Since the discovery of the hepatitis C virus (HCV) in the late 1980s, there has been an explosion of information regarding its natural history, treatment, and replication cycle. Nonetheless, there are still relatively few data regarding acute HCV infection. By convention, the term acute hepatitis refers to the presence of clinical signs or symptoms of hepatitis for a period of 6 months or fewer after the presumed time of HCV exposure. Early studies of posttransfusion patients who developed non-A, non-B hepatitis provide a clinical picture of early infection.1 Following the availability of specific serologic and virologic tests, most such patients were shown to have acute HCV infection. After acute infection, HCV RNA may become detectable in the serum/plasma in as little as 2 weeks (Fig. 1). Several weeks later, a high percentage of patients experience an increase in serum aminotransferase levels consistent with the development of acute hepatocellular injury. In the majority of cases, patients develop mild constitutional symptoms, including abdominal pain, nausea, vomiting, anorexia, and fatigue. During this acute infection, serum aminotransferases often peak below 1000 IU/mL and may return to normal levels. A minority develops sufficient elevations in bilirubin to lead to overt jaundice or the development of dark urine. Unless the clinical suspicion is high, few patients will be tested for HCV RNA or HCV antibody seroconversion. However, in the majority— but not all— of infected patients, HCV RNA persists, and a chronic disease state develops.
Fig. 1.
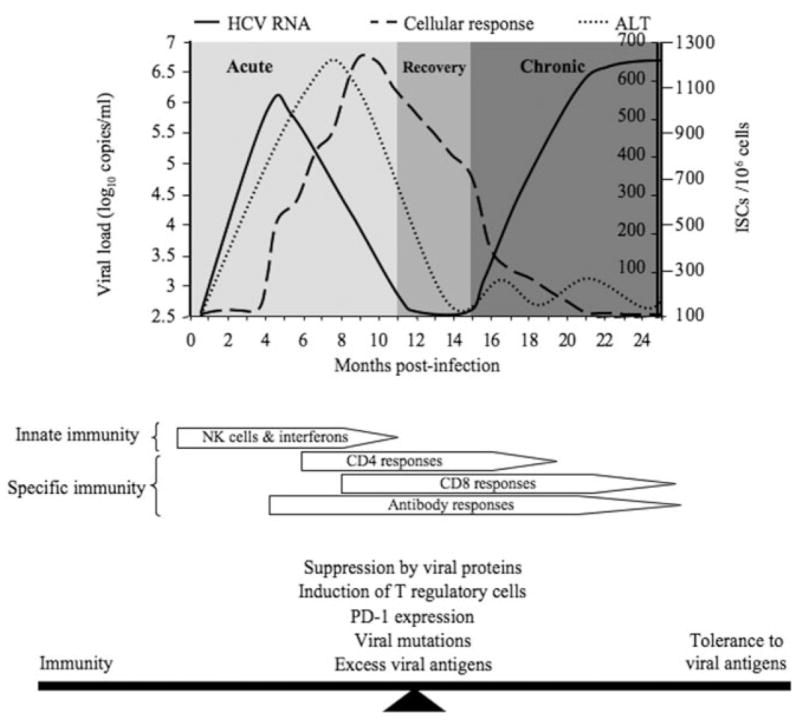
Natural history of HCV infection (upper panel) and immunologic responses to HCV infection (lower panel): (−) HCV RNA, (- - -) cellular response, and (· · ·) ALT. ALT indicates alanine aminotransferase; HCV, hepatitis C virus; ISC, interferon secreting cell; NK, natural killer; and PD-1, programmed death 1.
The reasons for the general lack of data regarding acute HCV infection are multifactorial and include (1) the relatively high percentage of asymptomatic or unrecognized early infections, (2) the lack of large-scale identification of chronic carriers in the general population who serve as a reservoir for infection, and (3) the decreased number of acute infections that occur in controlled clinical settings such as that of blood transfusions. These factors and the lack of nonprimate animal models necessitate reliance on retrospective studies in chronic carriers, the use of limited historical collections of banked sera, and the extrapolation of outcomes based on small disease outbreaks in unique settings (for example, transmission from a physician to a patient in the operating room setting or following parenteral exposure in healthcare workers). Moreover, there exist only a limited number of population cohorts that continue to experience high rates of HCV transmission (for example, Egypt); nonetheless, there is a growing body of information regarding the clinical presentation, natural history, and treatment outcomes of acute HCV infection.
In this article, we review the current information regarding our understanding of the epidemiology, virology, and immunology of HCV with a particular emphasis on acute HCV infection. In addition, we review recent data related to interferon-based treatment intervention and propose an algorithm for the diagnosis and management of acute HCV infection.
Epidemiology and Natural History of HCV Infection
Data from the National Health and Nutrition Examination Survey estimated that more than 4.1 million people have evidence of HCV exposure, as measured by HCV antibody, in the United States.2 This number may be even higher when homeless and incarcerated persons are taken into account, as infection rates in these populations may exceed 40%3–6 and even 70% among human immunodeficiency virus–positive (HIV+) urban poor.7 Overall, approximately 85% of those with an acute infection will develop chronic disease,8 persistent viremia occurring at least 6 months after initial exposure, with estimates ranging from 55% in the Irish anti-D immune globulin cohort9 and 60% in a study of community-acquired HCV in the United States10 to 90% in a single-source outbreak from contaminated clotting factors in Austria.11 Fortunately, HCV incidence has dropped nearly 10-fold in the United States since the 1980s,12 largely because of improved screening of blood products, decreased injection drug use (IDU), and safer sexual practices. However, HCV still poses a significant public health threat, as most patients with an acute infection do not exhibit symptoms and therefore are unaware that they are infected and remain capable of transmitting the virus to others.
Transmission of Acute HCV Infection
It is believed that HCV can be acquired or transmitted via any blood-borne route. High-risk settings include IDU and the transfusion of unscreened blood and blood products. Other possible but less well characterized risk exposures include tattoos and piercings, needle sticks, and unsafe/traumatic sexual practices. Historically, sexual transmission has been considered a relatively inefficient route for HCV transmission. For instance, in a recent study of sexual transmission among monogamous couples in Italy, only 3 persons contracted HCV among 770 partners of persons with a chronic HCV infection who were observed during a 10-year period.13 Another cross-sectional analysis detected a 2.5% risk of spousal HCV transmission.14
Despite the relatively small risk of sexual transmission of HCV, coinfection with HIV may potentially increase this risk. For instance, the Swiss HIV Cohort Study calculated an HCV incidence rate of 6.4 per 1000 person years (PY) among HIV+ subjects who were HCV-sero-negative at the baseline.15 In contrast, the national rate in Switzerland is 0–4 cases per 100,000 PY. Factors increasing the risk of acute HCV infection included a history of IDU and unsafe sex. However, the association between unsafe sex and HCV seroconversion was not statistically significant in those with HIV transmission from heterosexual sex but was only among men who have sex with men (MSM). In a related study of acute HCV among HIV+ MSM, 29 cases were detected in a 3.5-year study period.16 All patients reported unprotected anal sex, whereas 6 described sexual practices with mucosal trauma. Forty-one percent had concomitant sexually transmitted diseases, although none reported IDU. Another study detected incident HCV infection in 11 of 308 HIV+ MSM during a 5-year follow-up.17 A detailed behavioral questionnaire revealed that fisting was the only significant predictor of acute HCV infection. Furthermore, an analysis of the HIV+ French PRIMO Cohort followed 379 subjects without HCV at the baseline for at least 18 months. Six subjects seroconverted, yielding an HCV incidence rate of 4.3 per 1000 PY.18 Four were male, and all reported high-risk, unprotected anal sex. Another analysis reported a statistically significant trend toward increasing HCV seroconversion, from 0.2 per 1000 PY in 1997 to 4.5 per 1000 PY in 2002 in HIV+ MSM in the United Kingdom.19 This trend was exaggerated (from 0.6–9.3 per 1000 PY) in those who received an HCV test because of elevated liver enzymes.
Collectively, these data suggest that HIV could mediate the risk of HCV transmission, particularly among MSM. There is mechanistic plausibility to support this hypothesis as well. For example, HCV viral loads are significantly elevated among individuals coinfected with HIV,20–22 whereas the viral half-life may also be prolonged.23 Furthermore, HCV RNA has been detected at higher rates in the semen from HIV/HCV-coinfected men24 versus HCV-monoinfected men.25 The practices of unprotected and traumatic anal sex increase the chance of semen-to-blood or blood-to-blood transmission, whereas concomitant sexually transmitted infections may further facilitate this process. These explanations may also help explain why similar effects of HIV on HCV transmission and acquisition are not generally observed in females.26
Natural History of Acute HCV
Most patients with newly acquired HCV do not exhibit symptoms of infection within the first 6 months. A prospective evaluation of 179 HCV antibody–negative injection drug users identified 62 seroconverters, yielding an incidence rate of 27.2 cases per 100 PY.27 Of the 40 cases with available follow-up data, 8 cleared the infection. None of the patients exhibited any clinical symptoms that would warrant medical attention. Symptomatic patients may exhibit jaundice but more often will complain of fatigue, nausea, abdominal pain, or flulike symptoms. In a study with a surprisingly high rate of symptoms (68%), Santantonio et al.28 noted jaundice in 57% and alanine aminotransferase (ALT) levels greater than 20 times the upper limit of normal in 73%.28 Seventy-three of the 203 subjects (36%) had spontaneous viral clearance— 80% within 3 months of disease onset. Although symptomatic infection was not associated with clearance in this study, it has nonetheless been postulated that symptomatic patients have a higher rate of spontaneous clearance than asymptomatic patients. For instance, Gerlach et al.29 observed a spontaneous clearance rate of 52% (24 of 46) in symptomatic acute HCV–infected patients with HIV, but clearance was not evident in any asymptomatic patients in that study. Likewise, a retrospective analysis from a non-HIV clinic population reported symptoms in 26 of 28 acute HCV cases.30 The authors observed spontaneous clearance in 25% (7 of 28) of subjects, all of whom were symptomatic. Interestingly, this study included 4 patients, each with 2 distinct instances of acute HCV; each infection was symptomatic, and each was cleared. However, in another small study of 9 HIV+ men with acute HCV, of whom 7 were symptomatic, 2 had spontaneous clearance, 3 responded to interferon-based treatment, and 4 developed a chronic infection.31 Similarly, among incarcerated injection drug users, McGovern and colleagues32 detected 21 cases of acute HCV infection. Of 17 individuals observed for more than 6 months, 8 spontaneously cleared the virus (6 of 13 patients with symptoms and 2 of 4 patients without symptoms). One patient had de novo HCV reinfection through IDU after spontaneous clearance and normalization of the liver enzyme, as demonstrated by sequence analysis. Although the initial infection caused jaundice, the patient remained asymptomatic during the second infection, and this was consistent with the increased likelihood of spontaneous clearance during symptomatic acute infection.
Several studies have shown that a wide spectrum of clinical, virologic, and immunologic outcomes may be exhibited after exposure, even during common source outbreaks.9,33,34 This suggests complex interactions among various factors that result in self-limiting acute infection versus chronic infection (Table 1).
Table 1.
Factors Potentially Associated with the Clearance or Persistence of an Acute HCV Infection
| Type of exposure |
| Size of inoculum/HCV viral load |
| Gender |
| Age |
| Prior HCV exposure |
| Prior exposure to interferon therapy |
| HCV genotype |
| Quasispecies diversity/complexity |
| Other coinfections |
| Immunologic response |
| Innate immune response: viral evasion |
| Neutralizing antibody response: viral epitope recognition or escape |
| CD8+ cytotoxic T lymphocyte response: viral epitope recognition or escape |
HCV Diversity
Hepatitis C viral replication is extremely robust, producing an estimated 10 trillion viral particles per day.35 A hallmark of RNA viruses is their extreme genetic diversity. The nonstructural 5B protein of HCV is an RNA-dependent RNA polymerase that lacks a proofreading mechanism. Thus, mutations within the HCV genome are generated at a rate of approximately 1 mutation per genome per replication cycle. This results in a population of distinct but closely related viral variants, termed the viral quasispecies, that exist within a single individual.
Given the diverse nature of HCV, it has been suggested that the emergence of particular viral variants may permit HCV to circumvent the host immune response and maintain persistent infection.36–40 Moreover, several studies have explored potential associations between immunologic selection pressure and clinical outcome in vivo. For instance, Ray et al.38 investigated viral diversity in 5 individuals who spontaneously cleared viremia and 10 individuals with persistent viremia. Persistent viremia was associated with a higher hypervariable region 1 (HVR1) nonsynonymous/synonymous rate ratio, a lower E1 non-synonymous/synonymous rate ratio, higher quasispecies complexity, and fewer positively charged residues in HVR1. Spontaneous clearers also differed from individuals with persistent viremia at 8 amino acid positions, although no residues were completely predictive of clinical outcome. Farci et al.39 similarly examined viral diversity among 12 patients with different clinical outcomes. Acute resolving hepatitis was associated with relative stasis of the viral quasispecies, whereas progressing hepatitis correlated with HCV evolution, particularly in HVR1.
Innate Immunity to HCV Infection
An acute viral infection triggers the activation of several antiviral effectors in mammalian cells. This innate antiviral response is an early host defense mechanism that occurs prior to adaptive immune responses.41 The recent discovery of pathogen-associated molecular patterns that are recognized by specific toll-like receptors have dramatically advanced our understanding of the innate host response to viral infection.42 For example, HCV RNA contains pathogen-associated molecular pattern motifs43,44 that could bind to toll-like receptor 3 at the cell surface and intracellularly through retinoic acid–inducible gene 1 to induce type I interferons (interferon alpha and interferon beta) in hepatocytes.43,45 Type I interferons regulate the antigen-processing machinery through the induction of immunoproteasome subunits, their incorporation into the proteasome complex, and the generation of an immunoproteasome-dependent CD8 T cell epitope.46 Moreover, type I interferons activate the expression of more than 300 interferon-stimulated genes that also have antiviral functions. The best characterized include the RNA-dependent protein kinase (PKR), 2′5′-oligoadenylate synthetase, RNase L, adenosine deaminase (adenosine deaminase, RNA-specific), and the Mx protein GTPases.
It is generally thought that only a minority of hepatocytes are infected with HCV.47 Nonetheless, the gene products secreted by this small number of infected cells can produce a transient antiviral state in neighboring uninfected cells. Although such a scenario would limit the potential replicative space within the liver, it is rare that this innate antiviral response completely eradicates the virus as the majority of persons exposed to HCV develop a chronic infection. Thus, the ability of HCV to antagonize these antiviral responses is crucial to viral persistence. Several HCV proteins, including core, E2, nonstructural 3/4A, and nonstructural 5A proteins, have been implicated in the inhibition of interferon-inducible genes and/or key components of interferon signaling pathways via multiple mechanisms.48 Thus, HCV can both trigger and control the hepatic response to infection (Fig. 1).
Role of Natural Killer (NK) Cells
NK cells are the major effector cells of the innate immune system and play an important role in the activation and maintenance of subsequent adaptive immune responses. The antiviral role of NK cells during HCV infection has been demonstrated by the induction of an HCV-associated, perforin/granzyme-dependent lysis of human hepatoma cells by cytokine-activated NK cells.49 The ligation of CD81 (a potential receptor for HCV) on NK cells inhibits interferon gamma production and results in decreased anti-HCV activity. In addition, antibodies to interferon gamma or interferon gamma receptors abolish the anti-HCV activity of NK cells.50 Furthermore, genes encoding an inhibitory NK cell receptor (killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 3) and its human leukocyte antigen C group 1 ligand directly influence the resolution of HCV infection,51 and this suggests that inhibitory NK cell interactions are important determinants of antiviral immunity and that diminished inhibitory responses confer protection against HCV.
Humoral Immune Responses
Humoral immune responses are generated against multiple HCV proteins, but the correlation of a single specific immune response with recovery from an acute infection has not been clearly established. Two HVRs (HVR1 and HVR2) in the E2 envelope glycoprotein have been identified.52,53 At least 1 study has suggested that the early appearance of HVR1 antibodies is associated with an acute self-limiting HCV infection.54 Additionally, mutations within certain regions, especially the E2 HVR, have been associated with the emergence of virus resistance to neutralization and the persistence of infection.55 Recently, discordance between the level of neutralizing anti-HCV antibodies and clearance of viremia has been reported.56 In fact, there exists a subpopulation of individuals exposed to HCV that clear the virus without the induction of anti-HCV antibodies; therefore, the only surrogate markers for HCV exposure in such persons are HCV-specific cell-mediated immune responses.57–63
Role of CD4 T Cells
A growing body of evidence indicates that the spontaneous clearance of HCV is associated with a strong HCV-specific CD4+ T cell response.56,64,65 A number of studies have indicated that successful cellular immune responses in recovered patients appear to be multispecific and sustained, with CD4+ T cells playing major roles.66–73 The role of CD4+ T cells in acute HCV infection has been examined by depletion studies in chimpanzees, in which the loss of CD4+ T cells resulted in persistent infection.74 CD4+ T cell levels also appear to be important during acute HCV infection, as the level of CD4+ T cell proliferative responses is associated with viral clearance,65,75,76 whereas the loss of such responses often results in the recurrence of viremia.77 Moreover, it has also been shown that patients who clear the infection respond to higher numbers of HCV epitopes in comparison with chronically infected patients.66,78
Cytokines are also important for the clearance or persistence of viremia. For instance, a vigorous HCV-specific type 1 helper T cell CD4+ response, particularly against nonstructural proteins, is associated with viral clearance65 or a successful response to therapy.76,79–82 In fact, the lack of type 1 helper T effector cells within the first months of acute HCV is a predictor of viral persistence and could thus serve as a criterion for selecting candidates for early antiviral treatment.65 The cause of dysfunctional CD4+ T cells in chronic HCV infection is not clear. However, exhaustion from continuous T cell stimulation,83 the induction of T-cell anergy,84 the induction of T regulatory cells that inhibit the immune responses,85,86 and the suppression of immune responses by HCV proteins48,87–93 represent several intriguing hypotheses. Without sufficient HCV-specific CD4 help, HCV-specific CD8+ T cell and heterologous neutralizing antibody responses may develop but fail to clear viremia.56
Role of CD8 T Cells
CD8 T cells could respond to HCV viral infection through 2 main mechanisms: the killing of infected hepatocytes or the secretion of antiviral cytokines. In comparison with CD4+ T cells, the role of CD8+ T cells in an acute HCV infection is less well defined because their detection during an acute infection coincides with viral clearance in some94,95 but not all studies.96 The reason for this discrepancy is not clear; however, most studies have used peripheral blood lymphocytes stimulated with selected epitopes previously identified or predicted from protein sequences. Therefore, this approach may not adequately evaluate the local intrahepatic immune responses to naturally processed HCV peptides. A comprehensive analysis of the CD8 responses using overlapping peptides covering the entire HCV genome has revealed multiple unpredicted epitopes that stimulate CD8-specific T cells even in chronically HCV-infected patients.97 Moreover, differences also exist between intrahepatic T cell responses and those in the peripheral blood.98–100 However, strong, multispecific interferon gamma–producing, HCV-specific CD8+ T cells are one of the characteristic features of patients who recover from an acute HCV infection.66,68,69,72,101–114 In a chronic HCV infection, although the frequencies of HCV-specific CD8+ T cells may be normal, the cells exhibit a dysfunctional or stunned phenotype.66,100,115,116 Recent data indicate that most HCV-specific CD8+ T cells express the inhibitory receptor programmed death 1 (PD-1) at the time of acute infection.117 Interestingly, levels of PD-1 decline in patients with a resolved HCV infection but remain high during viral persistence.117 The high expression of PD-1 is associated with dysfunctional CD8+ T cells and may partially explain the defective phenotypes of CD8+ T cells reported in chronically HCV-infected patients. Blocking the PD-1/programmed death ligand 1 interaction improved the functional activity of HCV-specific CD8+ T cells and restored CD8 function in vitro.118 Supporting data for the role of PD-1 expression in chronic HCV infection has been recently published with chimpanzees models.119 However, there are also data suggesting that most intrahepatic CD8+ T cells are toleragenic and express PD-1.120 One of the evasion mechanisms used by HCV to escape immune responses, especially CD8 responses, is the development of viral escape mutants.121 Finally, a significant correlation has been demonstrated between the number of lobular CD8+ T cells and ALT levels, suggesting a prominent role for T cell–mediated cytotoxicity in the genesis of hepatocellular damage.122
Treatment of Acute HCV Infection
Treatment decisions related to acute HCV infection must be considered in light of the natural history of the disease process. Although early treatment may increase the likelihood of improved treatment outcome, this must be balanced against the possibility that spontaneous clearance during the acute phase of infection could render treatment interventions needless and potentially harmful. Therefore, treatment paradigms must effectively balance these outcomes and lead to optimal patient selection without significant loss of treatment efficacy.
As in chronic infection, all current acute HCV treatment paradigms are interferon-based. Mechanistically, issues related to both viral evolution and immune response must be considered in the context of interferon use. Several studies now suggest that quasispecies diversity is an independent predictor of HCV treatment response. For instance, Chambers et al.123 noted that an early treatment response to pegylated interferon alpha 2a and ribavirin (RBV) was associated with lower baseline HCV RNA complexity in the envelope coding region, although this was not the exclusive predictor of sustained viral response (SVR). Among subjects with advanced liver disease, the treatment response was also reduced in subjects with increased baseline quasispecies complexity.124 Shire et al.125 examined the relationship between baseline and early viral selection pressures in HCV-monoinfected and HIV/HCV-coinfected subjects with hemophilia who were treated with pegylated interferon plus weight-based RBV. Lower baseline quasispecies complexity was associated with SVR. At the end of the phase 1 decline in HCV viremia, subjects whose decrease was greater than 90% also had a strong trend toward lower baseline complexity. The presence of HIV coinfection further mediated changes in the complexity over time. Thus, low pretreatment quasispecies complexity may predict the pegylated interferon response. These data do not, however, permit the ascertainment of the latest time following acute exposure at which HCV infection can be treated with maximum effect.
Multiple clinical trials report the arbitrary selection of start times for treatment intervention after the recognition of HCV infection. However, because of the variability among treatment regimens, current clinical recommendations are derived from diverse population cohorts with data extrapolated to individual patients. Jaeckel et al.126 described the treatment response among 44 German patients with acute HCV. Patients had known or suspected exposure within 4 months, as documented by HCV seroconversion or a serum ALT greater than 20 times the upper limit of normal with evidence of previously normal ALT for 1 year. Patients were treated with interferon alpha 2b at a dose of 5 MU per day for 4 weeks followed by 5 MU 3 times per week for 20 weeks. Sixty-eight percent met the first entry criteria for documented seroconversion; 61% were infected with HCV genotype 1. Fully 98% of the subjects achieved SVR. Subsequently, another German study evaluated a 24-week course of pegylated interferon alpha 2b.127 The overall SVR was 71%, although an 89% SVR rate was achieved among subjects classified as adherent with prescribed therapy. A third German experience by Gerlach et al.29 included 60 patients with acute HCV by either seroconversion or acute hepatitis with ALT greater than 10 times the upper limit of normal. There was no randomization or fixed time for treatment intervention, and patients were offered the most effective therapy at the time of diagnosis. Fifty-two percent spontaneously cleared HCV RNA less than 12 weeks after diagnosis. Only 26 subjects were treated with an interferon-based regimen; 12 had therapy started more than 6 months after the diagnosis of acute HCV. Twenty received pegylated interferon, and half of those had coadministration of RBV. Viral clearance was observed in 21 of 26 (81%) treated subjects, leading to SVR. Kamal et al.81 described 54 patients screened after either the first positive HCV RNA or the onset of symptoms. Laboratory studies were performed for 12 weeks, and then HCV RNA–positive subjects were offered interferon-based therapy [either pegylated interferon alpha 2a (180 μg/week) ± RBV (800 mg/day) or pegylated interferon alpha 2b (1.5 μg/kg/week) ± weight-based RBV]. Only 4 subjects cleared the virus during the 12-week period without treatment. Ten subjects refused therapy, and 1 of these cleared at week 14. All subjects had either genotype 1 or 4, with a slight genotype 4 predominance. Among the treated subjects, 33 of 40 (82.5%) achieved SVR. There was a nonstatistically significant advantage to subjects treated with regimens containing RBV. Subsequently, Kamal et al. evaluated the optimization of the treatment duration and time of initiation in 2 randomized treatment trials. Among 102 subjects randomized to receive pegylated interferon alpha 2b for 8, 12, or 24 weeks, the highest rate of response was observed in the 24-week arm (91.2%). Although stratification by genotype led to relatively small subsets (only 15–16 subjects per arm), 88% of genotype 1 subjects who were treated for 24 weeks achieved SVR. Excellent results were reported for shorter therapy in genotype 2 and 3 subjects; however, each treatment arm contained only 2 or 3 subjects, and this limited interpretation. Genotype 4 was nearly as common as genotype 1 and had the highest response (100% SVR) in the 24-week treatment group.128 In a second study, Kamal et al.129 followed patients for 8 weeks after the identification of acute HCV. Subjects were then randomized to receive pegylated interferon alpha 2b, beginning at 8, 12, or 20 weeks, and they underwent a 12-week treatment regimen. SVR was higher for subjects with shorter waiting times.
Reports of acute HCV within the United States are limited. The largest experience involves a retrospective analysis of clinical practices reported by Corey et al.30 Acute HCV was diagnosed in 24 patients; 15 received interferon-based therapy. All treated patients cleared the virus on therapy, and all but 1 (93%) achieved SVR; 5 of 6 patients not offered therapy remained viremic. A similar study by Rahman et al.130 included 7 patients who were treated with an interferon-based regimen; 6 of 7 achieved SVR. Interestingly, the only patient to not achieve SVR did not receive RBV; however, the authors noted that this patient was also an African American male with poor prognostic likelihood of viral clearance, obscuring potential conclusions regarding the cause of treatment failure.
As mentioned previously, patients with acute HCV infection in the setting of HIV coinfection represent a unique and increasingly important subset of this disease process.19 A prospective evaluation for liver function test abnormalities and HCV antibody seroconversion was performed every 3 months in an HIV clinic in Great Britain. Fifty acute HCV infections were identified and confirmed with HCV RNA testing, and this was followed every 4 weeks with HCV RNA quantitative assays. After 12 weeks of positive HCV RNAs, patients were offered therapy with pegylated interferon and weight-based RBV. During the 12-week window, 12 of 50 (24%) cleared HCV RNA and remained aviremic. Eleven subjects declined therapy, and all progressed to chronic HCV infection. The remaining 25 were treated for 24 weeks. SVR was 59% among the treated patients, and this is clearly lower than reports during HCV monoinfection. All treatment failures occurred among patients with HCV genotype 1 and relatively low CD4 counts (median: 276 cells/mm3). However, better response rates were reported among 11 acutely infected German patients, with 10 having SVR.131 Ten patients with genotype 4 HCV and HIV were treated in France following a suspected common source sexually transmitted infection cluster. Treatment was provided within a mean time of 49 days from the onset of acute hepatitis, and a variety of interferon-based regimens were used. No patient achieved SVR.132 Finally, 9 acute HCV infections were identified in HIV-infected patients in California.31 Only 4 were treated with pegylated interferon and RBV; 3 achieved SVR. Among 5 untreated patients, 2 demonstrated spontaneous clearance, and 3 developed chronic liver disease.
On the basis of the available (albeit insufficient) data, several conclusions may be drawn. First, the optimal timing to initiate treatment remains unclear. Different definitions of decision trees used in clinical trials contribute to some of this confusion because some studies define the estimated time from acute exposure, whereas others define the starting point as seroconversion or acute hepatitis, using arbitrary serum aminotransferase criteria. The only study to prospectively evaluate this issue used 12 weeks of therapy, although the same group reported better efficacy with 24 weeks of therapy in another cohort. These inconsistencies argue for a formal definition to be used in future prospective trials (Fig. 2). Although the first HCV RNA–positive result may be useful, in unsuspected, community-acquired cases, clinical findings are often used to reach an eventual diagnosis. It is reasonable to suggest that treatment should be considered at least 12 weeks after seroconversion or acute hepatitis. In the future, increased availability of more sensitive HCV assays (for example, transcription-mediated amplification) may require reassessment of this algorithm because of the potential for earlier diagnosis. The latest time at which the treatment of acute infection may be initiated has not been precisely defined; however, the data support the concept that earlier treatment may be more effective than later treatment. Although excellent responses have been observed with standard interferon, clinical experience suggests that the use of once weekly agents improves adherence and is preferred.133 Therefore, pegylated interferon use is recommended. Second, the need for RBV with an interferon-based treatment remains controversial. European experts suggest that RBV use is unnecessary on the basis of excellent response rates observed with monotherapy.63 However, response rates have not been as high in the United States. The extrapolation of data from the treatment of chronically HCV-infected patients would support RBV use in the United States. Pending further data, a dose of 800 mg of RBV per day combined with pegylated interferon seems ideal, although patients with poor response characteristics may require weight-based dosing regimens. Treatment durations of 24 weeks have been shown to be more effective in genotype 1 patients in 1 prospective trial; therefore, this treatment cycle should be used in HCV-monoinfected patients because excellent overall responses have been observed. These recommendations are similar to those promulgated by the American Association for the Study of Liver Diseases,134 although that body felt that RBV use should be individualized. Finally, patients with HIV/HCV coinfection seem to have poorer outcomes following acute HCV infection. At this time, there are no data to support either longer treatment durations or higher doses of interferon products, and no specific recommendations can be made.
Fig. 2.
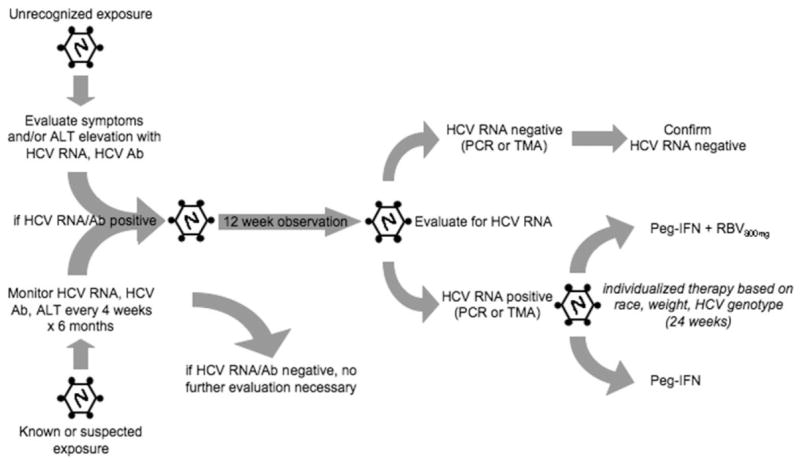
Proposed algorithm for the diagnosis and treatment of an acute HCV infection. Ab indicates antibody; ALT, alanine aminotransferase; HCV, hepatitis C virus; PCR, polymerase chain reaction; PEG-IFN, pegylated interferon; and RBV, ribavirin.
Conclusions
Acute HCV infection remains a significant clinical problem because of the difficulty of early case recognition and the inability to predict the risk of clearance versus chronicity of infection with a high degree of accuracy during early infection. Research focused on either virologic or immunologic events that signal spontaneous clearance should be vigorously pursued. Similarly, a lack of large, randomized clinical trials limits our ability to make informed treatment decisions and leads to the development of treatment paradigms that are often vigorously defended by their proponents yet poorly supported by the data available. Studies of timing, duration, and dose are needed. However, these studies are difficult to perform, and investment is often tempered by the rapid evolution of agents used in more common chronic infection scenarios. Therefore, it seems likely that existing regimens will dominate the acute HCV scene for several years to come.
Acknowledgments
Supported in part by a National Institute of Diabetes and Digestive and Kidney Diseases K24 award (DK 070528-01) to K.E.S. and by a National Institute on Drug Abuse R21 award (DA022148-01) to J.T.B.
Abbreviations
- Ab
antibody
- ALT
alanine aminotransferase
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HVR
hypervariable region
- IDU
injection drug use
- ISC
interferon secreting cell
- MSM
men who have sex with men
- NK
natural killer
- PCR
polymerase chain reaction
- PD-1
programmed death 1
- PEG-IFN
pegylated interferon
- PY
person years
- RBV
ribavirin
- SVR
sustained viral response
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Berman M, Alter HJ, Ishak KG, Purcell RH, Jones EA. The chronic sequelae of non-A, non-B hepatitis. Ann Intern Med. 1979;91:1–6. doi: 10.7326/0003-4819-91-1-1. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Williams IT, Maga UA, Viali S, Kuhnert WL, Mc-Garvey ST. Hepatitis C virus infection in Samoa and American Samoa. Am J Trop Med Hyg. 2006;74:261–262. [PubMed] [Google Scholar]
- 3.Baillargeon J, Wu H, Kelley MJ, Grady J, Linthicum L, Dunn K. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 4.Spaulding A, Greene C, Davidson K, Schneidermann M, Rich J. Hepatitis C in state correctional facilities. Prev Med. 1999;28:92–100. doi: 10.1006/pmed.1998.0418. [DOI] [PubMed] [Google Scholar]
- 5.Zalumas JC, Rose CD. Hepatitis C and HIV in incarcerated populations: fights, bites, searches, and syringes! J Assoc Nurses AIDS Care. 2003;14:S108–S115. doi: 10.1177/1055329003255590. [DOI] [PubMed] [Google Scholar]
- 6.Cheung RC, Hanson AK, Maganti K, Keeffe EB, Matsui SM. Viral hepatitis and other infectious diseases in a homeless population. J Clin Gastroenterol. 2002;34:476–480. doi: 10.1097/00004836-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Hall CS, Charlebois ED, Hahn JA, Moss AR, Bangsberg DR. Hepatitis C virus infection in San Francisco’s HIV-infected urban poor. J Gen Intern Med. 2004;19:357–365. doi: 10.1111/j.1525-1497.2004.30613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeff LB. Natural history of hepatitis C. Am J Med. 1999;107:S10–S15. doi: 10.1016/s0002-9343(99)00374-5. [DOI] [PubMed] [Google Scholar]
- 9.Kenny-Walsh E for the Irish Hepatology Research Group. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 10.Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, et al. for the Sentinel Counties Chronic Non-A, Non-B Hepatitis Study Team. The natural history of community-acquired hepatitis C in the United States. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 11.Datz C, Cramp M, Haas T, Dietze O, Nitschko H, Froesner G, et al. The natural course of hepatitis C virus infection 18 years after an epidemic outbreak of non-A, non-B hepatitis in a plasmapheresis centre. Gut. 1999;44:563–567. doi: 10.1136/gut.44.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prevention CfDCa. Viral hepatitis C fact sheet. [accessed July 25, 2007]; Available at http://www.cdc.gov/ncidod/diseases/hepatitis/c/fact.htm.
- 13.Vandelli C, Renzo F, Romano L, Tisminetzky S, De Palma M, Stroffolini T, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 14.Neumayr G, Propst A, Schwaighofer H, Judmaier G, Vogel W. Lack of evidence for the heterosexual transmission of hepatitis C. QJM. 1999;92:505–508. doi: 10.1093/qjmed/92.9.505. [DOI] [PubMed] [Google Scholar]
- 15.Rauch A, Rickenbach M, Weber R, Hirschel B, Tarr PE, Bucher HC, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 16.Gambotti L, Batisse D, Colinde-Verdiere N, Delaroque-Astagneau E, Desenclos JC, Dominguez S, et al. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro Surveill. 2005;10:115–117. [PubMed] [Google Scholar]
- 17.Turner JM, Rider AT, Imrie J, Copas AJ, Edwards SG, Dodds JP, et al. Behavioural predictors of subsequent hepatitis C diagnosis in a UK clinic sample of HIV positive men who have sex with men. Sex Transm Infect. 2006;82:298–300. doi: 10.1136/sti.2005.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosn J, Deveau C, Goujard C, Garrigue I, Saichi N, Galimand J, et al. Increase in hepatitis C virus incidence in HIV-1-infected patients followed up since primary infection. Sex Transm Infect. 2006;82:458–460. doi: 10.1136/sti.2006.021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browne R, Asboe D, Gilleece Y, Atkins M, Mandalia S, Gazzard B, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–327. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonacini M, Govindarajan S, Blatt LM, Schmid P, Conrad A, Lindsay KL. Patients co-infected with human immunodeficiency virus and hepatitis C virus demonstrate higher levels of hepatic HCV RNA. J Viral Hepat. 1999;6:203–208. doi: 10.1046/j.1365-2893.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 21.Matthews-Greer JM, Caldito GC, Adley SD, Willis R, Mire AC, Jamison RM, et al. Comparison of hepatitis C viral loads in patients with or without human immunodeficiency virus. Clin Diagn Lab Immunol. 2001;8:690–694. doi: 10.1128/CDLI.8.4.690-694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman K, Shire N, Rouster S, Peters M, Koziel M, Chung R, et al. Viral kinetics in hepatitis C or hepatitis C/human immunodeficiency virus-infected patients. Gastroenterology. 2005;128:313–327. doi: 10.1053/j.gastro.2004.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torriani FJ, Ribeiro RM, Gilbert TL, Schrenk UM, Clauson M, Pacheco DM, et al. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) dynamics during HCV treatment in HCV/HIV coinfection. J Infect Dis. 2003;188:1498–1507. doi: 10.1086/379255. [DOI] [PubMed] [Google Scholar]
- 24.Pasquier C, Bujan L, Daudin M, Righi L, Berges L, Thauvin L, et al. Intermittent detection of hepatitis C virus (HCV) in semen from men with human immunodeficiency virus type 1 (HIV-1) and HCV. J Med Virol. 2003;69:344–349. doi: 10.1002/jmv.10295. [DOI] [PubMed] [Google Scholar]
- 25.Briat A, Dulioust E, Galimand J, Fontaine H, Chaix ML, Letur-Konirsch H, et al. Hepatitis C virus in the semen of men coinfected with HIV-1: prevalence and origin. AIDS. 2005;19:1827–1835. doi: 10.1097/01.aids.0000189847.98569.2d. [DOI] [PubMed] [Google Scholar]
- 26.Augenbraun M, Goedert J, Thomas D, Feldman J, Seaberg E, French A, et al. Incident hepatitis C virus in women with human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1357–1364. doi: 10.1086/379075. [DOI] [PubMed] [Google Scholar]
- 27.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 28.Santantonio T, Medda E, Ferrari C, Fabris P, Cariti G, Massari M, et al. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–1159. doi: 10.1086/507640. [DOI] [PubMed] [Google Scholar]
- 29.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ul-senheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 30.Corey KE, Ross AS, Wurcel A, Schulze Zur Wiesch J, Kim AY, Lauer GM, et al. Outcomes and treatment of acute hepatitis C virus infection in a United States population. Clin Gastroenterol Hepatol. 2006;4:1278–1282. doi: 10.1016/j.cgh.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luetkemeyer A, Hare CB, Stansell J, Tien PC, Charlesbois E, Lum P, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–36. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGovern BH, Wurcel A, Kim AY, Schulze zur Wiesch J, Bica I, Zaman MT, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–1670. doi: 10.1086/504327. [DOI] [PubMed] [Google Scholar]
- 33.Larghi A, Zuin M, Crosignani A, Ribero ML, Pipia C, Battezzati PM, et al. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology. 2002;36:993–1000. doi: 10.1053/jhep.2002.36129. [DOI] [PubMed] [Google Scholar]
- 34.Itakura J, Nagayama K, Enomoto N, Hamano K, Sakamoto N, Fanning LJ, et al. Viral load change and sequential evolution of entire hepatitis C virus genome in Irish recipients of single source-contaminated anti-D immunoglobulin*. J Viral Hepat. 2005;12:594–603. doi: 10.1111/j.1365-2893.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 35.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 36.Martell M, Esteban J, Quer J, Genesca J, Weiner A, Esteban R, et al. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmonds P. Genetic diversity and evolution of hepatitis C virus—15 years on. J Gen Virol. 2004;85:3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 38.Ray S, Wang Y, Laeyendecker O, Ticehurst J, Villano S, Thomas D. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J Virol. 1999;73:2938–2946. doi: 10.1128/jvi.73.4.2938-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder J, et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 40.Manzin A, Solforosi L, Petrelli E, Macarri G, Tosone G, Piazza M, et al. Evolution of hypervariable region 1 of hepatitis C virus in primary infection. J Virol. 1998;72:6271–6276. doi: 10.1128/jvi.72.7.6271-6276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katze MG. Interferon, PKR, virology, and genomics: what is past and what is next in the new millennium? J Interferon Cytokine Res. 2002;22:283–286. doi: 10.1089/107999002753675695. [DOI] [PubMed] [Google Scholar]
- 42.Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 43.Sumpter R, Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCormick C, Challinor L, Macdonald A, Rowlands D, Harris M. Introduction of replication-competent hepatitis C virus transcripts using a tetracycline-regulable baculovirus delivery system. J Gen Virol. 2004;85:429–439. doi: 10.1099/vir.0.19676-0. [DOI] [PubMed] [Google Scholar]
- 45.Li K, Chen Z, Kato N, Gale M, Jr, Lemon SM. Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem. 2005;280:16739–16747. doi: 10.1074/jbc.M414139200. [DOI] [PubMed] [Google Scholar]
- 46.Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, et al. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanford R, Bigger C. Advances in model systems for hepatitis C virus research. Virology. 2002;293:1–9. doi: 10.1006/viro.2001.1316. [DOI] [PubMed] [Google Scholar]
- 48.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 49.Larkin J, Bost A, Glass JI, Tan SL. Cytokine-activated natural killer cells exert direct killing of hepatoma cells harboring hepatitis C virus replicons. J Interferon Cytokine Res. 2006;26:854–865. doi: 10.1089/jir.2006.26.854. [DOI] [PubMed] [Google Scholar]
- 50.Takehara T, Hayashi N. Natural killer cells in hepatitis C virus infection: from innate immunity to adaptive immunity. Clin Gastroenterol Hepatol. 2005;3:S78–S81. doi: 10.1016/s1542-3565(05)00702-0. [DOI] [PubMed] [Google Scholar]
- 51.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 52.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, et al. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farci P, Alter HJ, Wong DC, Miller RH, Govindarajan S, Engle R, et al. Prevention of hepatitis C virus infection in chimpanzees after antibody–mediated in vitro neutralization. Proc Natl Acad Sci U S A. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zibert A, Kraas W, Meisel H, Jung G, Roggendorf M. Epitope mapping of antibodies directed against hypervariable region 1 in acute self-limiting and chronic infections due to hepatitis C virus. J Virol. 1997;71:4123–4127. doi: 10.1128/jvi.71.5.4123-4127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, et al. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217–8229. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 57.Shata MT, Tricoche N, Perkus M, Tom D, Brotman B, McCormack P, et al. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–616. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semmo N, Barnes E, Taylor C, Kurtz J, Harcourt G, Smith N, et al. T-cell responses and previous exposure to hepatitis C virus in indeterminate blood donors. Lancet. 2005;365:327–329. doi: 10.1016/S0140-6736(05)17787-3. [DOI] [PubMed] [Google Scholar]
- 59.Post JJ, Pan Y, Freeman AJ, Harvey CE, White PA, Palladinetti P, et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846–1855. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- 60.Freeman AJ, Ffrench RA, Post JJ, Harvey CE, Gilmour SJ, White PA, et al. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190:1093–1097. doi: 10.1086/422605. [DOI] [PubMed] [Google Scholar]
- 61.Al-Sherbiny M, Osman A, Mohamed N, Shata MT, Abdel-Aziz F, Abdel-Hamid M, et al. Exposure to hepatitis C virus induces cellular immune responses without detectable viremia or seroconversion. Am J Trop Med Hyg. 2005;73:44–49. [PubMed] [Google Scholar]
- 62.Kamal SM, Amin A, Madwar M, Graham CS, He Q, Al Tawil A, et al. Cellular immune responses in seronegative sexual contacts of acute hepatitis C patients. J Virol. 2004;78:12252–12258. doi: 10.1128/JVI.78.22.12252-12258.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubitschke A, Bahr MJ, Aslan N, Bader C, Tillmann HL, Sarrazin C, et al. Induction of hepatitis C virus (HCV)-specific T cells by needle stick injury in the absence of HCV-viraemia. Eur J Clin Invest. 2007;37:54–64. doi: 10.1111/j.1365-2362.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 64.Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aberle JH, Formann E, Steindl-Munda P, Weseslindtner L, Gurguta C, Perstinger G, et al. Prospective study of viral clearance and CD4(+) T-cell response in acute hepatitis C primary infection and reinfection. J Clin Virol. 2006;36:24–31. doi: 10.1016/j.jcv.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Shata MT, Anthony DD, Carlson NL, Andrus L, Brotman B, Tricoche N, et al. Characterization of the immune response against hepatitis C infection in recovered, and chronically infected chimpanzees. J Viral Hepat. 2002;9:400–410. doi: 10.1046/j.1365-2893.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 67.Schirren CA, Jung M, Worzfeld T, Mamin M, Baretton GB, Gruener NH, et al. Cytokine profile of liver- and blood-derived nonspecific T cells after liver transplantation: T helper cells type 1/0 lymphokines dominate in recurrent hepatitis C virus infection and rejection. Liver Transpl. 2000;6:222–228. doi: 10.1002/lt.500060204. [DOI] [PubMed] [Google Scholar]
- 68.Prince AM, Shata MT. Immunoprophylaxis of hepatitis C virus infection. Clin Liver Dis. 2001;5:1091–1103. doi: 10.1016/s1089-3261(05)70211-7. [DOI] [PubMed] [Google Scholar]
- 69.Klenerman P, Lucas M, Barnes E, Harcourt G. Immunity to hepatitis C virus: stunned but not defeated. Microbes Infect. 2002;4:57–65. doi: 10.1016/s1286-4579(01)01510-6. [DOI] [PubMed] [Google Scholar]
- 70.Ferrari C, Valli A, Galati L, Penna A, Scaccaglia P, Giuberti T, et al. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology. 1994;19:286–295. [PubMed] [Google Scholar]
- 71.Farci P, Orgiana G, Purcell RH. Immunity elicited by hepatitis C virus. Clin Exp Rheumatol. 1995;13(suppl 13):S9–S12. [PubMed] [Google Scholar]
- 72.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 73.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 74.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 75.Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 76.Diepolder HM, Zachoval R, Hoffmann RM, Jung MC, Gerlach T, Pape GR. The role of hepatitis C virus specific CD4+T lymphocytes in acute and chronic hepatitis C. J Mol Med. 1996;74:583–588. doi: 10.1007/s001090050062. [DOI] [PubMed] [Google Scholar]
- 77.Pape GR, Gerlach TJ, Diepolder HM, Gruner N, Jung M, Santantonio T. Role of the specific T-cell response for clearance and control of hepatitis C virus. J Viral Hepat. 1999;6(suppl 1):36–40. doi: 10.1046/j.1365-2893.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 78.Day C, Lauer G, Robbins G, McGovern B, Wurcel A, Gandhi R, et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol. 2002;76:12584–12595. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lasarte JJ, Garcia-Granero M, Lopez A, Casares N, Garcia N, Civeira MP, et al. Cellular immunity to hepatitis C virus core protein and the response to interferon in patients with chronic hepatitis C. Hepatology. 1998;28:815–822. doi: 10.1002/hep.510280332. [DOI] [PubMed] [Google Scholar]
- 81.Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology. 2004;39:1721–1731. doi: 10.1002/hep.20266. [DOI] [PubMed] [Google Scholar]
- 82.Rosen HR, Miner C, Sasaki AW, Lewinsohn DM, Conrad AJ, Bakke A, et al. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology. 2002;35:190–198. doi: 10.1053/jhep.2002.30293. [DOI] [PubMed] [Google Scholar]
- 83.Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 84.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, et al. Foxp3+CD4+CD25+ T cells control virus–zspecific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424–4432. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 87.Yao ZQ, Shata MT, Tricoche N, Shan MM, Brotman B, Pfahler W, et al. gC1qR expression in chimpanzees with resolved and chronic infection: potential role of HCV core/gC1qR-mediated T cell suppression in the outcome of HCV infection. Virology. 2006;346:324–337. doi: 10.1016/j.virol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 88.Yao ZQ, Nguyen DT, Hiotellis AI, Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J Immunol. 2001;167:5264–5272. doi: 10.4049/jimmunol.167.9.5264. [DOI] [PubMed] [Google Scholar]
- 89.Gale MJ, Jr, Korth MJ, Katze MG. Repression of the PKR protein kinase by the hepatitis C virus NS5A protein: a potential mechanism of interferon resistance. Clin Diagn Virol. 1998;10:157–162. doi: 10.1016/s0928-0197(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 90.Gale M, Tan S-L, Wambach M, Katze M. Interaction of the interferon-induced PKR protein kinase with inhibitory proteins p58IPK and vaccinia virus K3L is mediated by unique domains. Mol Cell Biol. 1996;16:4172–4181. doi: 10.1128/mcb.16.8.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gale M, Jr, Kwieciszewski B, Dossett M, Nakao H, Katze MG. Anti-apoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J Virol. 1999;73:6506–6516. doi: 10.1128/jvi.73.8.6506-6516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gale M, Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 93.Gale M, Blakely C, Kwieciszewski B, Tan SL, Dossett M, Tang NM, et al. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 96.Lauer GM, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, et al. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005;79:12979–12988. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lauer G, Ouchi K, Chung R, Nguyen T, Day C, Purkis D, et al. Comprehensive analysis of CD8+-T-cell responses in hepatitis C virus reveals multiple unpredicted specificities. J Virol. 2002;76:6104–6113. doi: 10.1128/JVI.76.12.6104-6113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vrolijk JM, Tang TJ, Kwekkeboom J, Haagmans BL, Herscheid AJ, Kusters JG, et al. Monitoring intrahepatic CD8+ T cells by fine-needle aspiration cytology in chronic hepatitis C infection. J Viral Hepat. 2004;11:342–348. doi: 10.1111/j.1365-2893.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 99.Koziel MJ, Walker BD. Characteristics of the intrahepatic cytotoxic T lymphocyte response in chronic hepatitis C virus infection. Springer Semin Immunopathol. 1997;19:69–83. doi: 10.1007/BF00945026. [DOI] [PubMed] [Google Scholar]
- 100.Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, et al. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388–2394. doi: 10.1002/1521-4141(200108)31:8<2388::aid-immu2388>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 101.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392–11400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pancholi P, Liu Q, Tricoche N, Zhang P, Perkus ME, Prince AM. DNA prime-canarypox boost with polycistronic hepatitis C virus (HCV) genes generates potent immune responses to HCV structural and non-structural proteins. J Infect Dis. 2000;182:18–27. doi: 10.1086/315646. [DOI] [PubMed] [Google Scholar]
- 103.Nelson DR, Marousis CG, Davis GL, Rice CM, Wong J, Houghton M, et al. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158:1473–1481. [PubMed] [Google Scholar]
- 104.Lechmann M, Ihlenfeldt HG, Braunschweiger I, Giers G, Jung G, Matz B, et al. T- and B-cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and in healthy anti-hepatitis C virus—positive blood donors without viremia. Hepatology. 1996;24:790–795. doi: 10.1002/hep.510240406. [DOI] [PubMed] [Google Scholar]
- 105.Koziel MJ, Dudley D, Afdhal N, Choo QL, Houghton M, Ralston R, et al. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol. 1993;67:7522–7532. doi: 10.1128/jvi.67.12.7522-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koziel MJ. The role of immune responses in the pathogenesis of hepatitis C virus infection. J Viral Hepat. 1997;4(suppl 2):31–41. doi: 10.1111/j.1365-2893.1997.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 107.Kowalski H, Erickson AL, Cooper S, Domena JD, Parham P, Walker CM. Patr-A and B, the orthologues of HLA-A and B, present hepatitis C virus epitopes to CD8+ cytotoxic T cells from two chronically infected chimpanzees. J Exp Med. 1996;183:1761–1775. doi: 10.1084/jem.183.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung MC, Diepolder HM, Pape GR. T cell recognition of hepatitis B and C viral antigens. Eur J Clin Invest. 1994;24:641–650. doi: 10.1111/j.1365-2362.1994.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 109.Jin Y, Fuller L, Carreno M, Zucker K, Roth D, Esquenazi V, et al. The immune reactivity role of HCV-induced liver infiltrating lymphocytes in hepatocellular damage. J Clin Immunol. 1997;17:140–153. doi: 10.1023/a:1027326415164. [DOI] [PubMed] [Google Scholar]
- 110.Inchauspe G. Protection and defence mechanisms in HCV infection. Nephrol Dial Transplant. 1996;11(suppl 4):6–8. doi: 10.1093/ndt/11.supp4.6. [DOI] [PubMed] [Google Scholar]
- 111.He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, et al. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci U S A. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fiore G, Angarano I, Caccetta L, Serrone M, Jirillo E, Schiraldi O, et al. In-situ immunophenotyping study of hepatic-infiltrating cytotoxic cells in chronic active hepatitis C. Eur J Gastroenterol Hepatol. 1997;9:491–496. doi: 10.1097/00042737-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 113.Erickson AL, Houghton M, Choo QL, Weiner AJ, Ralston R, Much-more E, et al. Hepatitis C virus-specific CTL responses in the liver of chimpanzees with acute and chronic hepatitis C. J Immunol. 1993;151:4189–4199. [PubMed] [Google Scholar]
- 114.Cerny A, McHutchison JG, Pasquinelli C, Brown ME, Brothers MA, Grabscheid B, et al. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A21 binding motif . J Clin Invest. 1995;95:521–530. doi: 10.1172/JCI117694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rehermann B, Chang KM, McHutchinson J, Kokka R, Houghton M, Rice CM, et al. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lechner F, Wong D, Dunbar P, Chapman R, Chung R, Dohrenwend P, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 119.Rollier CS, Paranhos-Baccala G, Verschoor EJ, Verstrepen BE, Drexhage JA, Fagrouch Z, et al. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602–613. doi: 10.1002/hep.21573. [DOI] [PubMed] [Google Scholar]
- 120.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 121.Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ballardini G, Groff P, Pontisso P, Giostra F, Francesconi R, Lenzi M, et al. Hepatitis C virus (HCV) genotype, tissue HCV antigens, hepatocellular expression of HLA-A,B,C, and intercellular adhesion-1 molecules. Clues to pathogenesis of hepatocellular damage and response to interferon treatment in patients with chronic hepatitis C. J Clin Invest. 1995;95:2067–2075. doi: 10.1172/JCI117893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chambers TJ, Fan X, Droll DA, Hembrador E, Slater T, Nickells MW, et al. Quasispecies heterogeneity within the E1/E2 region as a pretreatment variable during pegylated interferon therapy of chronic hepatitis C virus infection. J Virol. 2005;79:3071–3083. doi: 10.1128/JVI.79.5.3071-3083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morishima C, Polyak SJ, Ray R, Doherty MC, Di Bisceglie AM, Malet PF, et al. Hepatitis C virus-specific immune responses and quasi-species variability at baseline are associated with nonresponse to antiviral therapy during advanced hepatitis C. J Infect Dis. 2006;193:931–940. doi: 10.1086/500952. [DOI] [PubMed] [Google Scholar]
- 125.Shire NJ, Horn PS, Rouster SD, Stanford S, Eyster ME, Sherman KE. HCV kinetics, quasispecies, and clearance in treated HCV-infected and HCV/HIV-1-coinfected patients with hemophilia. Hepatology. 2006;44:1146–1157. doi: 10.1002/hep.21374. [DOI] [PubMed] [Google Scholar]
- 126.Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 127.Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]
- 128.Kamal SM, Moustafa KN, Chen J, Fehr J, Abdel Moneim A, Khalifa KE, et al. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006;43:923–931. doi: 10.1002/hep.21197. [DOI] [PubMed] [Google Scholar]
- 129.Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130:632–638. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 130.Rahman F, Heller T, Sobao Y, Mizukoshi E, Nascimbeni M, Alter H, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 131.Vogel M, Bieniek B, Jessen H, Schewe CK, Hoffmann C, Baumgarten A, et al. Treatment of acute hepatitis C infection in HIV-infected patients: a retrospective analysis of eleven cases. J Viral Hepat. 2005;12:207–211. doi: 10.1111/j.1365-2893.2005.00580.x. [DOI] [PubMed] [Google Scholar]
- 132.Serpaggi J, Chaix ML, Batisse D, Dupont C, Vallet-Pichard A, Fontaine H, et al. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS. 2006;20:233–240. doi: 10.1097/01.aids.0000200541.40633.56. [DOI] [PubMed] [Google Scholar]
- 133.Karnam US, Reddy KR. Pegylated interferons. Clin Liver Dis. 2003;7:139–148. doi: 10.1016/s1089-3261(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 134.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]


