Abstract
Exposure to persistent organic pollutants, such as polychlorinated biphenyls (PCBs) can cause endothelial cell (EC) activation by inducing pro-inflammatory signaling pathways. Our previous studies indicated that linoleic acid (LA, 18:2), a major omega-6 unsaturated fatty acid in the American diet, can potentiate PCB77-mediated inflammatory responses in EC. In addition, omega-3 fatty acids (such as α-linolenic acid, ALA, 18:3) are known for their anti-inflammatory properties. We tested the hypothesis that mechanisms of PCB-induced endothelial cell activation and inflammation can be modified by different ratios of omega-6 to omega-3 fatty acids. EC were pretreated with LA, ALA, or different ratios of these fatty acids, followed by exposure to PCB77. PCB77-induced oxidative stress and activation of the oxidative stress sensitive transcription factor nuclear factor κB (NF-κB) were markedly increased in the presence of LA and diminished by increasing the relative amount of ALA to LA. Similar protective effects by increasing ALA were observed by measuring NF-κB-responsive genes, such as vascular cell adhesion molecule-1 (VCAM-1) and cyclooxygenase-2 (COX-2). COX-2 catalyzes the rate limiting step of the biosynthesis of prostaglandin E2 (PGE2). PCB77 exposure also increased PGE2 levels, which were down-regulated with relative increasing amounts of ALA to LA. The present studies suggest that NF-κB is a critical player in the regulation of PCB-induced inflammatory markers as modulated by omega-6 and omega-3 fatty acids.
Keywords: atherosclerosis, inflammation, polyunsaturated fatty acid, PCB, vascular endothelial cell
1. Introduction
Substantial evidence from epidemiological studies suggests that cardiovascular diseases are linked to environmental pollution. For example, there was a significant increase in mortality from cardiovascular diseases among Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) for at least five years [1], and most excess deaths were due to cardiovascular disease in power workers exposed to phenoxy herbicides and PCBs in waste transformer oil [2]. Furthermore, an increase in hospitalization rates was reported for coronary heart disease in populations residing near areas contaminated with persistent organic pollutants [3]. A recent study still found excessive concentrations of PCBs in the serum of Yusho victims, 35 years after accidental poisoning with PCBs in Nagasaki, Japan [4]. Interestingly, lipid peroxidation was markedly enhanced in these victims as well. These studies suggest that populations near contaminated sites are at increased risk to develop cardiovascular diseases, and in particular in the presence of additional risk factors, such as hypertriglyceridemia and an associated persistent state of oxidative stress. There is evidence linking the aryl hydrocarbon receptor (AhR) with mechanisms associated with cardiovascular diseases [5] and that AhR ligands may be atherogenic by disrupting the functions of endothelial cells in blood vessels.
Dysfunction of endothelial cells is a critical underlying cause of the initiation of cardiovascular diseases such as atherosclerosis [6]. Severe endothelial cell activation and injury can lead to necrotic and apoptotic cytotoxicity, and ultimately to disruption of endothelial integrity. The mechanisms by which environmental chemicals induce endothelial cell activation, oxidative stress and inflammation are not fully understood. Oxidative stress-induced transcription factors, which regulate inflammatory cytokine and adhesion molecule production, play critical roles in the induction of inflammatory responses. One of these transcription factors, nuclear factor κB (NF-κB), plays a significant role in these regulatory processes [7]. Binding sites for NF-κB and related transcription factors were identified in the promoter regions of a variety of inflammatory genes [8, 9] such as interleukin 6 (IL-6), vascular cell adhesion molecule-1 (VCAM-1) or cyclooxygenase-2 (COX-2), all of which are up-regulated during PCB toxicity [4, 10-12].
Of increasing recognition is the paradigm that nutrition can modulate the toxicity of environmental pollutants and thus affect health and disease outcome associated with chemical insult [13]. Evidence suggests that nutrition can influcence the lipid milieu, oxidative stress and antioxidant status within cells, and thus modulate mechanisms of cytotoxicity mediated by environmental pollutants [14]. For example, certain dietary fats may increase the risk to environmental insult induced by PCBs, while fruits and vegetables, rich in antioxidant and anti-inflammatory nutrients or bioactive compounds, may provide protection [13].
Specific fatty acids rich in plant oils, such as linoleic acid (the parent omega-6 fatty acid), can amplify PCB toxicity in vascular endothelial cells [15]. There is also evidence that elevated levels of linoleic acid may enhance the cellular availability of PCBs [16]. Furthermore, coplanar PCBs can suppress delta 5 and 6 desaturase activities, thus disrupting the synthesis of fatty acid precursors for eicosanoid metabolism [17]. Our own data from plasma and livers of LDL receptor-deficient mice support the hypothesis that treatment with PCBs can facilitate clearance of linoleic acid from plasma into vascular tissues [18]. Such a change in lipid milieu could exacerbate fatty acid- and/or PCB-induced oxidative stress and a vascular inflammatory response.
In contrast to omega-6 fatty acids, omega-3 fatty acids can influence cardiovascular disease pathology by beneficially modulating inflammation. Epidemiological and interventional studies have shown a dose-dependent decrease in risk of cardiovascular disease endpoints with increased dietary consumption of moderate amounts of omega-3 fatty acids, either plant or marine derived [19]. Cardio-protective properties of omega-3 fatty acids include down-regulation of proinflammatory and proatherogenic genes, including adhesion molecules and cytokines, during early atherogenesis and possibly also during later stages of plaque development and plaque rupture [20]. For example, an α-linolenic acid-rich oil decreased oxidative stress and CD40 ligand in patients with mild hypercholesterolemia [21], reduced levels of soluble cell adhesion molecules in plasma [22] and recurrence of coronary heart disease [23].
The current study was designed to test the hypothesis that PCB-induced endothelial cell inflammation can be enhanced by omega-6 fatty acids and antagonized by omega-3 fatty acids. We focused on omega-6 and omega-3 fatty acids, which are most commonly consumed in the average U.S. diet [24].
2. Materials and Methods
2.1. Cell culture and experimental media
Endothelial cells were isolated from porcine pulmonary arteries and cultured as previously described [25]. Arteries obtained during routine slaughter were donated from the College of Agriculture, University of Kentucky. The basic culture medium consisted of medium 199 (M-199) (GIBCO Laboratories, NY) containing 10% (v/v) fetal bovine serum (FBS, HyClone Laboratories, UT). The experimental media were composed of M-199 enriched with 5% (v/v) FBS and with different ratios of linoleic acid (LA) to α-linolenic acid (ALA) (□ 99% pure; Nu-Chek Prep, MN). Preparation of experimental media with LA and ALA were performed as described earlier [26]. Different ratios of LA to ALA were 2:1, 1:1, and 1:2 (v/v), and the total concentration of fatty acids in all the cultures did not exceed 20 μM. Following the pretreatment with fatty acids for 18 h, cells were exposed to coplanar PCB77 for indicated times (6 to 24 h). PCB77 was solubilized in DMSO (sterile-filtered, Sigma-Aldrich, MO) and the final concentration in the cell culture media was 3.4 μM. This level was chosen because it has been reported in serum after acute exposure to PCBs [27, 28]. The final concentration of DMSO in the culture media did not exceed 0.03%. All vehicle controls and PCB treated cultures contained the same amount of DMSO. On the other hand, controls for fatty acid experiments did not contain DMSO. In NF-κB inhibition studies, cells were first pre-enriched with 50 μM PDTC (EMD Biosciences, Inc., CA) for 4 h, then treated with PCB77 or LA.
2.2. Measurement of oxidative stress
Cellular oxidation was determined by 2′, 7′-dichlorofluorescein (DCF) fluorescence as described earlier [29]. This method is based on the conversion of 2′, 7′-dichlorofluorescin into fluorescent 2′, 7′-dichlorofluorescein by oxygen reactive species, primarily peroxyl radicals and peroxides. Cells were pretreated with different fatty acids followed by PCB77 treatment. Then cells were incubated with 100 μM 2, 7-dichlorofluorescin diacetate (Molecular Probes, Inc., OR) for 30 min. A multi-well fluorescent plate reader (Molecular Devices, CA) was utilized for the imaging study. Excitation and emission wavelengths were 490 and 520 nm respectively.
2.3. Transcription factor NF-κB activation studies: electrophoretic mobility shift assay (EMSA)
Nuclear extracts containing active proteins were prepared from cells according to the method of Beg et al. [30]. Binding reactions were performed in a 20 μL volume containing 7 μg of nuclear protein extracts. Nuclear extracts were incubated for 25 min with 32P-end-labeled oligonucleotide probes containing enhancer DNA element NF-κB (5′ AGTTGAGGGGACTTTCCCAGGC 3′) (Santa Cruz Biotech, CA). Following binding, the protein-DNA complexed and uncomplexed DNA in the mixture were resolved on native 5% polyacryamide gels using 0.5□ TBE buffer (50 mM Tris-Cl, 45 mM boric acid, 0.5 mM EDTA, pH 8.4) and visualized by autoradiography. Control reactions using supershift assay were performed to demonstrate the specificity of the shifted DNA-protein complexes for NF-κB.
2.4. Analysis of VCAM-1 and COX-2 gene expression by real-time PCR
Total RNA was extracted from endothelial cells using RNA-STAT-60 (TEL-TEST, Friendswood, TX) according to the manufacturer’s protocol. Reverse transcription was performed using the AMV reverse transcription system (Promega, WI). The levels of mRNAs and the PCR-product were then assessed by real-time PCR using 7300 Real Time PCR System (Applied Biosystems, CA). Real-time PCR samples were mixed with SYBR Green Master Mix (Applied Biosystems, CA) and VCAM-1 or COX-2 specific primers. The sequences for porcine VCAM-1 and COX-2 gene were designed by Primer Express Software 3.0 for real-time PCR (Applied Biosystems, CA). VCAM-1 sequences: sense, 5′-TGGAAAGACATGGCTGCCTAT-3′; antisense, 5′-ACACCACCCCAGTCACCATA TC-3′. COX-2 sequences: sense, 5′-TGCTGAAGCCCTATCGATCA-3′; antisense, 5′-TACAGCTCCATGGCATCAATG-3′ (Integrated DNA Technologies, Inc., IA). β-actin was used as a housekeeping gene in both VCAM-1 and COX-2 studies. β-actin sequences: sense, 5′-TCATCACCATCGGCAACG-3′; antisense, 5′-TTCCTGATGTCCACGTCG-3′ (Integrated DNA Technologies, Inc., IA).
2.5. Measurement of VCAM-1and COX-2 protein levels by western blot
Cells were harvested using cell lysis buffer as previously described [31]. Protein samples were resolved by SDS-PAGE using 10% gradient gels and transferred electrophoretically to nitrocellulose membranes using a Bio-Rad immunoblot transfer apparatus (Bio-Rad Laboratories, CA) according to the manufacturer’s instructions. The nonspecific sites on the membrane were blocked for 1 h at room temperature with 5% nonfat dry milk in TBST followed by incubation with VCAM-1 or COX-2 primary antibodies (Santa Cruz Biotech, CA) overnight at 4 °C. Bands were visualized using the appropriate horseradish peroxidase-conjugated secondary antibody followed by ECL immunoblotting detection reagents (Amersham Biosci, UK). Detection and quantitative analysis were performed using a digitizing system (UN-SCAN-IT, Silk Scientific Corporation, UT).
2.6. PGE2 determination
Cells were seeded in 60 mm culture dishes (Becton Dickinson Labware, NJ) and grown to confluence. After pre-treatment with LA, ALA, or different ratios of these fatty acids, cells were exposed to PCB77. Supernatants of cell cultures were collected into microcentrifuge tubes (Isc BioExpress, UT), centrifuged at 4 °C to remove cellular debris and stored at -80 °C. PGE2 levels were assessed using a PGE2-specific enzyme immunoassay (EIA) (Cayman Chemicals, MI) following the manufacturer’s protocol. Absorbance at 405 nm was detected using a microplate spectrophotometer SpectraMaxPro M2 (Molecular Devices Corporation, CA). Results were expressed in ng/mL.
2.7. Statistical analysis
Values are reported as mean ± standard error of the mean (SEM) of at least three independent experiments performed on triplicate sets of endothelial cells derived from the same porcine artery. Data were analyzed using Sigma Stat software (Jandel Corp., Wan Rafael, CA). One way ANOVA followed by post hoc least significant difference(LSD)’s pairwise multiple comparison procedure were used for statistical analysis of the original data. A statistical probability of p< 0.05 was considered significant.
Results
Different ratios of linoleic acid (LA) to α-linoleic acid (ALA) modulate cellular oxidative stress induced by exposure to PCB77
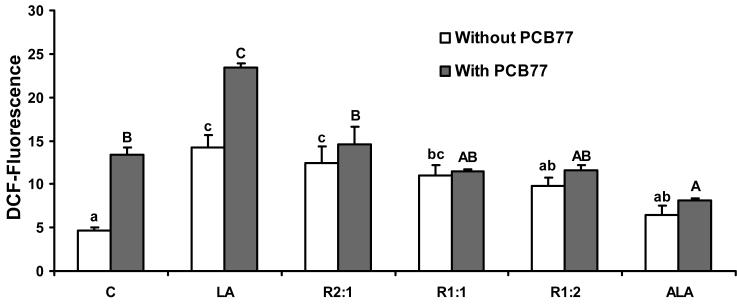
To assess the effects of changing relative amounts of LA to ALA on oxidative stress generation in the absence and presence of PCB77, EC were incubated with different ratios of LA to ALA for 24 h without PCB77 exposure, or pre-incubated with different ratios of LA to ALA for 18 h and then exposed to PCB77 for an additional 6 h. As indicated in Fig. 1, in the absence of PCB77, LA significantly induced oxidative stress as observed by DCF fluorescence compared with control cells. When increasing the relative amount of ALA, a significant decrease in oxidative stress was observed in all groups containing more ALA than LA. In the PCB77 treatment group, oxidative stress was increased significantly compared with the control group, and pretreated cells with LA followed by PCB77 further induced oxidative stress compared with cells that were treated only with PCB77 or LA. Replacing LA with relative increasing amounts of ALA significantly reduced the additive effect of both LA and PCB on oxidative stress. Furthermore, cells pretreated with ALA followed by exposure to PCB77 blocked the oxidative stress induced with PCB77 treatment alone.
Fig. 1.
Effect of different ratios of linoleic acid (LA) to α-linoleic acid (ALA) on PCB77-induced oxidative stress. Cultures were incubated in media supplemented with 20 μM fatty acids, either all LA, all ALA, or different ratios of LA to ALA (R2:1, R1:1, R1:2) for 24 h. Some cultures were first pre-treated with these different ratios of fatty acids for 18 h, followed by coexposure to 3.4 μM PCB77 for additional 6 h. C = vehicle control. Values are means ± SEM (n = 3). Statistical comparisons of the two experimental settings (without and with added PCB77) are indicated by small and capital letters. Different letters represent significant differences among the treatment groups.
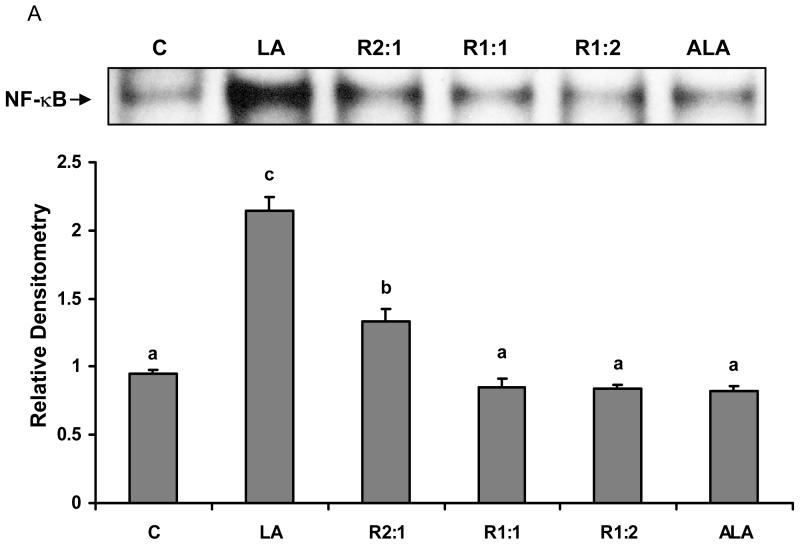
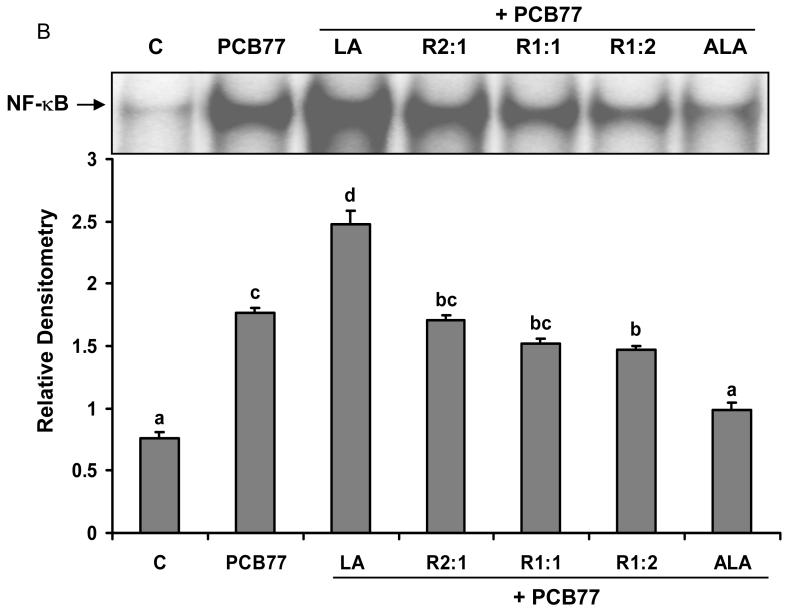
Different ratios of linoleic acid (LA) to α-linoleic acid (ALA) modulate PCB77-induced NF-κB DNA binding activity
Oxidative stress can alter gene expression via the activation of redox sensitive transcription factors such as NF-κB [32]. We further investigated the effects of changing the relative amounts of LA to ALA on PCB77-induced NF-κB activation. First, EC were incubated with different ratios of LA to ALA for 6 h. As shown in Fig. 2A, LA significantly induced NF-κB activation. When introducing ALA into the media, the activity of NF-κB significantly decreased compared with cells treated with only LA. As indicated in Fig. 2B, cells were also pretreated first with different ratios of LA to ALA for 18 h, followed by exposure to PCB77 for an additional 6 h. Compared to the control group, exposure to PCB77 significantly increased NF-κB DNA binding activity and this effect of PCB77 was further increased in the presence of supplemental LA. When introducing ALA into the media, the activity of NF-κB significantly decreased compared with cells treated with only LA plus PCB77. When cultures were pretreated only with ALA, a PCB77-induced increase in NF-κB DNA binding was completely blocked when compared with the control group.
Fig. 2.
Effect of different ratios of linoleic acid (LA) to α-linoleic acid (ALA) on PCB77-induced activation of NF-κB. Cells were treated with 20 μM of LA, ALA, or different ratios of LA to ALA (R2:1, R1:1, R1:2) for 6 h (Fig. 2A), or cells were treated with these fatty acids for 18 h prior to exposure to 3.4 μM PCB77 for an additional 6 h (Fig. 2B). C = vehicle control. Experiments were repeated three times, and the blots shown are a representative of one of the experiments. The bar graph shows the corresponding densitometric analysis of the blots. Values are means ± SEM. Different letters represent significant differences among treatment groups.
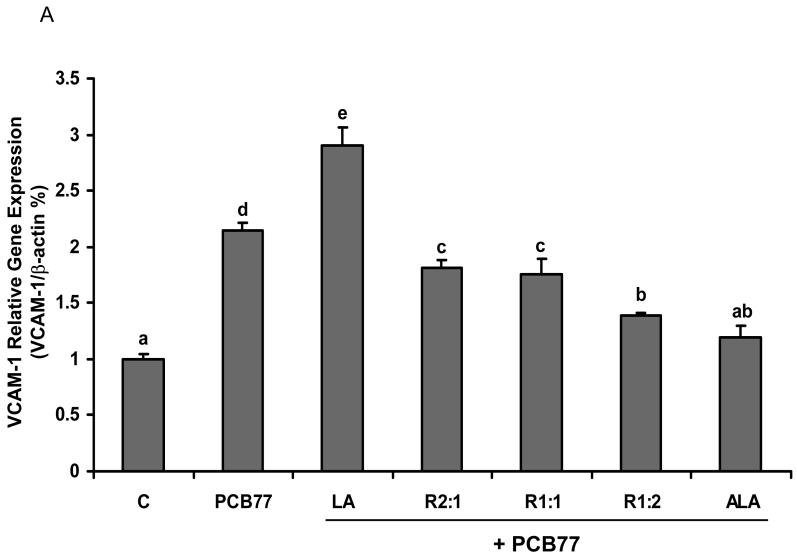
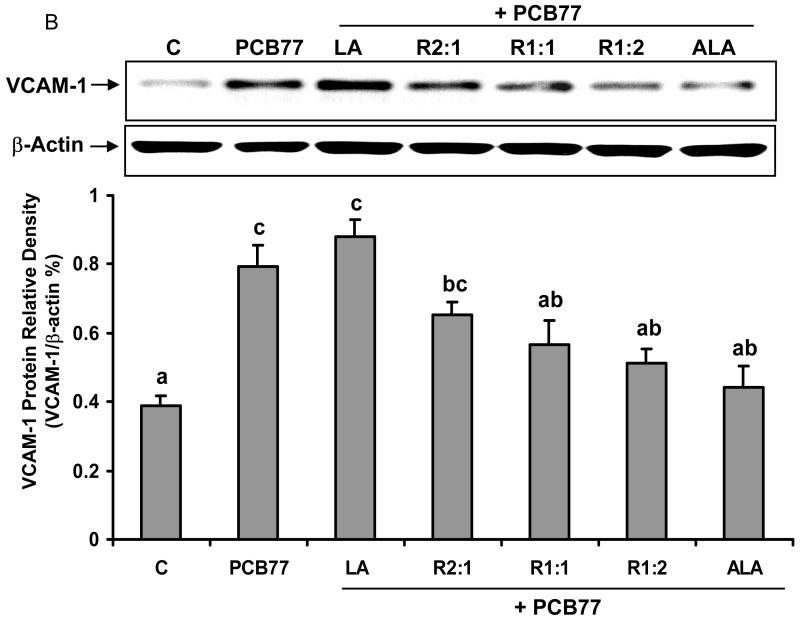
Different ratios of linoleic acid (LA) to α-linoleic acid (ALA) modulate PCB77-induced VCAM-1 expression
NF-κB is critical in the regulation and expression of inflammatory genes, such as adhesion molecule VCAM-1. Expression of VCAM-1 on EC represents one of the early pathological changes in immune and inflammatory diseases, such as atherosclerosis [33]. We investigated whether PCB77-induced VCAM-1 expression was mediated by changing the ratios of LA to ALA. As indicated in Fig. 3A, VCAM-1 gene expression, measured by real-time PCR, was up-regulated after exposure to PCB77. LA pre-treatment alone followed by PCB77 increased VCAM-1 gene expression, which was reduced with a relative increase in ALA. This real-time PCR data was confirmed by measuring VCAM-1 protein levels by western blot (Fig. 3B).
Fig. 3.
Effect of different ratios of linoleic acid (LA) to α-linoleic acid (ALA) on PCB77-induced expression of VCAM-1. Endothelial cells were pretreated with 20 μM of LA, ALA, or different ratios of LA to ALA (R2:1, R1:1, R1:2) for 18 h and then exposed to 3.4 μM PCB77 for additional 6 h for VCAM-1 mRNA measurement (Fig. 3A) or 8 h for VCAM-1 protein level measurement (Fig. 3B). C = vehicle control. β-actin was used as a housekeeping gene in both mRNA and protein measurements. Experiments were repeated three times, and the blots shown are a representative of one of the experiments. Values are means ± SEM. Different letters represent significant differences among treatment groups.
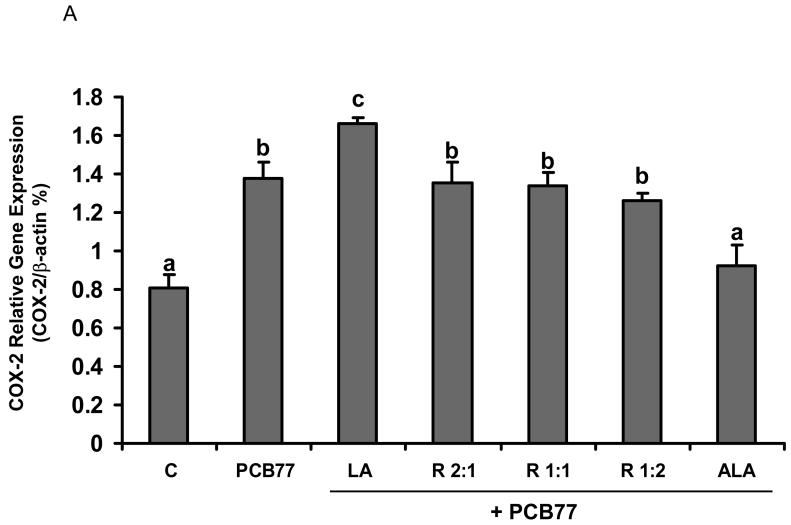
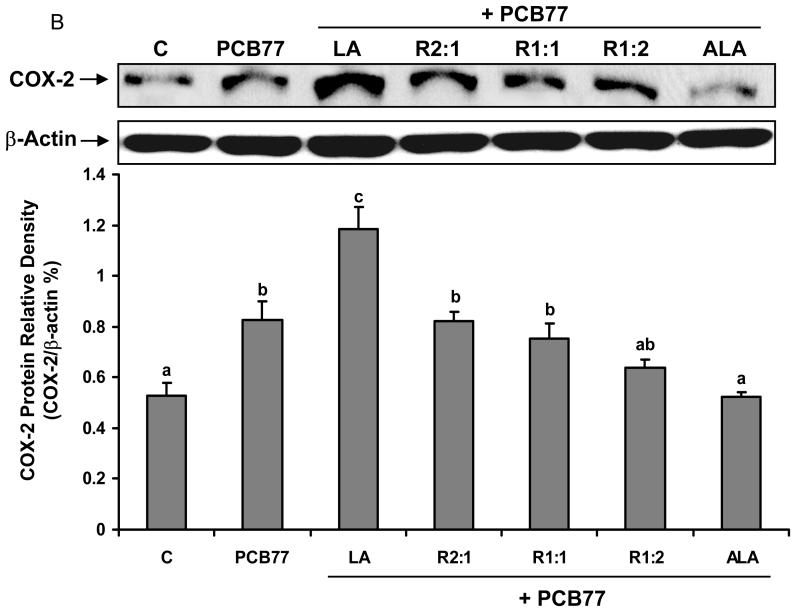
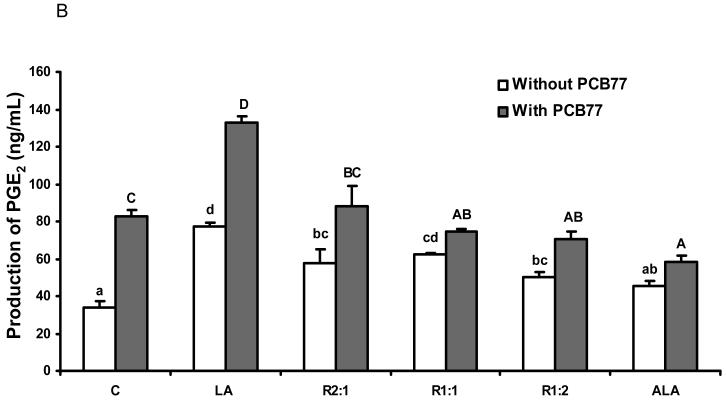
Different ratios of linoleic acid (LA) to α-linoleic acid (ALA) modulate PCB77-induced COX-2 expression and subsequent PGE2 production
Another inducible pro-inflammatory enzyme controlled by NF-κB is COX-2 [34]. To assess whether different ratios of LA to ALA can modulate PCB77-induced COX-2 up-regulation, real time RT-PCR was performed on total cell RNA. Similar to the NF-κB data, PCB77-induced COX-2 mRNA levels were attenuated by increasing relative amounts of ALA (Fig. 4A). These data were confirmed by measuring protein levels of COX-2 by western blot (Fig. 4B).
Fig. 4.
Effect of different ratios of linoleic acid (LA) to α-linoleic acid (ALA) on PCB77-induced expression of COX-2. Cells were pretreated with 20 μM of LA, ALA, or different ratios of LA to ALA (R2:1, R1:1, R1:2) for 18 h and then exposed to 3.4 μM PCB77 for additional 6 h for COX-2 mRNA measurement (Fig. 4A) or 8 h for COX-2 protein level measurement (Fig. 4B). C = vehicle control. β-Actin was used as a housekeeping gene in both mRNA and protein measurements. Experiments were repeated three times, and the blots shown are representative of one of the experiments. Values are means ± SEM. Different letters represent significant difference among treatment groups.
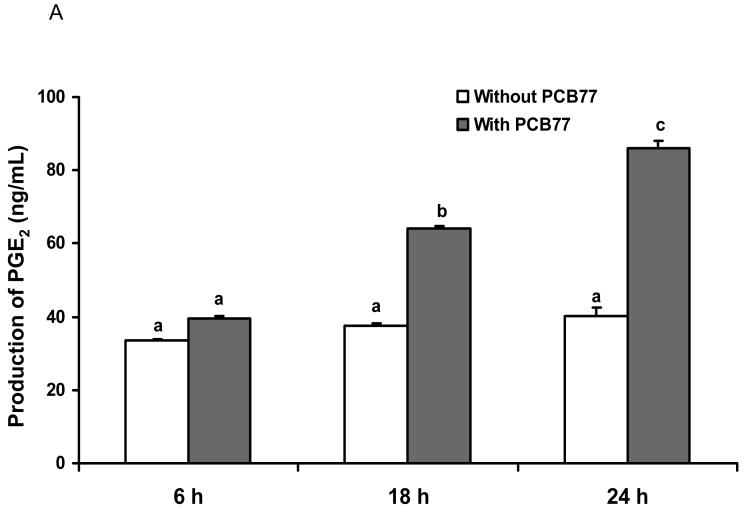
The inducible COX-2 isoform plays a key role in inflammation and is the rate-limiting enzyme for prostaglandin synthesis. In order to assess COX-2 activity, we measured PGE2 production. As shown in Fig. 5A, PCB77 significantly induced PGE2 production in a time-dependent manner. We also pretreated cells with different ratios of LA to ALA for 18 h followed by exposure to PCB77 for an additional 24 h. As indicated in Fig. 5B, compared to the control group, LA significantly increased PGE2 production, which was further enhanced by PCB77 exposure. Substituting relative amounts of LA with ALA markedly decreased the PCB-induced PGE2 production.
Fig. 5.
Effect of different ratios of linoleic acid (LA) to α-linoleic acid (ALA) on PCB77-stimulated release of PGE2 from EC. Cells were exposed to 3.4 μM PCB77 for 6, 18 and 24 h (Fig. 5A). In Fig. 5B, cells were incubated in media supplemented with 20 μM fatty acids, either all LA, all ALA, or different ratios of LA to ALA (R2:1, R1:1, R1:2) for 18 h. Some cultures were first pre-treated with these different ratios of fatty acids for 18 h, followed by co-exposure to 3.4 μM PCB77 for additional 24 h. C = vehicle control. Supernatants of cell cultures were collected and PGE2 levels were measured by enzyme immunoassay. Bars represent means ± SEM from three independent experiments. Statistical comparisons of the two experimental settings (without or with added PCB77) are indicated by small or capital letters.
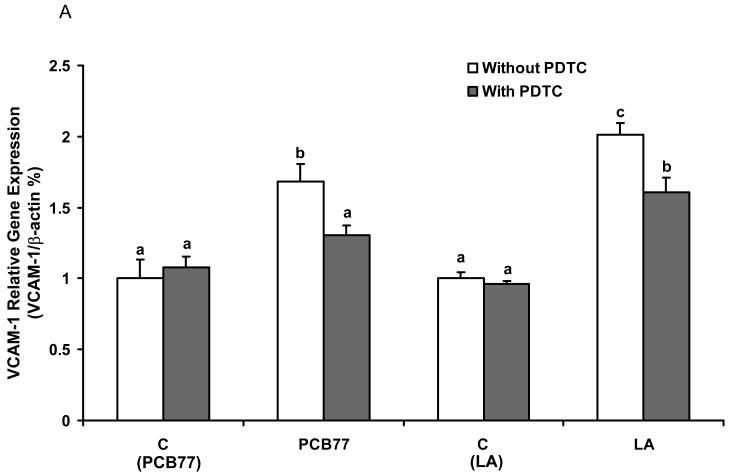
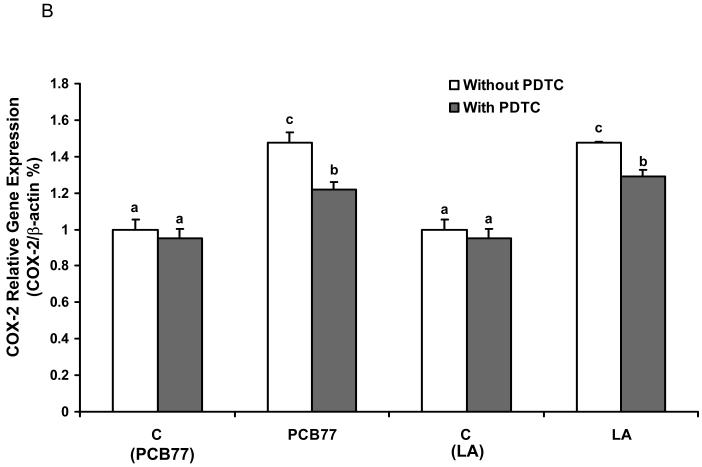
Inhibition of NF-κB decreases PCB77-and linoleic acid (LA)-induced VCAM-1 and COX-2 expression
In order to confirm that NF-κB is a critical transcription factor in PCB77-induced endothelial inflammatory response as modulated by fatty acids, we studied the effect of PDTC (a potent and specific inhibitor of NF-κB) on PCB77- and LA-mediated VCAM-1 and COX-2 expression by real time RT-PCR. As shown in Fig. 6, PDTC significantly decreased PCB77- and LA-induced VCAM-1 (Fig. 6A) and COX-2 (Fig. 6B) expression, respectively.
Fig. 6.
Effect of NF-κB inhibitor on PCB77- or linoleic acid (LA)-induced VCAM-1 and COX-2 expression. Cells were exposed to 3.4 μM PCB77 or 20 μM LA alone for 6 h, or first pre-enriched with 50 μM PDTC for 4 h followed by coexposure to PCB77 or LA for additional 6 h. C (PCB77) = vehicle control for PCB77; C (LA) = control for linoleic acid. PCB77- or LA-induced VCAM-1 (Fig. 6A) and COX-2 (Fig. 6B) mRNA levels, as measured by real-time RT-PCR. β-Actin was used as a housekeeping gene. Experiments were repeated three times. Values are means ± SEM. Different letters represent significant difference among treatment groups.
4. Discussion
Exposure to environmental toxicants such as persistent organic pollutants can significantly compromise heath, and there is evidence that PCBs are proatherogenic. In fact, epidemiological studies with humans demonstrate a link between cardiovascular diseases and exposure to environmental pollutants. For example, an increase in hospitalization rates was reported for coronary heart disease in populations residing near areas contaminated with persistent organic pollutants [3]. Endothelial cells which line the inner layer of blood vessels are an important cell type involved in the regulation of metabolic events associated with the pathology of atherosclerosis. Dysfunction of the vascular endothelium is considered to be a critical underlying cause of the initiation of cardiovascular diseases such as atherosclerosis [6]. Environmental toxicants, once absorbed, distribute themselves to tissues, especially adipose, where they are in dynamic equilibrium with the blood. Thus, risk factors of pollutants such as PCBs are chronic and can continuously amplify pathologies of diseases that are associated with endothelial dysfunction. Data from our present study confirm that endothelial exposure to PCB77 provides a prooxidative cellular environment, sufficient to induce oxidative stress-sensitive transcription factors such as NF-κB and associated inflammatory events characteristic of early events in the pathology of atherosclerosis.
The diet is a major route of exposure to environmental toxic pollutants, such as persistent organic pollutants, including PCBs. Since many of these pollutants are fat soluble, fatty foods are not only unhealthy risk factors by themselves, but they usually contain higher levels of persistent organics than vegetable matter [35]. Thus, high-fat foods may pose as multiple risk factors in contributing to diet-derived proatherogenic lipids as well as environmental toxicants. We have published previously that linoleic acid uptake is enhanced in the presence of PCB77, both in vitro and in vivo [18, 36]. Our recent data also support the hypothesis that membrane domains called caveolae play a critical role in PCB-mediated endothelial activation [37]. Furthermore, we have evidence that PCBs can accumulate in the caveolae fraction of endothelial cells (unpublished data). These data may suggest an interaction of fatty acids and PCBs at the caveolae level, which also might explain possible facilitated cellular uptake of PCBs in the presence of specific fatty acids.
Since omega-6 rich oils are prooxidative and can amplify PCB-mediated dysfunction of endothelial cells [15], and since omega-3 rich oils exhibit cardio-protective properties [19, 20], we were interested in comparing the effect of linoleic acid (the parent omega-6 fatty acid) and α-linolenic acid (the parent omega-3 fatty acid) on modulating PCB-mediated endothelial inflammatory events. Linoleic acid is the major fatty acid in common vegetable oils, and current estimates indicate that over 90% of the omega-3 consumed by U.S. citizens is in the form of α-linolenic acid, not the longer chain omega-3 fatty acids found in fish oils [24]. Nevertheless, many types of fish which are an excellent source of long-chain omega-3 fatty acids are often contaminated with persistent organic pollutants such as PCBs. Whether the cardio-protective omega-3 fatty acids in fish neutralize or markedly decrease the potential health risks associated with exposure to persistent organic pollutants is not known. There appears to be some consensus that regular fish consumption should be encouraged for its overwhelming cardio-protective properties [38, 39].
Using an established cell culture model, our data clearly demonstrate that increasing the relative amount of α-linolenic acid (ALA) over linoleic acid (LA) protects against PCB-mediated endothelial activation. We have previously observed that linoleic acid can amplify PCB-mediated dysfunction of vascular endothelial cells [15]. We also have demonstrated that antioxidants such as vitamin E can protect against PCB-mediated endothelial cell activation [40]. There is increasing evidence that nutrition can dictate the lipid milieu, oxidative stress and antioxidant status within cells, and thus modulate mechanisms of cytotoxicity mediated by environmental pollutants [14]. Many environmental pollutants induce signaling pathways that respond to oxidative stress and lead to inflammatory events; and many of these same pathways are associated with the etiology and early pathology of many chronic diseases [41].
Our data support the hypothesis that the lipid milieu within the vascular endothelium can either upregulate or downregulate inflammatory events induced by PCB77. We have shown previously that 18-carbon fatty acids differing in degree of unsaturation selectively induce an inflammatory environment in human endothelial cells [42]. Of the fatty acids studied, linoleic acid stimulated NF-κB transcriptional activation the most. In addition, treatment with this fatty acid markedly enhanced mRNA levels of TNF-α, monocyte chemoattractant protein 1 (MCP-1) and adhesion molecules such as VCAM-1 [42]. In contrast, treatment with α-linolenic acid had minimal effects on these inflammatory markers. Interestingly, in the current study, a relative increase in α-linolenic acid over linoleic acid markedly reduced inflammatory markers induced by exposure to PCB77.
Mechanisms of the lipid and PCB interactions on endothelial inflammatory events are not clear, but our data suggest that NF-κB is a critical player in the overall regulation of these events. We have previously demonstrated that PCB77-mediated activation of NF-κB can be blocked by pretreatment with pyrrolidine dithiocarbamate (PDTC), suggesting regulatory functions of PCB-induced endothelial cell activation through NF-κB signaling [40]. In the present study, we demonstrated that inhibition of NF-κB markedly reduced both PCB and linoleic acid -mediated endothelial inflammation. NF-κB binds to and affects the function of several genes encoding proteins mediating inflammation, and inhibition of NF-κB by PDTC resulted in reduced neutrophil infiltration in lungs, liver and hearts of rabbits infused with lipopolysaccharide (LPS) [43]. A recent study demonstrated that inhibition of NF-κB in aged vessels significantly attenuated inflammatory gene expression and inhibited monocyte adhesiveness [44].
Protective mechanisms of omega-3 fatty acids on down-regulation of PCB-induced vascular inflammation are not well understood. PCBs have been reported to induce arachidonic acid release in human platelets [45], and in rat amnion fibroblast cells [46] with a subsequent increase in eicosanoid formation. Our data suggest that linoleic acid can enhance and α-linolenic acid inhibit these events. One inhibitory mechanism may be a relative decrease in substrate availability for PGE2 formation during cellular enrichment with α-linolenic acid [47]. Others have reported that eicosapentaenoic acid can inhibit TNF-α induced endothelial cell inflammation via PI3K/Akt signaling pathways [48], pathways which also can regulate NF-κB DNA binding [31]. Omega-3 fatty acids also have been shown to inhibit NF-κB activation via peroxisome proliferators-activated receptor alpha signaling [49].
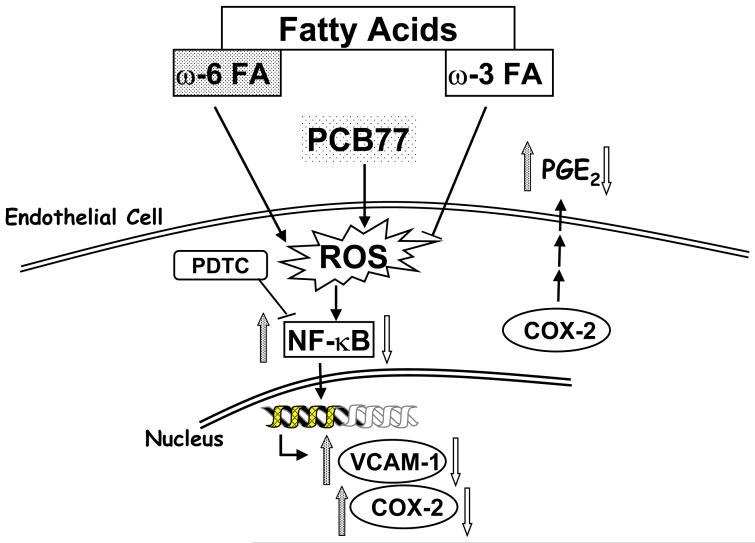
In summary, the current study demonstrates that diet-derived lipids can modulate PCB77 stimulated activation of endothelial cells by affecting oxidative stress sensitive signaling pathways (Figure 7), including activation of NF-κB and up-regulation of inflammatory genes. Our data contribute to the paradigm that nutrition can modulate the toxicity of environmental pollutants and thus affect health and disease outcome associated with chemical insult [13]. Whether an increase in the consumption of omega-3 polyunsaturated fatty acids can be used therapeutically against inflammation that is mediated in part by environmental pollutants warrants further study.
Fig. 7.
Proposed mechanism for fatty acid-mediated modulation of endothelial cell activation induced by PCB77. Omega-6 or omega-3 fatty acids can either enhance or reduce PCB77-induced up-regulation of oxidative stress and activation of NF-κB. Relative activation of NF-κB will further regulate VCAM-1, as well as COX-2 enzyme activity and subsequent PGE2 release from endothelial cells.
Acknowledgments
This study was supported by grants from the NIEHS/NIH (P42 ES 07380), and NIEHS Training Grant (T32 ES 07266), with additional support from the University of Kentucky Agricultural Experiment Station. Conflict of interest: none declared. The authors thank Elizabeth Oesterling and Zuzana Majkova for valuable comments and editing of the manuscript.
Abbrevations
- PCB
polychlorinated biphenyl
- EC
endothelial cell
- LA
linoleic acid
- ALA
α-linolenic acid
- NF-κB
nuclear factor κB
- VCAM-1
vascular cell adhesion molecule-1
- COX-2
cyclooxygenase-2
- PGE2
prostaglandin E2
- AhR
aryl hydrocarbon receptor
- IL-6
interleukin 6
- DCF
dichlorofluorescein
- EMSA
electrophoretic mobility shift assay
- MCP-1
monocyte chemoattractant protein 1
- TNF-α
tumor necrosis factor-alpha
- PDTC
pyrrolidine dithiocarbamate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32(3):234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [2].Hay A, Tarrel J. Mortality of power workers exposed to phenoxy herbicides and polychlorinated biphenyls in waste transformer oil. Ann N Y Acad Sci. 1997;837:138–156. doi: 10.1111/j.1749-6632.1997.tb56871.x. [DOI] [PubMed] [Google Scholar]
- [3].Sergeev AV, Carpenter DO. Hospitalization rates for coronary heart disease in relation to residence near areas contaminated with persistent organic pollutants and other pollutants. Environ Health Perspect. 2005;113(6):756–761. doi: 10.1289/ehp.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shimizu K, Ogawa F, Thiele JJ, Bae S, Sato S. Lipid peroxidation is enhanced in Yusho victims 35 years after accidental poisoning with polychlorinated biphenyls in Nagasaki. Japan, J Appl Toxicol. 2007;27(2):195–197. doi: 10.1002/jat.1205. [DOI] [PubMed] [Google Scholar]
- [5].Savouret JF, Berdeaux A, Casper RF. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutr Metab Cardiovasc Dis. 2003;13(2):104–113. doi: 10.1016/s0939-4753(03)80026-1. [DOI] [PubMed] [Google Scholar]
- [6].Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- [7].de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25(5):904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- [8].Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85(8):753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- [9].Muller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997;11(3):301–312. doi: 10.1006/meth.1996.0424. [DOI] [PubMed] [Google Scholar]
- [10].Kwon O, Lee E, Moon TC, Jung H, Lin CX, Nam KS, Baek SH, Min HK, Chang HW. Expression of cyclooxygenase-2 and pro-inflammatory cytokines induced by 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) in human mast cells requires NF-kappa B activation. Biol Pharm Bull. 2002;25(9):1165–1168. doi: 10.1248/bpb.25.1165. [DOI] [PubMed] [Google Scholar]
- [11].Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicology and applied pharmacology. 2002;181(3):174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- [12].Choi W, Eum SY, Lee YW, Hennig B, Robertson LW, Toborek M. PCB 104-induced proinflammatory reactions in human vascular endothelial cells: relationship to cancer metastasis and atherogenesis. Toxicol Sci. 2003;75(1):47–56. doi: 10.1093/toxsci/kfg149. [DOI] [PubMed] [Google Scholar]
- [13].Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, Silverstone A, Watkins B, Suk WA. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environmental health perspectives. 2007;115(4):493–495. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hennig B, Ormsbee L, Bachas L, Silverstone A, Milner J, Carpenter D, Thompson C, Suk WA. Introductory comments: nutrition, environmental toxins and implications in prevention and intervention of human diseases. J Nutr Biochem. 2007;18(3):161–162. doi: 10.1016/j.jnutbio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [15].Hennig B, Slim R, Toborek M, Robertson LW. Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. J Biochem Mol Toxicol. 1999;13(2):83–91. doi: 10.1002/(sici)1099-0461(1999)13:2<83::aid-jbt4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [16].Doi AM, Lou Z, Holmes E, Li C, Venugopal CS, James MO, Kleinow KM. Effect of micelle fatty acid composition and 3,4,3′, 4′-tetrachlorobiphenyl (TCB) exposure on intestinal [(14)C]-TCB bioavailability and biotransformation in channel catfish in situ preparations. Toxicol Sci. 2000;55(1):85–96. doi: 10.1093/toxsci/55.1.85. [DOI] [PubMed] [Google Scholar]
- [17].Matsusue K, Ishii Y, Ariyoshi N, Oguri K. A highly toxic coplanar polychlorinated biphenyl compound suppresses Delta5 and Delta6 desaturase activities which play key roles in arachidonic acid synthesis in rat liver. Chem Res Toxicol. 1999;12(12):1158–1165. doi: 10.1021/tx990104r. [DOI] [PubMed] [Google Scholar]
- [18].Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environmental health perspectives. 2005;113(1):83–87. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98(4A):3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- [20].De Caterina R, Zampolli A, Del Turco S, Madonna R, Massaro M. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83(2):421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
- [21].Alessandri C, Pignatelli P, Loffredo L, Lenti L, Del Ben M, Carnevale R, Perrone A, Ferro D, Angelico F, Violi F. Alpha-linolenic acid-rich wheat germ oil decreases oxidative stress and CD40 ligand in patients with mild hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26(11):2577–2578. doi: 10.1161/01.ATV.0000242795.08322.fb. [DOI] [PubMed] [Google Scholar]
- [22].Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36(11):1183–1193. doi: 10.1007/s11745-001-0831-4. [DOI] [PubMed] [Google Scholar]
- [23].Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med. 2005;11(3):24–30. quiz 31, 79. [PubMed] [Google Scholar]
- [24].Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- [25].Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4(5):489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- [26].Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- [27].Jensen AA. Polychlorobiphenyls (PCBs), polychlorodibenzo-p-dioxins (PCDDs) and polychlorodibenzofurans (PCDFs) in human milk, blood and adipose tissue. Sci Total Environ. 1987;64(3):259–293. doi: 10.1016/0048-9697(87)90250-6. [DOI] [PubMed] [Google Scholar]
- [28].Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- [29].Mattson MP, Barger SW, Begley JG, Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- [30].Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hennig B, Lei W, Arzuaga X, Ghosh DD, Saraswathi V, Toborek M. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem. 2006;17(11):766–772. doi: 10.1016/j.jnutbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [32].Altavilla D, Deodato B, Campo GM, Arlotta M, Miano M, Squadrito G, Saitta A, Cucinotta D, Ceccarelli S, Ferlito M, Tringali M, Minutoli L, Caputi AP, Squadrito F. IRFI 042, a novel dual vitamin E-like antioxidant, inhibits activation of nuclear factor-kappaB and reduces the inflammatory response in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2000;47(3):515–528. doi: 10.1016/s0008-6363(00)00124-3. [DOI] [PubMed] [Google Scholar]
- [33].Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. The American journal of medicine. 1999;107(1):85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- [34].Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual review of biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- [35].Hennig B, Toborek M, Ramadass P, Ludewig G, Robertson L. Polychlorinated biphenyls, oxidative stress and diet. In: Preedy VR, Watson RR, editors. Reviews in food and nutrition toxicity. CRC Press; Boca Raton, FL: 2005. pp. 93–128. [Google Scholar]
- [36].Slim R, Hammock BD, Toborek M, Robertson LW, Newman JW, Morisseau CH, Watkins BA, Saraswathi V, Hennig B. The role of methyl-linoleic acid epoxide and diol metabolites in the amplified toxicity of linoleic acid and polychlorinated biphenyls to vascular endothelial cells. Toxicology and applied pharmacology. 2001;171(3):184–193. doi: 10.1006/taap.2001.9131. [DOI] [PubMed] [Google Scholar]
- [37].Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- [38].Sidhu KS. Health benefits and potential risks related to consumption of fish or fish oil. Regul Toxicol Pharmacol. 2003;38(3):336–344. doi: 10.1016/j.yrtph.2003.07.002. [DOI] [PubMed] [Google Scholar]
- [39].Ikonomou MG, Higgs DA, Gibbs M, Oakes J, Skura B, McKinley S, Balfry SK, Jones S, Withler R, Dubetz C. Flesh quality of market-size farmed and wild British Columbia salmon. Environ Sci Technol. 2007;41(2):437–443. doi: 10.1021/es060409+. [DOI] [PubMed] [Google Scholar]
- [40].Slim R, Toborek M, Robertson LW, Hennig B. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicol Sci. 1999;52(2):232–239. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- [41].Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol. 2005;5(2):153–160. doi: 10.1385/ct:5:2:153. [DOI] [PubMed] [Google Scholar]
- [42].Toborek M, Lee YW, Garrido R, Kaiser S, Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr. 2002;75(1):119–125. doi: 10.1093/ajcn/75.1.119. [DOI] [PubMed] [Google Scholar]
- [43].Boyle EM, Jr., Kovacich JC, Canty TG, Jr., Morgan EN, Chi E, Verrier ED, Pohlman TH. Inhibition of nuclear factor-kappa B nuclear localization reduces human E-selectin expression and the systemic inflammatory response. Circulation. 1998;98(19 Suppl):II282–288. [PubMed] [Google Scholar]
- [44].Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-{kappa}B activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- [45].Forsell PK, Olsson AO, Andersson E, Nallan L, Gelb MH. Polychlorinated biphenyls induce arachidonic acid release in human platelets in a tamoxifen sensitive manner via activation of group IVA cytosolic phospholipase A2-alpha. Biochem Pharmacol. 2005;71(12):144–155. doi: 10.1016/j.bcp.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brant KA, Caruso RL. PCB 50 stimulates release of arachidonic acid and prostaglandins from late gestation rat amnion fibroblast cells. Reprod Toxicol. 2006;22(4):591–598. doi: 10.1016/j.reprotox.2006.04.012. [DOI] [PubMed] [Google Scholar]
- [47].Horia E, Watkins BA. Comparison of stearidonic acid and alpha-linolenic acid on PGE2 production and COX-2 protein levels in MDA-MB-231 breast cancer cell cultures. J Nutr Biochem. 2005;16(3):184–192. doi: 10.1016/j.jnutbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [48].Wan M, Li Y, Xue H, Li Q, Li J. Eicosapentaenoic acid inhibits TNF-alpha-induced Lnk expression in human umbilical vein endothelial cells: involvement of the PI3K/Akt pathway. J Nutr Biochem. 2007;18(1):17–22. doi: 10.1016/j.jnutbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [49].Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24(9):1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]