Summary
Background
The international standard radiotherapy schedule for early breast cancer delivers 50 Gy in 25 fractions of 2·0 Gy over 5 weeks, but there is a long history of non-standard regimens delivering a lower total dose using fewer, larger fractions (hypofractionation). We aimed to test the benefits of radiotherapy schedules using fraction sizes larger than 2·0 Gy in terms of local-regional tumour control, normal tissue responses, quality of life, and economic consequences in women prescribed post-operative radiotherapy.
Methods
Between 1999 and 2001, 2215 women with early breast cancer (pT1-3a pN0-1 M0) at 23 centres in the UK were randomly assigned after primary surgery to receive 50 Gy in 25 fractions of 2·0 Gy over 5 weeks or 40 Gy in 15 fractions of 2·67 Gy over 3 weeks. Women were eligible for the trial if they were aged over 18 years, did not have an immediate reconstruction, and were available for follow-up. Randomisation method was computer generated and was not blinded. The protocol-specified principal endpoints were local-regional tumour relapse, defined as reappearance of cancer at irradiated sites, late normal tissue effects, and quality of life. Analysis was by intention to treat. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN59368779.
Findings
1105 women were assigned to the 50 Gy group and 1110 to the 40 Gy group. After a median follow up of 6·0 years (IQR 5·0–6·2) the rate of local-regional tumour relapse at 5 years was 2·2% (95% CI 1·3–3·1) in the 40 Gy group and 3·3% (95% CI 2·2 to 4·5) in the 50 Gy group, representing an absolute difference of −0·7% (95% CI −1·7% to 0·9%)—ie, the absolute difference in local-regional relapse could be up to 1·7% better and at most 1% worse after 40 Gy than after 50 Gy. Photographic and patient self-assessments indicated lower rates of late adverse effects after 40 Gy than after 50 Gy.
Interpretation
A radiation schedule delivering 40 Gy in 15 fractions seems to offer rates of local-regional tumour relapse and late adverse effects at least as favourable as the standard schedule of 50 Gy in 25 fractions.
Introduction
The international standard radiotherapy regimen after breast conservation surgery or mastectomy for early breast cancer delivers 25 daily doses (fractions) of 2·0 Gy to a total dose of 50 Gy over 5 weeks.1–4 Alternative schedules based on a lower total dose delivered in fewer, larger fractions (hypofractionation) were introduced in the UK and Canada several decades ago5 on an empirical basis, and 40 Gy in 15 fractions over 3 weeks is a commonly used regimen.5,6 Results of retrospective studies of hypofractionated radiotherapy in early breast cancer suggest satisfactory outcomes in terms of tumour control and late adverse effects if modest increases in fraction size are combined with appropriate downward adjustments to total dose.7–14 The first results of a Canadian randomised trial testing 42·5 Gy in 16 fractions against 50 Gy in 25 fractions are consistent with these findings, suggesting equivalence in terms of local control and breast cosmesis for the 16-fraction regimen.15
Fractions of more than 2·0 Gy caused unacceptable rates of late adverse effects when inadequate downward adjustments to total dose were applied several decades ago.16–20 Despite widespread empirical use in the UK, 40 Gy in 15 fractions has never been formally compared with standard fractionation, raising concerns in the mid-1990s that it could be less effective or less safe than 50 Gy in 25 fractions. To address this uncertainty, the Standardisation of Breast Radiotherapy (START) Trials were initiated by the then UK Coordinating Committee for Cancer Research (now National Cancer Research Institute) to test the effects of radiotherapy schedules using fraction sizes larger than 2·0 Gy. START Trial A21 tested two dose levels of a 13-fraction regimen delivered over 5 weeks in order to measure the sensitivity of normal and malignant tissues to fraction size. START Trial B compared 40 Gy in 15 fractions of 2·67 Gy in 3 weeks with a control group of 50 Gy in 25 fractions of 2·0 Gy over 5 weeks. This paper presents the results of Trial B.
Methods
Participation was open to all UK centres that provided radiotherapy treatment to patients with early breast cancer. With START Trials A and B running in parallel, centres chose to participate in either Trial A (17 centres) or B (23 centres).
Patients
Women with operable invasive breast cancer (International Union Against Cancer stage pT1-3a pN0-1 M0) requiring radiotherapy after primary surgery (breast conserving surgery or mastectomy, with clear tumour margins ≥1 mm) were eligible for the trial if they were aged over 18 years, did not have an immediate reconstruction, and were available for follow-up. Patients from 21 of the centres participating in Trial B were also recruited into the quality of life and health economics studies (health economics data not presented here, baseline quality of life data have been published elsewhere22). 20 of the centres participating in the quality of life study recruited patients with breast conserving surgery into the photographic assessment of Trial B. Patients from 17 centres also consented to donate a 20 mL blood sample and to complete an associated family history questionnaire (substudy not reported here). The START Trials were approved by the South Thames Multi-Research Ethics Committee in September, 1998, and by the local ethics committees of all participating centres. Written informed consent was obtained for all patients.
Procedures
START Trial B patients were randomised to 50 Gy in 25 fractions over 5 weeks or to 40 Gy in 15 fractions over 3 weeks. Randomisation was arranged via telephone at the Clinical Trials and Statistics Unit at the Institute of Cancer Research (ICR-CTSU), Sutton, UK, where patient details were recorded and treatment was allocated. Randomisation was not blinded. Computer-generated random permuted blocks were used as the method of allocation, with patients stratified by hospital, type of surgery (breast conserving surgery or mastectomy), and intention to give a tumour bed boost dose or not. Use of adjuvant systemic treatment was recorded, with a requirement of at least a 2-week gap between exposure to chemotherapy and radiotherapy.
Patients lay in a supine treatment position. The planning target volume was defined as the whole breast with a 1 cm margin to palpable breast tissue; where regional radiotherapy was indicated, the planning target volume was supraclavicular nodes with or without axillary chain with a 1 cm margin. Most patients were treated with 6 MV x-rays, although treatment with higher energies or cobalt γ-rays was allowed after discussion with the START Trial radiotherapy quality assurance team. Planning protocols were specified at the time of notification of participation into the study and had to conform to the minimum quality criteria described in the protocol. Planning protocols varied slightly between centres, but within each centre they were identical in each fractionation group. Doses were prescribed to international reference points.23 Departments were required to have a protocol specifying whether patients who had had breast conserving surgery would receive a boost to the tumour bed, and to use an electron field of appropriate energy to deliver 10 Gy in five daily fractions to the 100% isodose, after initial radiotherapy.
All centres submitted details of the standard radiotherapy technique, after which a visit by the quality assurance team checked dosimetric measurements in a 2D and 3D breast phantom, including the junction region between supraclavicular fossa and tangential breast or chest wall fields.24–27 The mean difference between prescribed and measured dose in a phantom was 2·1%. Additionally, a third of the radiotherapy treatment plans were collected and analysed by the quality assurance team to ensure compliance with the protocol in terms of prescription point, dose homogeneity, and lung depth, and a random sample of patients had in-vivo thermoluminescent dosimeter measurements done.28–30 The protocol allowed for a dose variation (in the planning target volume) between 95% and 105% of that at the reference point on the central axis. Lung depth data was obtained by the radiotherapy quality assurance programme, and analysis indicated that most patients had less than 2 cm of lung within the treatment volume. These results confirmed a good compliance with the technical aspects of the trial protocol.
The principal endpoints specified in the protocol were local-regional relapse, normal tissue effects, and quality of life. Local-regional tumour relapse was defined as local relapse in breast or chest wall, and regional relapse in ipsilateral axilla or supraclavicular fossa if it had been within an irradiated target volume. Any ipsilateral regional relapse outside the radiotherapy target volume was excluded from the analysis of local-regional relapse. Normal tissue effects in the breast, arm, and shoulder were assessed by photographic comparison with baseline, patient self-reported assessments, and physician assessments. Other endpoints were disease-free and overall survival, second primary cancers, and health economic consequences. Disease-free survival was defined as time to any breast cancer-related event (local-regional or distant relapse, contralateral breast cancer, or death from breast cancer). Data relating to five key breast normal tissue effects from the patient quality of life self-assessments are presented here. Separate papers will present the full analysis of all self-assessments and physician assessments of normal tissue effects, and of quality of life. Cases of ischaemic heart disease, symptomatic rib fracture, and symptomatic lung fibrosis were recorded during follow-up; incidence with and without confirmation of diagnosis (eg, using imaging and further investigation) was included. Brachial plexopathy was reported if damage to the brachial plexus was suspected and the patient had symptoms of pain, parasthesia, numbness, or other sensory symptoms (graded on a 4-point scale). Suspected cases of brachial plexopathy were subject to confirmation by neurophysiological assessment and MRI.
Patients were reviewed every year for tumour relapse and radiotherapy-induced normal tissue effects. Clinical data were recorded on pre-printed case report forms and sent to the coordinating clinical trials office at the ICR-CTSU, Sutton, UK. Photographs were taken at baseline (post-surgery and pre-radiotherapy) and then at 2 and 5 years to assess changes to the breast based on change in size, shrinkage, and shape, and scored on a 3-point graded scale. Changes in breast appearance (photographic) were scored by three observers blind to patient identity, treatment allocation, and year of follow-up, and a final agreed score reached by consensus. Breast size and surgical deficit were both defined from the baseline photographs by the same three observers applying 3-point graded scales. Quality of life data were obtained using standardised questionnaires31–34 at baseline and at 6 months, 1, 2, and 5 years. Post-baseline quality of life questionnaires included an additional four protocol-specific items relating to changes in the affected breast after radiotherapy (skin changes in the area of the affected breast, overall change in breast appearance, firmness to touch of the affected breast, and reduction in size of the affected breast). Of these four items, patients who had had mastectomy only rated change in skin appearance after radiotherapy. Details of the quality of life study protocol and baseline data have been published elsewhere.22
The trial was coordinated by the ICR-CTSU, Sutton, UK. The trial was overseen by a Steering Committee of several independent experts joined by members of the ICR-CTSU, START Trial Management Group, and representatives of the funding bodies (as observers). The Trial Management Group was responsible for the day-to-day management of the trial, and the emerging safety and efficacy data was reviewed regularly by the Independent Data Monitoring Committee. Central statistical monitoring of data was done by ICR-CTSU, supplemented by selected on-site source document verification.
Statistical analysis
A 5-year local-regional tumour relapse rate of 10% in the 50 Gy group was predicted, based on the earlier Royal Marsden Hospital/Gloucestershire Oncology Centre (RMH/GOC) pilot trial.35 A target sample size of 1840 patients was defined in Trial B to provide 95% power to exclude an increase of 5% in the local-regional relapse rate for the 40 Gy group compared with 50 Gy (one-sided α=0·025). The protocol specified that if the true 5-year local-regional relapse rate in the 50 Gy group was lower than expected (eg, 5%), this sample size would give more than 95% power to detect an increase of 5% in the local-regional relapse rate in the 40 Gy group.
Survival analysis methods were used to compare rates of each endpoint between the fractionation schedules. Length of follow-up was calculated as time from randomisation until time of first event or last follow-up assessment, whichever occurred first. Patients were still evaluable for local-regional relapse after distant relapse, but were censored at date of death. For the photographic endpoint, patients were no longer evaluable for change in breast appearance after local-regional relapse. Kaplan-Meier estimates of 5-year relapse rates, rates of normal tissue effects, rates of any breast-cancer related event, and mortality rates were calculated (with 95% CIs). For the patient quality of life self-assessments of normal tissue effects an event was defined as the first occurrence of a moderate or marked symptom (graded “quite a bit” or “very much”). The scores from the photographic assessments of change in breast appearance at 2 and 5 years were dichotomised as none versus mild or marked change, and the first occurrence of such a change was taken as the endpoint for the survival analysis. There were too few patients with marked change in breast appearance to be able to analyse this category separately.
The log-rank test was used to compare fractionation schedules. Crude hazard ratios (with 95% CIs) comparing fractionation schedules for each endpoint were obtained from Cox proportional hazards regression models. The proportionality assumption of the Cox model was tested using Schoenfeld residuals and was found to be valid for all of the analyses. Since point estimates of differences in event rates can, by chance, be atypical of the overall pattern of differences between schedules, estimates of the absolute difference in 5-year event rates taking the whole range of observation times into account were obtained by applying the hazard ratios obtained from the Cox model to the Kaplan-Meier estimate of the rate in the 50 Gy control group.36 Both one-sided and two-sided 95% CIs were calculated for the absolute difference in local-regional relapse rates at 5 years, since the upper limit is of greater clinical interest, in view of concern about a possible excess risk caused by hypofractionated schedules. Kaplan-Meier survival curves and Nelson-Aalen cumulative hazard functions were plotted according to fractionation schedule. Plots were censored at the median length of follow-up (rounded to nearest year).
Analysis included all randomised patients on an intention-to-treat basis. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN59368779.
Role of the funding source
The funding sources provided peer-reviewed approval for the trial and have had representation (as observers) on the Trial Steering Committee, but had no other role in the design, conduct, data collection, data analysis or interpretation of the study or the results. The corresponding author had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Results
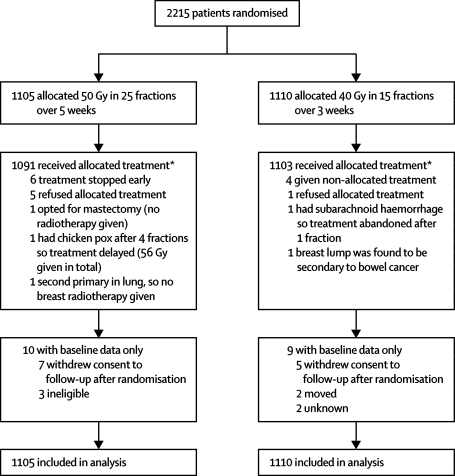
Between January, 1999, and October, 2001, 2215 patients were enrolled in START Trial B at 23 centres in the UK (figure 1). A total of 1079 patients were enrolled in the quality of life study and 1094 in the photographic assessment study (with 930 patients enrolled in both substudies).
Figure 1.
Trial profile for START Trial B
*Only major treatment deviations listed. Minor deviations due to public holidays, machine service days, and machine breakdowns not included.
Demographic and clinical characteristics were well balanced between the treatment groups (table 1). Of the women prescribed chemotherapy, 59·1% (290/491) received an anthracycline-containing regimen, which was balanced between randomised radiotherapy schedules (154/258 [59·7%] for 50 Gy and 136/233 [58·4%] for 40 Gy). Cyclophosphamide, methotrexate, and fluorouracil combination therapy alone was prescribed in 196 women (39·9% of those receiving chemotherapy), which was similarly balanced between randomised groups (103 [39·9%] for 50 Gy and 93 [39·9%] for 40 Gy). Nine women (three allocated 50 Gy and six allocated 40 Gy) received an adjuvant taxane. Of the 1955 women prescribed tamoxifen or another endocrine therapy, almost all were continuing treatment at randomisation (956/979 [97·6%] for 50 Gy and 963/976 [98·7%] for 40 Gy). The quality of life subgroup included 12·3% women who had undergone mastectomy. There were only 21 major treatment deviations (including early stopping of treatment, patient refusal of allocated treatment, and patients found to be ineligible for reasons including presence of second primaries), resulting in 99·0% compliance with allocated treatment (figure 1). Compliance with completion of quality of life questionnaires over 5 years was more than 90%.
Table 1.
Demographic and clinical characteristics at randomisation of the 2215 patients in START Trial B
|
Fractionation schedule |
Total n=2215 | |||
|---|---|---|---|---|
| 50 Gy in 25 fractions n=1105 | 40 Gy in 15 fractions n=1110 | |||
| Age (years) | ||||
| 20–29 | 7 (0·6) | 0 (0·0) | 7 (0·3) | |
| 30–39 | 62 (5·6) | 39 (3·5) | 101 (4·6) | |
| 40–49 | 179 (16·2) | 170 (15·3) | 349 (15·8) | |
| 50–59 | 427 (38·6) | 447 (40·3) | 874 (39·5) | |
| 60–69 | 304 (27·5) | 327 (29·5) | 631 (28·5) | |
| 70–79 | 117 (10·6) | 119 (10·7) | 236 (10·7) | |
| 80− | 9 (0·8) | 8 (0·7) | 17 (0·8) | |
| Mean (SD) | 57·0 (10·4) | 57·8 (9·5) | 57·4 (10·0) | |
| Time from surgery to randomisation (weeks); median (IQR) [range] | 7·3 (4·9–12·3) [0·9–45·3] | 7·1 (4·9–11·9) [0·6–49·3] | 7·3 (4·9–12·0) [0·6–49·3] | |
| Primary surgery | ||||
| Breast conserving surgery | 1020 (92·3) | 1018 (91·7) | 2038 (92·0) | |
| Mastectomy | 85 (7·7) | 92 (8·3) | 177 (8·0) | |
| Histological type | ||||
| Invasive ductal | 865 (78·3) | 843 (75·9) | 1708 (77·1) | |
| Invasive lobular | 122 (11·0) | 132 (11·9) | 254 (11·5) | |
| Mixed ductal/lobular | 20 (1·8) | 25 (2·3) | 45 (2·0) | |
| Other | 95 (8·6) | 103 (9·3) | 198 (8·9) | |
| Not known | 3 (0·3) | 7 (0·6) | 10 (0·5) | |
| Pathological node status | ||||
| Positive | 238 (21·5) | 266 (24·0) | 504 (22·8) | |
| Negative | 831 (75·2) | 804 (72·4) | 1635 (73·8) | |
| Not known (no axillary surgery) | 36 (3·3) | 39 (3·5) | 75 (3·4) | |
| Not known (missing data) | 0 (0·0) | 1 (0·1) | 1 (0·04) | |
| Tumour size (cm) | ||||
| <1 | 151 (13·7) | 167 (15·0) | 318 (14·4) | |
| 1− | 552 (50·0) | 542 (48·8) | 1094 (49·4) | |
| 2− | 287 (26·0) | 288 (25·9) | 575 (26·0) | |
| 3− | 113 (10·2) | 107 (9·6) | 220 (9·9) | |
| Not known | 2 (0·2) | 6 (0·5) | 8 (0·4) | |
| Tumour grade | ||||
| 1 | 306 (27·7) | 311 (28·0) | 617 (27·9) | |
| 2 | 518 (46·9) | 532 (47·9) | 1050 (47·4) | |
| 3 | 261 (23·6) | 248 (22·3) | 509 (23·0) | |
| Not known (not applicable)* | 15 (1·4) | 15 (1·3) | 30 (1·3) | |
| Not known | 5 (0·4) | 4 (0·4) | 9 (0·4) | |
| Adjuvant therapy | ||||
| None | 37 (3·3) | 47 (4·2) | 84 (3·8) | |
| Tamoxifen/no chemotherapy | 782 (70·8) | 810 (73·0) | 1592 (71·9) | |
| Chemotherapy/no tamoxifen | 77 (7·0) | 78 (7·0) | 155 (7·0) | |
| Tamoxifen+chemotherapy | 181 (16·4) | 155 (14·0) | 336 (15·2) | |
| Other endocrine therapy† | 16 (1·4) | 11 (1·0) | 27 (1·2) | |
| Not known | 12 (1·1) | 9 (0·8) | 21 (0·9) | |
| Lymphatic treatment | ||||
| None | 32 (2·9) | 36 (3·2) | 68 (3·1) | |
| Surgery/no radiotherapy | 980 (88·7) | 984 (88·6) | 1964 (88·7) | |
| Radiotherapy/no surgery | 5 (0·4) | 3 (0·3) | 8 (0·4) | |
| Surgery+radiotherapy | 74 (6·7) | 79 (7·1) | 153 (6·9) | |
| Not known | 14 (1·3) | 8 (0·7) | 22 (1·0) | |
| Boost (BCS patients only) | n=1020 | n=1018 | n=2038 | |
| Yes | 422 (41·4) | 446 (43·8) | 868 (42·6) | |
| No | 584 (57·3) | 565 (55·5) | 1149 (56·4) | |
| Not known | 14 (1·4) | 7 (0·7) | 21 (1·0) | |
| From baseline photographs | n=522 (%) | n=514 (%) | n=1036 (%) | |
| Breast size | ||||
| Small | 49 (9·4) | 42 (8·2) | 91 (8·8) | |
| Medium | 377 (72·2) | 390 (75·9) | 767 (74·0) | |
| Large | 96 (18·4) | 82 (16·0) | 178 (17·2) | |
| Surgical deficit | ||||
| Small | 307 (58·8) | 286 (55·6) | 593 (57·2) | |
| Medium | 164 (31·4) | 177 (34·4) | 341 (32·9) | |
| Large | 51 (9·8) | 51 (9·9) | 102 (9·8) | |
Data are n (%) unless otherwise stated. BCS=breast conserving surgery.
Lobular and other histological types.
Other endocrine therapies include combinations of tamoxifen/anastrozole/letrozole/goserelin mostly within randomised trials.
Median follow-up of surviving patients was 6·0 years (IQR 5·0 to 6·2), with a maximum follow-up of 8·0 years. At the time of analysis, 1872 patients (84·5%) were alive and without relapse, 34 (1·5%) were alive with local-regional relapse (without distant relapse), 45 (2·0%) were alive with distant relapse (including four with local-regional relapse), 245 (11·1%) had died (including 27 with local-regional relapse), and 19 (0·9%) had no follow-up (figure 1).
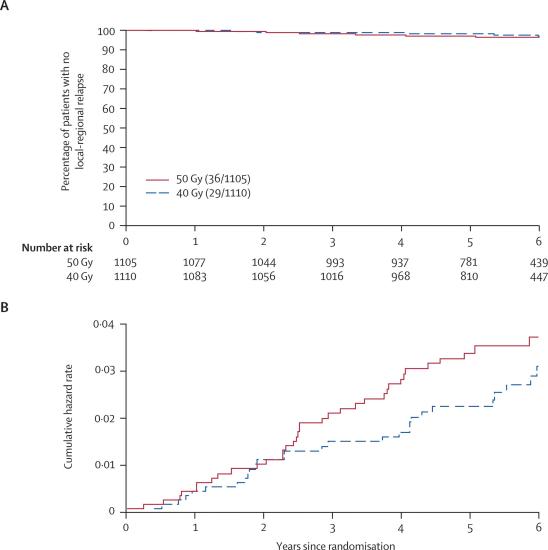
At the time of analysis, 65 (2·9%) patients had experienced a local-regional relapse. The hazard ratio for local-regional relapse after 40 Gy compared with 50 Gy was 0·79 (95% CI 0·48–1·29; table 2). The estimated absolute difference in local-regional relapse rates for 40 Gy compared with 50 Gy at 5 years was −0·7% (−1·7% to 0·9%), which indicates that the absolute difference between schedules is likely to be at worst 0·9% higher and at best 1·7% lower after 40 Gy in 15 fractions than after 50 Gy in 25 fractions. Since the main concern over hypofractionation is an excess risk rather than a possible benefit, a more precise estimate of the potential excess risk of local-regional relapse is obtained from the upper limit of the one-sided 95% CI for the absolute difference in 5-year local-regional relapse rates. This calculation indicated an upper limit of 0·6% excess risk associated with the 15-fraction schedule. The Kaplan-Meier and cumulative hazard rate plots for local-regional relapse according to fractionation schedule (figure 2) illustrate the low event rate in both randomised groups.
Table 2.
Survival analyses of relapse and mortality according to fractionation schedule
| Events/total (%) | Estimated % with event by 5 years (95% CI) | Crude hazard ratio (95% CI) | Log-rank test p value | |
|---|---|---|---|---|
| Local relapse* | ||||
| 50 Gy | 34/1105 (3·1) | 3·3 (2·2–4·4) | 1 | |
| 40 Gy | 25/1110 (2·2) | 2·0 (1·1–2·8) | 0·72 (0·43–1·21) | 0·21 |
| Local-regional relapse | ||||
| 50 Gy | 36/1105 (3·2) | 3·3 (2·2–4·5) | 1 | |
| 40 Gy | 29/1110 (2·6) | 2·2 (1·3–3·1) | 0·79 (0·48–1·29) | 0·35 |
| Distant relapse | ||||
| 50 Gy | 122/1105 (11·0) | 10·2 (8·4–12·1) | 1 | |
| 40 Gy | 87/1110 (7·8) | 7·6 (6·0–9·2) | 0·69 (0·53–0·91) | 0·01 |
| Any breast cancer-related event† | ||||
| 50 Gy | 164/1105 (14·8) | 14·1 (12·0–16·2) | 1 | |
| 40 Gy | 127/1110 (11·4) | 10·6 (8·7–12·4) | 0·75 (0·60–0·95) | 0·02 |
| All-cause mortality | ||||
| 50 Gy | 138/1105 (12·5) | 11·0 (9·1–12·9) | 1 | |
| 40 Gy | 107/1110 (9·6) | 8·0 (6·4–9·7) | 0·76 (0·59–0·98) | 0·03 |
Local relapse defined as ipsilateral local tumour relapse in breast parenchyma/breast skin/chest wall skin.
Local, regional, or distant relapse, breast cancer death, contralateral breast cancer (“disease-free survival”).
Figure 2.
Kaplan-Meier plot (A) and Nelson-Aalen cumulative hazard plot (B) of local-regional tumour relapse in 2215 patients
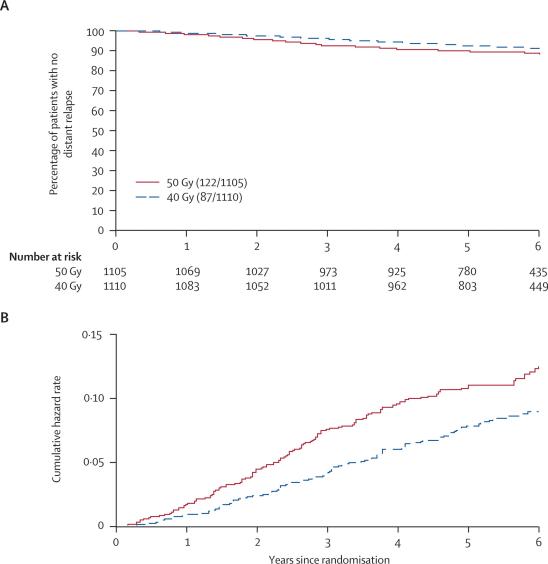
The 5-year rate of distant relapse was lower in the 40 Gy group (hazard ratio 0·69, 95% CI 0·53–0·91), which contributed to the higher rates of disease-free survival and overall survival than in the 50 Gy group (table 2). Analysis of the Kaplan-Meier and cumulative hazard rate plots indicated that the divergence in distant relapse between the schedules began at around 1 year after randomisation (figure 3).
Figure 3.
Kaplan-Meier plot (A) and Nelson-Aalen cumulative hazard plot (B) of distant relapse in 2215 patients
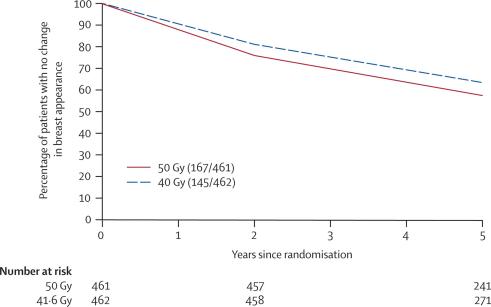
Change in breast appearance (photographic) was assessed on a 3-point graded scale (none, mild, marked) in 923 patients with a baseline and at least one follow-up image (461 for 50 Gy and 462 for 40 Gy). Not all patients had photographs available at both 2 and 5 years, for reasons including the 5-year assessment not yet being done at the time of scoring and analysis, patient refusal, and withdrawal from the photographic study due to relapse. There were no associations between score for change in breast appearance (photographic) at 2 years or patient demographic or treatment characteristics and whether or not the patient had a 5-year assessment (data not shown). Mild change was graded for 284 (30·8%) patients and marked change for 28 (3·0%) patients, by 5 years. Change in breast appearance (photographic) was less likely after 40 Gy than after 50 Gy, with a hazard ratio of 0·83 (95% CI 0·66–1·04, p=0·06; figures 4 and 5). Adjusting for breast size and surgical deficit made little difference to these results. Figure 4 shows that the treatment differences were evident at the first time point of 2 years, and persisted to 5 years.
Figure 4.
Kaplan-Meier plot of mild/marked change in breast appearance (photographic) in 923 patients with breast conserving surgery
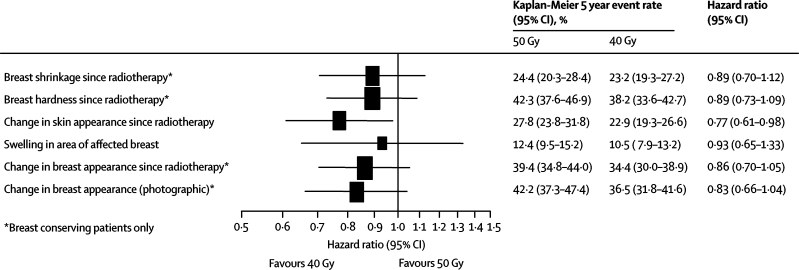
Figure 5.
Forest plot of late normal tissue effects assessed as moderate/marked by patients and mild/marked from photographs
Patient quality of life self-assessments of normal tissue effects were available for 1037 (96·1% of all patients in the quality of life study) patients with baseline information and at least one completed follow-up questionnaire (511 for 50 Gy and 526 for 40 Gy). Changes in breast appearance and breast hardness (patients with breast-conserving surgery) were the most common changes recorded. Analysis of patient self-assessments of five key normal tissue effects on the breast and breast area showed that rates of moderate or marked effects by 5 years tended to be lower after 40 Gy than after 50 Gy, with a significantly lower rate of change in skin appearance after radiotherapy for 40 Gy than after 50 Gy (p=0·02; figure 5). The results of the various assessments of normal tissue effects were consistently in favour of the 40 Gy group compared with 50 Gy (figure 5).
The incidence of ischaemic heart disease, symptomatic rib fracture, and symptomatic lung fibrosis was low at this stage during follow-up, and balanced between the schedules (table 3). There were no cases of brachial plexopathy in the 82 women given 40 Gy in 15 fractions or in the 79 women given 50 Gy in 25 fractions to the supraclavicular fossa, axilla, or both. An unusually marked acute reaction during radiotherapy was recorded for 16 (0·7%) patients (13 after 50 Gy [1·2%], three after 40 Gy [0·3%]). Of these, 14 cases were severe skin reactions (extensive moist desquamation), one was an infected seroma in the scar area, and one had severe pain in the breast tissue and ribs.
Table 3.
Incidence of ischaemic heart disease, symptomatic rib fracture, and symptomatic lung fibrosis according to fractionation schedule
|
Fractionation schedule |
Total n=2215 | ||
|---|---|---|---|
| 50 Gy n=1105 | 40 Gy n=1110 | ||
| Ischaemic heart disease* | |||
| Reported | 19 (1·7) | 15 (1·3) | 34 (1·5) |
| Confirmed† [left-sided]‡ | 12 (1·1) [4] | 7 (0·6) [3] | 19 (0·9) [7] |
| Symptomatic rib fracture§ | |||
| Reported | 17 (1·5) | 16 (1·4) | 33 (1·5) |
| Confirmed† | 2 (0·2) | 2 (0·2) | 4 (0·2) |
| Symptomatic lung fibrosis | |||
| Reported | 15 (1·4) | 16 (1·4) | 31 (1·4) |
| Confirmed† | 1 (0·1) | 3 (0·3) | 4 (0·2) |
Data are n (%) unless otherwise stated.
11 patients had pre-existing heart disease at randomisation and were excluded.
Cases confirmed following imaging and further investigations.
Confirmed cases of ischaemic heart disease in patients with left-sided primary tumours.
Reported cases include four with rib fracture after bone metastases and three after trauma.
There were 36 (1·6%) contralateral breast cancers (19 after 50 Gy (1·7%), 17 after 40 Gy [1·5%]), and 58 patients [2·6%] had other second primary cancers (32 after 50 Gy [2·9%], 26 after 40 Gy [2·3%]), the most frequent being lung (ten), endometrial (ten), ovarian (eight), and colon (five). The remaining 25 incidences of second primary cancers consisted of one or two cases of several different types.
Discussion
START trial B aimed to provide a robust evidence base for clinical practice in breast radiotherapy by comparing a commonly used 15-fraction schedule with the international standard based on 25 fractions of 2·0 Gy. The analysis of change in breast appearance (photographic) suggests that 40 Gy in 15 fractions over 3 weeks causes slightly less permanent damage to normal breast tissues than a standard regimen of 50 Gy in 25 fractions over 5 weeks. This observation is consistent with results of the RMH/GOC pilot trial35 and the START Trial A,21 which suggest that 40 Gy in 15 fractions over 3 weeks is equivalent in terms of late normal tissue effects in the breast to a total dose of 46 Gy delivered in 2·0 Gy fractions.21,37 The patient quality of life self-assessments of normal tissue effects in START Trial B are also consistent with this relation, suggesting 5-year estimates in favour of the 40 Gy group in most of the assessed normal tissue effects.
A preliminary analysis of the concordance of assessments of the normal tissue effects by patients, photographs, and physicians shows that in general patients tend to score effects more severely. A full description of patient-rated cosmesis and quality of life will be reported separately.
Physician assessments of normal tissue effects have not been presented in this paper. Preliminary analysis of these data produces estimates of the relative effects of the fractionation schedules, which are similar to those assessed by the photographic and patient self-assessments. Some variation existed, however, between centres in the practice used to complete the yearly case report forms. Most centres completed these forms in the presence of the patient, whereas others completed them afterwards from hospital case notes. Since the level of detail included in the hospital case notes varied within and between centres, this practice, although unbiased between treatment groups, could have led to underreporting of physician-assessed normal tissue effects. The results of the physician assessments will thus be the subject of a separate manuscript, reporting also the sensitivity of the endpoints according to method of completion of the forms.
A median follow-up of 5 years is too short to allow assessment of all the potential late normal tissue effects such as cardiac damage. Follow-up of all women within the trial is continuing in order to assess the long-term effects of the fractionation schedules. However, the RMH/GOC pilot data with a median of 10 years' follow-up showed that although estimates of absolute rates of normal tissue effects change with time, the relative effects of different fractionation schedules remain unchanged.37 15–20 years of follow-up will be needed to reliably measure cardiac effects. The short-term priority is to protect the heart from exposure to radiotherapy; something that is now possible with advanced radiotherapy technologies.
The local-regional relapse rate was lower than originally anticipated. A 5-year local-regional relapse rate of 10% in the 50 Gy group was originally assumed, on the basis of data from the pilot trial initiated in 1986. Since then, risks of local and metastatic breast cancer relapse have fallen because of improvements in the treatment and management of breast cancer patients. While accrual was still continuing, the emerging data presented in confidence to the Independent Data Monitoring Committee suggested that relapse rates were likely to be lower than predicted. At that time, adhering to appropriate governance procedures, the potential effects of the predicted lower than expected relapse rates were discussed by the independent Trial Steering Committee and the Independent Data Monitoring Committee. The consensus from these independent advisory committees was that there was no strong scientific rationale for changing the protocols and increasing the sample size.
Since the local-regional relapse rate was lower than expected (<4%), the study actually has greater power than originally planned to exclude a 5% absolute difference (99% power, assuming 4% in control group), since this represents a much larger relative treatment effect (4% in control group vs 9% in test group compared with 10% vs 15% as specified in the protocol). Alternatively, with the lower baseline local-regional relapse rate the same sample size provides sufficient power to exclude smaller absolute differences. For example with a 4% local-regional relapse rate in the control group, the study is able to exclude an absolute increase of 3·5% with a similar level (97%) of power as originally planned. The effect of 40 Gy in 15 fractions is unlikely to be worse than 50 Gy in 25 fractions.
In view of the hazard ratio for local-regional relapse of 0·79 (95% CI 0·48 to 1·29), the absolute difference in 5-year local-regional relapse rate suggests a benefit after 40 Gy corresponding to six fewer local-regional relapses per 1000 women. The 95% CI for the absolute difference indicates that the test schedule could prevent, at best, up to 17 relapses, or cause, at worst, an additional nine relapses per 1000 women. The apparent differences in disease-free and overall survival between treatment groups are unexpected. Such differences are unlikely to be due to differences in local tumour control between treatment groups, since these are too small and would translate into survival gains after 15 years,38 not at the early timepoint where the apparent divergence in distant metastasis rates is seen. Similarly, they are unlikely to be due to differences in the baseline characteristics or adjuvant therapy, since these were well-balanced between the treatment groups.
Data for oestrogen receptor status were not obtained as part of the trial, but routine policy in most of the centres during the accrual period was to prescribe tamoxifen only to oestrogen receptor-positive patients or those whose oestrogen receptor status was unknown. Hence tamoxifen use is a reasonable surrogate for oestrogen receptor status in the trial, which was balanced between the treatment groups. There are many factors which affect relapse and survival, including others which were unknown in the trial such as HER2 status; the purpose of randomisation is to ensure a balance in the unknown as well as the known prognostic factors. We cannot ascribe the survival difference to any biological or treatment-related factor, and can only conclude that this difference might be due to chance and could diminish with further follow-up. Long-term follow-up of these women is continuing, to verify whether the relative effects of the schedules remain stable over time, in terms of late normal tissue effects as well as relapse and survival.
The only other large trial with which START Trial B can be compared is a Canadian trial15 that tested 42·5 Gy in 16 fractions of 2·6 Gy fractions over 22 days against 50 Gy in 25 fractions over 35 days in 1234 women after tumour excision for early breast cancer. The 5-year local relapse rates were 2·8% after the shorter schedule and 3·2% after the standard 5-week regimen (absolute difference 0·4%, 95% CI −1·5 to 2·4%). The proportion of patients with clinically assessed excellent or good global cosmetic outcome was also similar between groups (absolute difference −0·6%, 95% CI −6·5 to 5·5%). The similarity of normal tissue effects is consistent with the results of START Trial B. On the same assumptions applied above, 42·5 Gy in 16 fractions is equivalent to 50 Gy in 25 fractions in terms of late-onset normal tissue effects in the breast and chest wall. Reliable comparison of tumour control is limited by the small number of relapses in both the Canadian study (44 events) and in START Trial B (65 events).
In conclusion, after surgery for early breast cancer, a radiotherapy schedule delivering 40 Gy in 15 fractions over 3 weeks seems to offer local regional tumour control and rates of late normal tissue effects at least as good as the accepted international standard of 50 Gy in 25 fractions over 5 weeks.
Acknowledgments
We thank all the patients who participated in this study, and the doctors, nurses, radiographers, physicists, and data managers at the participating centres. We acknowledge the support of The Royal College of Radiologists (UK) and Radiotherapy Action Group Exposure (RAGE), especially Margaret King, deceased, to whom this manuscript is dedicated. We acknowledge the contributions of colleagues who previously worked on the trial, including C Cruickshank, C Dawson, E Marriage, L Gamaldo, C Harper, D Hussey (all ex-ICR-CTSU) and A Deighton, S Griffiths, E Miles (all ex-Trial Management Group) We thank Cancer Research UK, the UK Medical Research Council, and the Department of Health for providing the funds to undertake this research (Grant G9600656). The Cancer Research UK number for the START Trial is CRUK/96/001.
START Writing Committee
The START Writing Committee are all members of the Trial Management Group, except S M Bentzen, who is a member of the Trial Steering Committee): R K Agrawal (Shrewsbury and Telford Hospital NHS Trust), E G A Aird (Mount Vernon Hospital, Northwood), J M Barrett (Royal Berkshire NHS Foundation Trust, Reading), P J Barrett-Lee (Velindre Hospital NHS Trust, Cardiff), S M Bentzen (University of Wisconsin Medical School, Madison, USA), J M Bliss (ICR-CTSU, Sutton), J Brown (Previously MRC Health Services Research Collaboration, University of Bristol, now Eli Lilly & Company Limited), J A Dewar (Ninewells Hospital, Dundee), H J Dobbs (Guys and St Thomas' NHS Trust, London), J S Haviland (ICR-CTSU, Sutton), P J Hoskin (Mount Vernon Hospital, Northwood), P Hopwood (Christie Hospital, Manchester), P A Lawton (Nottingham City Hospital), B J Magee (Christie Hospital, Manchester), J Mills (ICR-CTSU, Sutton), D A L Morgan (Nottingham University Hospital NHS Trust, J R Owen (Cheltenham General Hospital), S Simmons (ICR-CTSU, Sutton), G Sumo (ICR-CTSU, Sutton), M A Sydenham (ICR-CTSU, Sutton), K Venables (Mount Vernon Hospital, Northwood), and J R Yarnold (Chair and Chief Investigator, Institute of Cancer Research, Royal Marsden NHS Foundation Trust).
Contributors
J R Yarnold (Chief Investigator and Chair), B J Magee, and J M Bliss were responsible for the trial design, trial management, data interpretation and manuscript writing. J M Bliss oversaw all statistical analyses. J S Haviland and G Sumo did the main analyses and contributed to data interpretation and manuscript writing. SM Bentzen contributed to data interpretation and manuscript writing. S Simmons and M A Sydenham were responsible for the trial coordination and data collection, and contributed to data interpretation and manuscript writing. P Hopwood was responsible for the design of the quality of life study and contributed to the trial management, data interpretation, and manuscript writing. J Mills was responsible for the coordination of the quality of life study and contributed to data interpretation and manuscript writing. E G A Aird, P J Hoskin, and K Venables were responsible for the design and conduct of the quality assurance programme and contributed to the trial management, data interpretation, and manuscript writing. P J Barrett-Lee, J A Dewar, P A Lawton, and J R Owen contributed to trial design, trial management, data interpretation, and manuscript writing. R K Agrawal, J M Barrett, J Brown, H J Dobbs, and D A L Morgan contributed to trial design, trial management, and data interpretation. The Writing Committee accept full responsibility for the overall content of the START papers.
The START Trialists' Group
In addition to the Trial Management Group (named as Writing Committee above), the following groups are all part of the START collaboration:
Principal and main co-investigators according to centre (number of patients recruited): Addenbrooke's NHS Trust, Cambridge (135), M Daly, A M Moody, H Patterson, J Singer, M V Williams, C B Wilson; Christie Hospital, Manchester (166), B Magee, A Stewart, A Sykes; Clatterbridge Centre for Oncology, Bebington (68), D Errington, S Myint, I Syndikus, N Thorp; Cumberland Infirmary, Carlisle(82), P Dyson, J J Nicoll; Derriford Hospital, Plymouth (49), S Kelly; Guys and St Thomas' NHS Trust, London (120), J Dobbs, S Harris, E A MacDonald, M O'Connell, A R Timothy; Ipswich Hospital, Ipswich (97), J LeVay; James Cook University Hospital, Cleveland (21), P D J Hardman, N Storey, N Wadd; Leicester Royal Infirmary, Leicester (75), S Khanna, F Madden, A Osmond, I Peat; Maidstone Hospital, Maidstone (87), C Abson, J D Dubois, F McKinna, D Pickering, G Sadler; Mount Vernon Hospital, Northwood (128), R Ashford, E Grosch, M Harrison, P A Lawton, E J Maher, A Makris, P Ostler; New Cross Hospital, Wolverhampton (97), R Allerton, C Brammer, J Brown, M Churn, D Fairlamb, T Priestman; Norfolk and Norwich University Hospital, Norwich (22), A S Bulman, W M C Martin; Queens Hospital, Romford (ex-Oldchurch Hospital) (92), Gibbs, J Money-Kyrle, M Quigley; Royal Devon and Exeter Foundation Trust, Exeter (88), P Bliss, A G Goodman, A Hong, Rowland; Royal Preston Hospital, Preston (56), A Biswas, S Kumar, G Reed, G E Skailes; St Mary's Hospital, Portsmouth (63), J D Dubois, P F Golding, G G Khoury, E Low; Shrewsbury and Telford Hospital NHS Trust (237), R K Agrawal; Southend Hospital, Southend (130), A Robinson, C Trask; Sussex Cancer Centre, Brighton (113), D Bloomfield, G P Deutsch, N Hodson; Torbay Hospital, Torquay (58), P Bliss, A Goodman, A Hong; University Hospital of North Staffordshire, Stoke-on-Trent (84), A M Brunt, Cook; Weston Park Hospital, Sheffield (147), K Dunn, M Hatton, O Purohit, S Ramakrishnan, M Robinson.
Trial Steering Committee: Independent members: A Barrett (Chair, University of East Anglia), M Armitage (Royal Bournemouth & Christchurch NHS Trust), S M Bentzen (University of Wisconsin Medical School, Madison, USA), U Chetty (Western General Hospital, Edinburgh), P Mayles (Clatterbridge Centre for Oncology, Wirral), L Walker (University of Hull).
Independent Data Monitoring Committee: H Lucraft (Chair, Newcastle General Hospital), M Parmar (MRC Clinical Trials Unit, London), I Turesson (Akadamiska Sjukhuset, Uppsala, Sweden).
Consumers (observers to Trial Management Group): M Carling (RAGE), J Pritchard (independent), M King (ex-member of RAGE, deceased), E Parkin (ex-member of RAGE).
Funders (observers to Trial Steering Committee): K Law (Cancer Research UK), S Perkins (Medical Research Council), U Wells (Department of Health).
Conflict of interest statement
J Brown is an employee of Eli Lilly & Company, who are not a sponsor. We declare that we have no conflict of interest.
Correspondence to: Prof John Yarnold, Department of Clinical Radiotherapy, Royal Marsden Hospital, Sutton, Surrey, SM2 5PT, UK
References
- 1.Fisher B, Redmond C, Fisher ER. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JA, Bartelink H, Fentiman IS. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr. 1992;11:15–18. [PubMed] [Google Scholar]
- 3.Veronesi U, Luini A, Del Vecchio M. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328:1587–1591. doi: 10.1056/NEJM199306033282202. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino J, Redmond C. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 5.Paterson R. The treatment of malignant disease by radium and x-rays. First edn. Edward Arnold; London: 1948. [Google Scholar]
- 6.Williams MV, James ND, Summers ET, Barrett A, Ash DV. National survey of radiotherapy fractionation practice in 2003. Clin Oncol (R Coll Radiol) 2006;18:3–14. doi: 10.1016/j.clon.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Peters MV. Carcinoma of the breast. Stage II—radiation range. Wedge resection and irradiation. An effective treatment in early breast cancer. JAMA. 1967;200:134–135. doi: 10.1001/jama.200.2.134. [DOI] [PubMed] [Google Scholar]
- 8.Ash DV, Benson EA, Sainsbury JR, Round C, Head C. Seven-year follow-up on 334 patients treated by breast conserving surgery and short course radical postoperative radiotherapy: a report of the Yorkshire Breast Cancer Group. Clin Oncol (R Coll Radiol) 1995;7:93–96. doi: 10.1016/s0936-6555(05)80808-8. [DOI] [PubMed] [Google Scholar]
- 9.Olivotto IA, Weir LM, Kim-Sing C. Late cosmetic results of short fractionation for breast conservation. Radiother Oncol. 1996;41:7–13. doi: 10.1016/s0167-8140(96)91824-1. [DOI] [PubMed] [Google Scholar]
- 10.Clark RM, Whelan T, Levine M. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 11.Magee B, Stewart AL, Swindell R. Outcome of radiotherapy after breast conserving surgery in screen detected breast cancers. Clin Oncol (R Coll Radiol) 1999;11:40–45. doi: 10.1053/clon.1999.9007. [DOI] [PubMed] [Google Scholar]
- 12.Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for postlumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;47:1219–1228. doi: 10.1016/s0360-3016(00)00567-8. [DOI] [PubMed] [Google Scholar]
- 13.McBain CA, Young EA, Swindell R, Magee B, Stewart AL. Local recurrence of breast cancer following surgery and radiotherapy: incidence and outcome. Clin Oncol (R Coll Radiol) 2003;15:25–31. doi: 10.1053/clon.2002.0165. [DOI] [PubMed] [Google Scholar]
- 14.Fyles AW, McCready DR, Manchul LA. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351:963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 15.Whelan T, MacKenzie R, Julian J. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 16.Overgaard M, Bentzen SM, Christensen JJ, Madsen EH. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol. 1987;9:1–11. doi: 10.1016/s0167-8140(87)80213-x. [DOI] [PubMed] [Google Scholar]
- 17.Rodger A. Fears over radiotherapy fractionation regimens in breast cancer. Proposed UK trial needs to define techniques as well as numbers of treatments. BMJ. 1998;317:155–156. doi: 10.1136/bmj.317.7152.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams M. Inappropriate use of linear-quadratic analysis of tumor response. Int J Radiat Oncol Biol Phys. 1985;11:1570. doi: 10.1016/0360-3016(85)90349-9. 13P6. [DOI] [PubMed] [Google Scholar]
- 19.Dische S, Joslin CA, Miller S. The RAGE litigation. Radiation Action Group Exposure. Lancet. 1998;351:1967–1968. doi: 10.1016/s0140-6736(05)78663-3. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Ackerman I, Franssen E, MacKenzie RG, Thomas G. Does the dose fractionation schedule influence local control of adjuvant radiotherapy for early stage breast cancer? Int J Radiat Oncol Biol Phys. 1999;44:99–104. doi: 10.1016/s0360-3016(98)00507-0. [DOI] [PubMed] [Google Scholar]
- 21.The START Trialists' Group The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008 doi: 10.1016/S1470-2045(08)70077-9. published online March 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopwood P, Haviland J, Mills J, Sumo G, Bliss JM. The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2208 women recruited to the UK START Trial (Standardisation of Breast Radiotherapy Trial) Breast. 2007;16:241–251. doi: 10.1016/j.breast.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 23.ICRU. Prescribing, recording and reporting photon beam therapy. Bethsada: Interantional Commission on Radiation Units and Measurements (ICRU), 1999: Report 62. Supplement to ICRU Report 50.
- 24.Venables K, Winfield E, Deighton A, Aird E, Hoskin P. Breast radiotherapy phantom design for the START trial. START trial management group. Br J Radiol. 2000;73:1313–1316. doi: 10.1259/bjr.73.876.11205676. [DOI] [PubMed] [Google Scholar]
- 25.Venables K, Winfield E, Deighton A, Aird E, Hoskin P. The START trial-measurements in semi-anatomical breast and chest wall phantoms. Phys Med Biol. 2001;46:1937–1948. doi: 10.1088/0031-9155/46/7/314. [DOI] [PubMed] [Google Scholar]
- 26.Venables K, Winfield E, Deighton A, Aird E, Hoskin P. A survey of radiotherapy quality control practice in the United Kingdom for the START trial. Radiother Oncol. 2001;60:311–318. doi: 10.1016/s0167-8140(01)00376-0. [DOI] [PubMed] [Google Scholar]
- 27.Venables K, Winfield EA, Aird EG, Hoskin PJ. Three-dimensional distribution of radiation within the breast: an intercomparison of departments participating in the START trial of breast radiotherapy fractionation. Int J Radiat Oncol Biol Phys. 2003;55:271–279. doi: 10.1016/s0360-3016(02)03808-7. [DOI] [PubMed] [Google Scholar]
- 28.Venables K, Miles EA, Aird EG, Hoskin PJ. The use of in vivo thermoluminescent dosimeters in the quality assurance programme for the START breast fractionation trial. Radiother Oncol. 2004;71:303–310. doi: 10.1016/j.radonc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Venables K, Miles EA, Hoskin PJ, Aird EG. Verification films: a study of the daily and weekly reproducibility of breast patient set-up in the START trial. Clin Oncol (R Coll Radiol) 2005;17:337–342. doi: 10.1016/j.clon.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Venables K, Miles EA, Aird EG, Hoskin PJ. What is the optimum breast plan: a study based on the START trial plans. Br J Radiol. 2006;79:734–739. doi: 10.1259/bjr/80814021. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson NK, Ahmedzai S, Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 32.Sprangers MA, Groenvold M, Arraras JI. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 33.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. 2001;37:189–197. doi: 10.1016/s0959-8049(00)00353-1. [DOI] [PubMed] [Google Scholar]
- 34.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 35.Owen JR, Ashton A, Bliss JM. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 36.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarnold J, Ashton A, Bliss J. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Clarke M, Collins R, Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]