Abstract
Background
Vitellogenin (Vg), a major reproductive protein, has been associated with infection-resistant response in fish. However, the underlying mechanisms by which Vg is involved in anti-infectious response are not understood.
Methodology/Results
By both protein-microbe interaction analysis and enzyme-linked immunosorbent assay as well as phagocytosis test, we demonstrate for the first time that fish Vg acts as a pattern recognition molecule with multiple specificities that can recognize bacteria as well as fungus rather than self components from fish, and functions as an opsonin that can enhance macrophage phagocytosis.
Conclusions
This study shows that fish Vg plays an integrative function in regulating immunity via its pleiotropic effects on both recognizing pathogen-associated molecular patterns and promoting macrophage phagocytosis. It also supports the notion that factors normally involved in control of female reproduction are associated with immunity in organisms that rely on Vg for oocyte development.
Introduction
Vitellogenin (Vg), a phospholipoglycoprotein, is a member of the lipid transfer protein superfamily including apolipoprotein B (apoB), microsomal triglyceride transfer protein and Vg [1], and constitutes the precursor of major yolk proteins in all oviparous organisms. It is usually synthesized by the extraovarian tissues of female animals, secreted into bloodstream and transported to ovary, where it is internalized by growing oocytes and proteolytically cleaved to form yolk proteins that are later used as the nutrients by developing embryos and larvae [2]–[7]. However, Vg appears to have evolved pleiotropic functions in the advanced eusocial honeybee. It has been shown that honeybee Vg is associated with quite a few biological processes including social organization, temporal division of labor and foraging specilization, regulation of hormonal dynamics, and change in gustory responsiveness [8]–[11]. Besides, honeybee Vg has been shown to be capable of reducing oxidative stress by scavenging free radicals, thereby prolonging lifespan in the facultatively sterile worker castes and reproductive queen castes [12]. Similar antioxidant activity has also been observed for nematode (Caenorhabditis elegans) Vg [13].
Another novel function of Vg is linked with immune defense [14]. For example, Vg has recently been demonstrated to possess both hemagglutinating and antibacterial activities in the protochordate amphioxus (Branchiostoma belcheri) as well as the bony fish rosy barb (Puntius conchonius) [15], [16]. Moreover, the male rosy barb is able to produce Vg while challenged with Escherichia coli, implicating that Vg may be related to infection-resistant response. However, the mode of action by which Vg is involved in anti-infectious response remains unknown.
Non-self recognition and phagocytosis are two pivotal processes in innate immune response. Recognition of non-self in innate immunity is mediated by a set of germline-encoded proteins known as pattern-recognition receptors [17], [18], that recognize the microbial cell wall constituents called pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) of Gram-negative bacteria, lipoteichoic acid (LTA) of Gram-positive bacteria, and β-1,3-glucan of eukaryotic fungi [18], [19], [20]. Phagocytosis, a crucial defense mechanism against microbial infection, is performed by professional phagocytes such as macrophages and dendritic cells in vertebrates [21]. Humoral proteins such as antibody fragments and complement components from vertebrates can bind to the surface of pathogens and function as opsonins to promote phagocytosis [22], [23]. Scavenger receptors and cell surface C-type lectins also enhance phagocytosis [24]. Similarly, in invertebrates like insects, plasma proteins such as lectins and complement-like factor, and membrane proteins including peptidoglycan recognition protein LC also mediate phagocytosis [25], [26], [27]. Relatively, non-self recognition and phagocytosis have not as yet been given sufficient attention in fish.
The aims of this study were to determine if fish Vg is a pattern recognition molecule, and if so, to examine if it has an opsonic activity to promote macrophage phagocytosis. It is demonstrated for the first time that Vg from fish Hexagrammos otakii (Jordan & Starks) can not only act as a multivalent pattern recognition receptor binding to LPS, LTA, peptidoglycan (PGN), glucan and laminarin, but also function as an opsonin promoting macrophage phagocytosis.
Results
Purification and characterization of Vg
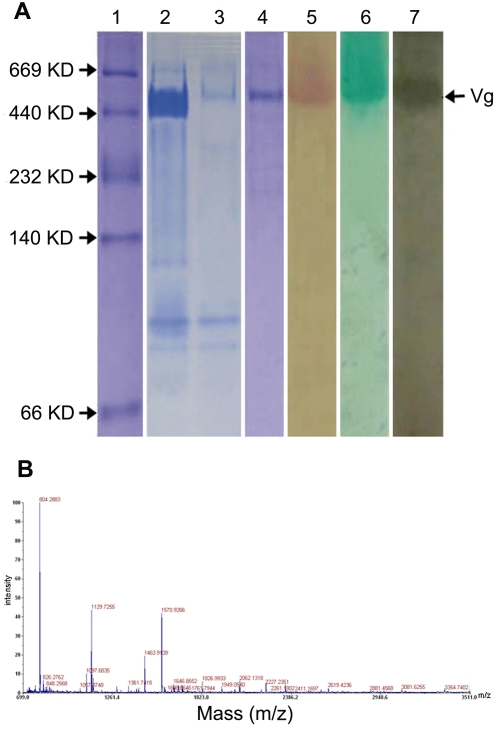
In fishes, Vg is a protein with a molecular weight ranging from 250 kDa to 600 kDa [28], [29]. Compared with untreated fish, plasma from 17-β-estradiol (E2)-injected H. otakii had an intense band of protein with a molecular mass of ∼450 kDa (Fig. 1A lanes 2 and 3), suggesting that it was E2-inducible protein. The protein from E2-injected fishes was purified by double-step chromatography (Fig. 1A lane 4), and the purified protein stained positive for carbohydrate, lipid, and phosphorus (Fig. 1A lanes 5, 6 and 7), thus establishing the protein as a phospholipoglycoprotein. Peptide mass mapping analysis performed on an Applied Biosystems 4700 proteomics analyzer further revealed that the protein purified was Vg from fish H. otakii (Fig. 1B ).
Figure 1. Purification and identification of Vg.
(A) Purification of Vg and determination of carbohydrate, lipid and phosphorus components. The proteins were run on a 7.5% native PAGE. lane 1, marker; lane 2, plasma (diluted 20-fold) of fish injected with E2; lane 3, plasma (diluted 20-fold) of fish injected with 0.9% NaCl; lane 4, purified Vg; lanes 5, 6 and 7, purified Vg stained with Schiff reagent, methyl green and Sudan black B, respectively. The protein induced by E2 stained positive for carbohydrate, lipid, and phosphorus. (B) Peptide mass mapping of the purified protein.
Vg binds to both Gram-negative and Gram-positive bacteria as well as fungus
The F/P ratios of FITC-labeled Vg, apoB and bovine serum albumin (BSA) were 4.9, 6.1 and 5.4, respectively, showing that they had similar F/P ratios. Antibacterial activity examination by colony forming unit assay showed that the growth of E. coli pre-incubated with 50 µg/ml of FITC-labeled Vg and unlabeled Vg was significantly inhibited, with the similar inhibitory rates of 27% and 29%, individually. In addition, FITC-labeled Vg appeared as a single band on a native 7.5% native-PAGE, suggesting that FITC-labeled Vg is able to dimerise (data not shown). These indicated that Vg activity is not affected by labeling with FITC.
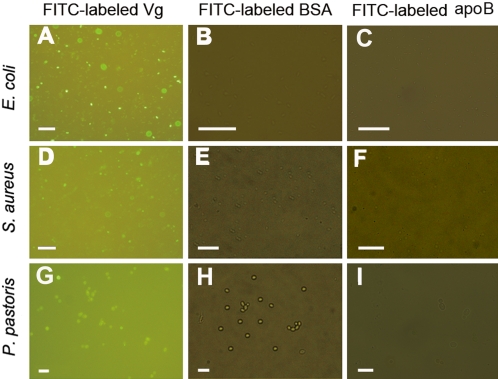
To test if Vg can bind to microbes, FITC-labeled Vg was incubated with Gram-negative bacterium E. coli, Gram-positive bacterium Staphylococcus aureus and fungus Pichia pastoris. It was found that Vg bound to both E. coli and S. aureus as well as P. pastoris (Fig. 2). In contrast, none of FITC-labeled apoB and FITC-labeled BSA employed as controls bound to the microbial cells tested. These revealed the specificity of Vg binding to the microbes.
Figure 2. Binding of FITC-labeled Vg to microbial cells.
Vg was labeled by FITC, and FITC-labeled Vg was incubated with Gram-negative bacterium E. coli, Gram-positive bacterium S. aureus and fungus P. pastoris, respectively. After washing three times with 25 mM Tris-HCl buffer containing 137 mM NaCl and 3 mM KCl (TBS; pH 7.6), the microbes were harvested by centrifugation, applied to microscope slides, and observed under an Olympus BX51 fluorescence microscope. The microbes treated with FITC-labeled BSA and FITC-labeled apoB were processed under the same conditions. (A, D and G) Binding of FITC-labeled Vg to E. coli, S. aureus and P. pastoris; (B, E and H) Non-binding of FITC-labeled BSA to E. coli, S. aureus and P. pastoris; (C, F and I) Non-binding of FITC-labeled apoB to E. coli, S. aureus and P. pastoris. A, D and G are the images under a fluorescent field, while B, E, H, C, F and H are the merged images under fluorescent and bright field channels. Scale bars: 20 µm.
Vg binds to various ligands
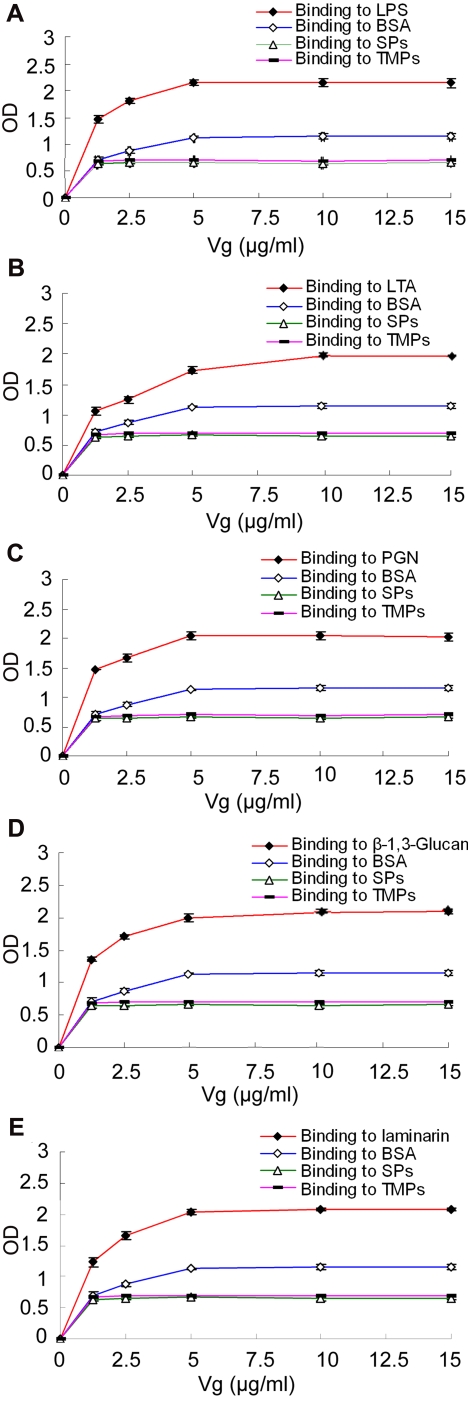
To better understand the mechanisms of binding activity, an enzyme-linked immunosorbent assay (ELISA) was carried out to investigate what molecules on the microbial surfaces are recognized by Vg. Although Vg slightly bound to BSA, it had a significantly stronger affinity to the immobilized ligands including LPS from Gram-negative bacteria, LTA from Gram-positive bacteria, PGN from both Gram-positive and Gram-negative bacteria, β-1,3-glucan from fungi and laminarin from brown algae (p<0.05; Fig. 3). Moreover, the affinity of Vg to the serum proteins (SPs) and total muscle proteins (TMPs) extracted from H. otakii itself was also markedly lower than that of Vg binding to the immobilized ligands (p<0.05). These demonstrated that Vg possesses a significantly higher affinity to the microbial surface molecules rather than BSA and the self components from H. otakii itself.
Figure 3. Binding of Vg to various ligands.
LPS, LTA, PGN, laminarin and β-1,3-glucan dissolved in re-distilled water were applied to a 96-well microplate, and air-dried overnight at room temperature, followed by ELISA. BSA, serum proteins (SPs) and total muscle proteins (TMPs) of H. otakii at the same concentrations were treated similarly as controls. Data were expressed as mean values±SEM (n = 3). The bars represent the standard error of mean values. Although Vg slightly binds to BSA and SPs and TMPs of H. otakii, its affinity to the immobilized ligands is significantly higher (p<0.05). (A–E) Binding of Vg to LPS, LTA, PGN, β-1,3-glucan and laminarin.
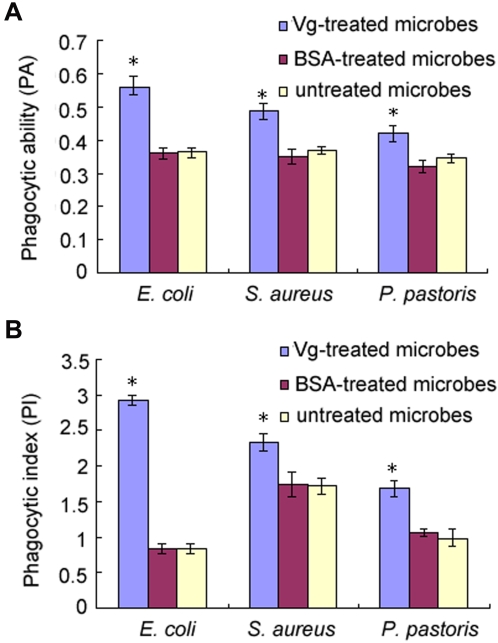
Vg enhances phagocytosis of microbes in vitro
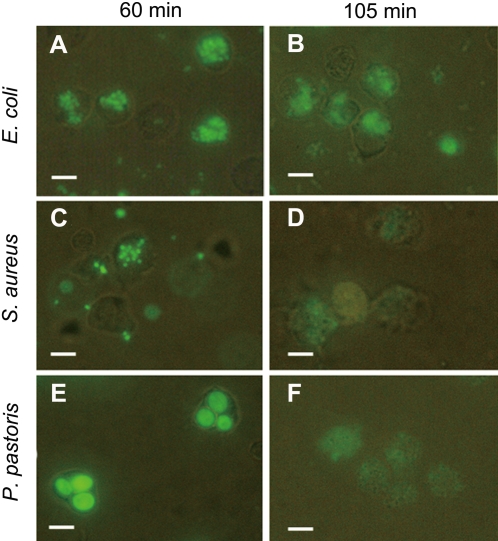
The macrophage population isolated from the head kidney (front part of fish kidney) of H. otakii had a viability of about 98%. To examine if Vg promotes macrophage phagocytosis, both Vg in PBS and BSA in PBS as well as PBS alone were pre-incubated with the FITC-labeled microbes, E. coli, S. aureus and P. pastoris, and then incubated with the macrophages freshly isolated. Pilot experiments showed that both phagocytic ability (PA) and phagocytic index (PI) reached maximum values at 60 min after incubation of macrophages with microbes, and the engulfed microbes became digested to pieces at 105 min (Fig. 4). Therefore, phagocytosis was observed at 60 min after mixing macrophages with microbes in subsequent experiments. Compared with the microbes pre-incubated with BSA and PBS alone, those pre-incubated with Vg were readily phagocytosed by the macrophages. The PA and PI values of the macrophages phagocytosing Vg-treated E. coli, S. aureus and P. pastoris were 56.0±3.5%, 49.0±2.0% and 42.0±2.4%, and 2.920±0.072, 2.333±0.113 and 1.677±0.118, respectively, while the PA and PI values of the cells engulfing BSA-treated and untreated E. coli, S. aureus and P. pastoris were 36.0±1.7%, 35.0±2.0% and 32.0±1.8%, and 36.3±1.8%, 36.9±1.2% and 34.5±1.3%, and 0.831±0.065, 1.740±0.179 and 1.060±0.049, and 0.836±0.049, 1.719±0.046 and 0.991±0.015, individually (Fig. 5). Apparently, the percent of macrophages phagocytosing Vg-treated microbes was significantly higher than that of cells phagocytosing BSA-treated and untreated microbes (p<0.05). Furthermore, the number of Vg-treated microbes engulfed by each macrophage was also significantly higher than that of BSA-treated and untreated microbes engulfed by each macrophage (p<0.05). In contrast, there was no significant difference in the PA and PI values of the cells engulfing BSA-treated and untreated microbes. These denoted that Vg is able to promote the macrophage phagocytosis.
Figure 4. Phagocytosis of E. coli, S. aureus and P. pastoris by macrophages.
Vg, FITC-labeled microbes and macrophages freshly isolated were mixed and incubated together at room temperature. The controls were performed in the presence of BSA instead of Vg and in the absence of Vg. Phagocytosis was observed at 60 min after mixing macrophages with microbes. (A, C and E) Phagocytosis of E. coli, S. aureus and P. pastoris by macrophages at 60 min; (B, D and F) Phagocytosis of E. coli, S. aureus and P. pastoris by macrophages at 105 min. Scale bars: 10 µm.
Figure 5. Phagocytic ability (PA) and phagocytic index (PI) of macrophages engulfing E. coli, S. aureus and P. pastoris.
Both Vg in PBS and BSA in PBS as well as PBS alone were pre-incubated with the FITC-labeled microbes, E. coli, S. aureus and P. pastoris, and then incubated with the macrophages freshly isolated. Phagocytosis was observed at 60 min after mixing macrophages with microbes. The microbes pre-incubated with Vg were readily phagocytosed by the macrophages, while those precubated with BSA or PBS alone were not. The PA and PI values of the macrophages phagocytosing Vg-treated E. coli, S. aureus and P. pastoris were significantly higher than those of the cells phagocytosing BSA-treated and untreated microbes (p<0.05). There was no significant difference in the PA and PI values of the cells engulfing BSA-treated and untreated microbes. Data are expressed as mean values±SEM from three experiments. The bars represent the standard error of the mean, and asterisks denote statistically significant differences (p<0.05). (A) PA of macrophages engulfing E. coli, S. aureus and P. pastoris. (B) PI of macrophages engulfing E. coli, S. aureus and P. pastoris.
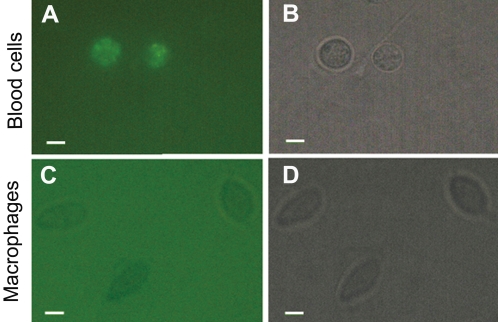
Vg binds to macrophages
To test if Vg can preferentially bind to macrophages, FITC-labeled Vg was incubated with the macrophages and red blood cells, respectively. It was found that Vg bound to the surface of macrophages, but not red blood cells (Fig. 6), suggesting that Vg is capable of binding to macrophage surfaces specifically.
Figure 6. Binding of FITC-labeled Vg to macrophages.
Macrophage suspensions and FITC-labeled Vg were incubated together at room temperature for 30 min, and then 60 µl of cell suspensions was sampled to make smears for microscopic examination under an Olympus BX51 fluorescence microscope. For control, red blood cells were used, and treated similarly. A and C are the images under a fluorescent field, and B and D are the images under a bright light field. (A and B) Macrophages. (C and D) Red blood cells. Scale bars: 10 µm.
Discussion
Vg, a major reproductive protein, has been proposed to perform pleiotropic functions including immune defense reaction. Our results suggest that fish Vg plays an integrative role in regulating innate immunity via its recognizing microbial cell wall constituents PAMPs and enhancing macrophage phagocytosis.
It is shown for the first time in this study that fish Vg binds to LPS from Gram-negative bacteria, LTA from Gram-positive bacteria, PGN from both Gram-positive and Gram-negative bacteria, β-1,3-glucan from eukaryotic fungi and laminarin from brown algae. In agreement, fish Vg also binds to both Gram-negative bacterium E. coli and Gram-positive bacterium S. aureus as well as fungus P. pastoris. However, Vg fails binding to self molecules including the serum components and total muscle proteins prepared from H. otakii. Recently, significant advances have been made in identifying the non-self-recognition molecules, and few of them have been tested for the existence of multiple specificities recognizing PAMPs [30]–[33]. It is clear that fish Vg is a novel pattern recognition receptor with a wide spectrum of specificity capable of identifying non-self components including LPS, LTA, PGN, glucan and laminarin.
It is of particular interest to note that fish Vg can bind to P. pastoris, and recognize β-glucan. As far as we are aware, β-glucan binding proteins have been documented only in invertebrates [34]. In contrast, only a few cell surface receptors including dectin-1 and scavenger receptors are known to mediate recognition of β-glucan in vertebrates [35], [36], while no plasma proteins recognizing β-glucan have ever been documented. Therefore, fish Vg appears the first plasma β-glucan binding molecule reported so far in vertebrates.
Plasma proteins that bind to both microbes and surface receptors of macrophages function as opsonins to promote phagocytosis. We show here that in addition to acting as a pattern recognition receptor with multiple specificities, fish Vg is able to bind to the surface of macrophages (Fig. 6) and enhance macrophage phagocytosis (Fig. 5). Enhanced phagocytosis by fish Vg suggests that Vg has an opsonic activity, which is apparently attributable to its ability to bind both microbes and macrophages (Fig. 2 and Fig. 6). Further study is needed to identify the surface molecules on macrophages to which Vg binds.
Overall, it is highly likely that fish Vg is physiologically involved in both the sensing of invading pathogens via interaction with their PAMPs and the recruitment of macrophages to phagocytose and digest the pathogens. The study of in vivo function of fish Vg is currently under way.
In summary, the present study highlights previously unreported roles for teleostean Vg in two key areas of fish innate immunity, namely, microbial molecular pattern recognition and macrophage phagocytosis. It also bolsters the notion that factors normally involved in control of female reproduction are associated with immunity in organisms that rely on Vg for oocyte development.
Materials and Methods
Purification and characterization of Vg
A total of 12 fishes H. otakii at the previtellogenic stage and with an average weight of 500 g were purchased from a local fish dealer, and reared at room temperature in two tanks with 50 L of seawater, which was changed once a day. Six fishes H. otakii were each injected intraperitoneally with 2 mg/kg body weight of 17-β-estradiol (E2) dissolved in ethanol plus 0.9% NaCl (1∶1, v/v). Two injections were given at 7-days intervals. Another 6 H. otakii received two intraperitoneal injections of only ethanol plus 0.9% NaCl. Seven days after the second injection, fish were anaesthetized with 0.05% 3-aminobenzoicacid ethyl ester (Sigma–Aldrich, Germany), and blood samples (totally about 12 ml) were collected via the caudal sinus with heparinized syringes, placed in heparinized tubes containing 0.5 mM phenylmethylsulfonyl flunoride (PMSF, Sigma), and centrifuged at 3,000 g at 4°C for 20 min. Serum was pooled, and Vg was purified as described previously [37].
Native-polyacrylamide gel electrophoresis (PAGE) was carried out on a 7.5% separation gel with a 4% spacer gel using the buffer system of Davis [38]. After electrophoresis, proteins were stained with Coomassie brilliant blue R-250. To test the presence of carbohydrate, lipid and phosphorus components, the gels were stained using periodic acid/Schiff reagent [39], Sudan black B [40] and methyl green [41], respectively. The target protein band was also excised from the gel, and subjected to trypsin digestion and mass spectrophotometry analysis [42].
Labeling Vg with fluorescein isothiocyanate (FITC)
Purified Vg (1 mg/ml) was adjusted to pH 9.8 by dialyzing against 200 mM carbonate buffered solution (CBS; pH 9.8) at 4°C for 24 h. To label Vg with FITC, Vg treated as above was dialyzed against CBS (pH 9.8) with 0.1 mg/ml FITC (Sigma) at 4°C for 24 h. To separate the FITC-labeled Vg from free FITC, the mixture was dialyzed against 10 mM phosphate buffered solution (PBS; pH 7.4), which was changed twice a day, at 4°C for about 4 days. The purity of the conjugate, FITC-labeled Vg, was confirmed by native-PAGE and Coomassie blue staining, and its F/P ratio (F/P ratio is defined as the ratio of moles of FITC to moles of protein in the conjugate) was calculated by the equation F/P = (2.77×A495)/[A280-(0.35×A495)] from the absorbance readings of the conjugate samples [43]. Bovine serum albumin (BSA) and human apolipoprotein B (apoB), a member of protein belonging to the same superfamily as Vg, were also labeled with FITC using the same method.
To assess if Vg activity is affected by labeling with FITC, the colony forming unit (CFU) assay was conducted to compare the growth inhibition rates of E. coli by FITC-labeled Vg and unlabeled Vg [16]. Briefly, an aliquot of 60 µl of FITC-labeled Vg (100 µg/ml) was mixed with 60 µl of E. coli suspension with 2×104 cells/ml, and the mixture was pre-incubated, with gentle stirring, at 25°C for 1 h, and plated onto 3 plates (40 µl each plate) with LB media. After incubation at 37°C for 12 h, the resulting bacterial colonies in each plate were counted. The controls were processed similarly except that FITC-labeled Vg was replaced with Vg or PBS alone.
Assay for binding of FITC-labeled Vg to microbial cells
Binding of Vg to microbes was assayed by the method of Jiang et al. [44] with slight modification. In brief, aliquots of 30 µl of FITC-labeled Vg (0.2 mg/ml) in PBS were mixed with 30 µl of Gram-negative bacterium E. coli (5×107 cells), Gram-positive bacterium S. aureus (5×107 cells), and eukaryotic fungus P. pastoris (5×106 cells), respectively, at 4°C overnight. The microbes were washed three times with 1.0 ml of 25 mM Tris-HCl buffer containing 137 mM NaCl and 3 mM KCl (TBS; pH 7.6), harvested by centrifugation at 3,000 g at room temperature for 15 min, and re-suspended in 1 ml of TBS. Aliquots of 10 µl of the microbial suspensions were applied to microscope slides, and binding of FITC-labeled Vg to the microbial cells was observed under an Olympus BX51 fluorescence microscope. The microbes treated with FITC-labeled BSA and FITC-labeled apoB under the same conditions were used as controls.
Labeling Vg with DIG
Labeling Vg with DIG was performed according to the instruction of DIG protein labeling kit (Roche). Purified Vg was dialyzed against 10 mM PBS (pH 7.4) at 4°C overnight, and adjusted to a concentration of 1 mg/ml with 10 mM PBS. A total of 1 mg/ml Vg in 10 mM PBS was mixed with 16.35 µl of 20 mg/ml DIG-NHS solution dissolved in dimethylsulfoxide (DMSO; Sigma) and incubated at 25°C for 2 h. The remaining non-reacted DIG-NHS was removed by gel filtration on a Sephadex G-25 column.
Preparation of serum and total muscle proteins
Fishes H. otakii were anaesthetized with 0.05% 3-aminobenzoicacid ethyl ester, and the blood was collected in 10 ml eppendorff tubes from the caudal vein, and the muscle dissected out. The blood samples were allowed to clot at room temperature for 1 h, placed at 4°C overnight, and the sera pooled by centrifugation at 200 g for 10 min.
Total muscle proteins (TMPs) were prepared by the method of Chen and Resh [45]. In brief, the muscle tissue was triturated in liquid nitrogen with mortar, lysed in RIPA buffer (150 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, 10 mM Tris, pH 7.4, 1 mM Na3VO4, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM PMSF) by 100 mg tissue/ml RIPA. Lysates were centrifuged at 23,700 g at 4°C for 15 min, and the supernatants collected. Protein concentrations were determined as above.
Assay for binding of DIG-labeled Vg to various ligands
LPS, LTA, PGN, β-1,3-glucan and laminarin (all from Sigma) were individually dissolved in re-distilled water, giving concentrations of 40 µg/ml, and a volume of 50 µl (2 µg) of each solution was applied to a 96-well microplate and air-dried overnight at room temperature. The plates were incubated at 60°C for 30 min to fix the ligands, and the wells were each blocked with 200 µl of 1 mg/ml BSA in 10 mM PBS (pH 7.4) at 37°C for 2 h. After washing four times with 200 µl of 10 mM PBS supplemented with 1% Tween-20, 50 µl of DIG-labeled Vg solutions with different concentrations of DIG-labeled Vg (0, 1.25, 2.5, 5, 10 and 15 µg/ml) was added into the wells. After incubation at room temperature for 3 h, the wells were each rinsed four times with 200 µl of 10 mM PBS supplemented with 1% Tween-20, 100 µl of anti-DIG-Fabs (Roche) diluted at 1∶1,000 with 10 mM PBS (pH 7.4) containing 1% dry milk powder was added into the wells. The wells were incubated at 37°C for 2 h, washed as above, and then reacted with 75 µl of 0.4 mg/ml O-phenylenediamine (OPD; Amresco) in 51.4 mM Na2HPO4, 24.3 mM citric acid and 0.045‰ H2O2 (pH 5.0) at 37°C for 20 min. Subsequently, 25 µl of 2 mM H2SO4 was added into each well to terminate the reactions, and absorbance at 492 nm was monitored by a microplate reader (GENios Plus, Tecan). For controls, BSA, serum proteins (SPs) and TMPs of H. otakii at the same concentrations were treated similarly.
The ELISA experiments were performed in triplicate, and repeated three times. Statistical calculations were performed using the computer program SPSS 13.0. The statistical significance of difference between mean values was determined by Student's two-tailed t-tests, and difference at p<0.05 was considered significant. All data were expressed as mean±SEM.
Isolation of head kidney-derived macrophages and red blood cells
The head kidney of teleostean fish is considered to be the functional and structural homologue of mammalian bone marrow [46], [47]. To procure macrophage population, head kidneys were dissected out from 3 fishes H. otakii aseptically, and placed in 10 mM PBS (pH 7.4) containing 300 U/ml penicillin and 300 µg/ml streptomycin. After washing three times with 10 mM PBS with 300 U/ml penicillin and 300 µg/ml streptomycin, the tissues were cut into 1 mm3 pieces, gently passed through an 80 µm sterile steel mesh, and rinsed with Leibovitz's L-15 medium (pH 7.2; Gibco) supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin and 2% foetal calf serum (FCS; Gibco). Cell suspensions were again gently passed through a 15 µm sterile steel mesh, and loaded onto a discontinuous gradient consisting of 5 ml of 45% Percoll (Pharmacia) overlaid with 5 ml of 31% Percoll. After centrifugation at 400 g at 4°C for 30 min, the macrophage fraction was collected from 31–45% interface. Macrophage cells were washed three times with Leibovitz's L-15 medium (pH 7.2) supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin and 0.1% FCS, harvested by centrifugation at 200 g at 4°C for 5 min, and suspended in 2 ml Leibovitz's L-15 medium supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin and 5% FCS. The concentration of cells was established by directly counting the cells in the cell suspension in a Burker cell counter, and adjusted to 2×107 cells/ml with Leibovitz's L-15 medium, and the cell viability was assessed by trypan blue exclusion. The resulting cell suspension was stored at 4°C, and used for the following experiments within 2 h.
To obtain red blood cells, a fish H. otakii with a weight of about 500 g was anaesthetized, surface-sterilized with alcohol and bled into anti-coagulant solution (10 mM PBS, 600 IU/ml heparin sodium salt, pH 7.4). The plasma was centrifugated at 200 g at 4°C for 5 min. After washing three times with 10 mM PBS, the red blood cells were suspended in Leibovitz's L-15 medium and adjusted to a density of 2×107 cells/ml.
Labeling microbes with FITC
Labeling microbes with FITC was carried out as described previously [48]. The densities of E. coli, S. aureus and P. pastoris were all adjusted to 5×108 cells/ml, and the microbes were heat-inactivated at 60°C for 15 min. Aliquots of 1 ml of the heat-inactivated samples were mixed with 100 µl of 1 mg/ml FITC in DMSO, and incubated for 30 min in dark with gentle shaking at room temperature. After washing three times with 10 mM PBS (pH 7.4), the FITC-labeled microbes were harvested by centrifugation at 3,000 g at room temperature for 15 min, re-suspended in 1 ml of 10 mM PBS (pH 7.4), and stored at −20°C until used.
Assay for effects of Vg on phagocytosis
To test the effects of Vg on phagocytosis, aliquots of 300 µl macrophage suspensions were mixed in 1.5 ml eppendorff tubes with 200 µl of 400 µg/ml Vg and 300 µl of FITC-labeled microbe suspensions. The mixtures were incubated at room temperature for 60 min and 105 min, shaking every 5 min to prevent sedimentation of macrophages and microbes, and the phagocytosis process was stopped by addition of 500 µl ice-cold 10 mM PBS (pH 7.4), followed immediately by centrifugation at 200 g at 4°C for 5 min to separate macrophages from non-phagocytosed microbes. The macrophage pellets were re-suspended in 200 µl of 10 mM PBS (pH 7.4), and a volume of 60 µl of the macrophage suspensions was sampled to make smears for microscopic examination under an Olympus BX51 fluorescence microscope. For each example, at least 100 macrophages were observed. The number of macrophages with phagocytosed microbes and the number of microbes in each macrophage were recorded. The phagocytic ability (PA) was defined as the percentage of macrophages with one or more engulfed microbes within the total cell population, and the phagocytic index (PI) as the number of engulfed microbes per cell. For controls, phagocytosis assay was also similarly performed in the presence of BSA instead of Vg and in the absence of Vg.
The phagocytosis experiments were performed in triplicate, and repeated three times. The number of macrophages with phagocytosed microbes and the number of microbes in each macrophage were subjected to statistical evaluation with Student's two-tailed t-tests, and difference at p<0.05 was considered significant. All data were expressed as mean±SEM.
Assay for binding of FITC-labeled Vg to macrophages
To test the binding of Vg to macrophages, aliquots of 300 µl macrophage suspensions were mixed in 1.5 ml eppendorff tubes with 200 µl of 400 µg/ml of FITC-labeled Vg. The mixtures were incubated at room temperature for 30 min, shaking every 5 min, and then centrifuged at 200 g at room temperature for 5 min to separate the macrophages from free FITC-labeled Vg. The macrophage pellets were re-suspended in 200 µl of 10 mM PBS (pH 7.4), and a volume of 60 µl of cell suspensions was sampled to make smears for microscopic examination under an Olympus BX51 fluorescence microscope. For control, red blood cells were used, and treated similarly.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants (2006CB101805; 30730072) of Ministry of Science and Technology (MOST) and Natural Science Foundation of China (NSFC).
References
- 1.Avarre JC, Lubzens E, Babin PJ. Apolipocrustacein, formerly vitellogenin, is the major egg yolk precursor protein in decapod crustaceans and is homologous to insect apolipophorin II/I and vertebrate apolipoprotein B. Bmc Evol Biol. 2007;7:3. doi: 10.1186/1471-2148-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne BM, Gruber M, Ab G. The evolution of egg yolk proteins. Prog Biophys Mol Biol. 1989;53:33–69. doi: 10.1016/0079-6107(89)90005-9. [DOI] [PubMed] [Google Scholar]
- 3.Sappington TW, Raikhel AS. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol. 1998;28:277–300. doi: 10.1016/s0965-1748(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu N, Cheek AO, Sullivan CV, Matsubara T, Hara A. Vitellogenesis and endocrine disruption. In: Mommsen TP, Moon TW, editors. Biochemistry and Molecular Biology of Fishes. Vol. 6. London: Elsevier Science; 2005. pp. 431–471. [Google Scholar]
- 5.Shyu AB, Raff RA, Blumenthal T. Expression of the vitellogenin gene in female and male sea urchin. Proc Natl Acad Sci USA. 1986;83:3865–3869. doi: 10.1073/pnas.83.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.della-Cioppa G, Engelman F. The vitellogenin of Leucophaea maderate: synthesis of a large phosphorylated precursor. Insect Biochem. 1987;17:401–415. [Google Scholar]
- 7.Dhadialla TS, Raikhel AS. Biosynthesis of mosquito vitellogenin. J Biol Chem. 1990;265:9924–9933. [PubMed] [Google Scholar]
- 8.Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc Natl Acad Sci USA. 2003;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amdam GV, Norberg K, Page RE, Erber J, Scheiner R. Downregulation of vitellogenin gene activity increases the gustatory responsiveness of honey bee workers (Apis mellifera). Behav Brain Res. 2006;169:201–205. doi: 10.1016/j.bbr.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Omholt S, et al. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007;5:0673–0677. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. PNAS. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura A, Yasuda K, Adachi H, Sakurai Y, Ishii N, et al. Vitellogenin-6 is a major carbonylated protein in aged nematode, Caenorhabditis elegans. Biochem Bioph Res Co. 1999;264:580–583. doi: 10.1006/bbrc.1999.1549. [DOI] [PubMed] [Google Scholar]
- 14.Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested by the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during over-wintering? J Econ Entomol. 2004;97:741–747. doi: 10.1093/jee/97.3.741. [DOI] [PubMed] [Google Scholar]
- 15.Shi XD, Zhang SC, Pang QX. Vitellogenin is a novel player in defense reactions. Fish Shellfish Immunol. 2006;20:769–772. doi: 10.1016/j.fsi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SC, Sun YN, Pang QX, Shi XD. Hemagglutinating and antibacterial activities of vitellogenin. Fish Shellfish Immunol. 2005;19:93–95. doi: 10.1016/j.fsi.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rem Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 20.Söderhäll K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 21.Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Garcia E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2002;72:1092–1108. [PubMed] [Google Scholar]
- 23.Roos A, Xu W, Castellano G, Nauta AJ, Garred P, et al. Mini-review: a pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol. 2004;34:921–929. doi: 10.1002/eji.200424904. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S. Pattern recognition receptors: doubling up for the innate immune responses. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 25.Jomori T, Natori S. Function of the lipopolysaccharide-binding protein of Periplaneta americana as an opsonin. FEBS Lett. 1992;296:283–286. doi: 10.1016/0014-5793(92)80305-z. [DOI] [PubMed] [Google Scholar]
- 26.Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, et al. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 27.Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002;416:644–648. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- 28.Arukwe A, Goksøyr A. Eggshell and egg yolk proteins in fish: hepatic proteins for the next generation: oogenetic, population, and evolutionary implications of endocrine disruption. Comp Hepatol. 2003;2:e4. doi: 10.1186/1476-5926-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler CR, Sumpter JP. Oocyte growth and development in teleost. Rev Fish Biol Fisheries. 1996;6:287–318. [Google Scholar]
- 30.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Kurata S, Ariki S, Kawabata S. Recognition of pathogens and activation of immune responses in Drosophila and horseshoe crab innate immunity. Immunobiology. 2006;211:237–249. doi: 10.1016/j.imbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Royet J. Infectious non-self recognition in invertebrates: lessons from Drosophila and other insect models. Mol Immunol. 2004;41:1063–1075. doi: 10.1016/j.molimm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Yu Y, Huang H, Feng K, Pan M, et al. A shot-form C-type lectin from amphioxus acts as a direct microbial killing protein via interaction with peptidoglycan and glucan. J Immunol. 2007;179:8425–8434. doi: 10.4049/jimmunol.179.12.8425. [DOI] [PubMed] [Google Scholar]
- 34.Yu XQ, Kanost MR. Manduca sexta lipopolysaccharide-specific immulectin-2 protects larvae from bacterial infection. Dev Comp Immunol. 2003;27:189–196. doi: 10.1016/s0145-305x(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 35.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 36.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Zhang S. Purification and characterization of a putative vitellogenin from the ovary amphioxus (Branchiostoma belcheri tsingtauense). Comp Biochem Physiol B. 2001;129:121–127. doi: 10.1016/s1096-4959(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 38.Davis R. Disc electrophoresis-II. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 39.Fairbanks G, Steck TL, Wallace DFH. Electrophoretic analysis of the major polypeptide of the human erythrocyte membrane. Biochem J. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 40.de Vlaming VL, Vodicnik MJ, Bauer G, Marquette T, Evans D. Estradiol-17 beta effects on lipid and on the induction of a yolk precursor in goldfish, Carassius auratus. Life Sci. 1977;20:1945–1952. doi: 10.1016/0024-3205(77)90172-2. [DOI] [PubMed] [Google Scholar]
- 41.Cutting JA, Roth TF. Staining of phosphoproteins on acrylamide gel electropherograms. Anal Biochem. 1973;54:386–394. doi: 10.1016/0003-2697(73)90367-9. [DOI] [PubMed] [Google Scholar]
- 42.Zou Y, He L, Huang SH. Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with Escherichia coli invasion protein IbeA. Biochem Biophys Res Commun. 2006;351:625–630. doi: 10.1016/j.bbrc.2006.10.091. [DOI] [PubMed] [Google Scholar]
- 43.Roque AC, Taipa MA, Lowe CR. A new method for the screening of solid-phase combinatorial libraries for affinity chromatography. J Mol Recognit. 2004;17:262–267. doi: 10.1002/jmr.661. [DOI] [PubMed] [Google Scholar]
- 44.Jiang H, Ma C, Lu ZQ, Kanost MR. β-1,3-Glucan recognition protein-2 (βGRP-2) from Manduca sexta: an acute-phase protein that binds beta-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem Mol Biol. 2004;34:89–100. doi: 10.1016/j.ibmb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Resh MD. Activation of mitogen-activated protein kinase by membrane-targeted Raf chimeras is independent of raft localization. J Biol Chem. 2001;276:34617–34623. doi: 10.1074/jbc.M103995200. [DOI] [PubMed] [Google Scholar]
- 46.Zapata A. Ultrastructural study of the teleost fish kidney. Dev Comp Immunol. 1979;3:55–65. doi: 10.1016/s0145-305x(79)80006-3. [DOI] [PubMed] [Google Scholar]
- 47.Zapata A. Lymphoid organs of teleost fist. II. Ultrastructure of renal lymphoid tissue of Rutilus rutilus and Gobio gobio. Dev Comp Immunol. 1981;5:685–690. doi: 10.1016/s0145-305x(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 48.DeVelasco EA, Dekker HA, Antal P, Jalink KP, van Strijp JA, et al. Adjuvant Quil A improves protection in mice and enhances the opsonic capacity of antisera induced by pneumoccocal polysaccharide conjugate vaccines. Vaccine. 1994;12:1419–1422. doi: 10.1016/0264-410x(94)90151-1. [DOI] [PubMed] [Google Scholar]