Abstract
Background
Current recommendations to prevent malaria in African pregnant women rely on insecticide treated nets (ITNs) and intermittent preventive treatment (IPTp). However, there is no information on the safety and efficacy of their combined use.
Methods
1030 pregnant Mozambican women of all gravidities received a long-lasting ITN during antenatal clinic (ANC) visits and, irrespective of HIV status, were enrolled in a randomised, double blind, placebo-controlled trial, to assess the safety and efficacy of 2-dose sulphadoxine-pyrimethamine (SP). The main outcome was the reduction in low birth weight.
Findings
Two-dose SP was safe and well tolerated, but was not associated with reductions in anaemia prevalence at delivery (RR, 0.92 [95% CI, 0.79–1.08]), low birth weight (RR, 0.99 [95% CI, 0.70–1.39]), or overall placental infection (p = 0.964). However, the SP group showed a 40% reduction (95% CI, 7.40–61.20]; p = 0.020) in the incidence of clinical malaria during pregnancy, and reductions in the prevalence of peripheral parasitaemia (7.10% vs 15.15%) (p<0.001), and of actively infected placentas (7.04% vs 13.60%) (p = 0.002). There was a reduction in severe anaemia at delivery of borderline statistical significance (p = 0.055). These effects were not modified by gravidity or HIV status. Reported ITN's use was more than 90% in both groups.
Conclusions
Two-dose SP was associated with a reduction in some indicators, but these were not translated to significant improvement in other maternal or birth outcomes. The use of ITNs during pregnancy may reduce the need to administer IPTp. ITNs should be part of the ANC package in sub-Saharan Africa.
Trial Registration
ClinicalTrials.gov NCT00209781
Introduction
Every year 50 million women become pregnant in areas where malaria is endemic, and at least half of them live in Africa [1]. Malaria during pregnancy is associated with maternal and foetal morbidity and mortality through maternal anaemia, low birth weight (LBW) and premature delivery [2]–[6]. Thus, prevention of malaria during pregnancy is a public health priority, particularly in sub-Saharan Africa.
Currently, prevention of malaria in pregnancy in Africa relies on intermittent preventive treatment (IPTp) and insecticide treated nets (ITNs) [1]. ITNs are associated with significant health benefits for both the mother and the newborn in sub-Saharan Africa [7]. IPTp entails the provision of an antimalarial at treatment doses irrespective of the presence of parasites or symptoms. The currently recommended regimen for IPTp is at least 2 treatment courses of SP given from the 2nd trimester onwards at least one month apart [1].
Although IPTp and ITNs have shown separately to be efficacious in reducing the harmful effects of malaria during pregnancy, limited information exists on the safety and efficacy of both interventions together [8], [9].
IPTp, is now being implemented throughout most malaria endemic areas of Africa, and has become somewhat controversial. This is fuelled by two elements; on the one hand, the spread of SP resistance, and the other, the overlap in some areas of malaria transmission and high HIV prevalence. IPTp with SP is not recommended to HIV-positive women receiving cotrimoxazole prophylaxis or antiretroviral drugs [10]. Thus, although HIV-positive women have an increased risk of malaria [11], current recommendations may fail to provide them adequate protection.
In order to evaluate the safety and efficacy of two intermittent doses of SP in women of all parities and regardless of HIV status, who had been given a long-lasting ITN (LLITN) through the antenatal clinic (ANC), we carried out a randomised, double blind, placebo-controlled trial of IPTp in Mozambican pregnant women. This information should help guide policy towards the rational use of control tools for malaria prevention in pregnancy.
Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1.
Study area and population
The study was undertaken at the Centro de Investigação em Saúde da Manhiça (CISM) in Manhiça District, southern Mozambique. A demographic surveillance system covering 36 000 inhabitants is carried out by the CISM and constitutes the study area. Adjacent to the CISM is the Manhiça District Hospital (MDH), a 110 bed health facility. The characteristics of the area have been described in detail elsewhere [12]. Perennial malaria transmission with some seasonality is mostly attributable to P falciparum. Anopheles funestus is the main vector, and the estimated entomological inoculation rate for 2002 was 38 infective bites per person per year. Data on the efficacy of SP in children in this area showed a therapeutic efficacy rate of 83%, with an in vivo parasitological sensitivity of 78.6% at day 14 [13]. Eighty percent of pregnant women have an institutional delivery. During the study, malaria control in pregnancy relied exclusively upon case management.
Study design
This double blind, individually randomised, placebo controlled trial had the primary objective of estimating the additive protective effect of two-dose IPTp with SP to that of ITNs on LBW prevalence. Based on previous estimates in the area, a LBW prevalence of 20% and an estimated 25% reduction to 15% in the presence of ITNs [14], 411 women per group were needed to show a lack of difference between the two groups with a confidence interval of 7 (8% to 22%), at the 5% level of significance with a 80% statistical power. The study protocol was approved by the National Mozambican Ethics Review Committee, and the Hospital Clinic of Barcelona Ethics Review Committee.
Enrolment and interventions
From August 2003 to April 2005 pregnant women were enrolled at the MDH ANC if their gestational age was ≤ 28 weeks, they did not report allergies to sulpha drugs, and they were permanent residents of the CISM study area. After written informed consent was obtained, the lowest available study number was assigned. A computer-generated sequential list contained the study numbers linked to treatment identification letters, randomly ordered in blocks of 10. Tablets of SP or placebo, identical in shape and colour, were stored in 10 bottles labelled only with a single treatment identification letter. Women were randomised to receive 3 tablets of SP (1500 mg sulphadoxine/75 mg pyrimetamine) or placebo. The project health nurse administered study drugs to women with at least 12 weeks of gestational age. Doses were given twice from the second trimester, at least one month apart. Regardless of their gestational age, all women at recruitment received a LLITN. Assessment of gestational age was made by bimanual palpation of the fundal height. Haemoglobin and the rapid plasma reagin test (RPR, Syphacard, Wellcome, USA) were assessed as part of the routine antenatal care. In accordance to the National Programme for HIV control, women were offered voluntary counselling and testing; HIV positive women and their babies were given Nevirapine prophylaxis and referred for further clinical follow-up.
Follow-up
Plastic photo cards of mother and child facilitated identification at every contact throughout the study (from recruitment until 8 weeks postpartum). A health facility-based, passive surveillance system was established at the MDH. At each consultation, a standardized questionnaire was completed documenting signs and symptoms. Blood films were prepared for malaria parasite examination and the packed cell volume (PCV) measured if there was a history of fever in the preceding 24 hrs, or the axillary temperature was ≥37.5°C. Clinical malaria episodes were treated with chloroquine or SP in the first and subsequent trimesters, respectively, for uncomplicated malaria, and parenteral quinine for severe malaria.
At delivery, venous blood was collected by venipuncture from the mother and the umbilical cord, and two thick blood smears and a filter paper prepared for haematological and parasitological determinations. Newborns were weighed on a digital scale, accurate to the nearest gram. The gestational age was assessed by the Dubowitz's method [15]. A placental biopsy was collected and two impression smears and blood placed onto filter paper were prepared from a placental biopsy sample.
Eight weeks after delivery a capillary blood sample was collected from the mother and the infant for parasite and haematological determinations. The axillary temperature was measured and the weight of the baby recorded.
ITN use was assessed by asking the women if they had slept under the LLITN the night before.
Laboratory Methods
Thick and thin blood films were stained and read according to standard, quality-controlled procedures [16], [17]. PCV was measured in a microcapillary tube after centrifugation. Blood samples were centrifuged and the plasma and erythrocyte pellets stored at −70°C.
HIV serostatus was assessed using a rapid test (Determine, Abbot Laboratories, USA), and positive results confirmed using Unigold rapid test (TM HIV, Trinity Biotech, Ireland).
Tissue samples were collected from the maternal side of the placentas and placed into 10% neutral buffered formalin. Biopsies were processed and stained following standard procedures [18]. Impression smears from the placenta, were stained with Giemsa and read following standard procedures [19].
Data Management, Statistical Methods and Definitions
Data were analysed by intention-to-treat (ITT) analysis whereby all randomised women were included regardless of whether or not they had received the intervention and the number of doses.
The risk of the first or only episode of clinical malaria between recruitment and 8 weeks postpartum, or censoring due to withdrawal or death, was estimated using Cox regression models. The protective effect (PE) of SP was estimated from the hazard ratio (HR) as PE = 100(1-HR) %.The effect of time on efficacy since dose 1 was estimated using time versus treatment interaction in a Cox time-dependent model. In the secondary analyses, crude and adjusted (for parity, HIV status and baseline variables) prevalences were calculated using the Risk Ratio. Likelihood Ratio tests were calculated to evaluate the interaction between the intervention group and parity, or HIV status. Differences in prevalence were estimated with the χ2 test and proportions with the Fisher's exact test. Continuous values were evaluated with the non-parametric Wilcoxon test. Data analysis was performed using Stata 8.2 (Stata Corporation, College Station, TX, USA).
Malaria infection was defined as the presence of asexual P. falciparum parasites of any density in a blood smear. A clinical malaria episode was defined as the latter plus an axillary temperature ≥37.5°C. The duration of a malaria episode was estimated as 28 days. Overall and severe anaemia were defined as a PCV lower than 33% and 21%, respectively.
Placental infection was classified according to a previous definition [20]. Any placental infection was defined as the presence of parasites and/or pigment in the histological examination, and/or in the impression smear.
Women with a multiple delivery (twins or triplets) were also included in the analysis.
Results
Baseline characteristics of study women
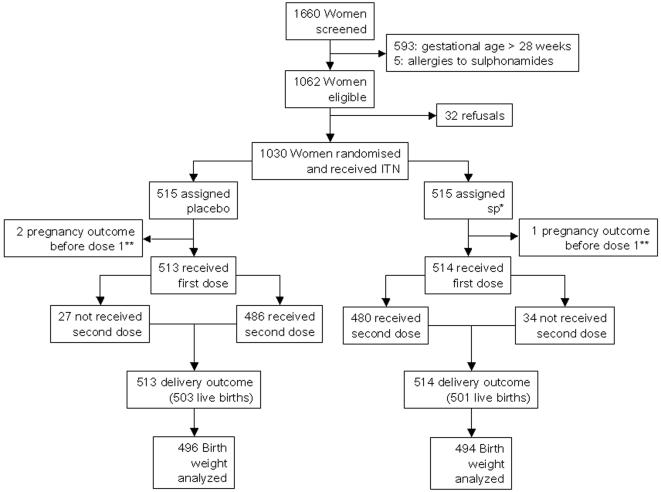
Figure 1 shows the trial profile and table 1 shows the baseline characteristics of participants. Reported ITN use at the end of pregnancy was similar between the groups (90.38% and 92.52% in the SP and placebo groups, respectively, p = 0.324). 85% of the women had an HIV test and 23.6% were HIV positive. Mean gestational age was 23.21 (SD 3.44) weeks at the first IPTp SP/placebo dose and 28.34 (SD 3.65) weeks at the second IPTp dose. Mean time between first and second IPTp SP/placebo dose was 36.53 (SD 11.55) days, and mean time between last dose and delivery was 76.91 (SD 31.19) days.
Figure 1. Trial profile.
There were 3 and 7 twins births in the SP and placebo groups respectively. There was one triplet birth in the SP Group.* sulphadoxine-pyrimethamine ** 1 woman in the SP group and 2 in the placebo group had a miscarriage before receiving dose 1
Table 1. Characteristics of the study women at recruitment.
| Placebo | SP* | |||||
| (n = 515) | (n = 515) | |||||
| Mean | (SD) † | Mean | (SD) † | p-value | ||
| Age (years) | 24.31 | (6.55) | 24.00 | (6.60) | 0.366 | |
| Parity | 3.12 | (2.00) | 3.09 | (1.99) | 0.809 | |
| Height (cm) | 158.00 | (5.63) | 157.24 | (5.99) | 0.030 | |
| Haemoglobin (gr/dl) | 10.93 | (1.21) | 10.97 | (1.26) | 0.742 | |
| n | (%) | n | (%) | |||
| Gestational age (weeks) ‡ | 1st term | 4 | (1) | 3 | (1) | 0.294 |
| 2nd term | 291 | (57) | 315 | (61) | ||
| 3rd term | 220 | (43) | 197 | (38) | ||
| Gravidity | primigravidae | 129 | (25) | 137 | (27) | 0.821 |
| 1 to 3 pregnancies | 205 | (40) | 204 | (40) | ||
| 4 or > pregnancies | 181 | (35) | 174 | (34) | ||
| HIV test | negative | 338 | (66) | 324 | (63) | 0.107 |
| positive | 91 | (18) | 117 | (23) | ||
| not done § | 86 | (17) | 74 | (14) | ||
| MUAC index (cm) ¶ | normal >22 | 396 | (98) | 401 | (98) | 0.475 |
| ≤22 | 10 | (2) | 7 | (2) | ||
| Literacy | reads and/or writes | 212 | (41) | 213 | (41) | 0.346 |
| neither reads nor writes | 300 | (58) | 302 | (59) | ||
| unknown | 3 | (1) | 0 | (0) | ||
| RPR Syphilis test ** | positive | 72 | (14) | 50 | (10) | 0.034 |
| negative | 442 | (86) | 465 | (90) | ||
| unknown | 1 | (0) | 0 | (0) | ||
sulphadoxine-pyrimethamine
stardard deviation
1st term: 0–12 weeks, 2nd term: 13–24 weeks,3rd term: 25–40 weeks
Refused voluntary testing
Mid-upper arm circunference
Rapid plasma reagin
Safety and morbidity
Only one woman from the SP group vomited after IPTp dose 1. There were no differences in the incidence of hospital admissions and outpatient visits during pregnancy or postpartum period, or in infant visits until age 8 weeks. Nine women, similarly distributed between the groups, had mild skin reactions within 27 days after any IPTp dose (table 2). No severe skin reactions were reported. There was one maternal death (SP group), who died of obstetric causes. There were 6 infants deaths in the SP group and 11 in the placebo group (p = 0.089). There was one newborn with a major congenital malformation (spina bifida) in the SP group.
Table 2. Safety of SP* during the study †.
| Placebo+ITNs | SP*+ITNs | ||||||
| Safety in the mother | episodes | Rate | episodes | Rate | RR | (95% CI) ‡ | p-value |
| Incidence of all hospital admissions during pregnancy | 54 | 0.35 | 52 | 0.32 | 0.89 | (0.61,1.30) | 0.539 |
| Incidence of outpatient visits during pregnancy | 345 | 1.83 | 327 | 1.64 | 0.94 | (0.81,1.10) | 0.444 |
| Incidence of all hospital admissions during post-partum | 17 | 0.30 | 20 | 0.35 | 1.06 | (0.56,2.03) | 0.856 |
| Incidence of outpatient visits during post-partum | 38 | 0.68 | 39 | 0.69 | 0.92 | (0.59,1.43) | 0.697 |
| n/N | (%) | n/N | (%) | total | |||
| Skin reactions § | 4/515 | (0.77) | 5/515 | (0.97) | 9 | 1.000 | |
| Maternal deaths | 0/515 | (0.00) | 1/515 | (0.19) | 1 | 1.000 | |
Sulphadoxine-pyrimethamine
From recruitment up to 2 months after delivery
Confidence interval
Within 27 days after any IPTp doses
Effect of SP plus ITNs on main outcomes
There were no significant differences between the intervention groups in either the prevalence of LBW (RR, 0.99 [95% CI, 0.70–1.39];p = 0.940) (table 3) or in mean birth weight [3033.0 (SD 477.1) in the SP group, 3003.5 (SD 522.6) in the placebo group; p = 0.637] (table 3). SP was associated with a statistically significant reduction in the prevalence of LBW in women with ≥4 pregnancies in the subgroup analysis. Similarly, there were no significant differences between the two groups in either the prevalence of prematurity (RR, 0.74 [95% CI, 0.39–1.43];p = 0.372), or the mean gestational age [39.7 weeks (SD 1.31) in the SP group, 39.5 weeks (SD 1.49) in the placebo group; p = 0.081) (table 3). Adjustment by confounding variables did not modify these results.
Table 3. Low birth weight and secondary fetal and neonatal outcomes. Crude and adjusted effect by gravidity and HIV status.
| Placebo+ITNs | SP*+ITNs | |||||||
| n/N | (%) | n/N | (%) | RR | (95% CI) † | p-value | ||
| Low birth weight (<2500 gr) ‡ | ||||||||
| crude analysis | 59/496 | (11.90) | 58/494 | (11.74) | 0.99 | (0.70–1.39) | 0.940 | |
| adjusted by gravidity | 0.96 | (0.67–1.38) | 0.828 | |||||
| adjusted by HIV status | 0.98 | (0.68–1.40) | 0.900 | |||||
| Low birth weight (<2500 gr) by gravidity | ||||||||
| Primigravidae | 25/121 | (20.66) | 29/133 | (21.80) | 1.06 | (0.66–1.70) | 0.824 | |
| 1 to 3 pregnancies | 13/195 | (6.67) | 20/194 | (10.31) | 1.55 | (0.79–3.02) | 0.197 | |
| 4 or more pregnancies | 21/180 | (11.67) | 9/167 | (5.39) | 0.46 | (0.22–0.98) | 0.038 | |
| Low birth weight (<2500 gr) by HIV status | ||||||||
| Negative | 38/327 | (11.62) | 34/313 | (10.86) | 0.93 | (0.60–1.45) | 0.762 | |
| Positive | 10/85 | (11.76) | 17/112 | (15.18) | 1.29 | (0.62–2.67) | 0.490 | |
| Not done § | 11/84 | (13.10) | 7/69 | (10.14) | 0.77 | (0.32–1.89) | 0.573 | |
| Pre-term birth (<37 weeks) | 20/407 | (4.91) | 15/411 | (3.65) | 0.74 | (0.39–1.43) | 0.372 | |
| Cord blood parasitaemia | 5/435 | (1.15) | 4/435 | (0.92) | 0.80 | (0.22–2.96) | 0.738 | |
| Cord blood anaemia (PCV<37%) ¶ ** | 45/435 | (10.34) | 22/435 | (5.06) | 0.49 | (0.30–0.80) | 0.004 | |
| Quantitative variables (Mean (SD)) | ||||||||
| Birth weight | 3003.55 | (522.69) | 3033.00 | (477.11) | 0.637 | |||
| Newborn gestational age (weeks) | 39.55 | (1.49) | 39.70 | (1.31) | 0.081 | |||
| Cord blood PCV (%) | 44.05 | (7.49) | 45.06 | (7.88) | 0.003 | |||
| Pregnancy outcomes | n | % | n | % | ||||
| Spontaneous abortions (birth weight<500gr) | 6 | 1 | 4 | 1 | 0.753 | |||
| Stillbirths (birth weight ≥500gr) | 11 | 2 | 14 | 3 | 0.686 | |||
| Early neonatal deaths (<8 days of life) | 5 | 1 | 0 | 0 | 0.062 | |||
| Perinatal deaths (Stillbirths and early neonatal deaths) | 16 | 3 | 14 | 3 | 0.716 | |||
| Late neonatal deaths (>8 and <29 days of life) | 0 | 0 | 2 | 0 | 0.500 | |||
Sulphadoxine-pyrimethamine
Confidence interval
Interaction between group and gravidity, p = 0.061; Interaction between group and HIV status, p = 0.682
Refused voluntary testing
Packed-cell volume
Interaction between group and gravidity, p = 0.626; Interaction between group and HIV status, p = 0.191
The risk of foetal anaemia was halved among women in the SP group compared to those who received placebo (RR, 0.49 [95% CI, 0.30–0.80]; p = 0.003) (table 3). A non-statistically significant reduction in the number of early neonatal deaths was found among women receiving SP (p = 0.062) (table 3).
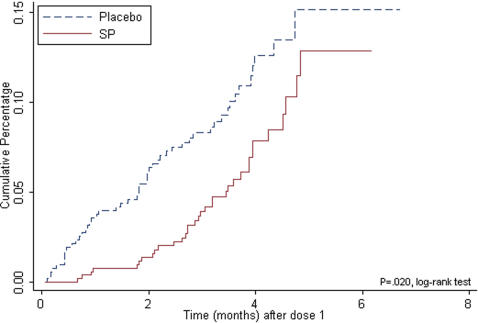
Overall, 7% of the women receiving two-dose SP and 10% receiving placebo had a first or only episode of uncomplicated malaria (protective efficacy (PE), 40% [95% CI, 7.40–61.20]; p = 0.020) (figure 2). The analysis of the incidence of clinical malaria within the month after each SP dose showed a PE of 74.1% (95% CI, 30.8–90.3; p = 0.003) after dose 1 and of 71.4% (95% CI, 13.10–90.60; p = 0.015) after dose 2.
Figure 2. Intention to treat cohort.
First or only episode of clinical malaria from IPTp dose 1 until delivery
There was no difference in the prevalence of anaemia at delivery between the intervention and control groups (RR, 0.92 [95% CI, 0.79–1.08]; p = 0.309). However, although not significantly, there were fewer women in the IPTp/SP than in the placebo group with severe anaemia at delivery (RR, 0.25 [95% CI, 0.05–1.16]; p = 0.055). This difference was not modified by adjustments for gravidity and HIV status (table 4).
Table 4. Severe anaemia and parasitological outcomes at delivery. Crude and adjusted effect by gravidity and HIV status.
| Placebo+ITNs | SP*+ITNs | |||||||
| n/N | (%) | n/N | (%) | RR | (95% CI) | p-value | ||
| Severe anaemia (PCV <21%) † ‡ | ||||||||
| Crude analysis | 8/495 | (1.62) | 2/493 | (0.41) | 0.25 | (0.05–1.16) | 0.055 | |
| adjusted by gravidity | 0.25 | (0.05–1.16) | 0.077 | |||||
| adjusted by HIV status | 0.24 | (0.05–1.13) | 0.070 | |||||
| Severe anaemia (PCV <21%) by gravidity | ||||||||
| Primigravidae | 3/127 | (2.36) | 0/133 | (0.00) | 0.00 | (.-.) | 0.072 | |
| 1 to 3 pregnancies | 3/193 | (1.55) | 1/192 | (0.52) | 0.33 | (0.03–3.16) | 0.312 | |
| ≥4 pregnancies | 2/175 | (1.14) | 1/168 | (0.60) | 0.51 | (0.05–5.63) | 0.579 | |
| Severe anaemia (PCV <21%) by HIV status | ||||||||
| Negative | 5/324 | (1.54) | 1/311 | (0.32) | 0.21 | (0.04–1.75) | 0.108 | |
| Positive | 2/87 | (2.30) | 1/112 | (0.89) | 0.39 | (0.04–4.25) | 0.424 | |
| Not done § | 1/84 | (1.19) | 0/70 | (0.00) | 0.00 | (.-.) | 0.351 | |
| Peripheral parasitaemia ¶ | ||||||||
| Crude analysis | 75/495 | (15.15) | 35/493 | (7.10) | 0.47 | (0.32–0.69) | 0.000 | |
| adjusted by gravidity | 0.46 | (0.31–0.69) | 0.000 | |||||
| adjusted by HIV status | 0.47 | (0.33–0.70) | 0.000 | |||||
| Peripheral parasitaemia by gravidity | ||||||||
| Primigravidae | 30/127 | (23.62) | 18/133 | (13.53) | 0.57 | (0.34–0.97) | 0.036 | |
| 1 to 3 pregnancies | 31/193 | (16.06) | 12/192 | (6.25) | 0.39 | (0.21–0.73) | 0.002 | |
| ≥4 pregnancies | 14/175 | (8.00) | 5/168 | (2.98) | 0.37 | (0.14–1.01) | 0.042 | |
| Peripheral parasitaemia by HIV status | ||||||||
| Negative | 43/324 | (13.27) | 22/311 | (7.10) | 0.53 | (0.33–0.87) | 0.010 | |
| Positive | 17/87 | (19.54) | 6/112 | (5.56) | 0.27 | (0.11–0.67) | 0.002 | |
| Not done § | 15/84 | (17.86) | 7/70 | (9.33) | 0.56 | (0.24–1.30) | 0.165 | |
| Any placental malaria infection ** | 219/419 | (52.27) | 222/426 | (52.11) | 1.00 | (0.88–1.13) | 0.964 | |
| Positive impression smears | 57/419 | (13.6) | 30/426 | (7.04) | 0.52 | (0.34–0.79) | 0.002 | |
| Positive histology | 213/419 | (50.84) | 218/426 | (51.17) | 1.01 | (0.88–1.15) | 0.922 | |
| Classification of placental infection by histology | n/N | (%) | n/N | (%) | ||||
| acute | 25/419 | (5.96) | 16/426 | (3.75) | 0.004 | |||
| chronic | 51/419 | (12.17) | 28/426 | (6.57) | ||||
| past | 137/419 | (32.69) | 174/426 | (40.84) | ||||
| not infected | 206/419 | (49.16) | 208/426 | (48.82) | ||||
Sulphadoxine-pirimethamine
Packed-cell volume
Interaction between group and gravidity, p = 0.409; Interaction between group and HIV status, p = 0.760
Refused voluntary counselling and testing
Interaction between group and gravidity, p = 0.622; Interaction between group and HIV status, p = 0.414
Either histology or impression smear
Table 4 shows the effect of IPTp on maternal parasitological endpoints. Peripheral parasitaemia was significantly lower among women in the SP group (7.10%, 35/493) compared to the placebo (15.15%, 75/495) (RR, 0.47 [95% CI, 0.32–0.69]; p = 0.000). This result was not modified by gravidity or HIV status.
Prevalence of any placental infection was comparable between the groups (RR, 1.00 [95% CI, 0.88–1.13]; p = 0.964), and so it was the proportion of placentas with only pigment deposition on histology (past infection). However, there were significantly less infected placentas with parasites in the SP group as assessed by impression smears (RR, 0.52 [95% CI, 0.34–0.79]; p = 0.002) or histology (acute and chronic infection) (table 4). This difference in the proportion of actively infected placentas was mainly accounted for the HIV positive women [6.12% (6/98) in the SP group versus 24.32% (18/74) in the placebo group; p = 0.001].
There were less women in the SP group than in the placebo group with malaria parasitaemia at 8 weeks after delivery (RR, 0.52 [95% CI, 0.27–0.99]; p = 0.044). There were no differences in the prevalence of women or infants with clinical malaria or anaemia (table 5). Similarly, there were no differences in the incidence of outpatient attendances or hospital admissions with malaria in either mothers or infants during the post-partum period (data not shown).
Table 5. Prevalence of parasitaemia and anaemia in the mother and infant at 2 months after delivery.
| Placebo+ITNs | SP*+ITNs | |||||||
| n/N | (%) | n/N | (%) | RR | (95% CI) † | p-value | ||
| Mother | ||||||||
| P falciparum parasitaemia ‡ | ||||||||
| crude analysis | 26/432 | (6.02) | 13/416 | (3.13) | 0.52 | (0.27–0.99) | 0.044 | |
| adjusted by gravidity | 0.50 | (0.25–0.97) | 0.040 | |||||
| adjusted by HIV status | 0.52 | (0.27–1.01) | 0.054 | |||||
| P falciparum parasitaemia by gravidity | ||||||||
| Primigravidae | 11/98 | (11.22) | 6/111 | (5.41) | 0.48 | (0.18–1.25) | 0.125 | |
| 1 to 3 pregnancies | 9/175 | (5.14) | 5/161 | (3.11) | 0.60 | (0.21–1.76) | 0.351 | |
| ≥4 pregnancies | 6/159 | (3.77) | 2/144 | (1.39) | 0.37 | (0.07–1.79) | 0.196 | |
| P falciparum parasitaemia by HIV status | ||||||||
| Negative | 20/286 | (6.99) | 8/263 | (3.04) | 0.43 | (0.19–0.97) | 0.036 | |
| Positive | 3/77 | (3.90) | 4/97 | (4.12) | 1.06 | (0.24–4.59) | 0.940 | |
| Not done § | 3/69 | (4.35) | 1/56 | (1.79) | 0.41 | (0.04–3.84) | 0.418 | |
| P falciparum parasitaemia+fever ¶ | 1/432 | (0.23) | 1/416 | (0.24) | 1.04 | (0.06–16.55) | 0.978 | |
| Overall anaemia (PCV<33%) ** | 89/432 | (20.60) | 95/416 | (22.84) | 1.10 | (0.85–1.42) | 0.461 | |
| Infant | ||||||||
| P falciparum parasitaemia | 5/417 | (1.20) | 2/404 | (0.50) | 0.41 | (0.08–2.12) | 0.273 | |
| Overall anaemia (PCV<33%) ** | 66/417 | (15.83) | 63/404 | (15.59) | 0.98 | (0.71–1.34) | 0.882 | |
Sulphadoxine-pyrimethamine
Confidence interval
Interaction between study group and gravidity, p = 0.876; Interaction between study group and HIV status, p = 0.575
Refused voluntary testing
Fever: Ta ≥37.5°C
Packed-cell volume
Discussion
This randomised placebo-controlled trial of IPTp in Mozambican pregnant women has shown that administering two IPTp-SP doses to women concurrently using LLITNs was safe and well tolerated, and there were no significant drug reactions despite the high prevalence of HIV among the study women. The intervention was associated with a moderate reduction in the incidence of clinical malaria during pregnancy, and with a statistically significant reduction in the prevalence of parasitaemia at delivery and at 8 weeks postpartum. However, these positive effects did not appear to translate in an improvement on relevant foetal birth outcomes such as birth weight or prematurity, nor on maternal anaemia.
It could be argued that parasite resistance to SP may explain the modest impact on some malariometric parameters and the lack of effect on the more significant birth outcomes. The available in vivo drug efficacy data in children in this area showed a parasitological sensitivity of 78,6% at day 14 [13], but the study was carried out 3 years before the start of the trial. Drug resistance can evolve quite rapidly, and it may be that by the time the study started, the true resistance level may have been higher. However, there is evidence that strongly suggests that SP was highly effective in the area during the study. SP was highly efficacious in participants for malaria prevention during the 30 days following each dose. A very similar effect was seen in a concurrent study of IPT in infants in the same area, whereby the efficacy of SP for malaria prevention varied between 60% and 90% in the month after SP administration [21, C. Menéndez et al., unpublished]. Furthermore, an ongoing study of placental malaria in this area has documented a sharp fall in the prevalence of placental infection following programme implementation of IPTp with SP in Mozambique (A. Mayor et al., unpublished). It is important to better understand the relationships between the assessment of in vivo drug resistance in sick children and the use of the same drug for prevention in asymptomatic individuals [22].
The risk of malaria during pregnancy seems to concentrate in primi and secundigravidae women [8], [23]–[27], and consequently previous IPTp studies have been carried out only in this population. There are two main reasons why we need to re-consider this approach. Firstly, targeting a specific group of women may prove difficult when scaling up this intervention to programme conditions. Secondly, our results suggest that in the presence of ITNs the effect of SP is independent of gravidity.
As previously reported, SP reduced the prevalence of malaria parasitaemia at delivery and post-partum [2], [9]. However, this reduction was not associated with a corresponding effect on the prevalence of anaemia. In this study, the prevalence of severe anaemia at delivery was much lower than expected, probably due to the effect of ITNs [14]. This might explain the insufficient power to see an effect of IPTp on this morbidity outcome. However, no effect was observed either in the prevalence of overall anaemia, despite its much higher prevalence. Further studies are needed to understand the lack of correlation between parasitological and morbidity outcomes frequently observed in malaria prevention trials during pregnancy.
The prevalence of active placental infection (i.e., containing parasites) in the SP group (7% overall and 6% in the HIV-positive women) was similar to the one reported in Kenyan and Malawian HIV-positive women who received monthly SP (7.1% and 7.8%, respectively) [23]; [26]. In these studies three or more SP doses were needed to show a comparable impact in HIV-positive and negative women. However, in these trials, ITNs were not provided as part of the study. Our data suggest that two-dose SP may protect against peripheral and placental parasitemia in HIV-positive women if they use an ITN.
Despite the fact that bednets are not traditionally used in this area, the delivery of ITNs through the ANC was well accepted and the compliance with their use was very high.
In recent years, IPTp with SP has been the cornerstone of malaria control in pregnant women living in stable transmission areas. However, the recent controversy has raised questions as to whether this was the most appropriate approach for malaria prevention in African pregnant women given the reported increased in parasite resistance to SP in some areas [22]. This has led WHO to question the adequacy of this recommendation in favour of other less well established strategies (such as indoor residual spraying) [28]. Another line of discussion recommended increasing the frequency of SP- IPTp to monthly doses to improve its efficacy. This controversy comes at a time when most endemic countries in Africa have already, or are in the process of, implementing IPTp, with all the logistical and economic efforts associated with the introduction of new public health measures in developing countries.
In conclusion, this study provides information of public health relevance in deciding the adequacy of adding IPTp when ITNs are used, and suggests that IPTp may not be required if an ITN is used. To confirm these findings, more studies of similar design are needed in areas of different malaria endemicity in Africa. In the meantime, efforts should be focused to promote the delivery of long-lasting ITNs as part of other routine ANC health interventions.
Supporting Information
Study Protocol
(0.10 MB DOC)
CONSORT Checklist
(0.05 MB DOC)
Acknowledgments
We are grateful to the women who participated in the study. We also thank the staff of the Manhiça Health Center, especially those at the maternity clinic, and the staff at the Manhiça Health Research Center (CISM). Special thanks to Sonia Amos for her dedication throughout the study. We would like to thank Marcel Tanner, Bernard Nahlen and Samba Cham for their comments on the protocol and their help to obtain the ITNs and the intervention drugs.
SP (Fansidar®) and its placebo were provided by Hoffman La Roche. Long-lasting insecticide treated nets (Permanet ® 2.0. Vestergaard Frandsen) were provided by WHO.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Cleofé Romagosa was supported by a grant from the “Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III” through a career development fellowship (number CM03/00125). The study received financial support from the Banco de Bilbao, Vizcaya, Argentaria Foundation (grant number BBVA 02-0). The Centro de Investigaçao em Saude de Manhiça (CISM), receives major core funding from the Spanish Agency for International Cooperation (AECI). The funding source did not have any involvement in study design, collection, analysis and interpretation of data; writing of the report, or in the decision to submit the paper for publication.
References
- 1.World Health Organization (WHO) Brazzaville, Democratic Republic of Congo: WHO Regional Office for Africa,2004; 2006. A strategic framework for malaria prevention and control during pregnancy in the African region: report AFR/MAL/04/01. [Google Scholar]
- 2.Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S, Hatibnjie AB. The Effects of Malaria Chemoprophylaxis Given by Traditional Birth Attendants on the Course and Outcome of Pregnancy. Trans R Soc Trop Med Hyg. 1989;83(5):589–94. doi: 10.1016/0035-9203(89)90362-3. [DOI] [PubMed] [Google Scholar]
- 3.Brabin B. An Assessment of Low-Birth-Weight Risk in Primiparae As An Indicator of Malaria Control in Pregnancy. Int J Epi. 1991;20(1):276–83. doi: 10.1093/ije/20.1.276. [DOI] [PubMed] [Google Scholar]
- 4.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181(5):1740–5. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 5.Granja AC, Machungo F, Gomes A, Bergstrom S, Brabin B. Malaria-related maternal mortality in urban Mozambique. Ann Trop Med Parasitol. 1998;92(3):257–63. doi: 10.1080/00034989859816. [DOI] [PubMed] [Google Scholar]
- 6.Romagosa C, Ordi J, Saute F, Quinto L, Machungo F, Ismail MR, et al. Seasonal variations in maternal mortality in Maputo, Mozambique: the role of malaria. Trop Med Int Health. 2007;12(1):62–7. doi: 10.1111/j.1365-3156.2006.01755.x. [DOI] [PubMed] [Google Scholar]
- 7.Gamble C, Ekwaru JP, Ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane.Database.Syst.Rev. 2006;(2):CD003755. doi: 10.1002/14651858.CD003755.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Njagi JK, Magnussen P, Estambale B, Ouma J, Mugo B. Prevention of anaemia in pregnancy using insecticide-treated bednets and sulfadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2003;97(3):277–82. doi: 10.1016/s0035-9203(03)90141-6. [DOI] [PubMed] [Google Scholar]
- 9.Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, et al. A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae. Trop Med Int Health. 2006;11(7):992–1002. doi: 10.1111/j.1365-3156.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 10.2006 WHO expert consultation on cotrimoxazol propphylaxis in HIV infection. [Google Scholar]
- 11.Ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, van Eijk AM, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71(2):41–54. [PubMed] [Google Scholar]
- 12.INDEPTH Network. Ottawa, Canada: International Development Research Centre,2002; 2006. Population and health in developing countries. [Google Scholar]
- 13.Abacassamo F, Enosse S, Aponte JJ, Gomez-Olive FX, Quinto L, Mabunda S, et al. Efficacy of chloroquine, amodiaquine, sulphadoxine- pyrimethamine and combination therapy with artesunate in Mozambican children with non-complicated malaria. Trop Med Int Health. 2004;9(2):200–8. doi: 10.1046/j.1365-3156.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 14.Ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kariuki SK, et al. Reduction of malaria during pregnancy by permethrin-treated bed nets in an area of intense perennial malaria transmission in western Kenya. Am J Trop Med Hyg. 2003;68(4 Suppl):50–60. [PubMed] [Google Scholar]
- 15.Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J.Pediatr. 1970;77(1):1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- 16.Alonso PL, Smith T, Schellenberg JR, Masanja H, Mwankusye S, Urassa H, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet. 1994;344(8931):1175–81. doi: 10.1016/s0140-6736(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 17.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 18.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22(8):1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Rogerson SJ, Mkundika P, Kanjala MK. Diagnosis of Plasmodium falciparum malaria at delivery: comparison of blood film preparation methods and of blood films with histology. J Clin Microbiol. 2003;41(4):1370–4. doi: 10.1128/JCM.41.4.1370-1374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31(1):85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 21.Macete E, Aide P, Aponte JJ, Sanz S, Mandomando I, Espasa M, Sigauque B, Dobaño C, Mabunda S, Dgedge M, Alonso P, Menendez C. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J Infect Dis. 2006;194(3):276–285. doi: 10.1086/505431. [DOI] [PubMed] [Google Scholar]
- 22.Ter Kuile FO, van Eijk AM, Filler SJ. Harare, Zimbabwe: World Health Organisation, Regional Office for Africa; 2005. Can the treatment response in children with symptomatic malaria be used to guide policy for case management and intermittent preventive treatment of malaria in pregnancy? A review of published and unpublished literature. [Google Scholar]
- 23.Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulfadoxlne-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. 1998;59(5):813–22. doi: 10.4269/ajtmh.1998.59.813. [DOI] [PubMed] [Google Scholar]
- 24.Schultz LJ, Steketee RW, Macheso A, Kazembe P, Chitsulo L, Wirima JJ. The efficacy of antimalarial regimens containing sulfadoxine-pyrimethamine and/or chloroquine in preventing peripheral and placental Plasmodium falciparum infection among pregnant women in Malawi. Am J Trop Med Hyg. 1994;51(5):515–22. doi: 10.4269/ajtmh.1994.51.515. [DOI] [PubMed] [Google Scholar]
- 25.Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. 1999;353(9153):632–6. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- 26.Filler SJ, Kazembe P, Thigpen M, Macheso A, Parise ME, Newman RD, et al. Randomized Trial of 2-Dose versus Monthly Sulfadoxine-Pyrimethamine Intermittent Preventive Treatment for Malaria in HIV-Positive and HIV-Negative Pregnant Women in Malawi. J Infect Dis. 2006;194(3):286–93. doi: 10.1086/505080. [DOI] [PubMed] [Google Scholar]
- 27.Challis K, Osman NB, Cotiro M, Nordahl G, Dgedge M, Bergstrom S. Impact of a double dose of sulphadoxine-pyrimethamine to reduce prevalence of pregnancy malaria in southern Mozambique. Trop Med Int Health. 2004;9(10):1066–73. doi: 10.1111/j.1365-3156.2004.01307.x. [DOI] [PubMed] [Google Scholar]
- 28.Menendez C, d'Alessandro U, Ter Kuile FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis. 2007;7(2):126–35. doi: 10.1016/S1473-3099(07)70024-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
(0.10 MB DOC)
CONSORT Checklist
(0.05 MB DOC)