Abstract
We studied the development of sexual dimorphism in resistance to NK-sensitive experimental metastasis under baseline conditions and following adrenoceptor stimulation. With increasing age, baseline resistance to MADB106 lung tumor retention (LTR) increased in both sexes, but also the susceptibility to the tumor-enhancing effects of a β-adrenergic agonist, metaproterenol. Beginning at 13 weeks, males exhibited a 2- to 3-fold greater increase in LTR than females following adrenoceptor stimulation. This adult dimorphism was robust to ovariectomy, and questionably related to androgens. The findings are consistent with reduced female responsiveness to sympathetic activation, and substantiate the importance of including both sexes when studying neuroimmunomodulation.
Keywords: β-adrenergic, testosterone, sex differences, MADB106
1. Introduction
Natural killer (NK) cells are well known to play an important role in the immune defense against viral infections and cancer, particularly along the metastatic process (Cerwenka and Lanier, 2001). NK function is also known to be modulated by stressful experiences, and the sympathetic nervous system has been shown to be a key mediator in stress-induced alterations in NK activity (e.g., Mills et al., 1995; Benschop et al., 1996; Ben-Eliyahu et al., 2000a; Jiang et al., 2004). We have previously observed that compared to females, male rats exhibit significantly greater suppression of NK function following a swim stress paradigm (Page et al., 2005) shown to suppress NK activity by β-adrenergic activation (Ben-Eliyahu et al., 2000a). Given that earlier work in our lab showed that prepubescent rats exhibit very low NK function (Page and Ben-Eliyahu, 1999) and no suppression of NK cytotoxicity following adrenoceptor-activation (Page and Ben-Eliyahu, 2000), the purpose of this study was threefold: (i) to assess whether the sexually dimorphic effects evident in adult rats are robust and have biological significance, as indicated by in vivo resistance to an NK-sensitive process of experimental metastasis; (ii) to identify the age at which such a sexually dimorphic response to adrenergic stimulation emerges; and (iii) discern possible involvement of gonadal hormones.
The NK-sensitive MADB106 cell line used in this study is a selected variant cell line obtained from a pulmonary metastasis of a mammary adenocarcinoma, MADB100, chemically induced in the inbred F344 rat (Barlozzari et al., 1985). Upon intravenous injection, MADB106 cells seed and colonize only in the lungs, and these processes are highly controlled by NK cells, but only in the first 24 h following injection (Barlozzari et al., 1985) and in a time dependent and decremental manner (Ben-Eliyahu and Page, 1992). Thus, the lung retention of MADB106 cells provides a sensitive in vivo index of NK function, and suggests host susceptibility to specific aspects of the complex metastatic process. Irrespective of NK cells, other mechanisms can also affect this index.
2. Methods
2.1. Animals
Fischer 344 (F344) male and female rats were either purchased from Harlan Sprague Dawley and used following a minimum of 4 weeks habituation to the environment (Experiment 1), or were bred and raised in our vivarium using Harlan Sprague Dawley F344 breeders every third generation to maintain the genetics of this inbred strain. Offspring were weaned on day 22 after birth, and all animals were single-sex sibling housed; mature animals used in experiments 2−5 were 16−49 weeks of age, age-matched within experiment. All animals had unlimited access to water and food and were maintained on a 12:12 h dark/light cycle at 22±1 °C. All experiments were conducted within the first 5 h of dark onset. The Johns Hopkins University Animal Care and Use Committee approved these experiments.
2.2. MADB106 Tumor Cell Maintenance, Radiolabeling, and Assessment of Lung Tumor Retention
MADB106 cells were maintained in 5% CO2 at 37° C in complete medium (RPMI 1640 media [Mediatech] supplemented with 10% heat-inactivated fetal calf serum [FCS], 0.05 mg/ml gentamicin, 2 mM L-glutamine, 0.1 mM non-essential amino acid and 1 mM sodium pyruvate). The DNA of MADB106 cells was labeled by adding 0.4 μCi [125I]iododeoxyuridine per ml medium to the growing MADB106 cell culture 24 h before cell harvest. The adherent MADB106 cells were separated from the flask using Trypsin 0.25%. After separation from the flask, cells were washed and reconstituted in phosphate buffered saline.
For all in vivo studies of lung tumor retention, at 1 h after drug/vehicle injection, animals were briefly anesthetized with halothane and injected with radiolabeled MADB106 tumor cells into the tail vein at a dose of 4×105 cells per kg body weight. Lungs were removed 22 h later and their radioactive content was assessed using a gamma counter (Packard Cobra 5002). The percent lung tumor retention (LTR) was calculated as [(lung count ÷ injectate count) × 100] (Ben-Eliyahu and Page, 1992).
2.3. Drugs
Metaproterenol (MP, Sigma), a β-adrenergic agonist was dissolved in saline and administered subcutaneously. MP has a higher affinity to β2 receptors than β1, and approximately a 2 h half-life (Dengler and Hengstmann, 1976; Muacevic, 1985). The concentration of MP was adjusted so that animals received 2 ml/kg body weight. Epinephrine (Sigma) was dissolved in saline and administered intraperitoneally, 0.6 mg/kg (Ben-Eliyahu et al., 2000a). Vehicle injected animals received saline.
2.4. Gonadectomy
Mature males underwent castration and mature females underwent bilateral ovariectomy (OVX) under halothane anesthesia at least 4 weeks prior to the experiment. Briefly, male gonads were clamped, tied and removed via bilateral scrotal incision. The incision was re-approximated using tissue adhesive. Females underwent a 3 cm midline abdominal incision, and the ovaries were clamped at the uterine horn, tied and excised. The abdominal skin and muscle was sutured using 5/0 monofilament wire; sutures were removed 10 days later. All animals were medicated with 10 mg/kg morphine in a slow release suspension (Page et al., 1998) upon the completion of the surgery. In an effort to minimize the number of animals exposed to the pain of undergoing and recovering from surgery, two pilot studies were undertaken to assess for possible differences between intact and sham castration males in this experimental paradigm. No significant differences between sham surgery and intact males in LTR were evident in either vehicle or in MP injected animals in both replications using the same experimental procedures reported herein. These findings are consistent with our previous studies showing that at adulthood, experimental abdominal surgery exerts no impact on LTR beyond the seventh postoperative day ( Ben-Eliyahu et al., 1999; Page et al., 1993). Therefore, and in order to avoid unnecessary pain and suffering, the studies involving gonadectomy include no sham operated animals.
2.5. Statistical Analyses
To accumulate sufficient numbers of animals, several replications were conducted of all experiments, each employing all experimental groups. Results were combined using standardized scores from all replications, thus neutralizing potential interassay variation in baseline levels. Given the markedly different variances between groups of saline and MP injected animals, which violates the ANOVA assumption of “homogeneity of variance”, standardized scores were Log10 transformed before employing ANOVA. Bonferroni post hoc pair-wise comparisons were used when indicated, and p < 0.05 was used in all analyses.
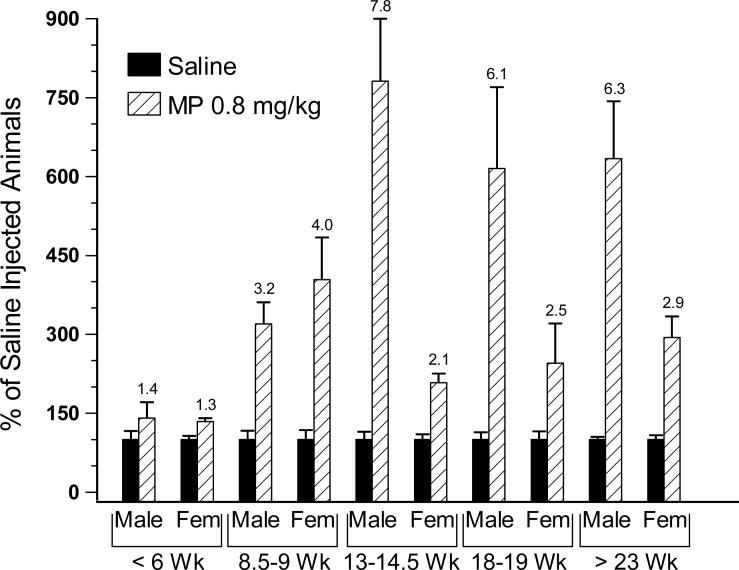
For graphic presentation, standardized scores were transformed back to “% tumor cell retention” based on the average of this raw index in all replications comprising an experiment. The single exception for such graphic presentation is Fig. 2, in which the magnitudes of MP effects are presented as percent control of the saline vehicle injected animals to facilitate the presentation of age- and sex-dependent alterations in the effects of two doses of MP. The absolute levels of these controls are presented in Fig. 1.
Fig. 2.
The development of sex differences in the impact of β-adrenergic activation on lung retention of MADB106 cells from prepubescence through maturity, expressed as percent of saline injected control levels of lung tumor retention in each age/sex condition (see Fig. 1). There were significant age by dose and sex by dose interactions such that at 13 weeks and older, the significant male-female difference in the magnitude of the metaproterenol (MP)-induced tumor-enhancing effect emerged. Error bars are SEM. The number above the 0.8 mg/kg MP dose bars indicates the magnitude of the MP effect compared to the sex/age matched saline injected group.
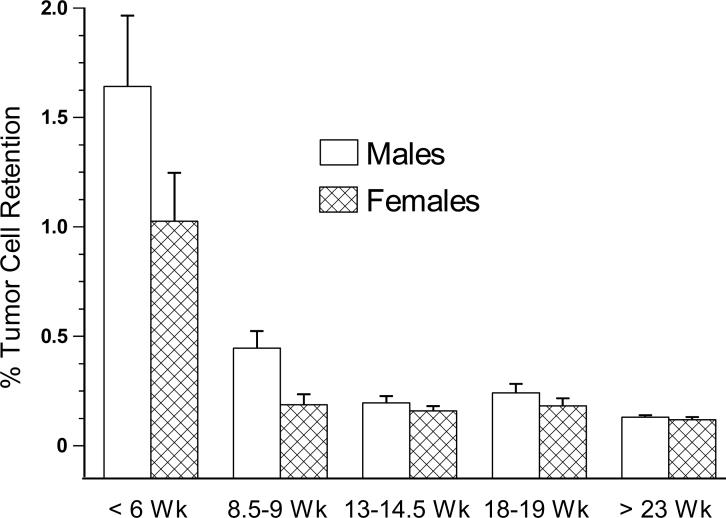
Fig. 1.
The development of resistance against lung tumor retention of NK sensitive MADB106 tumor cells in male and female Fischer 344 rats from prepubescence (≤ 40 days) through adulthood. These animals are the saline injected (control) groups seen in Fig. 2. The prepubescent animals retained a significantly greater percentage of MADB106 cells versus all other age groups, p < 0.05; error bars are SEM.
3. Results
3.1. Experiment 1: The effects of age, sex and MP dose on lung tumor retention (LTR)
3.1.1. Design and procedure
To learn whether there is a developmental component to the male – female differences in the impact of MP on lung tumor retention (LTR) of the NK-sensitive MADB106 tumor line, males and females of five age groups from prepubescence through maturity were studied. Specifically, male and female rats aged < 6 weeks (33−40 days, prepubescent), 8.5−9, 13−14.5, 18−19, and > 23 weeks (23−49 weeks) were randomly assigned to receive either MP in one of two doses (0.8 or 5.0 mg/kg) or vehicle (saline) 1 h before intravenous injection of radiolabeled MADB106 cells, n = 8−14 per age/sex saline control group and 5−8 per age/sex MP group.
3.1.2. Results
Among the saline injected animals, there was an age by sex interaction such that prepubescent animals (less than 6, and 8.5−9 weeks old) exhibited significantly greater lung tumor retention, more so among the males [F(4,104) = 2.684, p < 0.05, age comparisons p < 0.05, Bonferroni post hoc, Fig. 1]. With the addition of the MP injected animals and the analysis of the full model, there were significant age by dose and sex by dose interactions [F(8,232) = 5.699, p < 0.001, and F(2,238) = 4.159, p < 0.02, respectively (Fig. 2)]. Specifically, prepubescent animals exhibited smaller effects of MP, and once animals reached 13 weeks of age, the males exhibited significantly greater MP-induced increases in lung tumor retention compared to females. Among animals greater than 13 weeks of age, the MP-induced increase in LTR was 2- to 3-fold in the females versus 6- to 8-fold in the males at 0.8 mg/kg; and at 5 mg/kg (not shown), 13- to 25-fold versus 40- to 80-fold in females versus males, respectively.
3.2 Experiment 2: A comparison of the impact of MP versus epinephrine on LTR
3.2.1. Design and procedure
To learn whether the crossing of the blood-brain barrier by MP is a factor in the above observed male – female differences, a direct comparison of the impact of MP to the impact of epinephrine (of virtually no brain availability [Weil-Malherbe et al., 1959]) was undertaken in mature animals. A 2×3 experimental design was used in which mature males and females were assigned to receive MP (0.8 mg/kg), epinephrine (0.6 mg/kg), or vehicle injection 1 h before MADB106 tumor inoculation, n = 111 animals.
3.2.2. Results
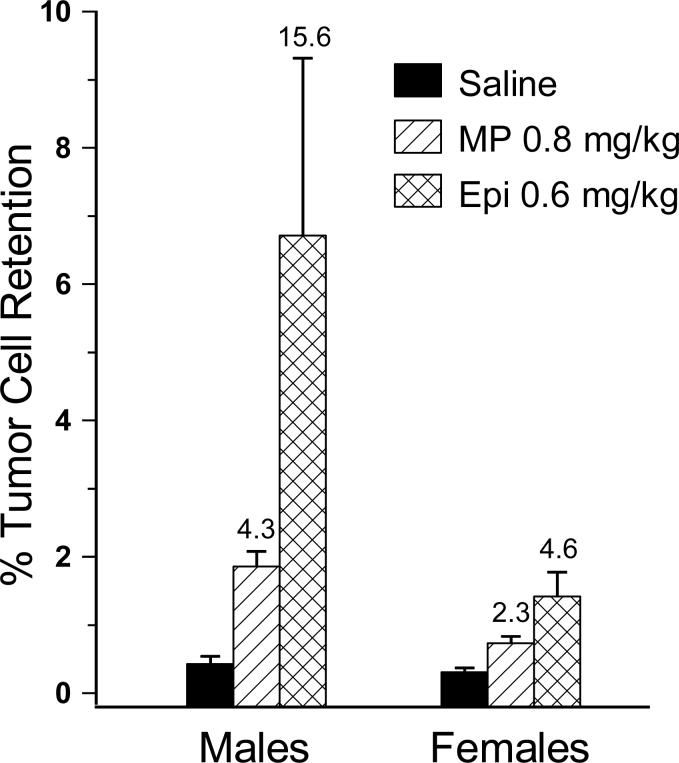
The significant male – female differences in response to β-adrenergic activation were similarly evident when epinephrine was used in comparison to MP. The significant sex by drug interaction was evident with both MP [F(1,82) = 4.974, p < 0.05] and epinephrine [F(1,68) = 8.801, p < 0.01] (Fig. 3). In particular, whereas the males exhibited a 4.3-fold and 15-fold effect of MP and epinephrine, respectively, the respective effects in the females were 2.3- and 4.6-fold.
Fig. 3.
Comparison of the impact of epinephrine (Epi) versus metaproterenol (MP) on lung tumor retention in mature male and female F344 rats. The significant male-female difference in the MP-induced increase in tumor retention was also evident in epinephrine injected animals. Error bars are SEM. The number above the MP and epinephrine bars indicates the magnitude of the respective effects compared to the sex-matched saline injected group.
3.3. Experiment 3: The effect of ovariectomy on sex differences in MP-induced increased LTR
3.3.1. Design and procedure
To test for a possible role of female gonadal hormones in the sexual dimorphic effects of MP observed in the previous studies, a 3×2 experimental design was employed: intact males and females and OVX females, were randomly assigned to receive either MP (0.8 mg/kg) or vehicle 1 h before tumor injection, n = 8−10 per group. All animals were 17−19 weeks old at the time of the experiment, and OVX was performed 6 weeks earlier, n = 8−10 per group.
3.3.2. Results
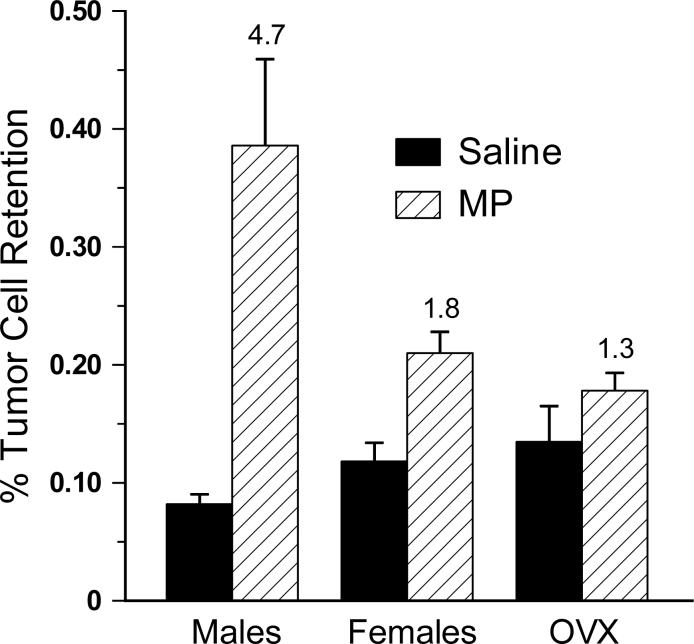
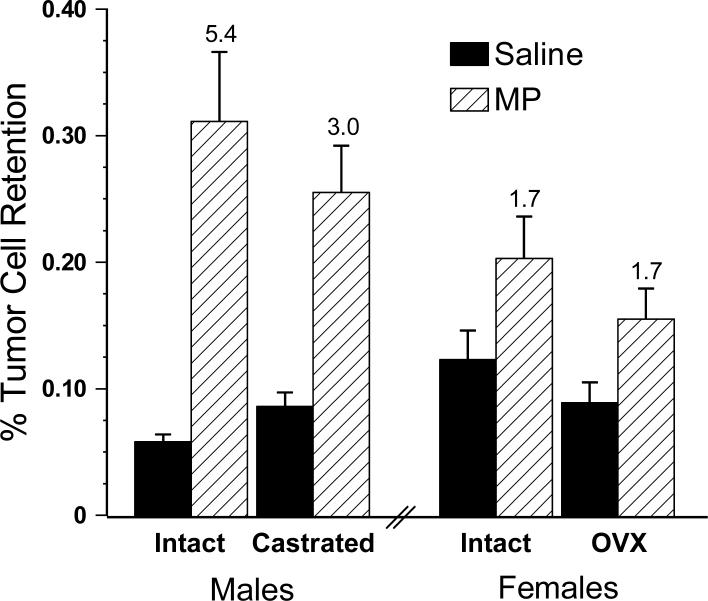
Ovariectomy did not significantly alter the tumor-enhancing effect of MP in the females. The large MP-induced increase in LTR observed in the males continued to account for the significant sex by drug interaction among males, females and OVX females [F(2,54) = 7.284, p < 0.01, Fig. 4]. Similar to Experiment 1, there was a 4.7-fold MP related increase in LTR in males compared to 1.8-fold in females and 1.3-fold in OVX females.
Fig. 4.
The effect of ovariectomy (OVX) on male-female differences in metaproterenol (MP)-induced increases in lung tumor retention. OVX did not significantly alter the tumor-enhancing effect of MP in the females. Error bars are SEM. The number above the MP bars indicates the magnitude of the MP effect compared to the sex/gonadal status matched saline injected group.
3.4. Experiment 4: The effects of gonadectomy in males and females on sex differences in MP-induced increased LTR
3.4.1. Design and procedure
To validate our OVX findings and to test for possible androgenic effects on MP-induced increases in LTR, a 2×2×2 design was used such that mature males and females either remained intact or underwent gonadectomy 11 weeks before the experiment (at age 11- 20 weeks), and one-half of each sex/gonadal status group was injected with either MP (0.8 mg/kg) or saline vehicle 1 h before tumor injection, n = 10−18 per group.
3.4.2. Results
The female-male differences in the MP-induced increases in lung tumor retention were clearly evident in this experiment, as indicated by a significant sex by drug (MP) interaction [F(1,108) = 12.113, p = 0.001]. As observed in Experiment 3, OVX did not affect MP-induced increases in LTR. On the other hand, there was a near significant interaction between MP and gonadal status in the males, p = 0.056, such that castration narrowed the magnitude of the MP-induced increase in LTR from 5.4-fold in the intact males to 3-fold in those that were castrated. Clearly, changes in baseline levels contributed to this trend (Fig. 5).
Fig. 5.
The impact of gonadectomy on metaproterenol (MP)-induced increases in MADB106 tumor retention. Whereas female OVX did not alter MP-induced increases in LTR, there was a trend of reduced MP effects in castrated males. Error bars are SEM. The number above the MP bars indicates the magnitude of the MP effect compared to the sex/gonadal status matched saline injected group.
4. Discussion
Findings from this study show that there is an age dependent sexual dimorphism in the effects of β-adrenoceptor stimulation, such that beginning at sexual maturity (approximately 90 days of age [Robb et al., 1978]), males are substantially more susceptible to the tumor promoting effects of MP than age-matched females. This large sex difference in mature rats was consistently evident throughout the experiments undertaken in this study as well as in a previous report (Page and Ben-Eliyahu, 2000). Specifically, by 13 weeks of age, whereas females exhibit a 1.7- to 3-fold tumor-enhancing effect of MP (median 2.3 fold), the magnitude of the MP effect in the males was far greater within each experiment, ranging from 4.3- to 7.8-fold above vehicle injected males (median 5.75 fold). The findings that ovariectomy in the mature female had no impact on the response to adrenoceptor stimulation rules out activational effects of female gonadal hormones as a mediating mechanism of this dimorphism. A reduced sensitivity to the MP effects on lung tumor retention (LTR) was apparent in castrated males as the magnitude of the MP effect reached the high end of that observed in the females; however, this change did not reach statistical significance. This trend may be consistent with the hypothesis that organizational or activational effects of androgens could contribute to the sexually dimorphic response to β-adrenergic activation. We are now conducting a more focused study addressing this hypothesis.
In adult animals, baseline (no MP) levels of MADB106 LTR naturally varied between experiments, as would be expected based upon our previous studies. Thus, all experiments included vehicle groups with which to assess the magnitude of the MP effects within each condition. Male-female differences in baseline levels of LTR were commonly not significant nor were they consistent. Our previous studies suggest that these small sex differences in baseline LTR do not directly translate to baseline NK activity (e.g., Page et al., 1995; Ben-Eliyahu et al., 1996; Page and Ben-Eliyahu, 1997; Page and Ben-Eliyahu, 1999). The effects of gonadectomy on baseline LTR were also not consistent or significant. Overall, the sexual dimorphism in the LTR response to MP and epinephrine was independent of baseline LTR levels – they are significant both in absolute levels of LTR in MP/epinephrine injected animals and as percent of baseline levels. We chose to present the raw data of baseline and MP levels of LTR rather than the percent increase in LTR resulting from MP compared to vehicle/control levels; thus enabling the assessment of the contribution of baseline levels in the results. In Fig. 2, the data are presented as percent control to facilitate efficient comparisons along the many ages/sex groups; however, the absolute baseline levels of the same experiment are presented in Fig. 1.
One may wonder whether some technical aspect of the study related to the sex differences in body weight underlies the findings observed herein. Specifically, both MP and MADB106 tumor cells were given per kg body weight which might lead one to question whether this common approach does not greatly overcompensate for the 1.6 ratio of male/female body weight. In other words, one could suggest that the body weight-based approach to administering the tumor or drugs is the source of the 2- to 3-fold greater effects of MP evident in the males; however, evidence from our previous studies refute such an hypothesis. For example, if the number of MADB106 cells injected per kg were doubled or quadrupled, the percent LTR, our main outcome, minimally increases (10−20%) (Shakhar et al., 2001; Ben-Eliyahu et al., 1996). Thus the 1.6-fold greater numbers of MADB106 cells injected to males (based on their greater body weight) would not account for the 2- to 3-fold greater impact of MP in males. MP was also given per body weight in a dose of 0.8 mg/kg in all experiments. Our previous studies employing MP doses ranging from 0.3 to 0.6, to 1.0, and to 3.0 mg/kg indicate that increasing the MP dose by 1.6 would result in approximately a 20−30% increase in percent LTR (Shakhar and Ben-Eliyahu, 1998). Taken together, even if we gave the same absolute amount of MP to both sexes (i.e., a 1.6-fold higher dose per body weight to the females), the herein evident 2- to 3-fold dimorphism would still remain in the same direction and would be both statistically and biologically significant. Thus, the evident male-female differences that we have observed are not derived from differences in body weight, but rather represent a real sexual dimorphism in the biological response to MP and epinephrine.
It is most likely that the evident sexual dimorphic effects of β-adrenergic activation are mediated through peripheral adrenoceptor activation, rather than via direct impact on brain substrate. First, unlike MP that penetrates the blood brain barrier (BBB), epinephrine which does not readily cross the BBB (Weil-Malherbe et al., 1959) induced the same dimorphic effects (Fig. 3). Second, in a previous study using MP and the same MADB106 tumor model in males, we found that nadolol, a β-adrenergic blocker which does not cross the BBB (Prichard and Tomlinson, 1986) completely prevented the effects of MP on MADB106 lung tumor retention (Shakhar and Ben-Eliyahu, 1998). Therefore peripheral adrenoceptor activation is a mediating mechanism of the dimorphism.
When taken with previous findings, it is also likely that the sexual dimorphic effects of MP are mediated, at least partly, through suppression of NK activity. As indicated in the introduction, the lung retention of MADB106 cells is highly sensitive to in vivo levels of NK activity (Ben-Eliyahu and Page, 1992; Shakhar and Ben-Eliyahu, 1998). Moreover, employing the exact same paradigms used herein, we have previously shown that the promotion of MADB106 metastasis by MP is almost exclusively mediated by suppression of NK activity. Specifically, MP caused marked suppression of NK activity, and a selective in vivo depletion of NK cells eliminated the tumor promoting effects of MP (Shakhar and Ben-Eliyahu, 1998) but not of other interventions (Ben-Eliyahu et al., 1999). Others have reported that NK cells express β2 receptors (van Tits et al., 1990), and that MP-induced β-adrenergic activation in vitro reduces granzyme-b and perforin levels, as well as IFN-γ mRNA levels in F344 rat splenocytes, effects that were also blocked by nadolol (Dokur et al., 2004). On the other hand, both B and T cells have been shown to have β-adrenoceptors (Edgar et al., 2002) and to be involved in early lung metastasis surveillance including that of MADB106 cells (Quan et al., 1999; Shingu et al., 2002).
Although the current study only addresses adrenoceptor activation by an agonist, various physiological and psychological stressors were shown to impact immunity through sympathetic nervous system activation. Specifically β-adrenergic activation has been shown to mediate outcomes reflective of NK suppression following repeated swim, social confrontation, cold exposure, and surgery (Stefanski and Ben-Eliyahu, 1996; Ben-Eliyahu et al., 2000a; Jiang et al., 2004; Melamed et al., 2005). Human studies have also shown NK modulation to be mediated by adrenoceptor activation, and those also accounting for changes in NK number have reported suppression of NK activity hours after stress (Kappel et al., 1991; Nieman et al., 1995), as in the current study. Finally, an in vitro study showed epinephrine-induced supression of NK activity to be mediated by β2 receptors on NK cells (Hellstrand and Hermodsson, 1989). Taken together, the current findings may be relevant to a variety of conditions that entail various stress responses and activation of adrenoceptors.
There is considerable evidence of sex differences in autonomic nervous system regulation. Whereas for women, vagal withdrawal is a key mechanism for increasing heart rate (Evans et al., 2001; Liu et al., 2003; Kajantie and Phillips, 2006), men increase heart rate via autonomic sympathetic activity balanced by parasympathetic activity (Evans et al., 2001; Allen et al., 1993). Compared to females, the male cardiovascular response to stress is characterized by greater increases in heart rate, blood pressure, and plasma catecholamine levels (Hinojosa-Laborde et al., 1999), consistent with less female responsiveness to adrenal medullary activation. Findings from studies employing laboratory stress in humans support this sex difference, as indicated by greater heart rate reactivity in men compared to women in response to ischemic pain (Fillingim et al., 2002) and isometric handgrip (Hogarth et al., 2007).
In the current study and in our previous studies in rats (Page and Ben-Eliyahu, 2000), the NK suppressive and tumor promoting effects of β-adrenoceptor activation markedly increased with age in both sexes, and, based on the current study became dimorphic between weeks 9 and 13, toward the time of complete sexual maturation of the males. The comparatively greater baseline LTR observed in the prepubescent animals has been a consistent finding (Page and Ben-Eliyahu, 1999; Page and Ben-Eliyahu, 2000) that was associated with reduced NK activity in prepubescent animals in both sexes (Page and Ben-Eliyahu, 1999). In humans, compared to 8−10 year old children, 15−17 year old adolescents exhibited greater β-adrenergic activation and greater sex-specific reactivity profiles in response to behavioral challenge, supporting a transition to a mature male adrenergic response to stress during human puberty (Allen and Matthews, 1997). The potential biological significance of this sexual dimorphism is that for a given amount of catecholamine secretion, males would assume greater risk for the potential maladaptive sequelae of adrenoceptor stimulation. This sexual dimorphism could become clinically relevant when a primary tumor is removed, and the immune suppression resulting from catecholamine release renders males more susceptible to metastatic development. Also intriguing is the known sexually dimorphic prevalence of both cardiovascular and autoimmune diseases. In both pathologies, the etiology or the manifestations are linked to sympathetic responses (Palatini, 2001; Marques-Deak et al., 2005; Straub, 2007) . Thus, the clinical significance of the observed sexual dimorphism in response to adrenoceptor stimulation should be further addressed in the context of these pathologies.
In conclusion, the robust in vivo β-adrenergic effects observed in these studies demonstrate a two- to three-fold greater impact in male compared to female rats. This phenomenon emerges beyond puberty and most likely reflects a female lower response to adrenoceptor stimulation. Our findings uniquely suggest possible involvement of androgen rather than estrogen driven mechanisms, and ongoing studies are focusing on this possibility. Most studies showing β-adrenergic mediation of stress-induced immune alteration and tumor progression have focused on males only. The findings herein further substantiate the great importance of including both males and females for investigating the immune and neuroendocrine consequences of stress.
Acknowledgements
We wish to acknowledge the excellent technical assistance of Wendy Blakely, Grace Ramaiah, and Deborah Wagner. This work was supported by NIH grant NR07742. Dr. Ben-Eliyahu was supported by NIH grant CA73056.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Reference List
- Allen MT, Matthews KA. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology. 1997;34:329–339. doi: 10.1111/j.1469-8986.1997.tb02403.x. [DOI] [PubMed] [Google Scholar]
- Allen MT, Stoney CM, Owens JF, Matthews KA. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosom. Med. 1993;55:505–517. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, Reynolds CW. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J. Immunol. 1985;134:2783–2789. [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG. The in vivo assessment of natural killer cell activity in rats. Prog. NeuroEndocrinImmunol. 1992;5:199–214. [Google Scholar]
- Ben-Eliyahu S, Page GG, Shakhar G, Taylor AN. Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: Possible role of oestradiol and natural killer cells. Br. J. Cancer. 1996;74:1900–1907. doi: 10.1038/bjc.1996.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: A role for adrenal catecholamines and β-adrenoceptors. Neuroimmunomodulation. 2000a;8:154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Jacobs R, Sommer B, Schurmeyer TH, Raab JR, Schmidt RE, Schedlowski M. Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. FASEB J. 1996;10:517–524. doi: 10.1096/fasebj.10.4.8647351. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Dengler HJ, Hengstmann JH. Metabolism and pharmacokinetics of orciprenaline in various animal species and man. Arch. Int. Pharmacodyn. Ther. 1976;223:71–87. [PubMed] [Google Scholar]
- Dokur M, Boyadjieva N, Sarkar DK. Catecholaminergic control of NK cell cytolytic activity regulatory factors in the spleen. J. Neuroimmunol. 2004;151:148–157. doi: 10.1016/j.jneuroim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Edgar VA, Cremaschi GA, Sterin-Borda L, Genaro AM. Altered expression of autonomic neurotransmitter receptors and proliferative responses in lymphocytes from a chronic mild stress model of depression: effects of fluoxetine. Brain Behav. Immun. 2002;16:333–350. doi: 10.1006/brbi.2001.0632. [DOI] [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J. Appl. Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Browning AD, Powell T, Wright RA. Sex differences in perceptual and cardiovascular responses to pain: the influence of a perceived ability manipulation. J. Pain. 2002;3:439–445. doi: 10.1054/jpai.2002.128067. [DOI] [PubMed] [Google Scholar]
- Hellstrand K, Hermodsson S. An immunopharmacological analysis of adrenaline-induced suppression of human natural killer cell cytotoxicity. Int. Arch. Allergy Appl. Immunol. 1989;89:334–341. doi: 10.1159/000234972. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin. Exp. Pharmacol. Physiol. 1999;26:122–126. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin. Sci. 2007;112:353–361. doi: 10.1042/CS20060288. [DOI] [PubMed] [Google Scholar]
- Jiang XH, Guo SU, Xu S, Yin QZ, Ohshita Y, Naitoh M, Horibe Y, Hisamitsu T. Sympathetic nervous system mediates cold stress-induced suppression of natural killer cytotoxicity in rats. Neurosci. Lett. 2004;357:1–4. doi: 10.1016/j.neulet.2003.11.075. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kappel M, Tvede N, Galbo H, Kjaer M, Linstow M, Klarlund K, Pedersen BK. Evidence that the effect of physical exercise on NK cell activity is mediated by epinephrine. J. Appl. Physiol. 1991;70:2530–2534. doi: 10.1152/jappl.1991.70.6.2530. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2188–H2193. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- Marques-Deak A, Cizza G, Sternberg EM. Brain-immune interactions and disease susceptibility. Mol. Psychiatry. 2005;10:239–250. doi: 10.1038/sj.mp.4001643. [DOI] [PubMed] [Google Scholar]
- Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav. Immun. 2005;19:114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Berry CC, Dimsdale JE, Ziegler MG, Nelesen RA, Kennedy BP. Lymphocyte subset redistribution in response to acute experimental stress: effects of gender, ethnicity, hypertension, and the sympathetic nervous system. Brain Behav. Immun. 1995;9:61–69. doi: 10.1006/brbi.1995.1006. [DOI] [PubMed] [Google Scholar]
- Muacevic G. Determination of bioavailability on the basis of tachycardia after intravenous and oral administration of fenoterol, orciprenaline and salbutamol in non-anesthetized rats. Arzneim Forsch. 1985;35:406–408. [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, Stone MH, Butterworth DE, Davis JM. The acute immune response to exhaustive resistance exercise. Int. J. Sports Med. 1995;16:322–328. doi: 10.1055/s-2007-973013. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S. Increased surgery-induced metastasis and suppressed natural killer cell activity during proestrus/estrus in rats. Br. Cancer Res. Treat. 1997;45:159–167. doi: 10.1023/a:1005826403235. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S. A role for NK cells in greater susceptibility of young rats to metastasis. Dev. Comp. Immunol. 1999;23:87–96. doi: 10.1016/s0145-305x(98)00040-8. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S. Natural killer cell activity and resistance to tumor metastasis in prepubescent rats: Deficient baselines, but invulnerability to stress and beta-adrenergic stimulation. Neuroimmunomodulation. 2000;7:160–168. doi: 10.1159/000026434. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Taylor AN. The development of sexual dimorphism in natural killer cell activity and resistance to tumor metastasis in the Fischer 344 rat. J. Neuroimmunol. 1995;63:69–77. doi: 10.1016/0165-5728(95)00132-8. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Page GG, Blakely WP, Kim M. The impact of early repeated pain experiences on stress responsiveness and emotionality at maturity in rats. Brain Behav. Immun. 2005;19:78–87. doi: 10.1016/j.bbi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Page GG, McDonald JS, Ben-Eliyahu S. Pre-operative versus postoperative administration of morphine: Impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br. J. Anaesth. 1998;81:216–223. doi: 10.1093/bja/81.2.216. [DOI] [PubMed] [Google Scholar]
- Palatini P. Sympathetic overactivity in hypertension: a risk factor for cardiovascular disease. Curr. Hypertens. Rep. 2001;3(Suppl 1):S3–S9. doi: 10.1007/s11906-001-0065-z. [DOI] [PubMed] [Google Scholar]
- Prichard BNC, Tomlinson B. The additional properties of beta adrenoceptor blocking drugs. J. Cardiovasc. Pharmacol. 1986;8:S1–S15. doi: 10.1097/00005344-198608004-00002. [DOI] [PubMed] [Google Scholar]
- Quan N, Zhang Z, Demetrikopoulos MK, Kitson RP, Chambers WA, Goldfarb AH, Weiss JM. Evidence for involvement of B lymphocytes in the surveillance of lung metastasis in the rat. Cancer Res. 1999;59:1080–1089. [PubMed] [Google Scholar]
- Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J. Physiol. 2005;563:265–274. doi: 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar G, Bar-Ziv I, Ben-Eliyahu S. Diurnal changes in lung tumor clearance and their relation to NK cell cytotoxicity in the blood and spleen. Int. J. Cancer. 2001;94:401–406. doi: 10.1002/ijc.1477. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J. Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- Shingu K, Helfritz A, Kuhlmann S, Zielinska-Skowronek M, Jacobs R, Schmidt RE, Pabst R, von Höersten S. Kinetics of the early recruitment of leukocyte subsets at the sites of tumor cells in the lungs: natural killer (NK) cells rapidly attract monocytes but not lymphocytes in the surveillance of micrometastasis. Int. J. Cancer. 2002;99:74–81. doi: 10.1002/ijc.10279. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and beta-adrenergic mechanisms. Physiol. Behav. 1996;60:277–282. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- van Tits LJ, Michel MC, Grosse-Wilde H, Happel M, Eigler F-W, Soliman A, Brodde OE. Catecholamines increase lymphocyte β2-adrenergic receptors via a β2-adrenergic, spleen-dependent process. Am. J. Physiol. Endocrinol. Metab. 1990;258:E191–E202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- Weil-Malherbe H, Axelrod J, Tomchick R. Blood-brain barrier for adrenaline. Science. 1959;129:1226–1227. doi: 10.1126/science.129.3357.1226. [DOI] [PubMed] [Google Scholar]