1. Structure
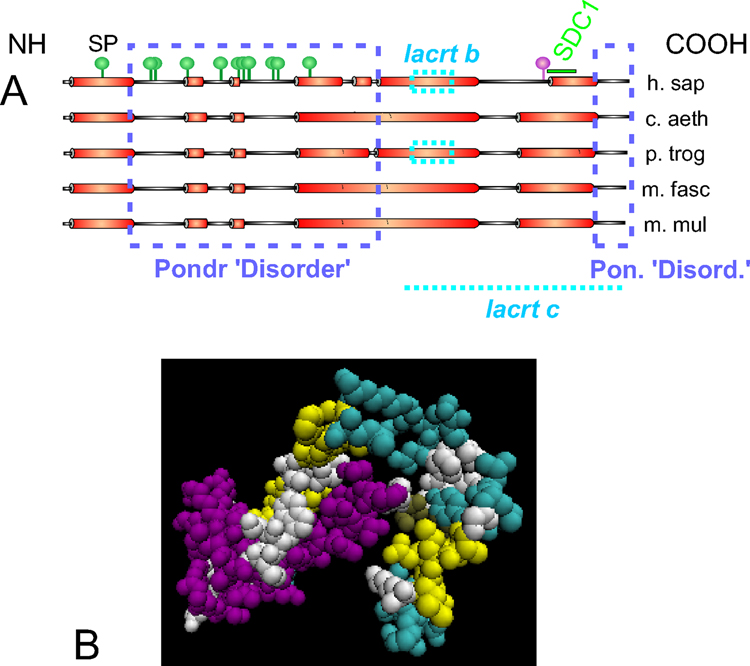
Lacritin is a 12.3 kDa secreted tear protein in human (accession Q9GZZ8 [Genpept; UniProt; Sanghi et al, 2001] and non-human primates. It consists of 119 aa after removal of the signal peptide (SP; Fig. 1) and has a predicted isoelectric point (pI) of 5. Predicted values for secreted lacritin in non-human primates include: 12.2 kDa and pI of 5 (C. aethiops), 12.3 kDa and pI of 4.8 (M. fascicularis), 12.2 kDa and pI of 4.8 (M. mulatta), and 12.2 kDa and pI of 5 (P. troglodytes). By SDS PAGE, lacritin mobility is greater than expected (bacterial recombinant w/o SP migrates at approximately 18 kDa [Wang et al., 2006]). Several conserved α-helices are predicted (Fig. 1). Two have been confirmed by circular dichroism using the synthetic peptides LKSIVEKSILLTEQALAKAGKGMH and KQFIENGSEFAQKLLKKFS (respectively amino acids 65 – 88 and 95 – 113 of human lacritin without SP; predicted α-helix underlined; Wang et al., 2006). ‘HelicalWheel’ predicts that the latter is strongly amphipathic with hydrophobic and hydrophilic residues on separate faces. Amphipathic α-helices support ligand-receptor and ligand-ligand binding (ie. respectively PTHLH-PTHR1 and VEGF-VEGF). Deletion analysis suggests that the predicted amphipathic α-helix is a binding domain for the N-terminus of deglycanated syndecan-1 (SDC1; Ma et al., 2006). SDC1 is a proteoglycan co-receptor for several different growth factors. NetOGlyc 3.1 suggests 11 sites of O-glycosylation, almost all in the N-terminal half (Fig. 1). One N-glycoslyation site is predicted at the C-terminus. Lacritin splice variants have been recently detected at very low levels in two normal lacrimal glands (NCBI Aceview). Lacritin-b (11.1 kDa; 108 aa; pI 5.3 [without SP]) lacks the sequence SIVEKSILTE from exon 4. Lacritin-c (10.7 kDa; 119 aa; pI 4.6 [without SP]) lacks exon 4 and 5. A novel 39 aa sequence from intron 3 forms its C-terminus. Lacritin-b and –c are conserved in P. troglodytes. Lacritin was proposed by one group to be a homologue of dermcidin (referenced in Wang et al, ’06). This has not been supported by NCBI Homologene. Sequence identity is 29% without the SP but regions of PONDR–predicted disorder approximately align. Both proteins are of similar size and function, and genes for each are immediately adjacent on human chromosome 12q13. An IPR003982 (LTB4R2) domain was assigned to lacritin between aa 10 and 114 (with SP) based on homology of lacritin aa 9 – 38 (with SP) with the third external loop motif 5 and of aa 98 – 114 (with SP) with C-terminal motif 7. The cytokine TNFSF8 also contains this domain. Lacritin crystals have been developed but are not yet suitable for X-ray diffraction.
Figure 1.
Structural features of lacritin. A. Linear model of lacritin from human and nonhuman primates showing alignment of PSIPRED-predicted α-helices (Wang et al, ’06) including the SP, regions of PONDR-predicted ‘disorder’ (dashed boxes), suggested post-translational O-linked (green pegs; NetOGlyc 3.1) and N-linked (purple peg; SeqWeb, PeptideStructure) glycosylation on human lacritin, lacritin-b and lacritin-c alternative splice forms, and the SDC1 binding domain (Ma et al., ’2006). B. VDW structural model (VMD) based on weak tertiary structure alignment to PDB structure 1usp B (tertiary prediction score 1.1; SamT02) by the GeneSilico Metaserver.
2. Function
Functional studies with recombinant human lacritin suggest that lacritin is a prosecretory mitogen. Most lacritin is generated by lacrimal gland from which it is apically released via acinar cell secretory granules to transit through ducts onto the ocular surface (Sanghi et al., 2001). Lacritin also appears to be a product of meibomian gland (Tsai et al., 2006). Only salivary and possibly thyroid gland also express lacritin but at much lower levels. The suggestion by one group that lacritin is expressed in normal human breast (see references in Ma et al., 2006) is not confirmed by Unigene. Lacritin’s eye specificity exceeds lens α-crystallin (Unigene Expression Profile). It is 1 of 29 ‘lacrimal-preferred genes’ and the 6th most common mRNA in NEIBank’s human lacrimal EST database. Lacritin appears to promote constitutive tear secretion in cultured lacrimal acinar cells, possibly via an autocrine stimulatory mechanism (Sanghi et al., 2001). Lacritin also stimulates human corneal epithelial (HCE-T) cell proliferation via a biphasic 1 – 10 nM dose optimum, but has no apparent mitogenic effect on fibroblasts, glia or erythroleukemic cells, and epithelia from epidermis, breast cancer, melanoma or testes (Wang et al., 2006). Cell specificity is generated by a unique ‘off-on’ switch mechanism in which heparanase deglycanation of cell surface SDC1 exposes a lacritin binding site in the N-terminus of SDC1 as a prerequisite for lacritin mitogenic signaling (Ma et al., 2006). Lacritin mitogenic signaling targets Gαi or Gαo/PKCα-PLC/Ca2+/calcineurin/NFATC1 and Gαi or Gαo/PKCα-PLC/PLD/mTOR (Wang et al., 2006). Thus, lacritin is an eye-specific growth factor that may play an important role in secretion and renewal of lacrimal and ocular surface epithelia.
3. Disease Involvement
Proteomics is now being applied to the question of whether ocular surface disease can be diagnosed by or even caused by an abnormal complement of tear proteins. Tear proteins from 19 patients suffering from chronic blepharitis vs 27 controls were examined. Of 491 different tear proteins only 8 were downregulated. 1 of 8 was lacritin (refs. in Tsai et al., 2006). Blepharitis is a complex inflammation of the eyelid associated with dry eye and meibomian gland dysfunction. One group reported amplification of lacritin and dermcidin genes in some invasive breast cancers and others suggested that lacritin is the second-most common SAGE marker for circulating breast cancer cells (refs in Ma et al., ’06). However a tumor marker role has not been supported by recent large-scale studies of breast and other human cancers.
4. Future Studies
Attention to a potential treatment or diagnostic role of lacritin in dry eye could be fruitful, ie. differential upregulation of lacritin-b or –c could alter the physiology of the ocular surface; and elements of lacritin’s SDC1/heparanase off/on switch could be defective. Continued development of deletion and point mutants is needed to precisely define active domains. Preclinical studies testing the utility of topically applied lacritin in rabbits and monkeys may open the door to new strategies towards the alleviation of dry eye.
Acknowledgments
Supported by NIH RO1 EY13143 to GWL.
Abbreviations
- LACRT
lacritin
- SDC1
syndecan-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ma P, Beck SL, Raab RW, McKown RL, Coffman GL, Utani A, Chirico WJ, Rapraeger AC, Laurie GW. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J. Cell Biol. 2006;74:1097–1106. doi: 10.1083/jcb.200511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghi S, Kumar R, Lumsden A, Dickinson D, Klepeis V, Trinkaus-Randall V, Frierson HF, Jr, Laurie GW. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J. Mol. Biol. 2001;310:127–139. doi: 10.1006/jmbi.2001.4748. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Evans JE, Green KM, Sullivan RM, Schaumberg DA, Richards SM, Dana MR, Sullivan DA. Proteomic analysis of human meibomian gland secretions. Br. J. Ophthalmol. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang N, Xie J, Walton SC, McKown RL, Raab RW, Ma P, Beck SL, Coffman GL, Hussaini IM, Laurie GW. Restricted epithelial proliferation by lacritin via PKCalpha-dependent NFAT and mTOR pathways. J Cell Biol. 2006;174:689–700. doi: 10.1083/jcb.200605140. [DOI] [PMC free article] [PubMed] [Google Scholar]