Abstract
Introduction
We describe a modified surgical technique for permanent, anterior tracheal-wall stoma for chronic, repeated respiratory studies in trained, conscious dogs. These cannula-free tracheostomies require minimal daily maintenance, permit repeated intubation with endotracheal tubes modified for airflow respiratory measurement, and facilitate up to 6 hour, continuous administration of aerosol agents during long-term, repeated respiratory studies.
Methods
In 20 dogs, during a 30-40 minute procedure, portions of tracheal rings 2-4 were removed to create an oval stoma, approximately 2×1 cm. The dermis was secured to the transected cartilage and tracheal mucosa in such a manner that skin covered the sternohyoid muscles and grew-in flush with the tracheal mucosa at the stomal opening. Stomas were cleaned daily, and fur was clipped weekly around the stomal site. No other maintenance procedures or environmental modifications were needed. Animals breathed through both the stoma and the upper airway and barked normally.
Results
Stomas remained viable in long-term animals (n=4) ongoing for 70.3±32.2 months (Mean±SEM), with an ongoing maximum of 126 months. Post-mortem examinations were performed on relatively short-term animals (n=16) sacrificed at 16.7±7.3 months. Thirteen showed no appreciable tracheal stenosis and three showed <10% stenosis at the level of the stoma. Histopathological examination of the stomal opening and surrounding tissue revealed minimal chronic inflammation and no evidence of necrosis or infection.
Conclusions
During long-term respiratory studies, this practical and dependable tracheal stoma provides a means for examining acute and chronic effects of environmental and pathophysiological influences on the respiratory system of conscious dogs.
Keywords: Tracheotomy, Permanent Tracheostomy, Chronic Respiratory Studies
Introduction
Tracheotomy is a valuable clinical procedure for emergency or elective airway management. It has become standard in our laboratory for environmental exposure studies with limited material for aerosol inhalation and for chronic, repeated respiratory measurement in laboratory animals. The continued use of tracheostomy in clinical medicine and respiratory physiology research is testament to its medical value. However, tracheotomy and tracheal stoma intubation present noteworthy hazards and complications. Some of the more common complications include tracheal stenosis secondary to cuffed endotracheal tube inflation [2-4,20-22], pneumonia [10,14], tracheobronchitis [13,14], tracheitis [11], hemorrhage from innominate artery dissection [9,24], laryngeal trauma including laryngeal nerve damage [14], stoma occlusion [14], stoma closure [12-14], and tracheo-esophageal fistula [23,24].
Surgical procedures in canines have been described for permanent tracheostomy [13-17]. The literature present different surgical approaches with much contention concerning direction and type of incision, location of stoma placement, and merit of cartilage excision [5,6,8,16,17]. However, most studies were designed to look at short-term changes in tracheal structure over a period of weeks or months and do not consider longer-term stoma viability.
We describe a modified surgical technique for long-term, permanent anterior tracheal wall stoma for repeated intubation, collection of respiratory measurements, and continuous administration of aerosol agents. This study contains a description of the surgical techniques, post-operative care, and ongoing maintenance of the tracheal stomas. Recommendations are made to prevent common complications of tracheotomy surgery and for ongoing tracheal stoma maintenance. Using this methodology, stomas were maintained for more than 10 years in individual animals.
Materials and Methods
Animals and Anesthesia
All animals received humane care and were handled in accordance with National Institute of Health and Harvard University animal care committee guidelines. Experimental procedures followed approved animal studies protocols at The Harvard School of Public Health in Boston, MA.
Twenty adult female mongrel dogs, weighing an average of 15.4 kg (range: 12-19.3 kg), were used for this study. These were all research purpose-bred animals obtained from either Butler Farms (USDA # 21-A-003, Clyde, NY) or from Marshall Laboratories. All surgeries were performed aseptically while animals were maintained under a surgical level of anesthesia. In the operating room, the animals were pre-anesthetized using ketamine (10 mg/kg bolus), xylazine (1.5 mg/kg bolus), and atropine (0.04 mg/kg bolus). Animals were placed in the supine position on the operating table. A trans-oral endotracheal tube was inserted to initiate mechanical ventilation with pure oxygen and 1.5% isofluorane for anesthesia.
Surgical Procedure
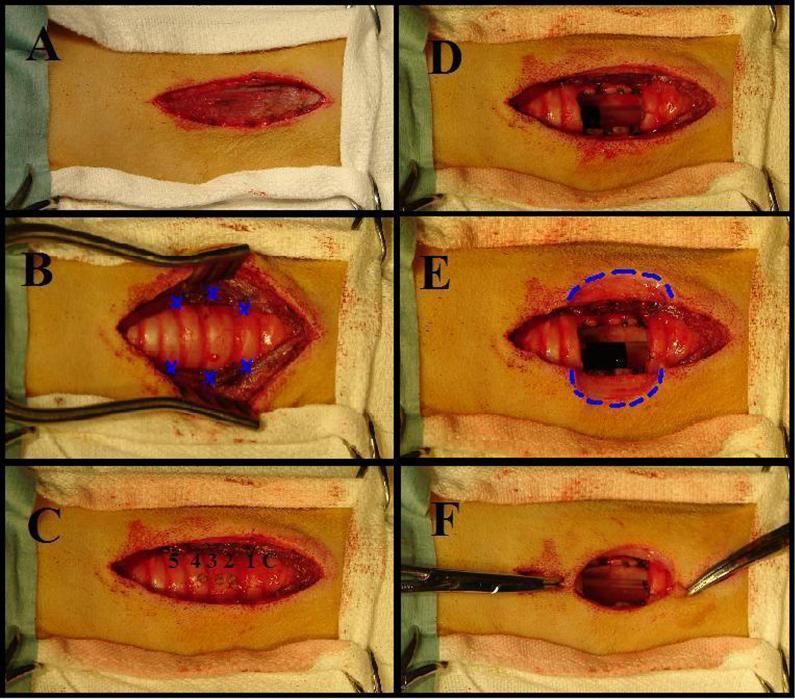
The surgical site, centered on the ventral midline of the neck, was prepared for surgery. A 6 cm anterior neck incision was made parallel to the trachea from just below the thyroid cartilage to the 6th tracheal ring (Figure 1A). The subcutaneous fascia was dissected. The sternohyoid muscles were exposed and divided down the midline. Three 3-0 Prolene stitches were used to fix the divided sternohyoid muscle to the lateral trachea in a hexagonal configuration (Figure 1B). This decreased tension on the skin-to-mucosa anastomosis and thereby decreased the tendency for dehiscence. Note that the central suture is more lateral than the top and bottom sutures.
Figure 1.
A: A 6 cm incision is made parallel to the trachea on the anterior neck. B: The trachea is exposed and the sternohyoid muscles are sutured to the lateral tracheal wall. Blue X’s mark the location of sutures. C: Tracheal rings 2,3,4 are identified and marked with electro-cautery. D: Rings 2,3,4 are removed creating a rectangular stoma, 2 × 1 cm in the anterior wall of the trachea. E: A four-leafed clover shaped incision was formed by removing a 2 cm × 1 cm semicircle of skin from either side of the tracheal opening (Dotted Blue Line). F: The edges of the four-leafed clover shaped incision approximate to the edges of the stomal opening.
Tracheal rings 2, 3, and 4 were identified and marked with cautery (Figure 1C). A # 11 blade was used to open the trachea at the 2nd intra-ring membrane with a vertical incision. Care was taken not to puncture the endotracheal tube used for ventilation and anesthesia. Anterior portions of the 2nd, 3rd, and 4th rings were removed to create a rectangular opening, 2 × 1 cm in the anterior wall of the trachea (Figure 1D). Minimal intra-ring membrane bleeding was cauterized as needed.
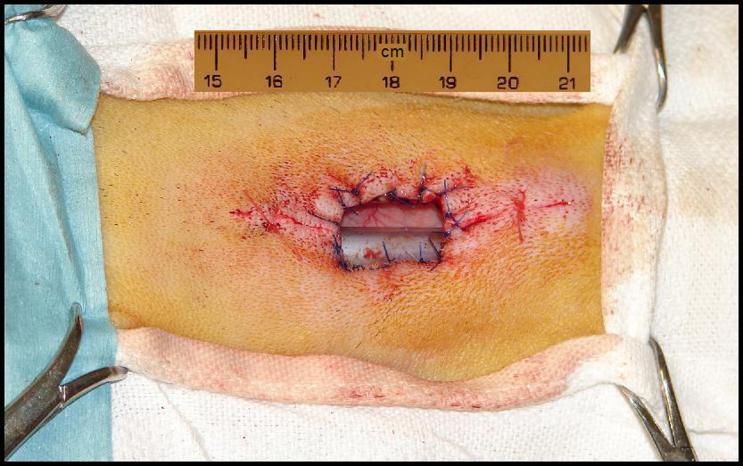
The skin flaps were elevated approximately 3 cm caudal to the cricoid cartilage and a 1-1.5 cm ellipse of skin was removed from either side of the tracheal opening (Dotted Blue Line, Figure 1E). The result is a four-leafed clover shaped incision (Figure 1E) which was closed above and below the stoma and secured to the tracheal mucosa at the edges of the stoma (Figure 1F). Non-absorbable sutures were used to secure the skin in direct apposition to the incised tracheal cartilage so that the sternohyoid muscles and exposed tracheal cartilage were covered with anterior neck skin that grew-in to form an anastomosis with the tracheal mucosa at the stomal opening. The small openings of skin above and below the stoma were loosely closed with a continuous 3-0 Vicryl suture. The final tracheal stoma is shown in Figure 2.
Figure 2.
Final surgical result. Fourteen 3-0 Prolene stitches were used to suture the anterior neck skin (four-leafed clover incision) to the edges of exposed tracheal cartilage.
Post-Operative Care
During the first 48 hours post-operatively, the animals received intra-muscular doses of buprenorphine (0.005 mg/kg bolus) every 8-12 hours. There was no significant post-operative morbidity, and sutures were removed 10-12 days post-operatively. Care was taken to avoid inhalation of suture material into the respiratory tract. The animals were typically used in studies beginning 3 weeks post-operatively.
Post-Operative Stomal Maintenance
Stomas were cleaned of tracheobronchial secretions daily. The skin and stomal area were lightly scrubbed with a 10% Chlorahexadine solution. Once per week, or as needed, fur was clipped around the stomal site. Care was taken to avoid inhalation of clipped hair into the respiratory tract.
Intubation And Data Acquisition
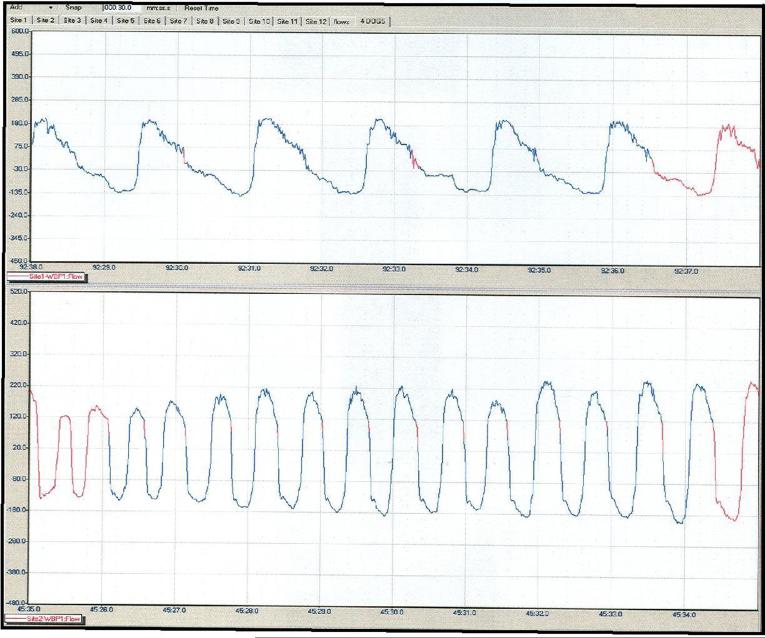
Stomas were lubricated with sterile surgical lubricant and intubated with a # 5-8 inflatable cuff tracheostomy tube (Portex, Keene, NH). During the collection of continuous respiratory data, tracheostomy tubes were fitted with a minimal flow restrictor across which the pressure differential was recorded by pressure transducers [26]. Thus, each breath throughout the five or six hours of experimentation was available for analysis [25-29]. A complete, automated hardware and software system (BUXCO Electronics, Troy, NY) collected and processed the signals generated by the transducers (Figure 3).
Figure 3.
Example of respiratory data collected from intubated tracheal stoma.
Histopathology
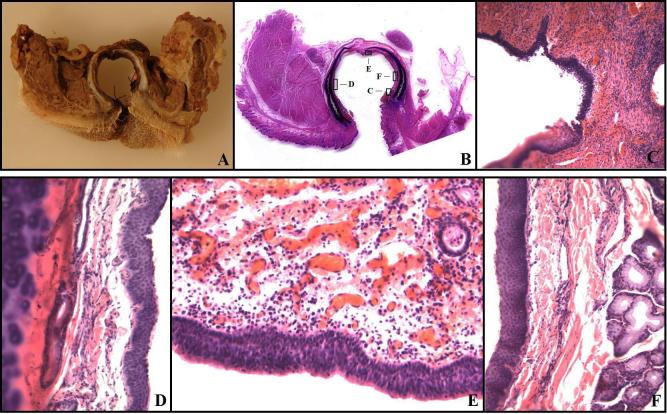
At sacrifice, the trachea and its surrounding structures were removed. Gross samples were photographed and sections at the level of the stoma and at the level of unaltered trachea (ring # 17) were taken. Sections were fixed in 10% buffered Formalin (Fischer Scientific, Pittsburg, PA), processed routinely for paraffin histology, sectioned at 4 μm thickness, stained with hematoxylin and eosin (H&E), and examined by light microscopy by a pathologist to asses the presence and extent of chronic inflammation and changes to tracheal and surrounding structures (Figure 4). In several instances, large whole-mounts were made of the trachea, surrounding muscles, and other tissues in the area. These sections were processed with longer time periods for each step of the histological process to assure penetration of the larger specimen. After paraffin embedding, sections were cut at 10 μm thickness, mounted on plate glass, and stained with H&E.
Figure 4.
Histopathology. A,B: The typical gross appearance of a cross-section at the stoma. A: The cross-section with fixation. B: A whole-mount large histological section showing the trachea, stoma, cutaneous mucosal junction, and the lateral muscle-trachea approximation. The boxes highlight areas illustrated at higher magnifications in the photomicrographs shown in C-F. C: The cutaneous-mucosal junction showing the transition from stratified squamous epithelium to respiratory mucosa. There is minimal chronic inflammation in both the subcutaneous tissue and the lamina propria of the trachea. Magnification 40X. D: The later tracheal wall. The respiratory mucosa has slight squamous metaplasia, but the lamina propria between the mucosa and cartilage has no pathological changes. Magnification 200X. E: The posterior tracheal wall opposite the stoma. Slight chronic inflammation is present in the lamina propria. This was the most severe chronic inflammation seen in any section of the trachea. The respiratory mucosa has no pathological changes. Magnification 200X. F: Lateral tracheal wall with squamous metaplasia, no chronic inflammation, and normal tracheal mucosal glands. There was neither an increase in tracheal mucosal gland thickness in the lamina propria, nor a pathological shift in the normal serous/mucous gland proportions. Magnification 200X.
Results
With this technique, tracheostomies were performed in 20 healthy dogs. Acute complications associated with tracheotomy surgery did not occur during the course of this study. In all animals, stridor was either absent or only demonstrable during exertion.
During surgery, the dermis was secured to the tracheal mucosa in such a manner that anterior neck skin covered the sternohyoid muscles and grew-in flush with the tracheal mucosa at the stomal opening. Granulation tissue sealed the skin to the tracheal cartilage lining the stomal opening. Excised cartilage did not regenerate. Stomas healed and were ready for intubation within three weeks of surgery.
In two animals, scratching caused the muco-cutaneous sutures to pull out. Swelling subsided within 10-14 days and repeat surgery was successfully performed.
In one animal, skin surrounding the stoma grew-in and narrowed the stomal opening. During experimentation with this animal, we uneventfully employed a smaller endotracheal tube for respiratory measurement.
Long Term Stoma Viability
In animals maintained for long-term studies (n=4), stomas remained viable, ongoing for an average of 70.3 ± 32.2 months (Mean± SEM), with an ongoing maximum of 126 months, during which time these animals have undergone repeated respiratory studies [27-29]. Figure 3 shows an example of continuous respiratory data simultaneously collected from two dogs—the top dog shows a resting breathing pattern while the bottom dog is panting. After experimentation, extubation has been accomplished without difficulty and none of the animals have developed clinical evidence of airway obstruction.
Post-Mortem Gross Anatomical Findings
In animals sacrificed during the course of our studies, gross anatomic and histopathologic examination (n=16) of the tracheas and surrounding tissues were carried out. These examinations were done at 16.7 ± 7.3 post-operative months. The neck skin attached contiguously with the luminal tracheal mucosa and completely covered the underlying sternohyoid muscles. The skin surrounding the stoma was removed and no gross deformities to the larynx or sternohyoid muscles surrounding the stoma were noted. Subcutaneous fascia secured the skin directly to the tracheal cartilage immediately surrounding the stoma. This tissue appeared healthy and well perfused. The sternohyoid muscles grew into an oval configuration surrounding the tracheal stoma as designed during surgery.
In 13 of the 16 animals sacrificed, after the sternohyoid muscles had been removed, no appreciable tracheal stenosis was noted. In the other 3 animals, minor stenosis was noted at the level of stomal opening. The defect occurred in the lateral wall of the trachea and accounted for a less than 10% stenosis. In the animal with the greatest stenosis, above and below the stoma, the tracheal diameter was 21 mm. At the level of stenosis, the tracheal diameter was 19 mm. This small indentation in the tracheal passage accounted for a less than 10% reduction in tracheal diameter. It should be noted that this was also the animal in which the skin surrounding the stoma grew-in and narrowed the stomal opening.
Histopathology Findings
In histological sections throughout the trachea and at the stomal site (Figure 4), minimal chronic inflammation was noted in the lamina propria of some sections, but most were without significant pathologic changes. In the tracheal mucosa, areas of squamous metaplasia were noted near the stoma and at the cuff site, but these were mild as illustrated below. No other pathologic changes were found. In Figure 4A,B, the typical gross appearance of the cross-section at the stoma is illustrated. Figure 4A is a gross section photograph of the cross-section including surrounding muscles after fixation. Figure 4B is a whole-mount section of the trachea with adjacent structures. Boxes illustrate the areas of sections shown at higher magnification in 4C-F. In Figure 4C, the junction of the skin and the tracheal mucosa illustrate the transition from squamous to respiratory mucosa. Figure 4D-F illustrate the respiratory mucosa at higher magnification and show minimal chronic inflammation. Figures 4D, F show that the mucosa has squamous metaplasia and no chronic inflammation. Figure 4E shows respiratory mucosa without metaplasia and minimal chronic inflammation at the lamina propria.
Discussion
Stomal Complications
Acute and chronic complications associated with tracheostomy did not occur during this study. Complications of tracheostomy have received extensive review in the medical literature, and tracheal stenosis within 6 weeks of tracheotomy surgery [7] has been especially emphasized [2-4,20,21,23]. Two factors can be considered: the endotracheal tube cuff pressure and the tracheal incision. Cuff pressure is an important etiologic factor in tracheal stenosis secondary to intubation with cuffed inflatable tracheostomy tubes. Studies have shown a progressive inflammatory response at the areas of maximum irritation from the tracheostomy tube and/or inflatable cuff [3,4,23]. Strictures developing at the level of the cuff are the result of circumferential mucosal ulceration of tracheal-mucosal membrane, which result from local ischemic change due to pressure exerted between the outside of the cuff and the tracheal wall [3,4]. The mucosa at these sites becomes hypoxic and ulcerates to expose the cartilaginous rings [3,4]. Destruction and absorption of the exposed underlying cartilage and subsequent healing by concentric fibrotic contracture narrow the tracheal passage [23]. As cited in previous literature [3,23], to avoid complications of stenosis, the use of high compliance-low pressure cuffs is recommended. These were used in our study. Furthermore, in previous studies with serious complications, the endotracheal tubes did remain in the stomas permanently [3,4,13,14]. During our studies, intubations were performed for a maximum of six hours per day, twice per week. Likely, our choice of cuff and intermittent rather than chronic intubation prevented the development of more severe inflammation and subsequent major tracheal stenosis.
In previous studies, the tracheal incision has received much scrutiny and has been a technical point of contention. Several types of tracheostomy incisions in dogs, including transverse and vertical, U-shaped flap through two tracheal rings, and excision of 1 cm segments of 1 and 2 rings have been compared [5,6,8,16,17,22]. Vertical tracheostomy shows the most consistent healing [5,6]. However, vertical incisions have been associated with higher incidence of stenosis [22]. In these studies, a stoma was fashioned around a single vertical slit made through the intra-ring membrane and held open with sutures fixed to the surrounding muscle. It is possible that this approach causes undue pressure-strain to the tracheal wall resulting in more substantial stenosis. As we have demonstrated, a better approach may be a vertical incision extended down across tracheal rings and the removal of a 2 × 1 cm portion of multiple rings. This approach minimizes pressure-strain to the tracheal wall while ensuring a sufficiently large stoma for intubation.
Airway Maintenance
During surgery, the dermis was secured to the tracheal mucosa in such a manner that skin covered the sternohyoid muscles and grew in flush with the tracheal mucosa at the stomal opening. Barking, coughing, and use of the tongue for thermoregulation were not affected. Contrary to previous reports [18,19], animals did not require humidification of the vivarium ambient air. To intubate the animal, no local anesthetic was required—only a lubricant. Animals displayed little reaction to intubation and in most cases resting measurements could be taken within 5 minutes. After training, dogs would lay unrestrained in a cubicle for repeated 5 or 6 hour respiratory measurement during aerosol administration. Results of respiratory and systemic effects of air pollution (which include 4 of the dogs reported in this paper) have been published [27-29].
Viscous, mucoid tracheo-bronchial secretions were noted lining the outside of the stoma, especially after prolonged intubation. These secretions did not cause complications such as pulmonary obstruction. On occasion, wood shaving bedding became stuck to the tracheobronchial secretions surrounding the stomas and posed risk of inhalation and pulmonary obstruction. These observations necessitate daily cleaning of the stomas to prevent complications.
In groups of dogs from previous studies from our laboratory [28,29], rarely, a few dogs, when excited, would occlude the stomal opening with the posterior membranous portion of the trachea causing apoplexy which immediately relieved the obstruction.
Respiratory Measurement
In the past, respiratory measurement in the conscious dog has been made with a face mask, head tent, or tracheostomy intubation [1]. The former two have been sources of dead space and gas leakage. Additionally, they tended to excite the animals and cause hyperventilation. Permanent tracheostomy cannulation was introduced as an alternative, however, this high-maintenance technique became associated with pressure necrosis, tracheal stenosis, and a high mortality rate [13,14]. As we have shown, cannula-free tracheostomy in conjunction with intermittent cuffed endotracheal tube intubation during long-term experimentation is a useful alternative which prevents major complications associated with tracheostomy intubation.
The presence of a tracheostomy has been shown to have no effect on airway mechanics [25]. Our finding of lack of pathological changes in the trachea and only minimal chronic inflammation at the stomal site is further evidence that tracheostomy as used in our studies is unlikely to alter lung physiology.
Conclusions
During long-term respiratory studies, this practical and dependable tracheal stoma provides a means for examining acute and long-term effects of environmental and pathophysiological influences on the respiratory systems of conscious dogs. With minimal post-operative and maintenance-related complications, individual subjects could be used for chronic experimentation for an ongoing period of 10 years.
Acknowledgments
The authors acknowledge and thank Dr. Gregory Wellenius, Sandra Verrier, Tracy Katz, Lani Lee, Jeffrey Pettit, and the Harvard veterinary staff for their support and assistance during the instrumentation and maintenance of these animals. We also thank Dr. Cheryl Killigsworth who made substantial contributions to the use of tracheostomy and development of the surgical technique used in our laboratory.
This study was supported in part by grants NIEHS R01 ES12972 and ES00002 from the National Institute of Health and through grant RD 83191701 from the USEPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Foss ML, Barnard RJ, Tipton CM. Use of tracheostomized dogs in chronic metabolic assessments of treadmill work. Lab Anim Sci. 1972 Jun;22(3):397–401. [PubMed] [Google Scholar]
- 2.Murphy DA, MacLean LD, Dobell AR. Tracheal stenosis as a complication of tracheostomy. Ann Thorac Surg. 1966 Jan;2(1):44–51. doi: 10.1016/s0003-4975(10)66536-8. [DOI] [PubMed] [Google Scholar]
- 3.Ching NP, Ayres SM, Paegle RP, Linden JM, Nealon TF., Jr The contribution of cuff volume and pressure in tracheostomy tube damage. J Thorac Cardiovasc Surg. 1971 Sep;62(3):402–10. [PubMed] [Google Scholar]
- 4.Shelly WM, Dawson RB, May IA. Cuffed tubes as a cause of tracheal stenosis. J Thorac Cardiovasc Surg. 1969 May;57(5):623–7. [PubMed] [Google Scholar]
- 5.Mendez-Picon G, Ehrlich FE, Salzberg AM. The effect of tracheostomy incisions on tracheal growth. J Pediatr Surg. 1976 Oct;11(5):681–5. doi: 10.1016/0022-3468(76)90090-7. [DOI] [PubMed] [Google Scholar]
- 6.Smith MM, Saunders GK, Leib MS, Simmons EJ. Evaluation of horizontal and vertical tracheotomy healing after short-duration tracheostomy in dogs. J Oral Maxillofac Surg. 1995 Mar;53(3):289–94. doi: 10.1016/0278-2391(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 7.Bryant LR, Mujia D, Greenberg S, Huey JM, Schechter FG, Albert HM. Evaluation of tracheal incisions for tracheostomy. Am J Surg. 1978 May;135(5):675–9. doi: 10.1016/0002-9610(78)90134-4. [DOI] [PubMed] [Google Scholar]
- 8.Bardin J, Boyd AD, Hirose H, Engelman RM. Tracheal healing following tracheostomy. Surg Forum. 1974;25(0):210–2. [PubMed] [Google Scholar]
- 9.Cooper JD. Trachea-innominate artery fistula: successful management of 3 consecutive patients. Ann Thorac Surg. 1977 Nov;24(5):439–47. doi: 10.1016/s0003-4975(10)63438-8. [DOI] [PubMed] [Google Scholar]
- 10.MacPhail CM, Monnet E. Outcome of and postoperative complications in dogs undergoing surgical treatment of laryngeal paralysis: 140 cases (1985-1998) J Am Vet Med Assoc. 2001 Jun 15;218(12):1949–56. doi: 10.2460/javma.2001.218.1949. [DOI] [PubMed] [Google Scholar]
- 11.Milo A, Eliachar I, Lane CJ, Myles JL, Munoz-Ramirez H. Clinical and histologic evaluation of an indwelling, inflatable, long-term laryngeal stent in the canine model. Otolaryngol Head Neck Surg. 1999 Sep;121(3):195–202. doi: 10.1016/S0194-5998(99)70171-3. [DOI] [PubMed] [Google Scholar]
- 12.Eliachar I, Levine SC, Tucker HM. A modified technique for tubeless tracheostomy. Otolaryngol Head Neck Surg. 1986 Jun;94(5):548–52. doi: 10.1177/019459988609400503. [DOI] [PubMed] [Google Scholar]
- 13.Ritter JW. Permanent tracheostomy for expired air collection in conscious dogs. Lab Anim Sci. 1984 Feb;34(1):79–81. [PubMed] [Google Scholar]
- 14.Miles WK. Tubeless canine tracheostomy for laryngeal experimentation. Arch Otolaryngol. 1970 Aug;92(2):124–7. doi: 10.1001/archotol.1970.04310020022006. [DOI] [PubMed] [Google Scholar]
- 15.Dalgard DW, Marshall PM, Fitzgerald GH, Rendon F. Surgical technique for a permanent tracheostomy in Beagle dogs. Lab Anim Sci. 1979 Jun;29(3):367–70. [PubMed] [Google Scholar]
- 16.Lulenski GC, Batsakis JG. Management of the flap tracheostomy. An experimental study. Arch Otolaryngol. 1979 May;105(5):260–3. doi: 10.1001/archotol.1979.00790170030008. [DOI] [PubMed] [Google Scholar]
- 17.Eliachar I, Levine SC, Tucker HM. A modified technique for tubeless tracheostomy. Otolaryngol Head Neck Surg. 1986 Jun;94(5):548–52. doi: 10.1177/019459988609400503. [DOI] [PubMed] [Google Scholar]
- 18.Leverment JN, Rae S. Cuffed tube tracheostomy in the dog. Lab Anim. 1978 Oct;12(4):203–6. doi: 10.1258/002367778781088495. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda T, Noguchi H, Takumi Y, Aochi O. Optimum humidification of air administered to a tracheostomy in dogs. Scanning electron microscopy and surfactant studies. Br J Anaesth. 1977 Oct;49(10):965–77. doi: 10.1093/bja/49.10.965. [DOI] [PubMed] [Google Scholar]
- 20.Miller DR, Sethi G. Tracheal stenosis following prolonged cuffed intubation: cause and prevention. Ann Surg. 1970 Feb;171(2):283–93. doi: 10.1097/00000658-197002000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg M, Pearson FG. Pathogenesis of tracheal stenosis following tracheostomy with a cuffed tube. An experimental study in dogs. Thorax. 1972 Nov;27(6):678–91. doi: 10.1136/thx.27.6.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lulenski GC, Batsakis JG. Tracheal incision as a contributing factor to tracheal stenosis. An experimental study. Ann Otol Rhinol Laryngol. 1975 Nov-Dec;84(6):781–6. doi: 10.1177/000348947508400609. [DOI] [PubMed] [Google Scholar]
- 23.Andrews MJ. The incidence and pathogenesis of tracheal injury following tracheostomy with cuffed tube and assisted ventilation. Analysis of a 3-year prospective study. Br J Surg. 1971 Oct;58(10):749–55. doi: 10.1002/bjs.1800581010. [DOI] [PubMed] [Google Scholar]
- 24.Stiles PJ. Tracheal lesions after tracheostomy. Thorax. 1965 Nov;20(6):517–22. doi: 10.1136/thx.20.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drazen JM, O’Cain CF, Ingram RH. Experimental induction of chronic bronchitis in dogs. Effects on airway obstruction and responsiveness. Am Rev Respir Dis. 1982;126:75–79. doi: 10.1164/arrd.1982.126.1.75. [DOI] [PubMed] [Google Scholar]
- 26.Gazula GK, Godleski JJ. Flow restrictor for measuring respiratory parameters. # 6224.560B1 US patent. 2001
- 27.Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. peer discussion. Research Reports of the Health Effects Institute; 2000. Mechanisms of morbidity and mortality from exposure to ambient air particles; pp. 5–88.pp. 89–103. Report number 91. [PubMed] [Google Scholar]
- 28.Clarke RW, Coull B, Reinisch U, Catalano P, Killingsworth CR, Koutrakis P, Kavouras I, Krishna Murthy GG, Lawrence J, Lovett EG, Wolfson JM, Verrier RL, Godleski JJ. Inhaled concentrated ambient particles are associated with hematological and broncho-alveolar lavage changes in canines. Env Health Perspectives. 2000;108:1179–1187. doi: 10.1289/ehp.001081179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wellenius GA, Coull BA, Godleski JJ, Koutrakis P, Okabe K, Savage ST, Lawrence JE, Krishna Murthy GG, Verrier RL. Inhalation of Concentrated Ambient Air Particles Exacerbates Myocardial Ischemia in Conscious Dogs. Env Health Perspectives. 2003;111:402–408. doi: 10.1289/ehp.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]