Summary
Background
Surgical resection alone is regarded as the standard of care for patients with liver metastases from colorectal cancer, but relapse is common. We assessed the combination of perioperative chemotherapy and surgery compared with surgery alone for patients with initially resectable liver metastases from colorectal cancer.
Methods
This parallel-group study reports the trial's final data for progression-free survival for a protocol unspecified interim time-point, while overall survival is still being monitored. 364 patients with histologically proven colorectal cancer and up to four liver metastases were randomly assigned to either six cycles of FOLFOX4 before and six cycles after surgery or to surgery alone (182 in perioperative chemotherapy group vs 182 in surgery group). Patients were centrally randomised by minimisation, adjusting for centre and risk score. The primary objective was to detect a hazard ratio (HR) of 0·71 or less for progression-free survival. Primary analysis was by intention to treat. Analyses were repeated for all eligible (171 vs 171) and resected patients (151 vs 152). This trial is registered with ClinicalTrials.gov, number NCT00006479.
Findings
In the perioperative chemotherapy group, 151 (83%) patients were resected after a median of six (range 1–6) preoperative cycles and 115 (63%) patients received a median six (1–8) postoperative cycles. 152 (84%) patients were resected in the surgery group. The absolute increase in rate of progression-free survival at 3 years was 7·3% (from 28·1% [95·66% CI 21·3–35·5] to 35·4% [28·1–42·7]; HR 0·79 [0·62–1·02]; p=0·058) in randomised patients; 8·1% (from 28·1% [21·2–36·6] to 36·2% [28·7–43·8]; HR 0·77 [0·60–1·00]; p=0·041) in eligible patients; and 9·2% (from 33·2% [25·3–41·2] to 42·4% [34·0–50·5]; HR 0·73 [0·55–0·97]; p=0·025) in patients undergoing resection. 139 patients died (64 in perioperative chemotherapy group vs 75 in surgery group). Reversible postoperative complications occurred more often after chemotherapy than after surgery (40/159 [25%] vs 27/170 [16%]; p=0·04). After surgery we recorded two deaths in the surgery alone group and one in the perioperative chemotherapy group.
Interpretation
Perioperative chemotherapy with FOLFOX4 is compatible with major liver surgery and reduces the risk of events of progression-free survival in eligible and resected patients.
Funding
Swedish Cancer Society, Cancer Research UK, Ligue Nationale Contre le Cancer, US National Cancer Institute, Sanofi-Aventis.
Introduction
Liver metastases are detected in 40–50% of the nearly one million patients who are diagnosed with colorectal cancer worldwide every year. When surgical resection of these metastases is possible, 5-year survival approaches 35%.1 However, relapse is common and occurs in 75% of patients.2
Chemotherapy and surgery combined can reduce the risk of relapse. Preoperative chemotherapy potentially allows surgery on small tumours that have become smaller after preoperative chemotherapy (or in response to chemotherapy).3 This method also allows the responsiveness of these liver metastases to chemotherapy to be assessed, and thus provides guidance about whether chemotherapy should be given after the resection of metastases. Postoperative chemotherapy should theoretically be effective in dormant cancer cells in the remnant liver. It improves the outcome of patients with stage III colon cancers4 and therefore might also be effective in stage IV disease after surgery.
Previous phase III trials comparing combined treatment to surgery alone did not recruit the targeted number of patients and thus did not have sufficient statistical power.5–9 However, these trials showed some benefit of postoperative chemotherapy based on fluorouracil combined with surgery. Some trials used intrahepatic arterial infusion and others intravenous chemotherapy. Consequently, a varied pattern of practice has evolved.
Although surgical resection alone is still regarded as the standard of care, many patients are given combined treatment. Others receive chemotherapy alone and are not referred to liver surgeons, even though their hepatic metastases are resectable. Thus, there remains a need for clear evidence for whether combined treatment with chemotherapy is better than surgery alone in patients with resectable liver metastases from colorectal cancer.
The present European Intergroup trial aimed to compare perioperative chemotherapy—ie, before and after surgery—with surgery alone in patients with one to four hepatic colorectal cancer metastases that are considered to be resectable on imaging. The trial design did not attempt to assess preoperative versus postoperative chemotherapy.
Methods
Patients
We recruited 364 patients from 78 hospitals (in Australia, Austria, Belgium, France, Germany, Hong Kong, Italy, Norway, Sweden, Netherlands, UK) between Oct 10, 2000, and July 5, 2004. To be eligible for enrolment, patients had to be aged between 18 and 80 years with a WHO performance status of 2 or less, histologically proven colorectal cancer, one to four liver metastases that were potentially resectable, and no detectable extrahepatic tumour. The primary tumour had to be either already resected (R0 resection) or judged to be resectable (in case of synchronous metastases) by the multidisciplinary team at the treating hospital. Patients with previous chemotherapy with oxaliplatin were excluded. We excluded those with any history of cancer in the past 10 years (except non-melanoma skin carcinoma or in-situ cervix cancer), major hepatic insufficiency, an absolute neutrophil count less than 1·5×109/L, platelet counts less than 100×109/L, serum creatinine more than twice the upper limit of normal, grade of common toxicity criteria10 more than 1 for peripheral neuropathy, uncontrolled congestive heart failure, angina pectoris, hypertension, arrhythmia, history of significant neurological or psychiatric disorders, or active infection. Pregnant or breastfeeding women were also excluded.
Clinical examination, chest radiography, abdomino-pelvic CT scan with contrast medium (spiral CT was recommended) or MRI, electrocardiogram, and standard laboratory work-up were undertaken within 14 days of study entry. The trial was approved by the medical ethics committees of all participating centres. Written informed consent was obtained from all patients before randomisation.
Procedures
Randomisation was done at the European Organisation for Research and Treatment of Cancer (EORTC) Headquarters in Brussels with the minimisation technique,11 and was stratified for centre, previous adjuvant chemotherapy to primary surgery for colorectal cancer, and a risk score derived from Nordlinger and colleagues.2
We chose the FOLFOX4 regimen for the study, since patients with metastatic colorectal cancer assigned to this treatment have previously shown a response rate above 50% and an increase in progression-free survival.12 Details of this regimen have been previously reported.13 Patients were randomly assigned to either six cycles of FOLFOX4 before and six cycles after surgery (perioperative chemotherapy group), which were given unless the tumour progressed during preoperative chemotherapy, or to surgery alone. Each cycle of chemotherapy lasted 14 days, with the subsequent cycle to start on day 15.12
In both groups, the study treatment had to start within 3 weeks of randomisation. In the perioperative chemotherapy group, liver resection was done 2–5 weeks after the last administration of preoperative chemotherapy, and whenever patients had completely recovered from side-effects of chemotherapy with a WHO performance status of 0 or 1, and adequate liver function.
Surgical exploration consisted of inspection of the peritoneal cavity to exclude extrahepatic involvement, and histological examination of frozen sections of any suspicious lesion. We used intraoperative ultrasonography to detect and localise all hepatic metastases. The type and extent of curative liver resection (wedge resection, or monosegmentectomy or plurisegmentectomy) was decided by the surgeon with the multidisciplinary team at the treating hospital at the time of randomisation, but was modified if previously undetected deposits were discovered, or if the tumour was larger than was expected.
Clinical and neurological examination and assessment of haematology, biochemistry, and toxic effects12 were undertaken before each chemotherapy cycle, and up to 30 days after treatment. An abdomino-pelvic CT scan (or MRI) was done after the first three chemotherapy cycles, and before and after liver surgery. The tumour response in the liver was assessed by contrast CT scan after three and six cycles of preoperative chemotherapy and was scored according to response criteria in solid tumour (RECIST)14 by the local radiologist; no confirmation of response was needed. Chest radiography, abdominal ultrasound or CT scan, and carcinoembryonic antigen concentrations were assessed every 3 months for 2 years after the end of treatment and every 6 months thereafter. Recurrence was diagnosed by imaging, cytology, or histology. When deemed unresectable or after recurrence, patients were treated at the physician's discretion.
The primary trial endpoint was progression-free survival, counted from randomisation to the date of either progressive or recurrent disease, surgery if metastases were deemed not resectable, or death of any cause. To address the lead-time bias that was inherent to the design, the event time to have occurred at 10 weeks was assigned in both treatment groups in the following circumstances: any patient who was operated upon but in whom the tumour was not actually resectable, any patient whose tumour was resected but recurred within week 1 and 20, or those who died between week 1 and 20 of follow-up. Week 10 was chosen as being in the middle of these 20 weeks.
The study was planned to detect a 40% increase in median progression-free survival, or equivalently an increase of the 3-year progression-free survival from 21·0% to 32·8%, in all patients randomly assigned to perioperative chemotherapy (hazard ratio [HR]=0·714) with 80% power at a two-sided 5% significance level, requiring 278 events. The trial was expected to provide this number of events after 6·5 years. However, at 6·5 years after trial start (Sept 20, 2006) the events had not accumulated at the pace anticipated, but the pressure from the medical community to have the trial results disclosed was very strong. Therefore, a stopping boundary for efficacy was implemented (on a gamma family alpha spending function with parameter γ=−4).15 An interim analysis was then undertaken in November, 2006, (at 235 events) and shown only to the EORTC independent data monitoring committee, who recommended to release updated results for the American Society of Clinical Oncology (ASCO) meeting in June, 2007, since the stopping boundary had been reached. The results were thus updated in March, 2007, for the ASCO late-breaking abstracts deadline (254 events, 4-year median follow-up) and presented at the two-sided 0·0434 significance level because of the interim analysis.
Statistical analysis
Rates of progression-free survival were estimated by the Kaplan-Meier method16 and compared by the logrank test.17 Effects are summarised by the HR and 95·66% CI. We used adjusted CIs for all analyses (adjusting for the interim analysis), which were set as 95·66% CIs, since the type I error rate was α=0·0434. Toxic effects and compliance rates were compared by the χ2 test. In all analyses, patients stayed assigned to the group that they had been randomly allocated to. The primary analysis was done in all randomised patients. Sensitivity analyses (not protocol-specified but decided before data analysis) were undertaken both in all eligible patients and all those with resectable liver metastases. Inference about overall survival is deferred until longer follow-up becomes available.
The trial is registered with ClinicalTrials.gov, number NCT00006479.
Role of the funding source
The study design, management, data analysis, and data interpretation were done at the EORTC headquarters (Brussels, Belgium) independently of any commercial interest and from all funding bodies. BN and LC had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
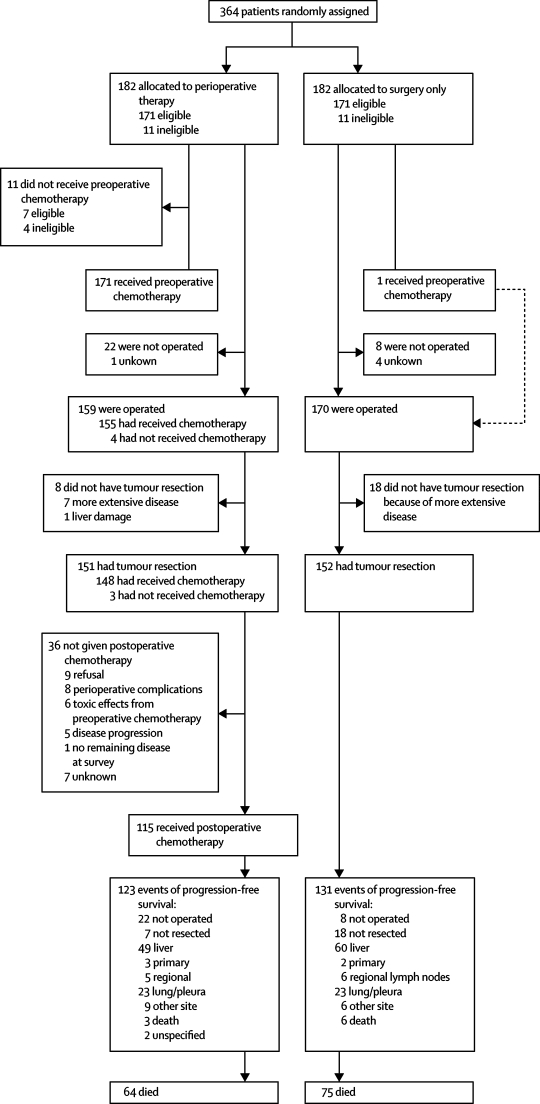
Figure 1 shows the trial profile. 364 patients were enrolled, 182 in each group. No log was kept of the number of patients who were screened for eligibility. Patient and tumour characteristics were much the same between the two groups at baseline (table 1). 11 patients in each group were ineligible. The reasons for ineligibility were more advanced disease than was allowed by the protocol (five in perioperative chemotherapy group and six in surgery group), primary liver cancer (one in both groups), no data (two in perioperative chemotherapy group and three in surgery group), second cancer (one in surgery group with colon cancer), late informed consent (one in perioperative chemotherapy group), high serum creatinine (one in perioperative chemotherapy group), and resection of primary less than 14 days of randomisation (one in perioperative chemotherapy group).
Figure 1.
Trial profile
Table 1.
Baseline characteristics
| PeriOpCT group (N=182) | Surgery group (N=182) | Total (N=364) | ||
|---|---|---|---|---|
| Age (years) | ||||
| Median (IQR) | 62 (29–79) | 64 (25–78) | 63 (25–79) | |
| Mean (SD) | 60·7 (9·35) | 62·4 (9·63) | 61·6 (9·5) | |
| Sex | ||||
| Men | 127 (70%) | 114 (63%) | 241 (66%) | |
| Women | 54 (30%) | 65 (36%) | 119 (33%) | |
| No data documentation (ineligible) | 1 (1%) | 3 (2%) | 4 (1%) | |
| WHO performance status | ||||
| 0 | 136 (75%) | 150 (82%) | 286 (79%) | |
| 1 | 44 (24%) | 31 (17%) | 75 (21%) | |
| 2 | 2 (1%) | 1 (1%) | 3 (1%) | |
| Number of liver metastases | ||||
| 1 | 92* (51%) | 95 (52%) | 188 (52%) | |
| 2 | 49 (27%) | 49 (27%) | 94 (26%) | |
| 3 | 27 (15%) | 27 (15%) | 50 (14%) | |
| 1–3 (exact number unknown) | 2 (1%) | 2 (1%) | 4 (1%) | |
| 4 | 12 (7%) | 14 (8%) | 26 (7%) | |
| >4 (ineligible) | 0 | 2 (1) | 2 (1%) | |
| Synchronicity of liver metastases | ||||
| Metachronous metastases | 121 (66%) | 115 (63%) | 236 (65%) | |
| Synchronous metastases | 61 (34%) | 67 (37%) | 128 (35%) | |
| Time from diagnosis of primary to diagnosis of liver metastases (years) | ||||
| <2 | 133 (73%) | 139 (76%) | 272 (75%) | |
| ≥2 | 49 (27%) | 43 (24%) | 92 (25%) | |
| T category of the primary cancer | ||||
| T1 | 3 (2%) | 2 (1%) | 5 (1%) | |
| T2 | 28 (15%) | 26 (14%) | 54 (15%) | |
| T3 | 124 (68%) | 129 (71%) | 253 (70%) | |
| T4 | 27 (15%) | 21 (12%) | 48 (13%) | |
| TX | 0 | 4 (2%) | 4 (1%) | |
| Lymphatic spread of the primary cancer | ||||
| N0 | 81 (45%) | 72 (40%) | 153 (42%) | |
| N1 | 69 (38%) | 67 (37%) | 136 (37%) | |
| N2 | 31 (17%) | 37 (20%) | 68 (19%) | |
| NX | 1 (1%) | 6 (3%) | 7 (2%) | |
| Location of primary cancer | ||||
| Colon | 95 (52%) | 107 (59%) | 202 (56%) | |
| Rectum | 84 (46%) | 68 (37%) | 152 (42%) | |
| Multiple | 1 (1%) | 3 (2%) | 4 (1%) | |
| Unknown | 2 (1%) | 4 (2%) | 6 (2%) | |
| Previous adjuvant chemotherapy for primary cancer | ||||
| No | 104 (57%) | 106 (58%) | 210 (58%) | |
| Yes (without oxaliplatin) | 78 (43%) | 76 (42%) | 154 (42%) | |
| Plasma CEA at diagnosis, if liver metastases (ng/mL) | ||||
| ≤5·0 | 66 (36%) | 68 (37%) | 134 (37%) | |
| 5·1–30·0 | 55 (30%) | 60 (33%) | 115 (32%) | |
| >30 | 61 (34%) | 54 (30%) | 115 (312%) | |
Data are number (%) unless otherwise indicated. PeriOpCT=perioperative chemotherapy with 5-fluorouracil/leucovorin and oxaliplatin. CEA=carcinoembryonic antigen.
One patient was randomised too early and was found to have seven metastases on a later CT scan, and thus was ineligible.
In the chemotherapy group, 143 (79%) patients completed the planned six cycles of preoperative chemotherapy, and 11 (6%) of 182 did not start treatment (four ineligible patients and seven eligible patients: four who refused, one with colostomy requiring immediate surgery, one with alcohol abuse, and one with previous raltitrexed treatment).
Table 2 shows compliance, treatment tolerance, and treatment response in the perioperative chemotherapy group, and table 3 the toxic effects. We recorded no deaths due to toxic effects. Partial or complete response (according to RECIST) was recorded in more than two-fifths of patients and the total lesion diameter was reduced by about a quarter after chemotherapy (table 2). 12 (7%) patients progressed during chemotherapy: eight after 3–4 cycles (three of whom underwent subsequent resection) and four after six cycles (one resected). Of the eight patients who could not undergo resection, unresectability was due to appearance of new lesions in four. None of these patients started the postoperative protocol chemotherapy. Apart from one patient randomly assigned to surgery alone who received the whole perioperative chemotherapy at his request, none of the patients in the surgery group received chemotherapy before recurrence.
Table 2.
Compliance, treatment tolerance, and treatment response to perioperative chemotherapy
| Preoperative chemotherapy (N=171) | Postoperative chemotherapy (N=115) | ||
|---|---|---|---|
| Administration of chemotherapy | |||
| Number of cycles | |||
| 1 | 6 (4%) | 7 (6%) | |
| 2 | 1 (1%) | 10 (9%) | |
| 3 | 8 (5%) | 5 (4%) | |
| 4 | 7 (4%) | 5 (4%) | |
| 5 | 6 (4%) | 8 (7%) | |
| 6 | 143 (84%) | 80 (70%)* | |
| Median (range) | 6 (1–6) | 6 (1–8) | |
| Relative dose intensity | |||
| 5-fluorouracil (%) | 92·3% (50·0 to 111·3) | 82·0% (36·9 to 112·2) | |
| Oxaliplatin (%) | 92·1% (54·6 to 106·0) | 79·1% (0·0 to 106·5) | |
| Dose reduction(s) | 58 (34%) | 69 (60%) | |
| Delayed cycle(s) | 75 (44%) | 73 (64%) | |
| Response to preoperative chemotherapy (RECIST)† | |||
| Complete response | 4 (3%) | .. | |
| Partial response | 63 (40%) | .. | |
| Stable disease | 60 (38%) | .. | |
| Progressive disease | 11 (7%) | .. | |
| After 3–4 cycles | 7‡ (64%) | .. | |
| After 6 cycles | 4§ (36%) | .. | |
| Not assessable | 21 (13%) | .. | |
| Ineligible | 5 (24%) | .. | |
| Benign lesion | 2 (10%) | .. | |
| <3 cycles | 10 (48%) | .. | |
| No follow-up measurements | 4 (19%) | .. | |
| Sum of the largest diameters of lesions on imaging | |||
| At entry (mm) | 50 (20 to 255) | .. | |
| After preoperative chemotherapy (mm) | 33 (0 to 230) | .. | |
| Relative reduction (%) | −25·6% (−100 to 228·6) | .. | |
Data are number (%) or median (range). RECIST=response criteria in solid tumours.13
Including one patient who received seven cycles and one who received eight cycles.
Assessed in patients with at least one baseline lesion 20 mm or more (N=159).
Three of seven patients underwent resection, one further patient who is not eligible for RECIST response assessment progressed after three cycles and did not undergo resection.
One of four patients underwent resection.
Table 3.
Adverse events during chemotherapy and postoperative complications
| PeriOpCT group | Surgery group | ||
|---|---|---|---|
| Tolerance to preoperative chemotherapy (N=171) | |||
| Allergy grade 3* | 1 (1%) | .. | |
| Diarrhoea grade 3* | 14 (8%) | .. | |
| Nausea grade 3* | 6 (4%) | .. | |
| Vomiting | |||
| Grade 3 | 4 (3%) | .. | |
| Grade 4 | 1 (1%) | .. | |
| Stomatitis/pharyngitis grade 3* | 11 (7%) | .. | |
| Hand-foot skin syndrome grade 3* | 0 | .. | |
| Sensory neuropathy grade 3* | 4 (2%) | .. | |
| Cholinergic syndrome grade 3* | 1 (1%) | .. | |
| Dysaesthesia grade 3* | 4 (2%) | .. | |
| Other neurological toxicity grade 3* | 10 (6%) | .. | |
| Hepatic grade 3* | 5 (3%) | .. | |
| Cardiovascular grade 3* | 4 (2%) | .. | |
| Febrile neutropenia | |||
| Grade 3 | 1 (1%) | .. | |
| Grade 4 | 1 (1%) | .. | |
| Infection grade 3* | 5 (3%) | .. | |
| Catheter-related infection grade 3* | 0 | .. | |
| Leucopenia | |||
| Grade 3 | 9 (5%) | .. | |
| Grade 4 | 1 (1%) | .. | |
| Neutropenia | |||
| Grade 3 | 19 (11%) | .. | |
| Grade 4 | 12 (7%) | .. | |
| Thrombocytopenia grade 3* | 2 (1%) | ||
| Haemoglobin grade 3* | 1 (1%) | .. | |
| Postoperative complications | |||
| Number in group | 159 | 170 | |
| Reversible postoperative complications† | 40 (25%) | 27 (16%) | |
| Cardio-pulmonary failure | 3 (2%) | 2 (1%) | |
| Bleeding | 3 (2%) | 3 (2%) | |
| Biliary fistula | 13 (8%) | 7 (4%) | |
| Output >100 mL/day for >10 days | 9 (6%) | 2 (1%) | |
| Hepatic failure | 11 (7%) | 8 (5%) | |
| Bilirubin >100 mg/day for >3 days | 10 (6%) | 5 (3%) | |
| Wound infection | 5 (3%) | 4 (2%) | |
| Intra-abdominal infection | 11 (7%) | 4 (2%) | |
| Need for reoperation | 5 (3%) | 3 (2%) | |
| Urinary infection | 4 (3%) | 0 | |
| Pleural effusion | 3 (2%) | 1 (1%) | |
| Pulmonary embolism/deep-venous thrombosis | 2 (1%) | 1 (1%) | |
| Pneumopathy | 2 (1%) | 0 | |
| Neutropenia | 2 (1%) | 0 | |
| Ascites | 1 (1%) | 1 (1%) | |
| Ileus | 2 (1%) | 1 (1%) | |
| Cardiac arrhythmia | 0 | 1 (1%) | |
| Renal failure | 0 | 1 (1%) | |
| Other | 4 (3%) | 4 (2%) | |
| Postoperative death | 1 (1%) | 2 (1%) | |
| Tolerance to postoperative chemotherapy (N=115) | |||
| Allergy | |||
| Grade 3 | 4 (4%) | .. | |
| Grade 4 | 1 (1%) | .. | |
| Diarrhoea grade 3* | 6 (5%) | .. | |
| Nausea grade 3* | 5 (4%) | .. | |
| Vomiting grade 3* | 3 (3%) | .. | |
| Stomatitis/pharyngitis grade 3* | 0 | .. | |
| Hand-foot skin syndrom grade 3* | 1 (1%) | .. | |
| Sensory neuropathy grade 3* | 11 (10%) | .. | |
| Cholinergic syndrome grade 3* | 1 (1%) | .. | |
| Dysaesthesia grade 3* | 5 (4%) | .. | |
| Other neurological toxicity grade 3* | 14 (12%) | .. | |
| Hepatic grade 3* | 6 (5%) | .. | |
| Cardiovascular grade 3* | 1 (1%) | .. | |
| Febrile neutropenia grade 3* | 4 (4%) | .. | |
| Infection grade 3* | 2 (2%) | .. | |
| Catheter-related infection grade 3* | 5 (4%) | .. | |
| Leucopenia grade 3* | 14 (12%) | .. | |
| Neutropenia | |||
| Grade 3 | 32 (28%) | .. | |
| Grade 4 | 8 (7%) | .. | |
| Thrombocytopenia grade 3* | 8 (7%) | .. | |
| Haemoglobin grade 3* | 1 (1%) | ||
Data are n (%) unless otherwise stated. Patients may have several complications, therefore number of complications does not add up to the total number of patients. Common toxicity criteria10 version 2.0 was used. PeriOpCT=perioperative chemotherapy with fluorouracil or leucovorin and oxaliplatin
No grade 4 reported.
p=0·04.
Surgery according to the protocol was undertaken at a median of 16·6 (range 0·1–30) weeks in the perioperative chemotherapy group and 2 (0·1–16) weeks in the surgery alone group. Surgery was done in a median of 4·1 (2·0–16·4) weeks after the last administration of preoperative chemotherapy. More patients received the operation in the surgery group than in the perioperative chemotherapy group (table 4). In both groups, a similar number of patients received potentially curative resection (table 4). The most frequent reason for non-resectability was disease that was more advanced than was expected (table 4). In one patient, resection was not done because of macroscopic liver damage, which was most probably related to chemotherapy. Reversible postoperative complications occurred more often after chemotherapy than after surgery alone (p=0·04; table 3). After surgery we recorded two deaths in the surgery alone group and one in the perioperative chemotherapy group. Further results regarding the translational research and pathology will be presented elsewhere.
Table 4.
Patients who received surgery and resection
| PeriOpCT group (N=182) | Surgery group (N=182) | |||
|---|---|---|---|---|
| Operated | 159 (87%) | 170 (93%) | ||
| Resected | 151 (83%) | 152 (84%) | ||
| Monosegmentectomy or wedge resection | 31 (20%) | 33 (22%) | ||
| Plurisegmentectomy* | 86 (57%) | 81 (53%) | ||
| Multiple resections | 33 (22%) | 36 (24%) | ||
| Unknown | 1 (1%) | 2 (1%) | ||
| Not resected | 8 (4%) | 18 (10%) | ||
| More extensive disease | 7 (88%) | 18 (100%) | ||
| Liver damage | 1 (13%) | 0 | ||
| Not operated | 22 (12%) | 8 (4%) | ||
| More advanced disease | 10 (45%) | 7 (88%) | ||
| Refusal | 4 (18%) | 0 | ||
| Poor condition/death | 3 (14%) | 0 | ||
| Other reason | 5 (23%) | 1 (13%) | ||
| Unknown | 1 (1%) | 4 (2%) | ||
| Time to surgery (weeks) | 16·6 (0·1–30) | 2 (0·1–16) | ||
Data are number (%) or median (range).
Major resection, two or more segments. PeriOpCT=perioperative chemotherapy with fluorouracil or leucovorin and oxaliplatin.
115 (63%) patients started postoperative protocol chemotherapy, of whom 80 (70%) received six cycles. Table 3 shows the tolerance to postoperative chemotherapy. Figure 1 shows the reasons why postoperative protocol chemotherapy was not started in the remaining patients.
As of March 2007, the median follow-up was 3·9 years. We recorded 254 events of progression-free survival (the primary endpoint) in all randomised patients (figure 1), including 240 events in eligible patients. 22 patients assigned to chemotherapy and 19 to surgery were alive without disease and had been followed up for less than 3 years. A total of 139 patients have died (figure 1).
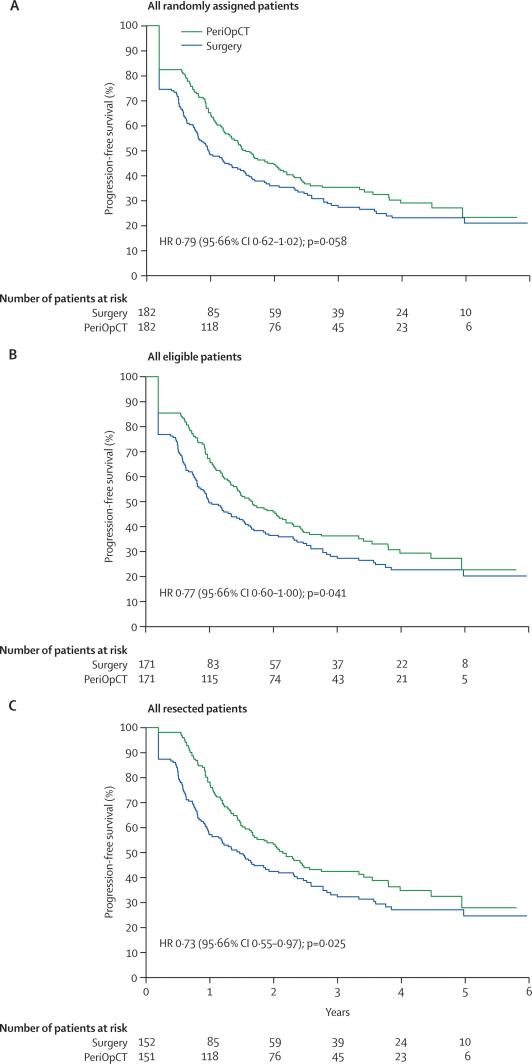
The HR for progression-free survival was 0·79 (95·66% CI 0·62–1·02; p=0·058) in all randomly assigned patients corresponding to a 7·3% increase in the rate of progression-free survival at 3 years from 28·1% (21·3–35·3) to 35·4% (28·1–42·7) with chemotherapy and to an increase of the median progression-free survival from 11·7 months to 18·7 months (figure 2). An analysis of only patients who were eligible to enter the trial showed an HR of 0·77 (0·60–1·00, p=0·041), corresponding to an 8·1% increase in the rate of progression-free survival at 3 years from 28·1% (21·2–36·6) to 36·2% (28·7–43·8) with chemotherapy (figure 2). In the 303 patients in whom resection was actually achieved after study entry, the analysis showed that the HR was 0·73 (0·55–0·97, p=0·025) and the rate of progression-free survival at 3 years was increased by 9·2% from 33·2% (25·3–41·2) to 42·4% (34·0–50·5) (figure 2).
Figure 2.
Progression-free survival by treatment group
(A) All randomly assigned patients. (B) All eligible patients. (C) All resected patients. For all patients randomly assigned and those who were eligible, no surgery or no resection were regarded as events for the primary endpoint of progression-free survival. PeriOpCT=perioperative chemotherapy with fluorouracil or leucovorin, and oxaliplatin.
When the usual definition of progression-free survival was used to compare treatments in all 364 randomised patients (ie, those not operated or not resected were not penalised as events until further disease progression or death), the HR was 0·76 (0·59–0·98, p=0·023) corresponding to a 7·3% increase in the rate of progression-free survival at 3 years from 28·6% (21·7–35·8) to 37·9% (30·5–45·3) with chemotherapy. Adjustment of the primary analysis for the stratification factors (risk grouping and previous adjuvant chemotherapy) did not change the results (data not shown).
Discussion
We have shown that perioperative chemotherapy with FOLFOX4 reduced the risk of progression-free survival events at 3 years by a quarter in patients with resectable liver metastases. In all patients randomly assigned to study treatments, the study showed a trend favouring administration of chemotherapy, which was not significant when a correction was used for the different timings of surgery in the two treatment groups. In all eligible and all resected patients the benefit was statistically significant. Our results have shown that perioperative chemotherapy was compatible with major liver surgery. Operative mortality was less than 1% in both treatment groups, which is very low after this type of liver surgery in a multicentre study. Reversible complications of surgery were more frequent in patients who had received preoperative chemotherapy than in those who had received surgery alone, but remained within the range commonly noted after resection of liver metastases.1,3,18
In patients with advanced colorectal cancer, several studies have compared various chemotherapy regimens. However, very few prospective studies have investigated the combination of chemotherapy with surgery, and none has assessed perioperative chemotherapy. Most trials did not achieve the planned recruitment for multifactorial reasons (table 5).5–9 Although no level I evidence for a benefit of combining chemotherapy and surgery was reported, individual opinions in favour of one or the other treatment option prevented some oncologists and surgeons from including patients in trials. This trial met its target accrual, thanks to an intercontinental collaboration involving Europe, Australia, and Hong Kong. Any future trial of this type is unlikely to have a surgery-only group.19
Table 5.
Previous studies of adjuvant chemotherapy in patients with resected liver metastases from colorectal cancer
| Number of resected metastases | Median follow-up (months) | Number of patients | Randomised postoperative treatments | Median TTP/PFS (months) | Median overall survival (months) | |
|---|---|---|---|---|---|---|
| Lorenz M (1998)5 | ≤6 liver metastases | ≥18 | 108 vs 111 | HAI FU/FA vs surgery alone | 14·2 vs 13·7 (NS) | 34·5 vs 40·8 (p=0·15) |
| Kemeny N (1999)6 | Any number of liver metastases | 62·7 | 74 vs 82 | Systemic FU/FA+HAI FUDR vs systemic FU/FA | 37·4 vs 17·2 (p=0·06) | 72·2 vs 59·3 (p=0·21) |
| Kemeny M (2002)7 | 1–3 liver metastases | 51 | 30 vs 45 | HAI FUDR+systemic FU vs surgery alone | 45·7%*vs 25·2%* (p=0·04) | 63·7 vs 49·0 (p=0·60) |
| Mitry E (2006)8 | ≤4 liver or lung metastases | NR | 138 vs 140 | Systemic FU/FA vs surgery alone | 26·4 vs 18·6 (p=0·059) | 61·1 vs 46·9 (p=0·125) |
| Portier G (2006)9 | Any number of liver metastases | 87 | 86 vs 87 | Systemic FU/FA vs surgery alone | 24·4 vs 17·6 (p=0·028) | 62·1 vs 46·4 (p=0·13) |
TTP=time to progression. PFS=progression-free survival. HAI=hepatic arterial infusion. FUDR=floxuridine. FU=fluorouracil. FA=folinic acid. NS=not significant. NR=not reported.
4-year progression-free survival rates.
The primary objective of this trial was to assess perioperative chemotherapy in patients qualifying for resection of their metastatic disease. Had we assessed postoperative chemotherapy only, randomisation could have been done after successful resection of the metastases, and no patient would have been excluded because of ineligibility or unresectability. Because of the specific objective in our trial, patients had to be randomly assigned imperatively before surgery—ie, without any certainty that metastases assessed by imaging were actually resectable. This uncertainty represents a fundamental difficulty for all studies assessing preoperative treatment and makes such studies difficult to undertake and analyse. For example, in the MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial13 that assessed perioperative chemotherapy in gastric cancer, many randomised patients did not undergo complete resection of the cancer. In our trial, some metastases that were initially considered to be resectable at randomisation were actually more advanced and not resectable at surgical examination. Therefore we examined not only all randomised and eligible patients, but also those who received resection. All analyses were done according to the allocated treatment group (ie, including the patients who did not receive the allocated treatment). The number of patients who finally underwent resection was much the same in both treatment groups.
The principal reason for non-resectability was more advanced disease than was expected, which was probably mostly due to a discrepancy between imaging and surgical examination. However, we noted a trend towards fewer failures to resect in the perioperative chemotherapy group than in the surgery group because of extensive disease, and a higher rate of failures to resect because of refusal or poor condition of the patient, which could introduce a selection bias. Results of progression-free survival in resected patients might be of interest in view of all other trials in this specialty, which assess postoperative chemotherapy only in patients with resected liver metastases since randomisation is done after surgery.5–9 Progress in imaging techniques (with spiral CT scan, MRI, PET scan, and contrast ultrasound) has further reduced the gap between imaging and surgery exploration when the resectability of liver metastases is assessed.20
In accordance with the statistical design in the protocol, unresected patients in both groups were counted as events for the primary endpoint of progression-free survival, which inevitably results in a dilution of the observed treatment difference in all analyses including the unresected patients. The protocol considered all events of non-resection to have occurred at week 10 to not bias results because of the different timing of surgery in the two groups.
In this study, metastases progressed during preoperative chemotherapy in 12 patients (7%), four of whom were subsequently non-resectable because of the appearance of new lesions and four because of progression of already known metastases. In the four patients with new lesions, immediate surgery would not have been beneficial since new metastases would have appeared anyway. In the four patients with progression of the known metastases, resection would also probably have been followed by progression.21 Progression during preoperative chemotherapy should rather be regarded as a biological marker for poor prognosis and an indication for administration of second-line chemotherapy before surgery is considered.
Chemotherapy does induce liver damage which varies according to the drugs given. Vascular lesions, but no steatohepatitis, have been noted after administration of oxaliplatin.22–24 Whether these liver lesions might affect the safety of subsequent surgery and thus counterbalance the potential benefits of treatment is unknown. Karoui and colleagues25 showed that the risk of surgical complications after preoperative chemotherapy is related to the number of chemotherapy cycles, and that this risk remains low if not more than six cycles are given preoperatively. We administered only six cycles preoperatively. The mortality rate was very low. The complication rate was higher in the chemotherapy group than in the surgery group but was similar to other series of patients undergoing hepatectomy.1,3,18 These complications were reversible. We believe that this moderate increase in the risks of liver surgery after chemotherapy does not compromise the potential benefits of the treatment.
In this study, chemotherapy was given before and after surgery. Previous trials5–9 showed a trend towards a benefit of postoperative-only chemotherapy; however, our trial was not designed to compare preoperative and postoperative chemotherapy. The improvement in progression-free survival with chemotherapy was recorded during the first 2 years but afterwards the curves seemed to remain parallel. Similarly, the MAGIC trial showed that perioperative chemotherapy increases overall survival, whereas most trials of postoperative chemotherapy alone did not show a benefit.13,26
This trial was restricted to patients with four or fewer metastases to reduce the proportion of patients that would be entered and later found to have more metastases than were detected on imaging, some of which would be unresectable. This restriction was not intended to serve as a definition of unresectability, but to serve as a selection criterion for the trial. These patients with a few metastases are those with best prognosis after surgical resection. We believe that the conclusions from this trial would probably also be valid for patients at higher risk. Future trials could thus investigate the potential benefit of intensified perioperative chemotherapy in resectable liver metastases from colorectal cancer. The combination of targeted agents with cytotoxic therapy has shown high response rates27–29 and thus warrants assessment in the perioperative setting. At present the EORTC 40051 BOS (Biologics, Oxaliplatin and Surgery) trial30 is assessing perioperative chemotherapy with FOLFOX6 and cetuximab with or without bevacizumab in patients with resectable hepatic metastases from colorectal cancer.
We conclude that perioperative FOLFOX4 chemotherapy reduced the risk of events of progression-free survival by a quarter and was compatible with major surgery. In all randomised patients the study showed a trend favouring administration of chemotherapy. In all eligible and in all resected patients, the benefit of administering chemotherapy was significant.
Acknowledgments
We thank all patients who consented to enter the study; from the EORTC Data Center: M A Lentz who was the study data manager, M Debois who contributed to the statistical analysis, and P Therasse (Director) for help with study management; I Tabah-Fisch and T Pearce who were responsible for this trial at Sanofi-Aventis; J Zalcberg, current chair of AGITG, and J Simes from NHMRC Clinical Trials Center who were in charge of the trial management for the Australian Group. This study was undertaken by the EORTC GITCG (European Organisation for Research and Treatment of Cancer Gastro-Intestinal Tract Cancer Group) in collaboration with AGITG (Australasian Gastro-Intestinal Trials Group), ALM-CAO (Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie), Cancer Research UK (formerly Cancer Research Campaign), and FFCD (Fédération Francophone de Cancérologie Digestive, formerly Fédération Française de Cancérologie Digestive). The sponsor of this trial was EORTC. This clinical trial was supported by grants from the Swedish Cancer Society (Sweden), Cancer Research UK (United Kingdom), the Ligue Nationale Contre le Cancer (France) and the National Cancer Institute (Bethesda, MD, USA; grants 5U10-CA11488-28 through 5U10 CA11488-37). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. Sanofi-Aventis provided free oxaliplatin and an educational grant. The trial management and data analysis were undertaken at the EORTC Data Center (Brussels, Belgium) independently of any commercial interest.
Contributors
BN was responsible for the design of the trial. BN, HS, BG, GJP, PMS, PR, WOB, JNP, ETW, MF-J, DJ, DM, RWP, EVC, WS, and TG contributed patients to the study and reviewed and approved the report. LC analysed the trial data, contributed to the writing of the report, and approved the final version. MP and UB contributed to the trial management, and reviewed and approved the final version of the report.
EORTC intergroup trial 40 983 participants
Investigators who participated through the collaborative groups—W Hohenberger, Universitätsklinikum Erlangen, Germany; T Iveson, University Department of Surgery, Southampton General Hospital, Southampton, UK; J Karner, Kaiser-Franz-Josef-Spital, Vienna, Austria; J Levi and T Hugh, Royal North Shore Hospital, New South Wales, Australia; J De Greve, Akademisch Ziekenhuis VUB, Brussels, Belgium; A Chan, Chinese University of Hong-Kong, Prince of Wales Hospital, Shatin, Hong Kong; B Davidson, Royal Free Hospital, London, UK; P Lindnér, Sahlgrenska Sjukhuset, Goteborg, Sweden; M Peeters, Universiteit Gent, Gent, Belgium; B Stein, Ashford Cancer Centre, South Australia, Australia; T Diamond, Mater Infirmorum Hospital, Belfast, UK; M Ducreux and P Lasser, Institut Gustave Roussy, Villejuif, France; U Graeven, Knappschaft Krankenhaus, Bochum-Langendreer, Germany; B Paillot, Hôpital Charles Nicolle, Rouen, France; J Doran, Queen's Medical Centre, Nottingham, UK; C Gouillat, Hotel Dieu, Lyon, France; I Iesalnieks and K W Jauch, Universitätskliniken Regensburg, Germany; P Jagot-Lacoussiere, Clinique du Pré Le Mans, France; R L Jansen, Academisch Ziekenhuis Maastricht, Netherlands; H Koehne and R Konopke, Universitätsklinikum Carl Gustav Carus, Dresden, Germany; F Otto, Klinikum der Albert-Ludwigs-Universität Freiburg, Freiburg, Germany; D Sherlock, North Manchester General Hospital, Manchester, UK; G Van Hazel, Sir Charles Gairdner Hospital, West Australia, Australia; S Ackland, Newcastle Mater Misericordiae Hospital, New South Wales, Australia; L Bedenne, Hôpital du Bocage, Dijon, France; E Bories, Centre Hospitalier de Beauvais, Beauvais, France; M C Clavero-Fabri, CMC Bligny, Briis sous Forges, France; T Conroy and M C Kaminsky-Forrett, Centre Alexis Vautrin, Vandoeuvre-Les Nancy, France; F Husseini, Hôpital Louis Pasteur, Colmar, France; C Karapetis, Flinders Medical Centre, South Australia, Australia; L Müller, Kreiskrankenhaus, Leer, Germany; T Price, Queen Elizabeth Hospital, South Australia, Australia; R Rosenberg, Klinikum Rechts der Isar/Technische Universität München, Munich, Germany; J Schott, Klinikum der Justus-Liebig-Universität Gießen, Germany; J Tschmelitsch, Krankenhaus der Barmherzigen Brüder, St Veit/Glan, Austria; J L Van Laethem, Hôpital Universitaire Erasme, Brussels, Belgium; J Wals, Atrium Medisch Centrum, Heerlen, Netherlands; A Weimann, Städtisches Klinikum Leipzig, Germany; J P Arnaud, Centre Hospitalier Universitaire d'Angers, France; D Arsene, Centre Hospitalier Régional Universitaire de Caen, France; D Auby, Hôpital Robert Boulin, Libourne, France; S Bhattacharya, Royal London Hospital, London, UK; E Cebon, Austin and Repatriation Medical Centre, Heidelberg, Victoria, Australia; D Cherqui, Centre Hospitalier Universitaire Henri Mondor, Créteil, France; C Confente, Hopital de Jolimont, Haine-Saint–Paul, Belgium; B Dousset, Hôpital Cochin, Paris, France; N Frickhofen, Doctor Horst-Schmidt-Kliniken, Wiesbaden, Germany; A Frilling, Universitätsklinikum Essen, Germany; P Evan and V Ganju, Frankston Hospital, Victoria, Australia; K Höffken, Klinikum der Friedrich-Schiller-Universität Jena, Germany; F Lazorthes, Centre Hospitalier Universitaire de Purpan, Toulouse, France; C Letoublon, Centre Hospitalier Régional de Grenoble–La Tronche, France; A Madroszyk, Centre Hospitalier Régional de Besançon–Hôpital Jean Minjoz, France; D Nitti, Università di Padova, Padova, Italy; B Orr, Weston Park Hospital, Sheffield, UK; E A Pariente, Centre Hospitalier de Pau, France; J C Pector, Institut Jules Bordet, Brussels, Belgium; J L Raoul, Centre Eugène Marquis, Rennes, France; M Rees, North Hampshire Hospital, Basingstoke, UK; K Ridwelski, Städtisches Klinikum Magdeburg-Krankenhaus Olvenstedt, Magdeburg, Germany; P Rouanet, CRLC Val d'Aurelle, Montpellier, France; G J Toogood, St James's University Hospital, Leeds, UK; P Vergauwe, CAZK Groeninghe—Campus St-Niklaas, Kortrijk, Belgium; H J Wilke, Kliniken Essen-Mitte, Essen, Germany
EORTC independent data monitoring committee—Richard Kaplan, Chairman, Cookridge Hospital, Leeds, UK; Jean-Claude Horiot, Centre Georges-François Leclerc, Dijon, France; Bo Littbrand, Umea Universitet, Umea, Sweden; Ahmad Awada, Institut Jules Bordet, Brussels, Belgium (Belgium); Sally Stenning, Medical Research Council Clinical Trials Office, London, UK; Ferdy Lejeune, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; and three anonymous external reviewers appointed by the independent data monitoring committee.
Conflict of interest statement
WS has received consultancy fees from Baxten and Ebewe, and lecture fees from Ebewe and Sanofi-Aventis. ETW has received travel and research grants from and has served on the advisory boards for Sanofi-Aventis, Merck, and Novartis. JNP has served on advisory boards for Sanofi-Aventis. WOB has received research grants and honoraria from Sanofi-Aventis. PR has received travel grants, served on the advisory board, and consults for Sanofi-Aventis and Pfizer. GJP has received grants from Sanofi-Aventis, Pfizer, Merck-Serono, Novartis, and Ipsen. BN has received an honorarium from Sanofi-Aventis. HS has received honoraria from Roche and Pfizer and has served on the advisory board for Sanofi-Aventis. BG, TG, EVC, UB, MP, LC, RWP, DM, DJ, MF-J, and PMS declare that they have no conflict of interest.
References
- 1.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordlinger B, Guiguet M, Vaillant J-C. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 3.Giacchetti S, Itzhaki M, Gruia G. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol. 1999;10:663–669. doi: 10.1023/a:1008347829017. [DOI] [PubMed] [Google Scholar]
- 4.Andre T, Boni C, Mounedji-Boudiaf L. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz M, Muller HH, Schramm H. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen) Ann Surg. 1998;228:756–762. doi: 10.1097/00000658-199812000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemeny N, Huang Y, Cohen AM. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 7.Kemeny MM, Adak S, Gray B. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 8.Mitry E, Fields A, Bleiberg H. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer. A meta-analysis of two randomized trials. ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24:152s. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 9.Portier G, Elias D, Bouche O. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Common toxicity criteria version 2.0. April 30, 1999. http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf (accessed July 30, 2007). [PubMed]
- 11.Freedman LS, White SJ. On the use of Pocock and Simon's method for balancing treatment numbers over prognostic factors in the controlled clinical trial. Biometrics. 1976;32:691–694. [PubMed] [Google Scholar]
- 12.de Gramont A, Figer A, Seymour M. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Allum WH, Stenning SP. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer E. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Hwang IK, Shih WJ, DeCani JS. Group sequential designs using a family of type I error probability spending functions. Stat Med. 1990;9:1439–1445. doi: 10.1002/sim.4780091207. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. Statistical analysis of failure time data. Wiley; New York: 1980. pp. 163–178. [Google Scholar]
- 18.Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883. doi: 10.1097/01.sla.0000098112.04758.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokudo N, Hasegawa K, Makuuchi M. Control arm for surgery alone is needed but difficult to obtain in randomized trials for adjuvant chemotherapy after liver resection for colorectal metastases. J Clin Oncol. 2007;25:1299–1300. doi: 10.1200/JCO.2006.09.9069. [DOI] [PubMed] [Google Scholar]
- 20.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 21.Adam R, Pascal G, Castaing D. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubbia-Brandt L, Audard V, Sartoretti P. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 23.Bilchik AJ, Poston G, Curley SA. Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol. 2005;23:9073–9078. doi: 10.1200/JCO.2005.03.2334. [DOI] [PubMed] [Google Scholar]
- 24.Vauthey JN, Pawlik TM, Ribero D. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 25.Karoui M, Penna C, Amin-Hashem M. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janunger KG, Hafstrom L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168:597–608. doi: 10.1080/11024150201680005. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz H, Fehrenbacher L, Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham D, Humblet Y, Siena S. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 29.Hoff PM, Ellis LM. Targeted therapy trials: approval strategies, target validation, or helping patients? J Clin Oncol. 2007;1:1639–1641. doi: 10.1200/JCO.2006.09.8384. [DOI] [PubMed] [Google Scholar]
- 30.National Cancer Institute EORTC protocol 40051 BOS: randomized phase II trial evaluating the feasibility and tolerance of the combination of FOLFOX with cetuximab and the combination of FOLFOX with cetuximab and bevacizumab as perioperative treatment in patients with resectable liver metastases from colorectal cancer. http:\www.cancer.gov\clinicaltrials\EORTC-40051 (accessed July 30, 2007).