recording and was checked periodically within the course of the experiments. Miniature synaptic currents were analyzed with software provided by P. Vincent (University of California at San Diego). Data in the text and figures are presented as mean ± SEM unless otherwise indicated.
Western Blots.
Membrane preparations prepared from cell cultures were subjected to SDS/PAGE and immunoblot analysis. Antibodies to SNAP-25 (Cl71.2, generously supplied by P. DeCamilli, Yale University) and to syntaxin (HPC-1, generously provided by C. J. Barnstable, Yale University) were used. Immunoreactive bands were detected by using enhanced chemiluminescence (Amersham).
cAMP Radioimmunoassay.
Detection of cAMP was performed by using the Biotrak cAMP assay kit (Amersham) according to the manufacturer’s instructions. Samples and standards were acetylated to increase the sensitivity caused by the small quantities of cultured cells used. Because our experiments were performed in mixed cultures containing both neurons and glia, we repeated these measurements in neuron-enriched embryonic hippocampal cultures. As in the mixed cultures, we found that BontA did not impair the ability of forskolin to stimulate the production of cAMP (data not shown).
RESULTS
Action of BontA on Neurotransmitter Release.
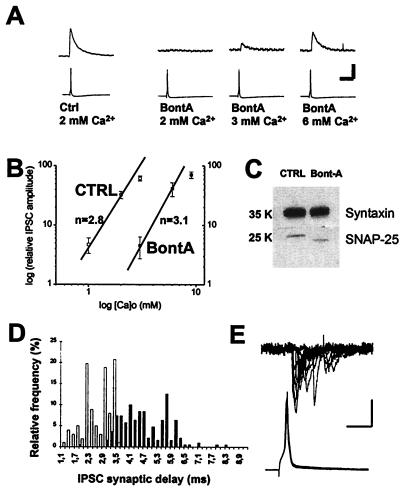
Using rat hippocampal neurons in primary culture, we confirmed that BontA significantly attenuates action potential-evoked neurotransmitter release both at γ-aminobutyric acid-mediated (Fig. 1A) and glutamate-mediated (data not shown) synapses. After overnight treatment with BontA, IPSCs were undetectable at 19 of 19 potential synaptic pairs in regular external saline (containing 2 mM Ca2+). By comparison, in control cultures, 10 of 10 cell pairs tested were connected synaptically. In accordance with previous work at the neuromuscular junction (14, 15) and with a recent report in hippocampal slice culture (16), the block of action potential-evoked synaptic currents by BontA could be rescued partially by increasing extracellular Ca2+ levels (Fig. 1A). We determined quantitatively the relationship between external Ca2+ concentrations and evoked IPSC amplitudes. In control cultures, synaptic currents were undetectable at 0.5 mM Ca2+, but their amplitude increased at concentrations between 1 and 5 mM. The mean amplitudes of IPSCs in control synapses were 21 ± 9 pA, 166 ± 57 pA, 341 ± 136 pA, 489 ± 92 pA, and 778 ± 315 pA at Ca2+ concentrations of 1, 2, 3, 5, and 10 mM, respectively (n = 4). The slope of this relationship on a logarithmic (log)–log plot was 2.8 (between 1 and 2 mM Ca2+) (Fig. 1B), which is compatible with previous reports of slopes of 2 to 4 (17–22). Concentrations of Ca2+ >2 mM were not used to determine the slope because it is known that this slope decreases as Ca2+ reaches higher levels. In BontA-treated cultures, IPSCs were undetectable at 2 mM Ca2+ but increased in amplitude between 3 and 9 mM. The mean amplitudes of IPSCs in BontA-treated synapses were 7 ± 3 pA, 67 ± 23 pA, 103 ± 24 pA, and 128 ± 33 pA, at Ca2+ concentrations of 3, 6, 9, and 15 mM, respectively (n = 6). The slope of this relationship on a log–log plot was 3.1, which is very similar to that found in control synapses (Fig. 1B). BontA thus induces a shift to the right in the dose–response relationship without a change in apparent Ca2+ cooperativity. Although these results are compatible with the idea that cleavage of SNAP-25 interferes with a regulatory but not obligatory function of this protein in the release process, it remains possible that the observed phenotype is caused by incomplete cleavage of SNAP-25. To directly determine the efficacy of toxin action, we isolated membrane fractions from cultures and asked how effectively the pool of SNAP-25 was cleaved. All detectable SNAP-25 was cleaved by BontA whereas the integrity of syntaxin, used here as a control, was preserved (Fig. 1C).
Figure 1.
SNAP-25 cleavage by BontA perturbs action potential-evoked neurotransmitter release. (A) Simultaneous pre- and postsynaptic whole-cell patch-clamp recordings obtained from cultured hippocampal neurons. Lower traces represent the action potential (in current clamp) whereas upper traces represent γ-aminobutyric acid-activated, chloride-mediated IPSCs. At control synapses, evoked synaptic currents were detected readily at an external Ca2+ concentration of 2 mM (Left). At BontA-treated synapses, no IPSCs were detectable in 2 mM Ca2+, but these became detectable as Ca2+ was increased gradually to higher concentrations (Right). (Calibration = 150 pA or 55 mV, 60 ms.) (B) The relative amplitude of IPSCs are plotted on a log–log plot for control synapses (at 1, 2, and 3 mM Ca2+) and BontA-treated synapses (at 3, 6, and 9 mM Ca2+). The data are plotted relative to the amplitude of IPSCs measured in 5 mM Ca2+ in control cultures and 15 mM Ca2+ in BontA-treated cultures. A shift to the right is observed without a notable change in slope (n). (C) Protein extracts from control and BontA-treated cultures were separated by SDS/PAGE. Blots were probed with anti-SNAP-25 and anti-syntaxin antibodies. All detectable SNAP-25 was cleaved by BontA and was shifted to a lower molecular weight compatible with complete cleavage whereas syntaxin was unaffected. (D) Synaptic delay measurements from five experiments were pooled for both control and BontA-treated inhibitory synapses. The relative distribution of synaptic delays in BontA-treated cultures (in 6 mM Ca2+; represented by black columns) and in control cultures (at 5 mM Ca2+; represented by white columns) is illustrated. Delays are longer and more variable after BontA. (E) These increased synaptic delays also are observed in excitatory synapses in which the variability in synaptic delay is made more striking by the faster rise times and decay times of these events. Paired recordings from 12 successive action potential-evoked excitatory synaptic currents in a cell pair are shown from a BontA-treated culture. (Calibration = 25 pA or 40 mV, 10 ms.)
The observed shift in the relationship between external Ca2+ and release is compatible with the idea that SNAP-25 regulates some aspect of the efficacy of the coupling between the Ca2+-sensing mechanism and the basic secretory apparatus. Additional support for this model is provided by our observation that the synaptic delay is perturbed at BontA-treated synapses. We found that, at control inhibitory synapses, the synaptic delay was 2.7 ± 0.6 ms (mean ± SD in 3 mM Ca 2+, n = 234 events at five synapses). However, in BontA-treated cultures, this was increased significantly to 4.7 ± 1.0 ms (in 6 mM Ca2+, n = 190 events at five synapses; Fig. 1D). Extensive variability in the synaptic delay usually was observed at BontA-treated synapses, as reflected by a near doubling of the standard deviation of synaptic delays. Very similar results were seen at excitatory synapses in which significant spike-to-spike variability in synaptic latency clearly was resolved (Fig. 1E). Furthermore, this effect was seen equally at all concentrations of external Ca2+, from 3 to 15 mM. In control cultures, the synaptic delay of IPSCs was 2.7 ± 0.5 ms at 2 mM external Ca2+ (n = 119 events at five synapses), 2.7 ± 0.6 ms at 3 mM external Ca2+ (n = 234 events at five synapses), 2.6 ± 0.6 ms at 5 mM external Ca2+ (n = 101 events at five synapses), and 2.7 ± 0.4 ms at 10 mM external Ca2+ (n = 51 events at five synapses). In BontA-treated cultures, the synaptic delay was 4.7 ± 1.0 ms at 6 mM external Ca2+ (n = 190 events at five synapses), 4.6 ± 0.8 ms at 9 mM external Ca2+ (n = 140 events at five synapses), and 4.4 ± 0.8 ms at 15 mM external Ca2+ (n = 111 events at five synapses). These results show that the perturbation in synaptic delay cannot be attributed to an indirect effect of high Ca2+ concentrations per se.
Calcium-Independent Release Is Unaffected by Botulinum A.
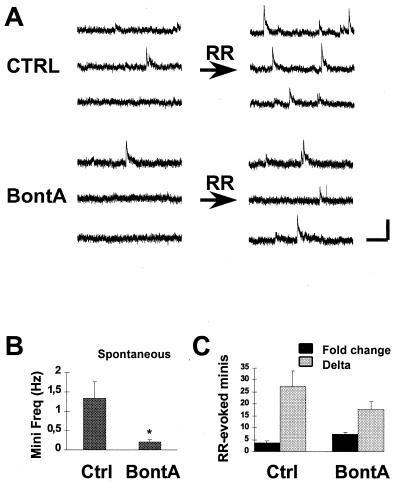
If SNAP-25 acts in coupling Ca2+ detection with exocytosis per se, we predicted that BontA treatment should not affect the ability of ruthenium red (RR), a Ca2+-independent secretagogue (13) that acts downstream of the Ca2+ receptor, to induce transmitter release. Although cleavage of synaptobrevin or syntaxin by tetanus toxin (10) and Botulinum C (L.-E.T. and P.G.H. unpublished results), respectively, blocks RR-evoked exocytosis, cleavage of SNAP-25 by BontA does not interfere with the ability of RR to evoke secretion. The basal frequency of minis was decreased significantly by BontA (Fig. 2 A and B), presumably because of a reduced baseline response to resting Ca2+ levels [from 1.3 ± 0.4 Hz (n = 10) to 0.2 ± 0.1 Hz (n = 15); P < 0.05]. However, the ability of RR to elevate the frequency of these events was not prevented. When expressed as a difference score obtained by subtracting the number of minis before RR from the number of minis in the presence of RR within 15-s periods of time for each cell, 27 ± 7 minis were found to be released by RR in control preparations, and 18 ± 3 minis were found to be released in BontA-treated cultures (Fig. 2 A and C). The difference was not statistically significant (n = 10 and n =15 cells; P > 0.05). The effectiveness of RR in BontA-treated cultures even was enhanced when expressed as an increase from baseline (3.7 ± 0.8-fold increase vs. 7.4 ± 0.8-fold increase in mini frequency for control and BontA-treated cultures, respectively) (Fig. 2C). These results were obtained under conditions in which large changes in the amplitude of miniature IPSCs were not observed (24.5 ± 4.6 pA vs. 19.2 ± 3.8 pA for control and BontA-treated cells, respectively; n = 7 experiments each; P > 0.05). Because BontA shifts the Ca2+/release relationship to the right and increases synaptic delay and variability without impairing the ability of RR to evoke fusion, we suggest that the target of this toxin action, SNAP-25, regulates the Ca2+ responsiveness of the secretory machinery by enhancing coupling between the Ca2+ detection apparatus and fusion machinery per se.
Figure 2.
Late steps in the secretory process are unimpaired by BontA. (A) Miniature inhibitory postsynaptic currents (minis) were evoked by puff-application of RR from a local pipette (30 μM). In control preparations, the basal frequency of minis was elevated rapidly by RR (Top). In BontA-treated preparations, the basal frequency of minis was significantly lower (∗, P < 0.05) (B), but RR was still effective at stimulating exocytosis (A Bottom), whether expressed as an increase in basal mini frequency (Fold change) or as a difference score obtained by subtracting the number of minis before RR from that in its presence within 15-s periods of time (Delta) (C). (Calibration = 15 pA, 350 ms.)
Perturbation of PKA-Mediated Synaptic Plasticity by Botulinum A.
We made use of the ability of BontA to selectively hamper coupling between Ca2+ detection and fusion to dissect the mechanism of PKA-mediated enhancement of release. We predicted that, if activation of PKA facilitates release by modulating either fusion or the number of functionally primed vesicles prepared for exocytosis, then both RR-evoked release and action potential-evoked release (rescued by increasing Ca2+) would be facilitated by the adenylyl cyclase activator forskolin in BontA-treated synapses. However, if PKA facilitates the interaction of the Ca2+ detection module with the secretory machinery, then the facilitation of RR-evoked but not action potential-evoked release should be reduced or prevented by BontA.
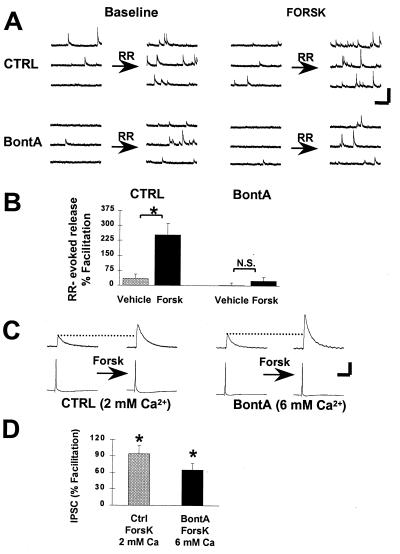
In control preparations, activation of PKA by using 20 μM forskolin produced a 254 ± 58% (n = 6; P < 0.05) facilitation of the RR-evoked secretory response (Fig. 3 A and B) This facilitatory action of forskolin is mediated by PKA because selective PKA inhibitors block forskolin-induced synaptic modulation (10). By contrast, in BontA-treated cultures, RR-evoked release was not modulated by forskolin (Fig. 3 A and B) [24 ± 20% (n = 9)]. To control for the possibility that BontA interfered with adenylyl cyclase activity, we measured forskolin-stimulated cAMP production in our cultures by radioimmunoassay. We found that cyclase activation was unhampered by BontA. A 6.0-fold increase in cAMP was found in control cultures (from 61 ± 4 fmol to 363 ± 17 fmol; n = 3 coverslips), as compared with a 6.9-fold increase in BontA-treated cultures (from 64 ± 13 fmol to 438 ± 12 fmol; n = 3 coverslips; P > 0.05).
Figure 3.
Evidence for PKA-mediated modulation of an early step in the release process. (A) Activation of cAMP production and PKA by forskolin facilitates Ca2+-independent, RR-evoked release in control cultures (Top). This facilitation is expressed as an enhancement of the efficacy of RR to accelerate the frequency of minis in the presence of forskolin (FORSK). In BontA-treated neurons (Bottom), RR still increases the occurrence of minis, but this action of RR is not facilitated by forskolin. (Calibration = 100 pA, 500 ms.) (B) Summary data illustrates that forskolin produced a significant enhancement of RR-evoked release in control preparations (n = 6) (∗, P < 0.01) whereas it was ineffective in BontA-treated cells (n = 7) (N. S., not significant). Exposure of cells to the vehicle solution (dimethyl sulfoxide) failed to change RR-evoked release in both control (n = 4) and BontA-treated (n = 5) preparations (Vehicle). (C) Dual whole-cell recordings were performed to measure facilitation of action potential-evoked IPSCs. In control cell pairs recorded in the presence of 2 mM Ca2+ (Left), IPSCs were facilitated readily by perfusion of 20 μM forskolin (Forsk). In BontA-treated pairs, no IPSCs were detectable at 2 mM Ca2+, but increasing Ca2+ to 6 mM permitted recovery of IPSCs. These rescued synapses were facilitated by forskolin (20 μM) (Right). (Calibration = 60 pA or 45 mV, 50 ms.) (D) Summary data of the synaptic facilitation expressed as a percent increase of IPSC amplitude above baseline. Significant facilitation was detected for both control (n = 9) and BontA-treated (n = 5) synapses (∗, P < 0.05).
Synaptic Facilitation After Rescue of Calcium-Evoked Neurotransmitter Release.
In control preparations, action potential-evoked transmitter release was facilitated readily by forskolin (20 μM) (94 ± 16% increase; n = 9; P < 0.05) (Fig. 3 C and D). In BontA-treated neurons, despite the fact that modulation of RR-evoked transmitter release was blocked, action potential-evoked transmitter release still was facilitated by forskolin. In 6 mM extracellular Ca2+, a condition that allows IPSCs to be detected, forskolin (20 μM) increased action potential-evoked synaptic currents by 65 ± 12% (n = 5; P < 0.05) (Fig. 3 C and D). In separate experiments, we determined that under these conditions of elevated external Ca2+, RR-evoked release still is not facilitated by forskolin in BontA-treated cultures (−18 ± 17% change; n = 3; data not shown). These results suggest that the PKA substrate(s) involved in the modulation of release is not destroyed by cleavage of SNAP-25 with BontA. Additionally, because RR-evoked release is not modulated by PKA after BontA treatment, we can conclude that PKA-dependent synaptic modulation does not result from an increase in the number of functionally docked or releasable vesicles (10) because this would be detected by the RR stimulus.
PKA-Mediated Modification of the Calcium-to-Release Relationship.
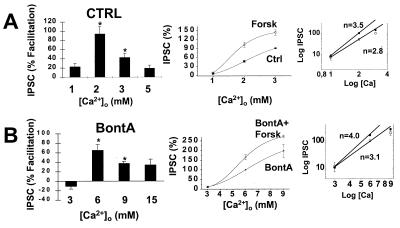
Our data point to the possibility that PKA modulates the secretory machinery by controlling some aspect of the coupling of the Ca2+ sensing module to exocytosis. To investigate this possibility further, we examined the ability of forskolin to facilitate action potential-evoked release at a number of different extracellular Ca2+ levels. We found an “inverted U” relationship whereby there was little facilitation under conditions of low release (1 mM Ca2+), large facilitation at intermediate levels (2 and 3 mM Ca2+), and less facilitation at higher levels of Ca2+ (5 mM) (Fig. 4A Left). The percent facilitation was 23 ± 7%, 94 ± 16%, 43 ± 9%, and 19 ± 7% at 1, 2, 3 and 5 mM Ca2+, respectively. Similar results were obtained in BontA-treated cultures at higher Ca2+ levels (Fig. 4B Left). The percent facilitation was −11 ± 6%, 65 ± 12%, 34 ± 4%, and 34 ± 13% at 3, 6, 9 and 15 mM Ca2+, respectively. The observation that there is little facilitation in lower Ca2+ is not compatible with the idea that PKA simply is increasing the Ca2+ sensitivity of the secretory machinery. Rather, these results suggest that the apparent Ca2+ cooperativity also may be changing. This possibility was examined more directly by multiplying the relative IPSC amplitudes in control cultures by the degree of forskolin-induced facilitation at each of these concentrations of Ca2+ to reconstruct a Ca2+ to release relationship in the presence of forskolin (Fig. 4 A Center and B Center). A comparison of the two curves (Ctrl vs. Forsk and BontA vs. BontA+Forsk) demonstrates that forskolin is producing a complex modification of the relationship between Ca2+ and release. Examination of log–log plots of theses results demonstrate that forskolin produced an increase in slope from 2.8 to 3.5 in controls (Fig. 4A Right) and from 3.1 to 4.0 in BontA treated cultures (Fig. 4B Right). Because an increased Ca2+ cooperativity for release would be predicted to decrease transmitter release (21), we must conclude that PKA has at least two actions on the secretory machinery: an increase in the apparent Ca2+ cooperativity for release and an additional action, such as an enhancement of the efficacy of the coupling of the Ca2+ sensing module to the secretory machinery (see Discussion).
Figure 4.
PKA changes the relationship between Ca2+ and transmitter release. The ability of forskolin (20 μM) to facilitate action potential-evoked IPSCs was evaluated at different concentrations of Ca2+ in control (A) and BontA-treated synapses (B). Forskolin caused the largest degree of facilitation at 2 and 6 mM Ca2+ in control and BontA treated synapses, respectively (Left). (Data are expressed as mean ± SEM. ∗, P < 0.05; n = 3–9). A Ca2+ to release relationship in the presence of forskolin was reconstructed by multiplying the relative IPSC amplitude of control cultures by the degree of forskolin-induced facilitation (Center) (Right, the same data on a log–log format). The data demonstrate that forskolin-dependent facilitation is accompanied by an increase in the slope of this relationship. Although BontA shifts the Ca2+ to release relationship to the right, it does not affect the ability of forskolin to increase the slope of the log–log plot of the Ca2+ to release relationship.
DISCUSSION
We have taken advantage of the previously described (11, 12) ability of BontA to cleave the synaptic protein SNAP-25 to further investigate the mechanism by which PKA activation produces a facilitation of neurotransmitter release at synapses. The experimental findings presented in the first part of the present report provide information on the mechanism of action of BontA on nerve terminals. Our results first show that BontA shifts the relationship between external Ca2+ concentration and neurotransmitter release to the right (i.e., higher concentrations of Ca2+), without changing the slope of this relationship (Fig. 1B). These results confirm the recent findings of Capogna et al. (16) on cultured hippocampal slices and suggest that SNAP-25 plays some role in the coupling of Ca2+-sensing to synaptic vesicle exocytosis. Second, we show that BontA produces a perturbation of the synaptic delay (Fig. 1 D and E). To our knowledge, this observation has not been reported previously. The observation also is consistent with the idea that SNAP-25 may play a regulatory or “coupling” function in the release process. Finally, we show that, even though action potential-evoked neurotransmitter release is blocked by BontA under conditions in which the concentration of extracellular Ca2+ is normal, quantal secretion induced by the Ca2+-independent secretagogue RR is not prevented (Fig. 2). This provides further evidence in favor of the hypothesis that SNAP-25 plays a regulatory role in some early steps of the secretory process, as opposed to late steps in which vesicles are ready to fuse. These latter results also are very interesting in light of the recent observation that quantal release induced by an hyperosmotic challenge (high sucrose) is blocked by BontA (16). Like RR, this secretagogue has been suggested to act independently of Ca2+ influx (23). The finding that sucrose-evoked release is blocked by SNAP-25 cleavage under conditions in which the Ca2+ to-evoked release relationship is shifted thus raises the question of whether release induced by hyperosmotic challenges is really Ca2+-independent or Ca2+-dependent as suggested (24).
Our previous results (10) have provided support for the hypothesis that PKA facilitates neurotransmitter release by a direct action on the secretory machinery and not through an enhancement of morphological vesicle docking or through an increase in the number of functional terminals (10). The experimental results described in the second part of this study present data compatible with the hypothesis that PKA modulates an early step in the release machinery. Although our interpretations partially depend on the postulate that action potential-evoked and RR-evoked release are mediated by a similar pool of vesicles, this is a plausible assumption because both forms of quantal neurotransmitter release are blocked by tetanus toxin and BontC, which cleave synaptobrevin and syntaxin, respectively, and both forms of release are modulated in the same way by activating A1 adenosine receptors (13) or PKA (10). The observation that forskolin is unable to facilitate RR-evoked transmitter release after BontA treatment (Fig. 3 A and B) yet still can facilitate evoked release (Fig. 3 C and D) is critical for an interpretation of the site of modulation by PKA. First, this data supports our previous hypothesis that PKA acts to directly facilitate the secretory machinery and indicates that PKA does not act through increasing the number of functional “primed” vesicles available for release. If this were the case, RR-evoked release still would have been facilitated in BontA-treated synapses in response to forskolin. Rather, we suggest, PKA facilitates neurotransmitter release by acting at an early step in the secretory process, which, given that the calcium cooperativity of release was modified by forskolin, is likely to be at the level of calcium sensing.
Although there may be several possible interpretations of the findings described here, one interesting possibility is that PKA activation reveals a new Ca2+-binding site that serves to enhance the efficacy of the Ca2+-evoked release process. This site could be different from the putative Ca2+-binding sites that have been proposed to cooperatively trigger neurotransmitter release (17). This would be expected to cause an apparent increase in Ca2+ cooperativity such as we have observed (Fig. 4 A and B) and would be insensitive to SNAP-25 cleavage by BontA. This component of the facilitation thus could be expressed at synapses rescued by elevated calcium after BontA treatment. To explain why exocytosis induced independently from Ca2+ by RR also is facilitated by forskolin in intact nerve terminals, it must be hypothesized that an additional consequence of the action of PKA on the calcium-sensing module is to facilitate the interaction of the Ca2+-sensing module with other components of the machinery mediating vesicle fusion. By itself, this enhanced interaction could facilitate vesicle fusion whether or not it is triggered by Ca2+ influx. We suggest that, after cleavage of SNAP-25 by BontA, the interaction between the Ca2+-sensing and fusion modules is weakened, and PKA now is unable to facilitate RR-evoked release.
Changes in apparent Ca2+-cooperativity have been suggested to underlie some aspects of synaptic plasticity in invertebrate preparations (21, 22). It was proposed (21) that, at the squid giant synapse, a decline in Ca2+ cooperativity could account for synaptic facilitation evoked by high frequency stimulation (21). At neuromuscular junctions of the cAMP-specific phosphodiesterase Drosophila mutant dunce, it has been shown that the enhanced neurotransmitter release observed also is associated with a decrease in apparent Ca2+ cooperativity (22). To our knowledge, there have been no previous demonstrations of such a phenomenon at synapses from mammalian preparations. Our observations differ from those obtained in squid and in Drosophila in that the synaptic facilitation induced by forskolin is associated with an increase rather than a decline in apparent Ca2+ cooperativity (Fig. 4 A and B). By itself, an increase in Ca2+-cooperativity would be expected to decrease rather than to increase neurotransmitter release. It thus seems more likely that, as suggested above, PKA acts on the Ca2+-sensing mechanism to reveal a new Ca2+-binding site that serves to facilitate release without interfering with “true” Ca2+ cooperativity.
Our results lead to the prediction that one or more regulatory proteins in the synaptic vesicle fusion complex can be phosphorylated by PKA, thereby regulating the efficacy of early steps in the regulated exocytotic process. Among candidate proteins, α-SNAP and rabphilin-3A should be considered because they have been shown to be phosphorylated by PKA in vitro (25–26), but one should not exclude SNAP-25 itself, which can be phosphorylated by PKC (27) but also can be phosphorylated weakly by PKA (25). Finally, the recently described (28) Rab-3-dependent regulator protein Rim also is known to contain consensus sequences for PKA (28). One can, however, exclude a synaptic protein such as Rab3A because it has been demonstrated that forskolin can still facilitate synaptic transmission in the hippocampus of Rab3A-deficient mice (29).
Acknowledgments
We thank Drs. Robert Zucker and Vladimir Parpura for helpful comments on the manuscript. This paper was supported by grants from the National Institutes of Health (NS26650, NS24233) (P.G.H.) and postdoctoral fellowships from the Human Frontier Science Program and the Medical Research Council of Canada (L.-E.T.) L.-E.T. is now a Michael Smith scholar of the Medical Research Council of Canada and is supported by grants from the Medical Research Council and the Fonds de la Recherche en Santé du Québec.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BontA, Botulinum toxin A; RR, ruthenium red; PKA, protein kinase A; IPSC, inhibitory synaptic current; log, logarithm.
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 3.Martinez J L, Jr, Derrick B E. Annu Rev Psychol. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Bouron A, Reuter H. Proc Natl Acad Sci USA. 1997;94:12224–12229. doi: 10.1073/pnas.94.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon D, Atwood H L. J Neurosci. 1989;9:4245–4252. doi: 10.1523/JNEUROSCI.09-12-04246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez-Noriega L E, Stevens C F. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M. Neuron. 1994;13:159–166. doi: 10.1016/0896-6273(94)90466-9. [DOI] [PubMed] [Google Scholar]
- 8.Weisskopf M G, Castillo P E, Zalutsky R A, Nicoll R A. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 9.Capogna M, Gähwiler B H, Thompson S M. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trudeau L-E, Emery D G, Haydon P G. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 11.Schiavo G, Rossetto O, Montecucco C. Semin Cell Biol. 1994;5:221–229. doi: 10.1006/scel.1994.1028. [DOI] [PubMed] [Google Scholar]
- 12.Ahnert-Hilger G, Bikalke H. Prog Neurobiol. 1995;46:83–96. doi: 10.1016/0301-0082(95)00003-e. [DOI] [PubMed] [Google Scholar]
- 13.Trudeau L-E, Doyle R T, Emery D G, Haydon P G. J Neurosci. 1996;16:46–54. doi: 10.1523/JNEUROSCI.16-01-00046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreyer F, Rosenberg F, Becker C, Bigalke H, Penner R. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;335:1–7. doi: 10.1007/BF00165027. [DOI] [PubMed] [Google Scholar]
- 15.Molgó J, Siegel L S, Tabti N, Thesleff S. J Physiol. 1989;411:195–205. doi: 10.1113/jphysiol.1989.sp017568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capogna M, McKinney R A, O’Connor V, Gähwiler B H, Thompson S M. J Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodge F A, Jr, Rahamimoff R. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlton M P, Smith S J, Zucker R S. J Physiol. 1982;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustine G J, Eckert R. J Physiol. 1984;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith S J, Augustine G J, Charlton M P. Proc Natl Acad Sci USA. 1985;82:622–625. doi: 10.1073/pnas.82.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley E F. J Neurosci. 1986;6:782–789. doi: 10.1523/JNEUROSCI.06-03-00782.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Y, Wu C-F. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- 23.Rosenmund C, Stevens C F. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 24.Brosius D C, Hackett J T, Tuttle J B. J Neurophysiol. 1992;68:1229–1234. doi: 10.1152/jn.1992.68.4.1229. [DOI] [PubMed] [Google Scholar]
- 25.Hirling H, Scheller R H. Proc Natl Acad Sci USA. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fykse E M, Li C, Südhof T C. J Neurosci. 1995;15:2385–2395. doi: 10.1523/JNEUROSCI.15-03-02385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimazaki Y, Nishiki T-I, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof T C. Nature (London) 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 29.Castillo P E, Janz R, Südhof T C, Tzounopoulos T, Malenka R C, Nicoll R A. Nature (London) 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]