Abstract
INTRODUCTION
For chronic, repeated hemodynamic studies in conscious dogs, we designed and tested a chronically instrumented canine, microsphere delivery model. The goals of this study were to investigate the accuracy of repeated estimations of blood perfusion using fluorescent-labeled microspheres and to develop and validate a chronic preparation that permits consecutive estimations in the same conscious animal over an extended protocol.
METHODS
Via thoracotomy, 9 dogs were instrumented with left atrial appendage and aortic vascular access catheters connected to subcutaneous vascular access ports (VAPs). Four animals received 7 serial injections of 1.6 million 15μm microspheres (total: 11.2 million), and five animals received 8 serial injections of 2.25 million microspheres (total: 18 million) over the course of 11 or 18 weeks.
RESULTS
All catheters have remained bidirectionally patent during protocol for 14.9±0.8 (Mean±SEM) weeks. Sphere accumulation did not significantly alter global myocardial (p=0.69, p=0.25), renal (p=0.92, p=0.12), hepatic (p=0.84, p=0.32), or splenic (p=0.33, p=0.70) blood perfusion in either set of animals.
CONCLUSIONS
Catheters remained bidirectionally patent for months, did not interfere with the hemodynamic responses of the preparation, and allowed repeat percutaneous injection of microspheres and withdrawal of reference arterial blood from within conscious canines. Eight serial injections totaling 18 million microspheres over 18 weeks did not alter regional myocardial, hepatic, renal, or splenic blood flow. This dependable, chronic, percutaneous arterial access preparation provides a means for examining acute and long-term effects of pathophysiological, pharmaceutical, and environmental influences on regional arterial blood perfusion in conscious, large animals.
Keywords: Subcutaneous Vascular Access Port, Chronic Arterial Catheter, Fluorescent Polystyrene Microspheres, Perfusion
Introduction
Since the late 1960s when Rudolph and Heymann [1] introduced the use of plastic, radioactive microspheres for measurement of regional organ perfusion and Makowski et al. [2] added the concept of the reference sample, the microsphere method has been used extensively to measure regional blood flow (RBF) in laboratory animals. Small diameter microspheres injected into the left atrium distribute throughout the body and become trapped in capillaries of target tissue based on RBF patterns. The quantity of microspheres lodged in the tissue of interest is proportional to the reference blood flow sample and allows the calculation of RBF in ml/g/min. Using this technique, radioactive-labeled microspheres have been used to determine RBF in both acute and chronic preparations. In acute experimentation, these microspheres may slightly underestimate RBF due to the difference in density between radioactive-labeled microspheres and erythrocytes [3]. Chronic studies are precluded due to the slow leaching of radioactive signal from radioactive-labeled spheres [4,5] which cause dramatic underestimation of organ blood flow of up to 20–50% over a 2-month protocol [6].
To address these and other complications with radioactive-labeled microspheres, techniques have been developed for the use of fluorescent-labeled microspheres that have a similar density to erythrocytes [3] and do not leach signal over time. Fluorescent-labeled microspheres are therefore more useful for chronic experimentation, do not pose health risks for investigators, and avoid the high disposal costs of radioactive tissues and cadavers [3,7].
Studies examining RBF with fluorescent-labeled microspheres often employ multiple colors of microspheres, but have been limited to either acute experimentation or short-term survival surgery. During these studies, investigators often inject 6 to 18 million 15 μm microspheres during a single injection [8–13], and have reported that serial injections of up to 48 million, 15 μm microspheres do not affect regional flow patterns in an acute canine model [14]. While these acute studies establish a limit for the number of microspheres that will not influence regional blood flow, they do not indicate whether increasing deposition of microspheres in capillaries will influence RBF patterns during an extended protocol designed for repeated RBF measurements in the same chronic preparation.
Repeated measurement of RBF is desirable during long-term studies examining pathophysiological, pharmaceutical, and environmental influences over regional hemodynamics. In dogs, hemodynamic parameters are most validly assessed in the conscious state [15,16]. Therefore, it is desirable to maintain chronically instrumented animals for repeated injection of multiple colors of microspheres for serial RBF measurement during long-term studies.
We developed and tested a technique for implantation and maintenance of left atrial and aortic catheters attached to totally implanted subcutaneous vascular access ports (VAP) for repeat percutaneous microsphere injections in canines. Up to 8 serial injections of 2.25 million microspheres per injection over an 18 week protocol do not significantly alter myocardial, renal, hepatic, or splenic arterial blood perfusion. This preparation may be used to examine the acute and long-term effects of pathophysiological, pharmaceutical, and environmental influences on regional arterial blood flow in conscious, large animals.
Methods
Animals and Anesthesia
All animals received humane care and were handled in accordance with National Institutes of Health and Harvard Medical School animal care committee guidelines. Experimental procedures followed animal studies protocols approved by Harvard Medical School.
Nine adult female mongrel dogs, weighing an average of 15.8 kg (range: 14.2–18.2 kg) were used for this study. These were all research purpose-bred animals obtained from either Butler Farms (USDA # 21-A-003, Clyde, NY) or from Marshall Laboratories. All surgeries were performed aseptically while animals were maintained under a surgical level of anesthesia. In the operating room, the animals were pre-anesthetized using ketamine (10 mg/kg bolus), xylazine (1.5 mg/kg bolus), and atropine (0.04 mg/kg bolus). An endotracheal tube was inserted to initiate mechanical ventilation with 1.5% isofluorane and pure oxygen for anesthesia. One-lung ventilation was performed during thoracotomy.
Thoracotomy
The animal was placed on the operating table in the right decubitus position. A 14 cm left chest incision was made above the 4th intercostal space. The latissimus muscle was dissected and spared. The scalenus muscle was transected. The serratus ventralis muscle was divided. The intercostal muscles were cut and the thorax was entered.
Catheter Implantation
After thoracotomy, the pericardium was opened. The left atrial appendage was retracted and a 4-0 Prolene suture was employed to set a purse-string 5 mm in diameter to secure the catheter entry site. A radio-opaque silicone catheter, 88% Dow Corning Silicone/12% BaSO4 (7 French; Access Technologies, Skokie, IL) with two adjustable silicone fastener loops was flushed with heparinized saline solution (~20 IU/ml). A 2-mm knick incision through the wall of the appendage was made with a #15 blade. The catheter was advanced for 4 cm into the left atrial appendage. This depth and the angle of catheter entry parallel to the surface of the atrial appendage ensured that the catheter did not interfere with mitral valve function. The purse string was tied and the catheter was secured to the appendage with the two fastener loops (Figure 1A). The catheter was tunneled out through a 3 mm knick incision on the pericardium, and the pericardium was closed.
Figure 1.
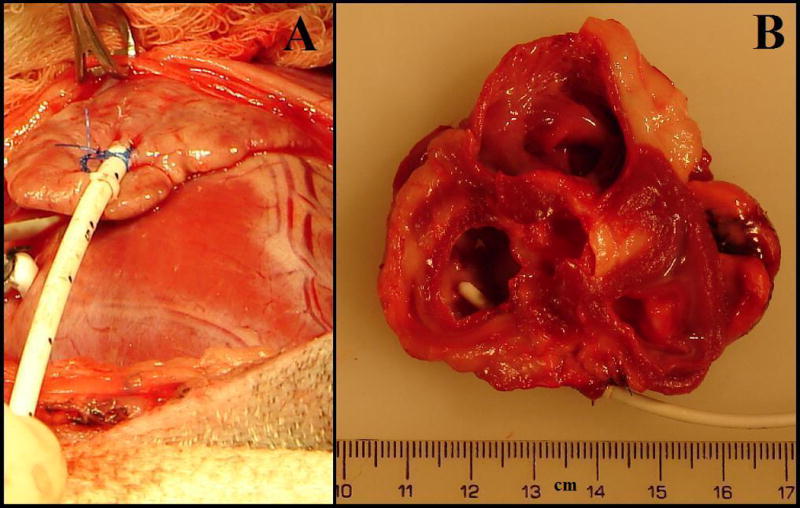
A Catheter Securement to Left Atrial Appendage. A 7-Fr, radio-opaque silicon catheter was inserted 4-cm into the left atrial appendage. After implantation, the catheter is secured to the surface of the left atrial appendage. The two purse-string sutures are tied and secured to the first fastener loop. The second fastener loop is secured near the tip of the left atrial appendage. B Intra-Atrial View of Catheter Tip Fifteen Weeks Post-Implantation. There was no overgrowth of tissue around the intra-atrial segment of catheter. Minimum fibrous growth and chronic inflammation, no necrosis, and no infection were observed at the catheter entry site into the left atrial appendage. This depth and the angle of catheter entry parallel to the surface of the atrial appendage ensured that the catheter did not interfere with mitral valve function.
An aortic catheter oriented down-stream was placed in the aorta using our previously described method (Figure 2A) [17].
Figure 2.
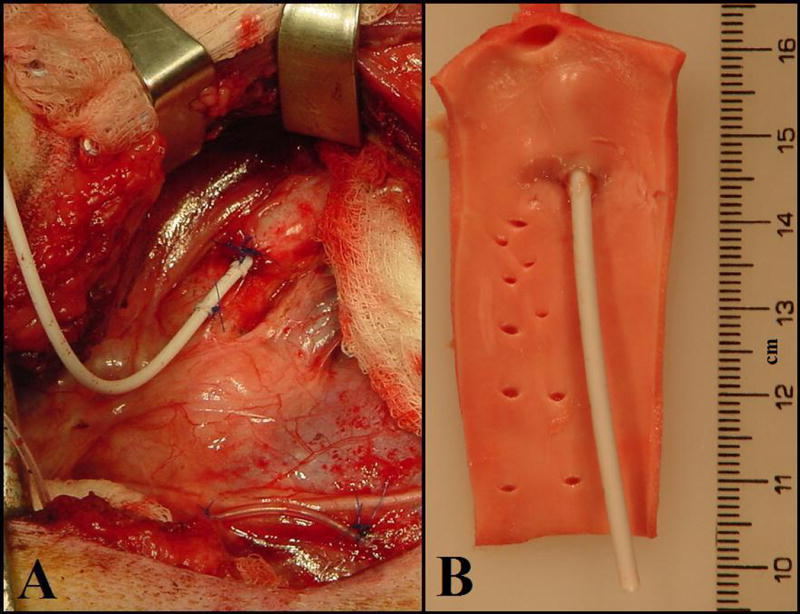
A Catheter Securement to Aorta. After implantation, the catheter is secured to the aortic wall. The two purse-string sutures are tied and secured to the first fastener loop. The second fastener loop is secured further up on the aorta. B: Intraluminal View of Intra-Aortic Catheter. There was no overgrowth of tissue around the intravascular segment of catheter. Minimum fibrous growth and chronic inflammation, no necrosis, and no infection were observed.
Port Implantation
A 3 cm incision was made at the nape of the neck just below the scapula. Subcutaneous fascia was dissected down to the surface of the back muscles. After aortic catheter implantation, a hollow trocar was used to tunnel the non-implanted segment of catheter to the VAP site just below the scapula. The catheter was attached to the nozzle of the VAP (Polysulfone GPVu; Access Technologies, Skokie, IL). The VAP was sutured to the back muscles. The catheter was checked for kinking and locked with 2 ml of heparinized saline (~20 IU/ml). The VAP was then buried in the fascia and the wound was closed in multi-layers.
Thoracotomy Closure
A chest tube was placed in the 5th intercostal space. The ribs were approximated and secured with multiple interrupted 1-0 Ticron sutures. The seratus ventralis muscle and the scalenus muscle were repaired with 2-0 and 3-0 Vicryl suture. The wound was closed in multi-layers with 3-0 Vicryl suture. Skin staples were used to close the skin. The chest tube was removed at the end of the operation with standard precautions to prevent pneumothorax.
Post-Operative Care
During the first 48 hours post-operatively, the animals received intra-muscular doses of buprenorphine (0.005 mg/kg bolus) every 8–12 hours. There was no significant post-operative morbidity and staples were removed 10–12 days post-operatively. The animals were typically used in studies beginning 3 weeks post-operatively.
Catheter Maintenance
At the suggestion of the manufacturer, catheters were flushed with heparinized bacteriostatic saline (2 ml,~20 IU/ml; Abbott Laboratories, North Chicago IL) once per day for three days post-operatively.
Post-Operative Port Access
Fur was clipped above the site of port implantation. The skin was scrubbed with a 0.05% Betadyne solution. To access the reservoir of the port, the skin was pierced with an 18-gauge butterfly needle with six inch catheter for mobility. To confirm catheter patency, 1 to 2 ml of heparinized bacteriostatic saline was injected into the port. After this flush, the port was used for injection of microspheres. After injection, the needle was retracted, and the skin was compressed with gauze.
Microsphere Protocols
Two sets of fluorescent polystyrene microspheres were used during this study. Fluosphere microspheres, 15.44 ± 0.93 μm (mean ± SD), were obtained from Molecular Probes, Eugene, OR. These microspheres are available with 11 different fluorescent color labels. We choose 7 colors that had no overlap between absorption and emission wavelengths. Vials obtained from the manufacturer contained 10 ml of solution with an approximate concentration of 1×106 microspheres/ml.
NuFlow microspheres, 15.5 ± 0.14 μm were obtained from IMT/Stason Laboratories, Irvine CA. These microspheres are available with eight different florescent color labels. We used all eight colors that had no overlap between absorption and emission wavelengths. Vials obtained from the manufacturer contained 5 ml of solution with an approximate concentration of 1×106 microspheres/ml.
For each experiment, vials were vortexed for 1 minute and then sonicated in an ultrasonic water bath for 15 minutes before injection. Animals underwent one of two protocols: 7 bolus injections of ~1.6 million microspheres (11.2 million Molecular Probes microspheres overall) injected over 11 weeks; 8 bolus injections of ~2.25 million microspheres (18 million NuFlow microspheres overall) injected over 18 weeks.
During injection, the subcutaneous ports were accessed with an 18-gauge butterfly needle as described above and clamped. Ten seconds before injection, a reference blood sample drawn from the aortic subcutaneous VAP was started. After 10 seconds, the microsphere bolus was injected over a period of less than 10 seconds, followed by a flush of 5 ml of warmed heparinized saline (~100° F, 20 IU/ml). This warm saline flush maintains vascular tone as well as clears the port/catheter system of lingering spheres. The reference withdrawal continued for an additional 80 seconds for a total of 100 seconds of withdrawal at a rate of 7.75 ml/min using a calibrated Harvard Apparatus withdrawal pump.
Quantification of Fluorescent Microspheres
After euthanasia, heart, kidney, liver, and spleen samples were harvested and weighed. Tissue and reference blood samples were sent to IMT/Stason Laboratories for automated digestion of tissue samples and counting of fluorescent microspheres using flow cytometry. Reference flows were calculated as milliliter per gram of tissue per minute.
Statistical Analysis
Baseline flow values from the initial blood flow determination were compared with the ultimate determination by paired t-tests. In the animals that received 18 million microspheres, a multiple outcome model incorporating endpoints from all four tissue types (liver, kidney, spleen, heart) was used to estimate a global pre versus post difference across the standardized outcomes. The model contained random animal terms to account for correlation among readings from the different tissue types recorded on the same animal. To ensure we maximized our power to statistically detect even small differences between initial and final baseline conditions, a multivariate analysis of variance (MANOVA) was performed on the readings from all tissue types, standardized by the outcome-specific standard deviations. This analysis tested for a global difference between standardized readings from the two conditions across the four tissue types.
Results
For long-term repeat microsphere studies to determine regional myocardial, renal, hepatic, and splenic blood flow in trained, conscious large animals, we implanted catheters into the aorta and left atrial appendage of 9 dogs. With minimum postoperative care, all 18 catheters have remained bidirectionally patent for 14.9 ± 0.8 weeks (Mean ± SEM) and have allowed repeated infusion of up to 8 different colors of fluorescent polystyrene microspheres in trained, conscious canines. Lack of tissue reaction to the catheters are illustrated in Figures 1B and 2B. During non-baseline injections, animals underwent different physiological interventions such as exposure to high concentrations of air pollution, α-adrenergic blockade, and coronary occlusion (data not shown).
Fluosphere Microspheres
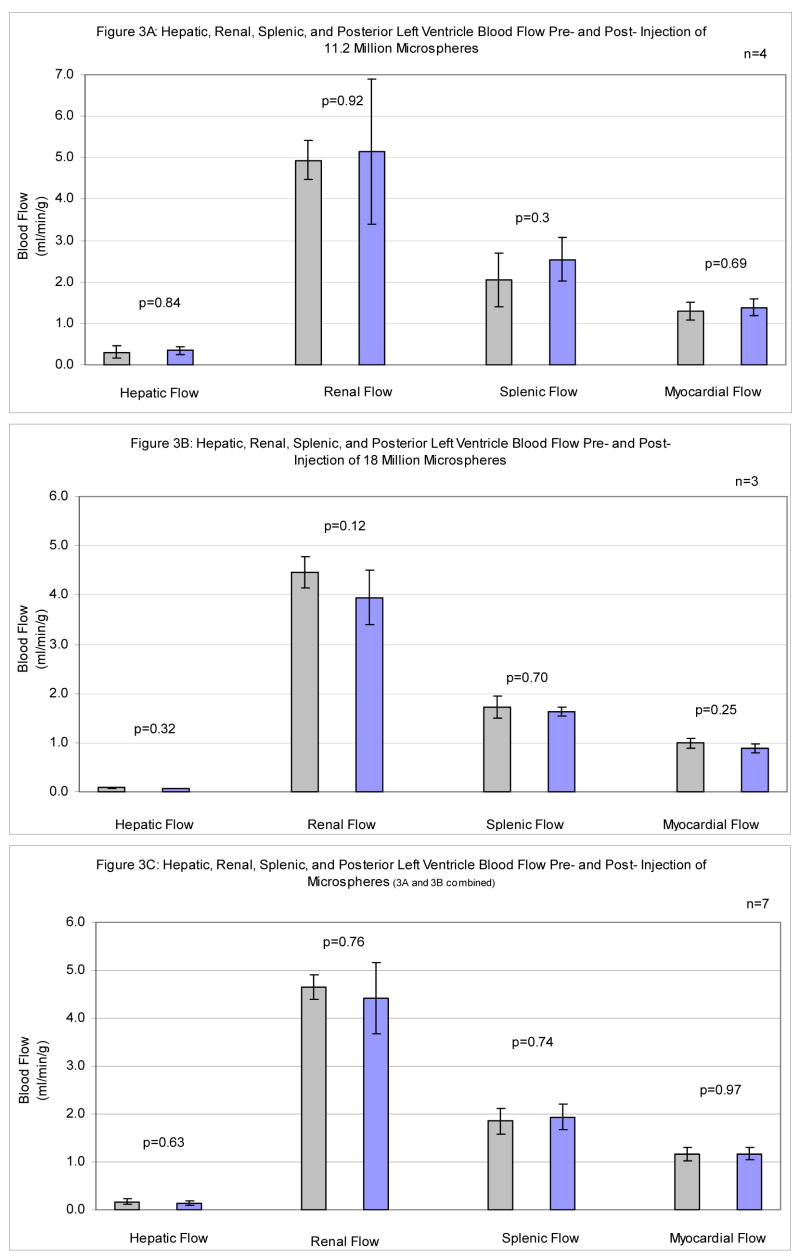
In 4 dogs, a total of 11.2 million Fluosphere microspheres (7 injections of 1.6 million microspheres) was injected over a period of 11 ± 2 weeks starting at three weeks post-operatively. Microsphere accumulation did not significantly alter global myocardial (p=0.69), renal (p=0.92), hepatic (p=0.84), or splenic (p=0.33) blood flow. As shown in Figure 3A, over the course of the study, there were no significant differences in organ perfusion.
Figure 3.
Individual Organ Perfusion ± SEM Before and After Multiple Injection of Polystyrene Microspheres A Hepatic, renal, splenic, and posterior left ventricle blood flow pre- and post- injection of 11.2 million microspheres. B Hepatic, renal, splenic, and posterior left ventricle blood flow pre- and post- injection of 18 million microspheres. C Combined results from Figure 3A and Figure 3B.
NuFlow Microspheres
In 5 dogs, a total of 18 million NuFlow microspheres (8 injections of 2.25 million microspheres) were injected over a period of 18 ± 1.1 weeks starting at three weeks postoperatively. Microsphere accumulation did not significantly alter global myocardial (p=0.25), renal (p=0.12), hepatic (p=0.32), or splenic (p=0.70) blood flow. As shown in Figure 3B, a slight trend toward decreases in flow was noted over the course of the study, but none of these comparisons in individual organs were statistically significant. Analysis of this trend using a multiple outcome model applied to all tissue types simultaneously did not show a statistically significant nor substantively significant overall difference between initial and final baselines (p=0.10).
Of the 5 animals injected with NuFlow spheres, 2 did not receive a final baseline injection. These animals were included in the study to demonstrate the reproducibility and utility of the surgical preparation for serial delivery of microspheres in a conscious, large animal model (n=9). The results from these animals were not included in the analysis (n=7).
Combined Results
When results from the two separate population of dogs were analyzed together (n=7), microsphere accumulation did not show a statistically significant nor a substantively significant change in global myocardial (p=0.97), renal (p=0.76), hepatic (p=0.63), or splenic (p=0.74) blood perfusion (Figure 3C).
Discussion
Catheter Patency
Subcutaneous VAPs allow repeated and atraumatic microsphere injections into the left atrium of chronically instrumented canines. This approach (i) circumvents the necessity of repeat thoracotomy for repeat intracardiac injections (ii) allows repetitive, percutaneous microsphere injections in various long-term experimental settings and (iii) without sedation or anesthetization of the preparation.
In previous studies, long-term catheter patency is a potential significant complication associated with chronically instrumented animals. Investigators must consider catheter entry site, catheter residence site, and direction of catheter in relationship to direction of blood flow. Numerous problems associated with chronic arterial catheterization include the integrity of the preparation’s hemodynamics [15], the maintenance of long-term patency of catheters residing opposite to the direction of blood flow [19], or inherent difficulty and danger of the procedure [20].
For example, ligation of the carotid artery for chronic repeated access to the arterial blood supply may compromise blood flow to the brain and alter baro- and chemoreceptor function [15]. Ligation and fluoroscopic catheterization of the femoral or iliac artery is an alternative that avoids complications associated with carotid ligation. However, in this preparation, the catheter tip resides opposite to the direction of aortic blood flow and requires a complicated flushing system to prevent clotting and obstruction of the lumen of the catheter [19].
For long-term arterial access in conscious canines, we previously developed and tested a technique for implantation of an aortic vascular access catheter [17]. Our technique enables arterial catheter implantation without interrupting the systemic circulation or compromising peripheral arterial flow, requires only a single penetration of the aortic wall, and results in a catheter oriented downstream in the aorta. Using this technique in a canine model, we implanted aortic vascular access catheters that are bidirectionally patent ongoing for 17 months. This aortic catheter, when paired with a left atrial catheter, provides the opportunity to perform serial microsphere injections in conscious animals during long-term perfusion studies.
Figure 1B and Figure 2B illustrates the lack of intraluminal fibrous overgrowth around these intra-arterial catheters. In our studies using vascular access catheters, long-term catheterization [17,18] did not stimulate fribrous tissue overgrowth around intra-aortic or intrapericardial segments of these silicone catheters. In this present study, we attribute this lack of reaction to the catheter material (88% Dow Corning Silicone/12% BaSO4) and to the catheter location in the arterial tree. Venous catheters residing in low flow velocity, low pressure environments often trigger fibrous overgrowth of the intravascular segment of catheter which may lead to catheter thrombosis and loss of catheter patency. Our catheters residing in the high flow velocity, high pressure arterial circulation, did not trigger fibrous overgrowth around the intraluminal catheter segments.
Microsphere Protocols
When appropriately sized microspheres are used, RBF is proportional to the number of spheres trapped in the region of tissue of interest [21,22]. Acute, tissue specific studies in small animal models suggest that 15 μm-diameter microspheres do not significantly alter RBF or vascular tone [21,23]. Chronic microsphere preparations in rabbits [6,7,24] which have allowed RBF determination up to 11 weeks post-operatively. However, in these studies, multiple determinations of RBF were not performed, and animals were sedated during the injection of microspheres.
Multiple colored fluorescently labeled polystyrene microspheres are increasingly used for serial injections of microspheres [7,13]. Eight fluorescent microsphere colors can be used in a single experiment to estimate RBF without correcting for spillover of emitted fluorescence. It has been reported that for 13 fluorescent colors, spillover error can be minimized so that all 13 colors provide accurate estimations of RBF [25]. In large animal studies, investigators often inject 6 to 18 million spheres during a single injection [8–13]. We demonstrated that eight serial injections totaling 18 million spheres administered over an extended protocol are feasible without concern for a statistically or substantively significant influence on RBF from excessive accumulation of microspheres in the capillaries after multiple, large injections. Therefore, using the 13-color technique [25], it may be possible to measure RBF in the same chronic preparation up to 13 times, provided that the cumulative injection of microspheres is less than 18 million spheres.
The implementation of animal models for chronic hemodynamic studies is important for investigating multiple areas of physiology. Chronic microsphere protocols in large animal models allow clinically relevant insights into microcirculatory disturbances during long-term pathophysiological, pharmaceutical, and environmental influences on regional arterial blood flow.
Limitations
In this study we did not assess regional blood flow patterns in nervous, muscular-skeletal, and connective tissues. Although our results may be extrapolated to these tissues, future studies must be performed to verify the effects of serial microsphere injections in various vascular beds.
It has been proposed that microsphere aggregation may stimulate the release of vasoactive, pro-inflammatory, angiogenic, and/or pro-coagulatory molecules which may influence regional hemodynamics during long-term studies [26]. During microsphere studies, as a vascular bed becomes plugged, remaining vessels may dilate to compensate or new vessels may form. Although we did not quantify humoral or tissue markers of inflammation, vasoactivity, or angiogenesis, our results suggest that fluorescent microspheres lodged in myocardial, renal, hepatic and splenic tissue for a period of months do not provoke localized hemodynamic changes.
Similarly, we did not determine local microsphere deposition patterns or investigate histological effects of resident microspheres. As part of a future study, we plan to examine with fluorescent microscopy the local distribution of microspheres in vascular beds and investigate histological effects such as vasodilatation and collateral growth.
Animals injected with 11.2 million FluoSphere microspheres showed slight, insignificant increases in flow over the course of the study whereas animals injected with 18 million NuFlow microspheres showed slight, insignificant decreases in flow over the course of the study. This difference may be attributable to the small number of animals that were used for this study or to the different microspheres used during the two protocols. Alternatively, our results may indicate a threshold in the neighborhood of 18 million spheres after which sphere aggregation or release of vasoactive agents or both may influence regional blood flow during chronic, serial injection of microspheres. In an acute study[14], it has been reported that up to 48 million, 15 μm microspheres do not affect regional flow patterns in a canine model [14]. However, future studies must be performed to determine the amount of microsphere accumulation at which regional blood flow does become significantly affected and to determine the point at which our model becomes invalid.
Despite these limitations, the implementation of animal models for chronic hemodynamic studies is important for investigating multiple areas of physiology. Chronic microsphere protocols in large animal models allow clinically relevant insights into microcirculatory disturbances during long-term pathophysiological, pharmaceutical, and environmental influences on regional arterial blood flow.
Conclusions
This dependable percutaneous arterial access system provides a means for examining the effects of environmental and pathophysiological influences during long-term hemodynamic studies in conscious dogs. Vascular access catheters properly introduced into the cardiovascular system that remain patent for extended periods of time provide the opportunity to perform repeated, percutaneous regional blood flow determinations in conscious canines during long-term microsphere protocols. Eight serial injections totaling 18 million microspheres did not interfere with the long-term hemodynamics of the preparation.
Acknowledgments
The authors acknowledge and thank Richard L. Verrier Ph.D., Gregory Wellenius D.Sc., Sandra Verrier, Tracy Katz, Lani Lee, Jeffrey Pettit, and the Harvard veterinary staff for their support and assistance during the instrumentation and maintenance of these animals and during the preparation of this manuscript. Joe Carlos of IMT Laboratories was instrumental in the processing of microsphere tissue samples. This study was supported in part by grants NIEHS R01 ES12972 and ES00002 from the National Institutes of Health, Bethesda MD, and grant RD 83191701 from the United States EPA, Washington D.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudolph AM, Heymann MA. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967 Aug;21(2):163–84. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- 2.Makowski EL, Meschia G, Droegemueller W, Battaglia FC. Measurement of umbilical arterial blood flow to the sheep placenta and fetus in utero. Distribution to cotyledons and the intercotyledonary chorion. Circ Res. 1968 Nov;23(5):623–31. doi: 10.1161/01.res.23.5.623. [DOI] [PubMed] [Google Scholar]
- 3.Hale SL, Alker KJ, Kloner RA. Evaluation of nonradioactive, colored microspheres for measurement of regional myocardial blood flow in dogs. Circulation. 1988 Aug;78(2):428–34. doi: 10.1161/01.cir.78.2.428. [DOI] [PubMed] [Google Scholar]
- 4.Glenny RW, Bernard S, Brinkley M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. doi: 10.1152/jappl.1993.74.5.2585. [DOI] [PubMed] [Google Scholar]
- 5.Prinzen FW, Glenny RW. Developments in non-radioactive microsphere techniques for blood flow measurement. Cardiovasc Res. 1994 Oct;28(10):1467–75. doi: 10.1093/cvr/28.10.1467. [DOI] [PubMed] [Google Scholar]
- 6.Van Oosterhout MF, Prinzen FW, Sakurada S, Glenny RW, Hales JR. Fluorescent microspheres are superior to radioactive microspheres in chronic blood flow measurements. Am J Physiol. 1998 Jul;275(1 Pt 2):H110–5. doi: 10.1152/ajpheart.1998.275.1.H110. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann JN, Steinhagen S, Kast C, Scheuber HP, Jochum M, Gippner-Steppert C, Inthorn D, Schildberg FW, Nolte D. Chronic left heart catheterization for microvascular blood flow determination in the rabbit: a minimally invasive technique using specially designed port devices. J Surg Res. 2002 Feb;102(2):119–25. doi: 10.1006/jsre.2001.6280. [DOI] [PubMed] [Google Scholar]
- 8.De Curzon OP, Ghaleh B, Tissier R, Giudicelli JF, Hittinger L, Berdeaux A. Myocardial stunning in exercise-induced ischemia in dogs: lack of late preconditioning. Am J Physiol Heart Circ Physiol. 2001 Jan;280(1):H302–10. doi: 10.1152/ajpheart.2001.280.1.H302. [DOI] [PubMed] [Google Scholar]
- 9.McCulloch AD, Sung D, Wilson JM, Pavelec RS, Omens JH. Flow-function relations during graded coronary occlusions in the dog: effects of transmural location and segment orientation. Cardiovasc Res. 1998 Mar;37(3):636–45. doi: 10.1016/s0008-6363(97)00290-3. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Friedman M, Lopez JJ, Wang SY, Li J, Prasad PV, Pearlman JD, Edelman ER, Sellke FW, Simons M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am J Physiol. 1996 May;270(5 Pt 2):H1791–802. doi: 10.1152/ajpheart.1996.270.5.H1791. [DOI] [PubMed] [Google Scholar]
- 11.Wouters PF, Van de Velde M, Van Aken H, Flameng W. Ischemic event characteristics determine the extent of myocardial stunning in conscious dogs. Basic Res Cardiol. 1996 Mar–Apr;91(2):140–6. doi: 10.1007/BF00799686. [DOI] [PubMed] [Google Scholar]
- 12.Neumann T, Heusch G. Myocardial, skeletal muscle, and renal blood flow during exercise in conscious dogs with heart failure. Am J Physiol. 1997 Nov;273(5 Pt 2):H2452–7. doi: 10.1152/ajpheart.1997.273.5.H2452. [DOI] [PubMed] [Google Scholar]
- 13.Kowallik P, Baumgart D, Skyschally A, Ehring T, Heusch G. Three-dimensional analysis of regional mechanical function, blood flow and electrophysiological parameters during early myocardial ischemia in dogs. Basic Res Cardiol. 1992 May–Jun;87(3):215–26. doi: 10.1007/BF00804331. [DOI] [PubMed] [Google Scholar]
- 14.Baer RW, Payne BD, Verrier ED, Vlahakes GJ, Molodowitch D, Uhlig PN, Hoffman JI. Increased number of myocardial blood flow measurements with radionuclide-labeled microspheres. Am J Physiol. 1984 Mar;246(3 Pt 2):H418–34. doi: 10.1152/ajpheart.1984.246.3.H418. [DOI] [PubMed] [Google Scholar]
- 15.Garner D, Laks MM. New implanted chronic catheter device for determining blood pressure and cardiac output in conscious dog. Am J Physiol. 1985 Sep;249(3 Pt 2):H681–4. doi: 10.1152/ajpheart.1985.249.3.H681. [DOI] [PubMed] [Google Scholar]
- 16.Muggenburg BA, Mauderly JL. Cardiopulmonary function of awake, sedated, and anesthetized beagle dogs. J Appl Physiol. 1974 Aug;37(2):152–7. doi: 10.1152/jappl.1974.37.2.152. [DOI] [PubMed] [Google Scholar]
- 17.Bartoli CR, Okabe K, Akiyama I, Verrier RL, Godleski JJ. Technique for implantation of chronic indwelling aortic access catheters. J Invest Surg. 2006 Nov–Dec;19(6):397–405. doi: 10.1080/08941930600985751. [DOI] [PubMed] [Google Scholar]
- 18.Bartoli CR, Akiyama I, Godleski JJ, Verrier RL. Catheter Cardiovasc Interv. Long-term pericardial catheterization is associated with minimum foreign body response. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Werdegar D, Johnson DG, Mason JW. A technique for continuous measurement of arterial blood pressure in unanesthatized monkeys. J Appl Physiol. 1964 May;19:519–21. doi: 10.1152/jappl.1964.19.3.519. [DOI] [PubMed] [Google Scholar]
- 20.Herd JA, Barger AC. Simplified technique for chronic catheterization of blood vessels. J Appl Physiol. 1964 Jul;19:791–2. doi: 10.1152/jappl.1964.19.4.791. [DOI] [PubMed] [Google Scholar]
- 21.Bassingthwaighte JB, Malone MA, Moffett TC, King RB, Little SE, Link JM, Krohn KA. Validity of microsphere depositions for regional myocardial flows. Am J Physiol. 1987 Jul;253(1 Pt 2):H184–93. doi: 10.1152/ajpheart.1987.253.1.H184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowallik P, Schulz R, Guth BD, Schade A, Paffhausen W, Gross R, Heusch G. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation. 1991 Mar;83(3):974–82. doi: 10.1161/01.cir.83.3.974. [DOI] [PubMed] [Google Scholar]
- 23.Glenny RW, Bernard SL, Lamm WJ. Hemodynamic effects of 15-microm-diameter microspheres on the rat pulmonary circulation. J Appl Physiol. 2000 Aug;89(2):499–504. doi: 10.1152/jappl.2000.89.2.499. [DOI] [PubMed] [Google Scholar]
- 24.Anetzberger H, Thein E, Loffler G, Messmer K. Fluorescent microsphere method is suitable for chronic bone blood flow measurement: a long-term study after meniscectomy in rabbits. J Appl Physiol. 2004 May;96(5):1928–36. doi: 10.1152/japplphysiol.00904.2003. [DOI] [PubMed] [Google Scholar]
- 25.Schimmel C, Frazer D, Glenny RW. Extending fluorescent microsphere methods for regional organ blood flow to 13 simultaneous colors. Am J Physiol Heart Circ Physiol. 2001 Jun;280(6):H2496–506. doi: 10.1152/ajpheart.2001.280.6.H2496. [DOI] [PubMed] [Google Scholar]
- 26.Pearse DB, Fessler HE, Wagner EM. Polystyrene microspheres decrease bronchial artery resistance in anesthetized sheep. Am J Physiol. 1995 Sep;269(3 Pt 2):H1037–43. doi: 10.1152/ajpheart.1995.269.3.H1037. [DOI] [PubMed] [Google Scholar]