Abstract
Phosphorylation of estrogen receptor-α (ERα) at specific residues in transcription activation function 1 (AF-1) can stimulate ERα activity in a ligand-independent manner. This has led to the proposal that AF-1 phosphorylation and the consequent increase in ERα activity could contribute to resistance to endocrine therapies in breast cancer patients. Previous studies have shown that serine 118 (S118) in AF-1 is phosphorylated by extracellular signal-regulated kinases 1 and 2 (Erk1/2) mitogen-activated protein kinase (MAPK) in a ligand-independent manner. Here, we show that serines 104 (S104) and 106 (S106) are also phosphorylated by MAPK in vitro and upon stimulation of MAPK activity in vivo. Phosphorylation of S104 and S106 can be inhibited by the MAP-erk kinase (MEK)1/2 inhibitor U0126 and by expression of kinase-dead Raf1. Further, we show that, although S118 is important for the stimulation of ERα activity by the selective ER modulator 4-hydroxytamoxifen (OHT), S104 and S106 are also required for the agonist activity of OHT. Acidic amino acid substitution of S104 or S106 stimulates ERα activity to a greater extent than the equivalent substitution at S118, suggesting that phosphorylation at S104 and S106 is important for ERα activity. Collectively, these data indicate that the MAPK stimulation of ERα activity involves the phosphorylation not only of S118 but also of S104 and S106, and that MAPK-mediated hyperphosphorylation of ERα at these sites may contribute to resistance to tamoxifen in breast cancer.
Introduction
Estrogen receptor-α (ERα) is a member of the nuclear receptor superfamily of ligand-regulated transcription factors, and is required for development and maintenance of the female and male reproductive systems, bone, and cardiovascular system, as well as being significant for certain brain functions (Couse & Korach 1999). Additionally, estrogens stimulate breast cancer progression and ∼70–80% of breast cancers express ERα. The presence of ERα correlates with likelihood of response to anti-estrogens, such as tamoxifen (Osborne 1998), and to aromatase inhibitors that inhibit estrogen biosynthesis (Henderson & Piccart-Gebhart 2005), demonstrating the importance of ERα in breast cancer progression. However, a proportion of patients with ERα-positive disease do not respond to endocrine therapy. Further, of those patients who respond, many who initially present with localized disease, and all with advanced breast cancer, relapse. Most resistant tumors remain ERα-positive and frequently respond to alternative anti-estrogen treatment, indicative of a continued role for ERα in breast cancer cell proliferation (Ali & Coombes 2002). Augmentation of ERα activity in the presence of estrogen or anti-estrogens, and/or ligand-independent activation of ERα, could provide an important mechanism for resistance to anti-estrogen therapies. In agreement with this possibility, a recent study shows that levels of S118 phosphorylation are increased following breast cancer recurrence following tamoxifen treatment (Sarwar et al. 2006).
ERα regulates gene expression through direct binding to estrogen-response elements within the promoters of estrogen-regulated genes, or by recruitment to DNA through interaction with other transcription factors (Gronemeyer 1991, Bjornstrom & Sjoberg 2005). Transcriptional regulation by ERα is partly mediated by transcription activation function, AF-2, which is intrinsic to the ligand-binding domain (LBD). Estrogen binding to the LBD results in a conformational change that allows recruitment of coactivators to the LBD (Brzozowski et al. 1997, Shiau et al. 1998, Glass & Rosenfeld 2000). A second transcription activation function, AF-1, is encoded within the N-terminal 180 amino acids. AF-1 activity is promoter and cellular context dependent (Tzukerman et al. 1994), and can be influenced by the expression profile of specific coactivators (Smith et al. 1997). Although there is evidence to suggest that phosphorylation of AF-1 alone can enhance activity (Bunone et al. 1996, Ignar-Trowbridge et al. 1996), ligand binding to AF-2 can also induce AF-1 activity (Metzger et al. 1992). This suggests that, in a basal state, AF-1 activity is blocked by AF-2. Finally, AF-1 and AF-2 can act synergistically in a promoter- and cell-specific manner (Gronemeyer 1991, Tsai & O'Malley 1994, Beato et al. 1995).
Tamoxifen is an example of a mixed estrogen agonist/antagonist, also termed selective ER modulators, which has been proposed to act by inhibiting the LBD/AF-2, whilst allowing activation of AF-1 (Berry et al. 1990, McDonnell et al. 1995, Brzozowski et al. 1997, Shiau et al. 1998). Structural studies have indicated that the inhibition of the LBD/AF-2 in the tamoxifen-bound ERα is due to the positioning of helix 12 in the hydrophobic cleft to which transcriptional coactivators, such as the p160 family members, are recruited, thereby preventing coactivator recruitment. The same cleft can also be involved in corepressor binding, and tamoxifen has been suggested to allow corepressor recruitment to the LBD (Nettles & Greene 2005). The nature of AF-1 activation by ligands such as tamoxifen, and in the absence of ligand, is unknown, but may involve phosphorylation of ERα. Previous studies have shown that in ovariectomized mice, uterine proliferation (which is normally estrogen- dependent) can be mediated by growth factors such as epidermal growth factor (EGF), and that the EGF-mediated uterine stimulation is ERα dependent (Ignar-Trowbridge et al. 1992, Curtis et al. 1996). ERα may become phosphorylated in response to EGF and other growth factors, resulting in ligand-independent ERα activation (Ignar-Trowbridge et al. 1993).
Phosphorylation site mapping has demonstrated that serine 118 (S118) in AF-1 is phosphorylated by Erk1/2 MAPK, resulting in stimulation of ERα activity (Kato et al. 1995, Bunone et al. 1996), whilst phosphorylation of serine 167 (S167) in AF-1 by AKT and p90RSK also stimulates ERα activity (Joel et al. 1998a, Campbell et al. 2001). The ERα AF-1 is additionally phosphorylated at serines 104 (S104) and/or 106 (S106) (Le Goff et al. 1994). Phosphorylation at these sites by Cdk2 (Trowbridge et al. 1997, Rogatsky et al. 1999) and GSK3 (Medunjanin et al. 2005) has been reported, and our own data have previously indicated that these sites might also be targets for MAPK (Chen et al. 2002). Increased ERα activity due to AF-1 phosphorylation mediated by MAPK and/or other kinases could therefore be at least partly responsible for resistance to endocrine therapy in breast cancer. This hypothesis is supported by findings that stimulation of growth factor-regulated pathways can augment the agonist properties of tamoxifen (Smith 1998) and inhibit tamoxifen-induced growth suppression and apoptosis in breast cancer cells (Benz et al. 1993, Pietras et al. 1995, Campbell et al. 2001). Here, we investigate phosphorylation at serines 104 and 106 by Erk2 MAPK in vitro and in response to MAPK activity in vivo, and determine their contribution to ERα activity. Transcription reporter assays using ERα phosphorylation site mutants suggest that phosphorylation at all three sites contribute significantly to AF-1 activity, and in particular are required for tamoxifen to act as an ERα agonist.
Materials and methods
Plasmids
The expression vectors pSG5-HEG0 and pSG5-HE15, encoding full length and AF-2-truncated human ERα (ERα-ΔLBD) respectively have previously been described (Tora et al. 1989a). pGEX1λT-ERα and pGEX1λT-ERα-ΔLBD were generated by cloning ERα encoding EcoRI fragments from pSG5 into pGEX1λT (GE Healthcare UK Ltd, Little Chalfont, UK). ERα mutants were generated by site-directed mutagenesis, according to manufacturer's protocols (Stratagene, La Jolla, CA, USA). pEF-RasV12, expression vector for constitutively active Ha-Ras, was kindly provided by Dr R Treisman (LRI, London, UK). pCMV-Raf CAAX and pCMV-Raf S621A were purchased from Clontech (Saint-Germaine-en-Laye, France).
Antibodies
Peptides corresponding to amino acids 97-112 of human ERα, having the sequence [C]-FPPLNSVPSPSPLMLLH (phospho-S104) or [C]-FPPLNSVSPPSPLMLLH (phospho-S106), were used to generate rabbit polyclonal antisera, as described (Chen et al. 2002). Antibodies specific for ERα phosphorylated at S118 (16J4, New England Biolabs, Hitchin, UK), ERα (6F11, Novocastra Laboratories, UK), MAPK (sc-93, Santa Cruz Biotechnology, Heidelberg, Germany) and P-MAPK (New England Biolabs) were obtained commercially. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse immunoglobulins were purchased from DAKO Ltd, Ely, UK.
Immunoblot analysis
Cells were grown in Dulbecco's modified Eagle's medium (DMEM) lacking phenol red and supplemented with 10% dextran-coated charcoal-stripped fetal calf serum (DSS) for 3 days prior to plating in six-well plates at 300 000 cells/well. Cells were transfected using Fugene 6 (Millipore, Wafford, UK), with 100 ng pSG5 empty vector or ERα expression construct, 5 ng empty vector or Ras/Raf expression construct, and 500 ng pBS+ carrier DNA, as appropriate. After 48 h the cells were treated with ethanol carrier, estradiol (E2; 10 nM), 4-hydroxytamoxifen (OHT; 100 nM) or ICI182,780 (ICI; 100 nM) 30 min prior to harvest, phorbol 12-myristate 13 acetate (PMA; 100 nM) was added 15 min, and U0126 (10 μM) was added 60 min, prior to harvesting, as appropriate. Cells were washed and harvested directly into Laemmli buffer and immunoblotting was performed, as described (Sarwar et al. 2006). For peptide competition experiments, the primary antibodies were pre-incubated with 10 μg/ml of the appropriate peptide (Chen et al. 2002). Quantitation of phospho-ERα signal was performed by densitometry of scanned films using ImageQuant software, and expressed relative to the respective total ER level.
Kinase assays
The glutathione-S-transferase (GST) fusion proteins, GST-ERα and GST-ERα-ΔLBD were expressed in Escherichia coli (Rosetta strain, Merck Chemicals Ltd, Nottingham, UK) following induction with 0. 1 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) for 3 h at 25 °C. Cells (50 ml) were lysed with 10 ml XTractor buffer (BD Biosciences, Oxford, UK) containing 1 mM dithiothreitol (DTT) and protease inhibitors, and the lysates cleared by high-speed centrifugation. The lysates were incubated with glutathione-sepharose beads at 4 °C for 1 h, and the beads were washed thrice with TBS containing 1 mM DTT and protease inhibitors. GST-ERα beads were resuspended in 1× kinase buffer, 200 μM ATP, ±E2 (10 nM) and kinase (Erk2 and GSK3, New England Biolabs, UK; Cdk2/cyclin A, Cdk2/cyclin E, Cdk4/cyclin D1, Cdk7/cyclin H/MAT1, AKT1, and AKT3; New England Biolabs), according to manufacturer's instructions. For radioactive kinase assays, 50 μM cold ATP and 10 μM 32PγATP (Amersham) was used. Reactions were incubated at 30 °C for 30 min and processed by SDS-PAGE followed by autoradiography or immunoblot.
Reporter assays
Cells were grown in DMEM lacking phenol red and supplemented with 10% DSS for 3 days prior to plating in 24-well plates at 50 000 cells/well. Cells were transfected using Fugene 6 (Roche), with 100 ng pERE3-TATA-luc and pRL-TK reporters, 10 ng pSG5 empty vector or ERα expression construct, 50 ng empty vector or Ras/Raf expression vector, and 500 ng pBS+ carrier DNA. After 4 h, the medium was replaced with fresh media containing ethanol carrier, E2, OHT or ICI 182 780, at concentrations indicated in figures. After a further 20 h, the cells were harvested and luciferase levels determined using Dual-Glo reagents (Promega). For experiments in which U0126 was used, 10 nM U0126 was added 1 h prior to the addition of ligands and the cells were harvested after a further 7 h. Firefly luciferase levels were corrected for transfection efficiency using corresponding renilla luciferase levels. The activity for wild-type ERα in the absence of ligand was taken as one, with all other activities shown relative to this. All experiments were independently repeated at least four times, and the data presented as mean values with s.e.m. error bars.
Results
Antisera display specificity for ERα phosphorylated at S104 and S106
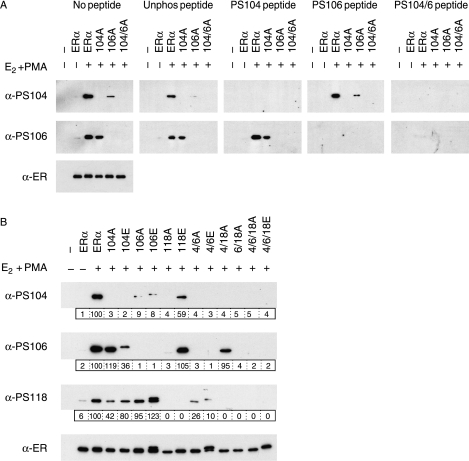
Serines 104 and/or 106 have been shown to be phosphorylated by Cdk2/cyclin A and Cdk2/cyclin E (Trowbridge et al. 1997, Rogatsky et al. 1999), and GSK3 (Medunjanin et al. 2005). To further characterize ERα phosphorylation at these residues, we generated rabbit antisera specific for phosphorylation at S104 (α-PS104) or at S106 (α-PS106). S104 phosphorylation was stimulated following treatment of ERα-transfected COS-1 cells with E2 and PMA (Fig. 1A). ERα mutants in which S104 was substituted by alanine were not detected by α-PS104, whilst mutation of S106 (106A) reduced but did not abolish S104 phosphorylation. S106 phosphorylation was also stimulated by E2 and PMA, and blocked by mutants of S106 but not S104. Competition with phosphorylated peptides further confirmed that α-PS104 and α-PS106 are specific for ERα phosphorylated at S104 and S106 respectively. Detection of phopho-S104 ERα and phospho-S106 ERα were blocked by pre-incubation of the respective antiserum with a 100-fold excess of peptides containing a phosphorylated S104 (PS104 and PS104/6), and phosphorylated S106 (PS106 and PS104/6) respectively.
Figure 1.
Characterization of phospho-specific antisera by peptide competition and phosphorylation site substitutions. Lysates prepared from COS-1 cells transiently transfected with an empty expression vector (−), or expression vectors for wild-type ERα or ERα in which S104, S106, and/or S118 had been substituted by alanine (A) or glutamic acid (E), as indicated, were immunoblotted using antibodies for total ERα (α-ER), or for ERα phosphorylated at S104 (α-PS104) or S106 (α-PS106). Cells were treated with ethanol solvent (−), or 17β-estradiol (E2; 10 nM) and 12-tetradecanoylphorbol-13-acetate (PMA; 100 nM), for 30 min prior to harvesting. (A) Replicate blots were incubated with primary antibody (no peptide), or antibody that had been pre-incubated with a 100-fold excess (10 μg/ml) of a peptide encompassing the ERα phosphorylation site; either unphosphorylated (unphos) or phosphorylated (PS104, PS106, or dual PS104/6) versions, as indicated. (B) Lysates were additionally immunoblotted using antibody for ERα phosphorylated at S118 (α-PS118). Levels of phospho-ERα were quantitated in relation to the respective total ERα level (boxed, below each immunoblot).
Investigation of phosphorylation using mutants in which S104, S106, and S118 were substituted by alanine or glutamic acid, either singly or together, showed that S104 phosphorylation was influenced by the status of S106 and S118 (Fig. 1B). This result could potentially be explained by a reduced efficacy of α-PS104 binding when S106 or S118 are not phosphorylated, but this is not supported by the fact that α-PS104 binding was competed similarly by both the PS104 and PS104/6 peptides (Fig. 1A). Hence, phosphorylation at S106 and S118 may be important for subsequent phosphorylation of S104. S106 phosphorylation was lower in the case of S118A, but not S104A, suggesting a role for S118 in S106 phosphorylation. In the case of S118, the data suggest that S104, but not S106 influences S118 phosphorylation. Together, these findings are indicative of crosstalk between S104, S106, and S118, which regulates phosphorylation at these sites.
Phosphorylation at S104 and S106 is induced by ERα ligands and PMA, and by activated Raf/Ras
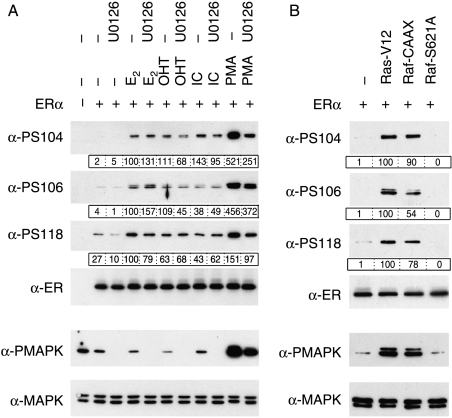
S118 phosphorylation is stimulated by E2, as well as anti-estrogens (Joel et al. 1995, 1998b) and can be mediated by Cdk7 (Chen et al. 2000, 2002). Further, S118 can be phosphorylated by MAPK in response to EGF and PMA (Kato et al. 1995, Bunone et al. 1996, Chen et al. 2002). Phosphorylation of S104 and S106 was also stimulated by E2, OHT, ICI, and PMA (Fig. 2A). In the case of PMA, this phosphorylation was decreased by the MEK1/2 inhibitor U0126, and corresponds to a decrease in activated Erk1/2 MAPK. The phosphorylation induced by ligands was mostly unaffected by the addition of U0126, except that of S104 in response to OHT and ICI, which were reduced. Co-expression of ERα with constitutively active Ha-Ras (Ras-V12) or Raf1 (Raf-CAAX) resulted in enhanced phosphorylation of S104, S106, and S118, whereas the kinase-dead mutant of Raf1 (Raf-S621A) did not (Fig. 2B). Together, these data indicate that S104, S106, and S118 can be phosphorylated by Erk1/2 MAPK in response to activation of the upstream pathways, and also though MAPK-independent pathways upon ligand binding.
Figure 2.
Phosphorylation of S104 and S106 is stimulated by ERα ligands and by activators of MAPK. Immunoblots of lysates prepared from COS-1 cells transfected with empty expression vector (−) or expression vector for ERα, were performed as for Fig. 1. Lysates were additionally immunoblotted using antibodies for total (α-MAPK) and phosphorylated (α-PMAPK) Erk1/2 MAPK. Levels of phospho-ERα were quantitated in relation to the respective total ERα level (boxed, below each immunoblot). (A) Cells were pre-incubated with U0126 (10 μM) for 1 h, followed by the addition of E2 (10 nM), 4-hydroxytamoxifen (OHT, 100 nM), ICI 182 780 (ICI; 100 nM) or PMA (100 nM), as indicated, and cells harvested 30 min later. (B) Cells were transfected with expression vector for ERα, together with expression vectors for Ras-V12, Raf-CAAX or Raf-S621A, as indicated.
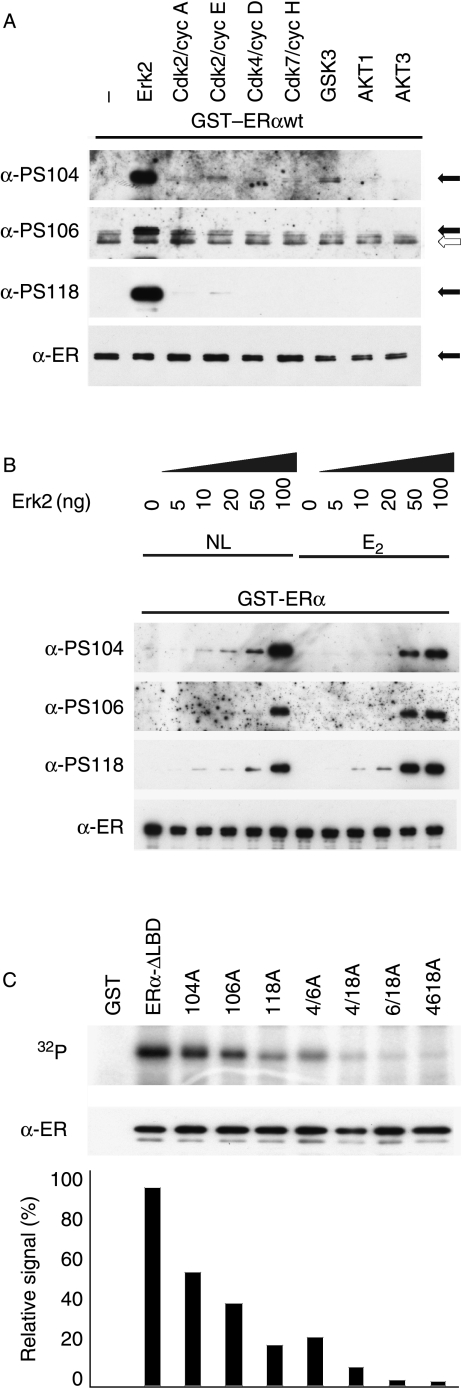
In agreement with the above findings, purified Erk2 readily phosphorylated GST-ERα at S104, S106, and S118 (Fig. 3A). Cdk2 also phosphorylated S104 and S118, but did not appear to phosphorylate S106. GSK3 also phosphorylated S104, with longer exposures showing phosphorylation at S106, but not S118. However, Cdk2 and GSK3-mediated ERα phosphorylation appeared to be less significant than that mediated by Erk2. Similar results were obtained using recombinant ERα purified from SF9 insect cells and transiently transfected ERα in crude COS-1 extracts (data not shown). The presence of estrogen provided a moderate enhancement of Erk2-dependent phosphorylation (Fig. 3B), but confirmed that ERα can be phosphorylated by MAPK in the absence of ligand. To investigate the relative levels of phosphorylation at these sites, GST-ERα-ΔLBD and alanine substitution mutants were incubated with Erk2 in the presence of 32P-γATP (Fig. 3C). Substitution of S104, S106, or S118 by alanine resulted in significant reductions in Erk2 phosphorylation, to ∼40, 60, and 80% respectively, relative to wild-type ERα, with negligible detectable phosphorylation when all three sites were mutated, suggesting that S104, S106, and S118 are the only significant Erk2 phosphorylation sites within amino acids 1–281 of ERα. These data further support the possibility that phosphorylation at each of these sites influences phosphorylation at the other sites.
Figure 3.
S104 and S106 are phosphorylated by Erk2 in vitro. (A) Purified GST-ERα was incubated with a panel of purified kinases, as indicated, according to manufacturer's instructions, followed by immunoblotting with phospho-specific antibodies (α-PS104, α-PS106, and α-PS118) or α-ER. The filled arrows indicate the position of GST-ERα, and the open arrow indicates a non-specific product seen with α-PS106 antisera. (B) Purified GST-ERα was incubated with increasing amounts of purified Erk2 MAPK (0, 5, 10, 20, 50, and 100 ng) in the absence of ligand (NL) or in the presence of E2 (10 nM), followed by immunoblotting as before. (C) Purified GST, or wild-type GSαT-ERα-ΔLBD (ERα-ΔLBD) or GST-ERα-ΔLBD in which S104, S106, and/or S118 had been substituted by alanine (A), as indicated, were incubated with Erk2 in the presence of 32P-γATP, followed by SDS-PAGE and autoradiography of the dried gel. Immunoblotting a duplicate gel with α-ER was used to determine the relative levels of each mutant. The bar chart shows quantification of each 32P signal relative to the respective total GST-ERα-ΔLBD level.
S104, S106, and S118 are important for ERα activity
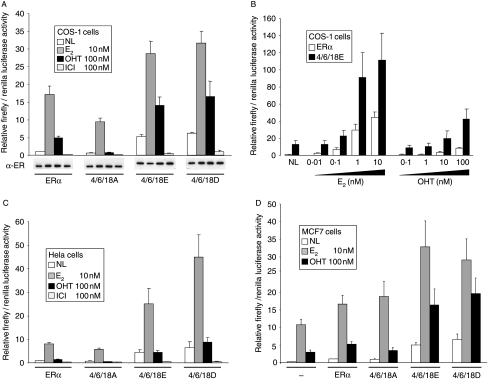
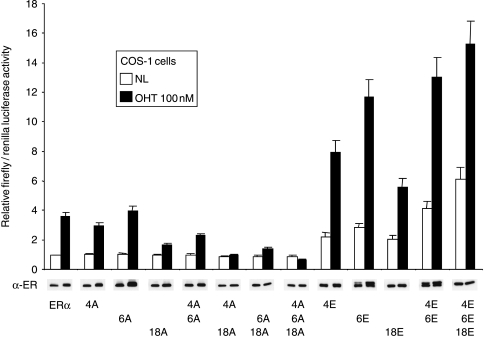
In reporter gene assays, substitution of S104, S106, and S118 by alanine (4/6/18A) resulted in a reduction in ERα activity in the presence of E2 by nearly 50%, whilst substitution with glutamic or aspartic acid, to ‘mimic’ phosphorylation at these sites, stimulated ERα activity two- to threefold (Fig. 4A). ERα activity for the alanine mutant in the presence of OHT was reduced by >80%, and was two- to threefold higher for the 4/6/18E and 4/6/18D mutants. Significant ERα activation (sevenfold relative to wild-type ERα) was also observed in the absence of ligand for 4/6/18E and 4/6/18D. These data suggest that phosphorylation at one or more of these residues is critical for AF-1 activity and for the agonist activity of OHT. Western analysis of total ERα levels indicated relatively equal levels of expression across the mutants and across different ligand treatments. ICI is generally thought to decrease ERα levels, but in many cases a change in solubility is mistaken for degradation (Lipfert et al. 2005); the assays here have measured total ERα levels.
Figure 4.
Effect of phosphorylation site mutations on ERα activity. (A and B) COS-1, (C) HeLa, and (D) MCF7 cells were co-transfected with the ERE-3-TATA-firefly luciferase reporter gene, a renilla luciferase control reporter gene, and expression vectors encoding wild-type ERα (ERα) or ERα in which S104, S106 and S118 were substituted by alanine (4/6/18A), glutamic acid (4/6/18E) or aspartic acid (4/6/18D), as indicated. Cells were treated with ethanol solvent (no ligand; NL), E2 (10 nM), OHT (100 nM) or ICI (100 nM), as indicated, except (B) where E2 was added at 0·01, 0·1, 1, and 10 nM and OHT at 0·1, 1, 10, or 100 nM. The cells were harvested for luciferase assays 20 h later. Results are presented as relative ratios of firefly to renilla control luciferase activities, as described in Materials and methods. (A) A parallel transfection series was assayed for ERα expression by immunoblot (α-ER). (D) MCF7 cells were additionally transfected with an empty vector control (−) in order to determine the contribution of endogenous ERα.
To examine whether acidic substitutions in ERα resulted in ligand hypersensitivity, we examined ERα activity by reporter assay in the presence of increasing concentrations of estrogen and OHT (Fig. 4B). The acidic ERα mutant displayed an enhanced activity, of similar magnitude compared with wild-type ERα, at all ligand concentrations. Hence, although the acidic mutant could yield equivalent levels of activity at lower ligand concentrations compared with that of wild-type ERα at higher ligand concentrations (compare 4/6/18E at 0·1 nM E2 with wt at 1 nM E2, and 4/6/18E at 1 mM OHT with wt at 100 mM OHT), there was no evidence for enhanced ligand sensitivity.
ERα AF-1 is only weakly active in HeLa cells, with the majority of ERα activity in HeLa cells being due to AF-2 (Bocquel et al. 1989). Further, the lack of AF-1 activity correlates with lack of agonist activity for OHT in HeLa cells (Bocquel et al. 1989, Berry et al. 1990). Triple acidic substitution stimulated ERα activity in the absence of ligand, as well as in the presence of E2 (Fig. 4C). Activity in the presence of OHT was also increased, but was not significantly greater than that obtained in the absence of ligand. Hence, acidic substitutions in AF-1 enhanced ERα activity in HeLa cells in a similar manner to COS-1 cells, but did not activate an OHT response. Finally, in the estrogen responsive and ERα-positive MCF7 breast cancer cell line, transfection of ERα stimulated reporter gene activity over and above the activity observed for endogenous ERα (Fig. 4D). Reporter gene activity for the glutamic and aspartic acid mutants was significantly greater in the absence of ligand or in the presence of E2 or OHT, compared with the activities obtained for wild-type ERα, generally following what was seen using COS-1 cells.
Phosphorylation at S104, S106, and S118 contributes to ERα activity
The above data demonstrate that serines 104, 106, and 118 are critical for AF-1 activity, and likely for AF-1 cooperativity with AF-2. In order to define the relative importance of these sites, single and double mutants were examined for their effect on ERα activity (Fig. 5). Substitution of S118 by alanine reduced OHT-stimulated ERα activity. Alanine substitution of 104A or 106A had little effect on OHT stimulation of ERα, although ERα-104A/106A was significantly less active than wild-type ERα. Interestingly, S104 or S106 mutations together with mutation of S118 reduced ERα activity more potently than the S118 substitution alone. On the basis of these findings, the order of importance of the three serines appears to be S118>S104>S106. Although mutation of S106 to alanine had very little effect on ERα activity, its substitution by glutamic acid resulted in the greatest stimulation in ERα activity, with the order of activity for the three mutants being S106>S104>S118, opposite to the relative activities observed with the alanine substitutions. Nevertheless, in agreement with the findings from the alanine mutants, multiple substitutions increased ERα activity correspondingly, again suggesting that all three phosphorylation sites are important for AF-1 activity.
Figure 5.
Effect of individual site mutations on ERα activity. COS-1 cells were co-transfected with ERE-3-TATA-luc, pRL-TK, and the wild-type ERα expression vector (ERα) or versions with alanine or glutamic acid substitutions, as indicated. Cells were treated and luciferase assays carried out as for Fig. 4. A parallel transfection series was assayed for ERα expression by immunoblot (α-ER).
Activated Ras/Raf enhances ERα activity
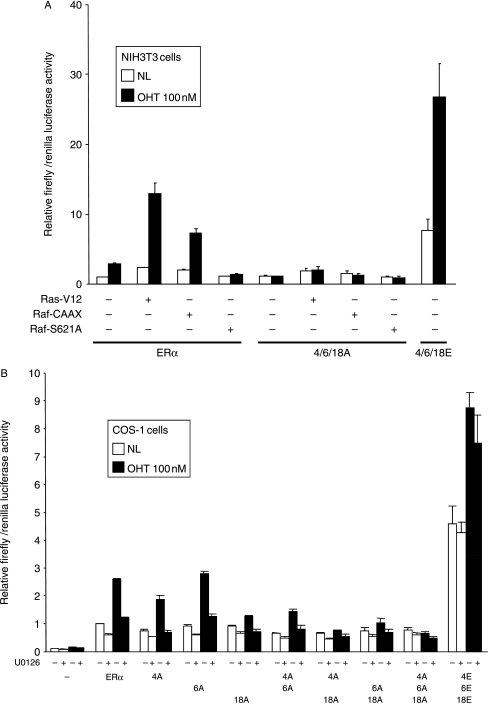
In order to confirm the role of MAPK in the phosphorylation of S104 and S106, NIH-3T3 cells were co-transfected with reporter constructs and ERα, together with expression vectors encoding Ras or Raf (Fig. 6A). ERα activity was significantly enhanced by constitutively active Ha-Ras (Ras-V12) and Raf1 (Raf-CAAX), and was inhibited by a kinase-dead mutant of Raf1 (Raf-S621A). By contrast, ERα-104A/106A/118A activity was not stimulated by Ras-V12 or Raf-CAAX. U0126 did not significantly inhibit the activities of ERα-104A/106A/118A or 104E/106E/118E, but did inhibit the activities of wt ERα and those encoding individual alanine mutants (Fig. 6B). Similar results were obtained by treatment with another MEK1/2 inhibitor, PD98059 (data not shown). These data suggest that the activation of the Ras/Raf/MEK/MAPK signal transduction pathway results in ERα activation through phosphorylation at S104, S106, and S118.
Figure 6.
Effect of MAPK signaling activators and inhibitors on ERα activity. (A) NIH3T3 cells were co-transfected with ERE-3-TATA-luc, pRL-TK, and the wild-type ERα expression vector (ERα), or versions in which S104, S106, and S118 were substituted by alanine (4/6/18A) or glutamic acid (4/6/18E), as indicated. Cells were additionally co-transfected with empty expression vector (−) or expression vectors for Ras-V12, Raf-CAAX, or Raf-S621A, as shown. Cells were treated and luciferase assays carried out as for Fig. 4. (B) COS-1 cells were transfected with ERE-3-TATA-luc, pRL-TK, and the wild-type ERα expression vector (ERα) or versions with alanine or glutamic acid substitutions, as indicated. Cells were pre-incubated with U0126 (10 μM), 16 h following transfection, as indicated, for 1 h prior to addition of OHT, and harvested for luciferase assays 7 h later.
Discussion
Phosphorylation at serine 118 in the AF-1 domain of ERα has been demonstrated by us and other researchers to be important for ERα activity (Ali et al. 1993, Le Goff et al. 1994, Kato et al. 1995, Bunone et al. 1996, Chen et al. 2000, 2002). Phosphorylation of serines 104 and 106 has also been shown to augment ERα activity (Le Goff et al. 1994), but their phosphorylation and function have not been investigated thoroughly.
In order to investigate phosphorylation of these residues in greater detail, we generated phosphorylation site-specific antisera. The antisera demonstrated appropriate specificity for ERα phosphorylated at S104 or S106, determined using multiple point mutants of S104 and S106 and peptide competition of antibody binding, and confirms that both S104 and S106 of ERα can be phosphorylated. Interestingly, phosphorylation at S104 appeared to be substantially reduced by substitutions at S106 and S118, and S106 phosphorylation reduced by S104E and S118A. S118 phosphorylation was also somewhat reduced by S104 substitutions. The epitopes for the α-PS104 and α-PS106 antisera may overlap, but the peptide competition studies of antibody specificity go some way to suggest that these antisera are not influenced by the phosphorylation status at the respective adjacent site. Thus, in general, phosphorylation at these sites appears to be partly interdependent. We cannot, of course, rule out the possibility that these substitutions simply affect an ERα conformation required for efficient phosphorylation.
Previous studies have indicated that the agonist activity of tamoxifen (OHT) is due to activation of AF-1, whilst AF-2 function is inhibited (Tora et al. 1989b, Berry et al. 1990). In reporter gene assays using OHT, alanine substitution of S104, S106, or S118 inhibited ERα activity, with the greatest inhibition being observed with substitution of all three residues. Similarly, substitution of the individual sites by glutamic acid augmented ERα activity, with the greatest activity being seen for the triple mutant. Additionally, these sites appear to be important for estrogen-induced and ligand-independent ERα activities. These data suggest that phosphorylation at some or all three sites is critical for AF-1 activity and for the agonist action of OHT. Substitution of S118 to alanine resulted in a greater loss of function compared with that of S104 and S106, but glutamic acid substitutions suggested that achieving a high level of phosphorylation at S104 and S106 might have a greater impact upon ERα function than that at S118. These apparently contradictory results may be explained by functionally stronger S104 and S106 sites being phosphorylated at lower levels in COS-1 cells, relative to that at S118. It is additionally difficult to assign levels of importance to these sites, since immunoblot analysis of ERα substitution mutants suggested that S104, S106, and S118 phosphorylation is partly interdependent. Our data instead suggest that these three sites, together, comprise a complex phosphorylated domain, involved in augmenting ERα activity.
Reporter gene assays indicated that the activity of the ERα-4/6/18E mutant (and 4/6/18D mutant) in the absence of ligand was at a level similar to that seen for wild-type ERα in the presence of OHT, suggesting that OHT-induced phosphorylation at these residues may be sufficient for AF-1 activity. However, the further stimulation of ERα-4/6/18E activity by tamoxifen in COS-1 cells, suggests that the agonist activity of OHT is not solely due to stimulation of phosphorylation at these sites. Indeed, acidic substitutions did not lead to a restoration of OHT-induced ERα activity in HeLa cells, in which ERα AF-1 activity is known to be low and associated with little or no stimulation by OHT (Tora et al. 1989b, Berry et al. 1990). These data further support the view that the agonist activity of OHT is not due simply to stimulation of AF-1 phosphorylation. Thus, although OHT agonist activity is dependent upon (and induces) phosphorylation, other factors – possibly cell context-dependent expression of coactivators and corepressors (Shang & Brown 2002) – are also likely to be involved.
Previous reports have shown that Cdk2 (Trowbridge et al. 1997, Rogatsky et al. 1999) and GSK3 (Medunjanin et al. 2005) can phosphorylate S104 and/or S106. However, whilst investigating the phosphorylation of S118, we had previously observed that mutation of S104 and S106 reduced the levels of ERα phosphorylation by MAPK in vitro (Chen et al. 2002). In agreement with this, we show here that S104 and S106 phosphorylation was stimulated by PMA and inhibited by the MEK1/2 inhibitor U0126, in transfected COS-1 cells. Further, co-transfection with activated Ras-V12 or Raf-CAAX, but not dominant-negative Raf-S621A, stimulated S104 and S106 phosphorylation. Together, these results suggest that serines 104 and 106 are phosphorylated by Erk1/2 MAPK. In vitro kinase experiments confirmed that, in addition to phosphorylating S118, Erk2 could also directly phosphorylate S104 and S106. Of the other kinases tested, Cdk2 was able to phosphorylate S104 and S118, and GSK3 able to phosphorylate S104, but to levels considerably lower than that achieved by Erk2. However, Cdk2 and/or GSK3 may phosphorylate S104 and/or S106 in vivo. Indeed, ligand-stimulated phosphorylation was in most cases insensitive to U0126, suggesting the involvement of other kinases. However, OHT- and ICI-induced phosphorylation of S104 did appear to partly involve MAPK, raising the possibility that multiple kinases are differentially involved, depending on the specific ligand.
In reporter gene assays, constitutively active Ras and Raf stimulated ERα activity in the absence of ligand or in the presence of OHT. Substitution of S104/S106/S118 by alanine prevented ERα activation by Ras and Raf, indicating that these sites are required for MAPK stimulation of ERα activity. Furthermore, U0126 inhibited ERα activity, as did dominant-negative Raf, but had little effect on triple S104, S106, and S118 mutants. These results suggest that the inhibitory effect of Raf-S621A and U0126 on ERα activity was due to the inhibition of phosphorylation at some or all of the three sites, and further implicates MAPK in the phosphorylation of S104, S106, and S118. Phosphorylation of S104, S106, and S118 could also be induced by ERα ligands; E2, OHT, and ICI in COS-1 cells. The in vitro kinase assays indicated that ligand-binding results in marginally more efficient phosphorylation of ERα by MAPK, perhaps due to the altered conformation of ERα and/or unmasking of potential MAPK docking site(s) (Obenauer et al. 2003). However, ligand-stimulated phosphorylation of ERα in vivo was largely insensitive to U0126, and may be mediated by Cdk2 and/or GSK3 (Trowbridge et al. 1997, Rogatsky et al. 1999, Medunjanin et al. 2005).
In conclusion, phosphorylation of S104, S106, and S118 is important for ERα AF-1 activity, as displayed by enhanced ligand-independent, and E2- and OHT-dependent, activities. This enhanced activity is not due to ligand hypersensitivity. No one site is critical, but lack of phosphorylation at all of the sites together results in near complete loss of AF-1 activity and prevents the agonist action of OHT. Additionally, phosphorylation of these sites occurs in a partially interdependent manner and phosphorylation at each site appears to act via a similar mechanism to enhance ERα activity, suggesting that this region constitutes a phospho-regulated domain of cooperative MAPK phosphorylation sites. Activation of the EGF receptor and ErbB2 pathways, which signal through MAPK, has been associated with more aggressive breast cancer phenotypes and poor patient prognosis (Ross & Fletcher 1998, Arteaga 2001). These pathways have additionally been linked to the tamoxifen resistance phenotype (Benz et al. 1993, Kurokawa et al. 2000, Gee et al. 2001, Kurokawa & Arteaga 2003, Shou et al. 2004). The evidence presented here suggests that modulation of ERα phosphorylation can determine whether or not tamoxifen acts as an ERα agonist or antagonist, and that hyperphosphorylation may result in tamoxifen-induced activities at levels high enough to support the growth of cells that depend upon ERα activity, such as those found in the majority of breast cancers.
Acknowledgements
We thank the members of the laboratory for helpful discussions, and Dr L Buluwela for discussion of the work and critical reading of this manuscript. This work was made possible by grants from Cancer Research UK and the Breast Cancer Research Trust. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nature Review Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- Ali S, Metzger D, Bornert JM, Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO Journal. 1993;12:1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. Journal of Clinical Oncology. 2001;19:32S–40S. [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Research and Treatment. 1993;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO Journal. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Molecular Endocinology. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bocquel MT, Kumar V, Stricker C, Chambon P, Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acid Research. 1989;17:2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO Journal. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor α: a new model for anti-estrogen resistance. Journal of Biological Chemistry. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Chen D, Riedl T, Washbrook E, Pace PE, Coombes RC, Egly JM, Ali S. Activation of estrogen receptor alpha by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Molecular Cell. 2000;6:127–137. [PubMed] [Google Scholar]
- Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly JM, et al. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine Reviews. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. PNAS. 1996;93:12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Robertson JF, Ellis IO, Nicholson RI. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. International Journal of Cancer. 2001;95:247–254. doi: 10.1002/1097-0215(20010720)95:4<247::aid-ijc1042>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes and Development. 2000;14:121–141. [PubMed] [Google Scholar]
- Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. Journal of Biological Chemistry. 1994;269:4458–4466. [PubMed] [Google Scholar]
- Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annual Review of Genetics. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- Henderson IC, Piccart-Gebhart MJ. The evolving role of aromatase inhibitors in adjuvant breast cancer therapy. Clinical Breast Cancer. 2005;6:206–215. doi: 10.3816/CBC.2005.n.022. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. PNAS. 1992;89:4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignar-Trowbridge DM, Teng CT, Ross KA, Parker MG, Korach KS, McLachlan JA. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Molecular Endocinology. 1993;7:992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge DM, Pimentel M, Parker MG, McLachlan JA, Korach KS. Peptide growth factor cross-talk with the estrogen receptor requires the A/B domain and occurs independently of protein kinase C or estradiol. Endocrinology. 1996;137:1735–1744. doi: 10.1210/endo.137.5.8612509. [DOI] [PubMed] [Google Scholar]
- Joel PB, Traish AM, Lannigan DA. Estradiol and phorbol ester cause phosphorylation of serine 118 in the human estrogen receptor. Molecular Endocinology. 1995;9:1041–1052. doi: 10.1210/mend.9.8.7476978. [DOI] [PubMed] [Google Scholar]
- Joel PB, Smith J, Sturgill TW, Fisher TL, Blenis J, Lannigan DA. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Molecular Cell Biology. 1998a;18:1978–1984. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel PB, Traish AM, Lannigan DA. Estradiol-induced phosphorylation of serine 118 in the estrogen receptor is independent of p42/p44 mitogen-activated protein kinase. Journal of Biological Chemistry. 1998b;273:13317–13323. doi: 10.1074/jbc.273.21.13317. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clinical Cancer Research. 2003;9:511S–515S. [PubMed] [Google Scholar]
- Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Research. 2000;60:5887–5894. [PubMed] [Google Scholar]
- Lipfert L, Fisher JE, Wei N, Scafonas A, Su Q, Yudkovitz J, Chen F, Warrier S, Birzin ET, Kim S, et al. Antagonist-induced, AF-2 independent estrogen receptor α phosphorylation. Molecular Endocinology. 2006;20:516–533. doi: 10.1210/me.2005-0190. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Clemm DL, Hermann T, Goldman ME, Pike JW. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Molecular Endocinology. 1995;9:659–669. doi: 10.1210/mend.9.6.8592512. [DOI] [PubMed] [Google Scholar]
- Medunjanin S, Hermani A, De Servi B, Grisouard J, Rincke G, Mayer D. Glycogen synthase kinase-3 interacts with and phosphorylates estrogen receptor α and is involved in the regulation of receptor activity. Journal of Biological Chemistry. 2005;280:33006–33014. doi: 10.1074/jbc.M506758200. [DOI] [PubMed] [Google Scholar]
- Metzger D, Losson R, Bornert JM, Lemoine Y, Chambon P. Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acid Research. 1992;20:2813–2817. doi: 10.1093/nar/20.11.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettles KW, Greene GL. Ligand control of coregulator recruitment to nuclear receptors. Annual Review of Physiology. 2005;67:309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acid Research. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK. Tamoxifen in the treatment of breast cancer. New England Journal of Medicine. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- Rogatsky I, Trowbridge JM, Garabedian MJ. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. Journal of Biological Chemistry. 1999;274:22296–22302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- Sarwar N, Kim JS, Jiang J, Peston D, Sinnett HD, Madden P, Gee JM, Nicholson RI, Lykkesfeldt AE, Shousha S, et al. Phosphorylation of ERα at serine 118 in primary breast cancer and in tamoxifen-resistant tumours is indicative of a complex role for ERα phosphorylation in breast cancer progression. Endocrine-Related Cancer. 2006;13:851–861. doi: 10.1677/erc.1.01123. [DOI] [PubMed] [Google Scholar]
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of National Cancer Institute. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Smith CL. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biology of Reproduction. 1998;58:627–632. doi: 10.1095/biolreprod58.3.627. [DOI] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Molecular Endocinology. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- Tora L, Mullick A, Metzger D, Ponglikitmongkol M, Park I, Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO Journal. 1989a;8:1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989b;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Trowbridge JM, Rogatsky I, Garabedian MJ. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. PNAS. 1997;94:10132–10137. doi: 10.1073/pnas.94.19.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual Review of Biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tzukerman MT, Esty A, Santiso-Mere D, Danielian P, Parker MG, Stein RB, Pike JW, McDonnell DP. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Molecular Endocinology. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]