Abstract
It was previously observed that cell confluence induced up-regulation of neutral sphingomyelinase 2 (nSMase2) and increased ceramide levels (Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM and Hannun YA. (2004) J Biol Chem, 279, 25101−11). In this study, we show that, in MCF7 cells, confluence induces the dephosphorylation of phosphorylated-β-catenin at threonine41/serine45. The effect of confluence on β-catenin dephosphorylation was prevented by down regulation of nSMase2 using siRNA; reciprocally, exogenous addition of short or very long chain ceramides induced dephosphorylation of β-catenin. The serine/threonine protein phosphatase inhibitors calyculin A and okadaic acid prevented β-catenin dephosphorylation during confluence. The specific phosphatase involved was determined by studies using siRNA against the major serine/threonine phosphatases, and the results showed that a specific siRNA against PP1cγ prevented dephosphorylation of β-catenin. Moreover, exogenous ceramides and confluence were found to induce the translocation of PP1cγ to the plasma membrane. All together these results establish: A) a specific intracellular pathway involving the activation of PP1 to mediate the effects of confluence-induced β-catenin dephosphorylation and B) PP1 as a lipid-regulated protein phosphatase downstream of nSMase2/ceramide. Finally, evidence is provided for a role for this pathway in regulating cell motility during confluence.
Keywords: β-catenin, neutral sphingomyelinase 2, protein phosphatase 1, confluence
Introduction
Ceramide, an increasingly recognized bioactive lipid, is involved in the regulation of diverse cellular functions including cell growth, apoptosis, differentiation and cell-cell adhesion [1, 2]. Many enzymatic pathways are involved in regulating ceramide levels, and neutral sphingomyelinase 2 (nSMase2) upon activation by extracellular agents serves as one such major intracellular regulator of ceramide [3, 4]. Indeed, a recent report revealed that p38 MAPK is an upstream regulator of nSMase2 and indicated a role for nSMase2 in pro-inflammatory responses induced by TNF-α as a regulator of adhesion proteins in lung epithelial cells [5]. Other recent research showed that downregulation of nSMase2 by small interference RNA (siRNA) completely blocked H2O2-induced apoptosis of human aortic endothelial cells and H2O2-induced nSMase2 trafficking to the plasma membrane (PM) whereas glutathione caused translocation to the perinuclear region, suggesting that oxidative stress can regulate nSMase2 localization [6]. In addition, it has been shown that nSMase2 is involved in IL-1beta-induced JNK activation in hepatocytes via a mechanism that involves activation of protein phosphatase 2A (PP2A) and alterations of the phosphorylation of IL-1β receptor-associated kinase (IRAK)-1, a key molecule that mediates IL-1β signaling [7]. Our previous studies showed that nSMase2 is up-regulated during cell growth, and is required for cells to undergo confluence-induced cell cycle arrest [8]. Furthermore, confluence-induced hypophosphorylation of the retinoblastoma protein (Rb) was inhibited by siRNA directed towards nSMase2 [8], suggesting a role for the generated ceramide in mediating this process, possibly via activation of a ceramide-activated serine/threonine phosphatases (PP1 and/or PP2A).
In addition, it was shown that nSMase2 in sub-confluent cells was primarily localized in Golgi and intracellular vesicles, and become enriched/restricted to the regions of cell-cell contact in confluent cells [5, 7, 8]. Several proteins that are regulated by cell contact have different localization depending on cell confluence. Indeed, previous studies have shown that β-catenin is translocated to the plasma membrane (PM) when cells reach confluence [9-11]. β-catenin at cell contact sites couples cadherins to the actin cytoskeleton strengthening adhesion and decreasing cell migration. On the other hand, in the cytosol, the axin/APC/GSK3β complex targets β-catenin for phosphorylation and degradation [12-16]. Recently, dephosphorylation of β-catenin has emerged as an alternative pathway that can protect β-catenin from degradation and may regulate its localization [17-20]. Interestingly, it was shown in vivo, as well as in vitro, that sphingolipids may regulate cytosolic accumulation of β-catenin [21]. However, the regulation of phospho- and β-catenin levels by ceramide or the mechanisms involved have not been determined.
In light of these results, we tested the hypothesis that the activation of nSMase2 during confluence functions as an endogenous regulator of phospho-β-catenin (Thr41/Ser45) and total β-catenin levels and localization. The goals of this study were: a) to determine if phospho-β-catenin phosphorylated at threonine41/serine45 and β-catenin levels and localization are regulated during confluence in MCF7 cells, b) to study the role of ceramide in mediating the dephosphorylation of phospho-β-catenin during confluence, c) to determine the mechanisms coupling ceramide action to β-catenin dephosphorylation, and d) to determined the physiological role of this pathway in the regulation of cell migration. The results from the study show a nSMase2-dependent dephosphorylation of β-catenin during confluence through a pathway that involves ceramide and the activation of PP1cγ resulting in decreased cell migration. Moreover, we find that exogenous ceramide mimics the effect of confluence in the translocation of PP1cγ and the dephosphorylation of β-catenin; thus demonstrating that ceramide is both necessary and sufficient to regulate this process in confluence.
Materials and Methods
Reagents
The MCF-7 cell line was purchased from American Tissue Culture Collection (Manassas, VA). D-erythro-C6-ceramide (D-e-C6-Cer), L-erythro-C6-ceramide (L-e-C6-Cer), D-threo-C6-ceramide (D-t-C6-Cer), and L-threo-C6-ceramide (L-t-C6-Cer) were synthesized in the Lipidomics Core at the Medical University of South Carolina. C24:1 and C2:0-ceramides were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). Antibody specific for phospho-β-catenin (Thr41/Ser45) (phosphorylated β-catenin at threonine41/serine45) was purchased from Cell Signaling Technology (Beverly, MA). Mouse monoclonal and rabbit polyclonal antibody specific for β-catenin was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Chicken polyclonal anti-PP1Cγ was from Abcam Inc. (Cambridge, MA). Goat polyclonal antibodies for PP2A catalytic subunit (PP2Ac), and isoforms of PP1 catalytic subunit (PP1c), were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) HRP-conjugated antibodies specific for mouse and rabbit IgG were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). HRP-conjugated antibodies specific for chicken IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA). Okadaic Acid (OA) and Calyculin A were obtained from Calbiochem (San Diego, CA) RPMI 1640 phenol and no-phenol red medium were obtained from Invitrogen.
Solubilization of Ceramides
C24:1-ceramide was made up in a 2% dodecane-ethanol solution and the solution was dissolved by incubation at 37 °C. After solubilization, the ceramide solution was kept at 37 °C until addition. C2 and C6-ceramides were dissolved in ethanol.
Cell Culture and siRNA
MCF7 cells were maintained in 10% fetal bovine serum in RPMI 1640 medium at 37 °C in 5% CO2. In experiments investigating the effects of confluence, cells were plated at low density (0.5 × 106 cells/100 mm dishes) and grown under conditions that achieved growth arrest by allowing cells to reach confluence (96 hr). For siRNA experiments, cells were seeded in 100-mm dishes (0.5 × 106/dish) and after 24 hr cells were transfected with scramble or specific siRNA (25 nM) using Oligofectamine according to the manufacturer's protocol. Cells were allowed to growth for 24 hr (sub-confluence) or 72 hr (confluence) prior to experiments. siRNA oligonucleotides for scrambled (SCR) and nSMase2 were as described previously [8]. Specific siRNAs for PP2Acβ (sc-39193) and PP2Acα (sc-39191) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The sequences of siRNAs for human PP1cα, PP1cγ, and A-SMase were as previously described [22, 23]. Real time RT-PCR for nSMase2 was performed as previously described [8] and samples were analyzed using Q-Gene® software, which expresses data as mean normalized expression. Mean normalized expression is directly proportional to the amount of RNA of the target gene (nSMase2) relative to the amount of RNA of the reference gene (β-actin).
Preparation of Cell Lysates
Cell lysates were obtained by syringe passage in buffer containing 50 mM Tris (pH 7.4), 5 mM EDTA, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 25 mM β–glycerophosphate, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml each chymostatin, leupeptin, antipain, and pepstatin A, plus a recommended amount of complete protease inhibitor mixture tablets (Roche Diagnostics, Indianapolis IN). Homogenate (2 μl) was used for protein estimation, using a Bio-Rad protein assay reagent. For cell fractionation studies, total cell lysate was centrifuged at 1000 × g (10 min) and the post-nuclear fraction was centrifuged at 100000 × g for 60 min to obtain a cytosolic and membrane fraction. Triton X-100 soluble fraction (TS) and Triton X-100 insoluble fraction (TI) fractions were obtained by incubating the 100000 × g pellet fraction in lysis buffer with the addition of 1 % Triton X-100 and 150 mM NaCl. After 1 hr of incubation at 4 °C, the homogenate was centrifuged at 14000 × g for 30 min. The collected supernatant contained the Triton X-100 soluble fraction.
Immunoblotting
Protein samples were separated on 4–20% gradient gels (Bio-Rad Criterion) at 100V before transfer to nitrocellulose membrane in Tris/glycine buffer (100 V, 30 min, 4 °C). Membranes were blocked (5% milk) and probed with primary antibody overnight (4 °C). Membranes were washed (3 × 0.1% Tween/Tris-buffered saline), probed with horseradish peroxidase-conjugated secondary antibody (1:5000 mouse, rabbit or chicken in 5% milk) for 60 min at room temperature and washed (3 × 0.1% Tween/Tris-buffered saline). Proteins were visualized by enhanced chemiluminescence (Pierce) with exposure to Biomax MR film.
Confocal Microscopy
For DNA transfection, confluent (1.5 × 105 cells/dish) and sub-confluent (0.5 × 105 cells/dish) MCF7 cells were plated onto 35-mm confocal dishes (MatTek) and transiently transfected with 0.3 μg of GFP-PP1cγ [24] using Effectene (Qiagen) according to the manufacturer's protocol. After 12 hr, medium was replaced, and cells allowed to grow for an additional 12 hr. Cells were fixed with 3.7% paraformaldehyde and 10% methanol for 15 min at 37 °C. For detection of phospho-β-catenin (Thr41/Ser45) and β-catenin, confluent or sub-confluent cells were fixed and permeabilized with 100% methanol (−20 °C) for 5 min and blocked in 3% FBS/PBS for 1 hr at room temperature. Primary antibody incubations were performed in 1.5% FBS/PBS with 0.15% saponin at 1:100 dilutions overnight at 4 °C. Cells were washed (3 × PBS) and incubated with the Alexa Fluor 488 or 633 goat anti-mouse or anti-rabbit secondary antibodies (Molecular Probes, Inc. Eugene, OR) in 1.5% FBS/PBS and 0.15% saponin at a dilution of 1:100 for 1 hr at room temperature. Nuclei were visualized via DRAQ-5 nuclear staining (red). After washing (3 × PBS), cells were viewed on a Zeiss LSM 510 Meta Confocal Microscope.
Cell Migration Assay
The directional migration of cells in Transwells (Costar, Cambridge, MA) was analyzed under serum-free conditions. Sub-confluent cells pretreated 72 hr with the PP1cγ siRNA or SCR control were preincubated with 5 μM of the celltracker green 5-chloromethylfluorescein diacetate (CMFDA, Invitrogen) during 30 min. After washing cells were serum starved for 4 hr and detached with enzyme-free buffer (Invitrogen). The indicated number of cells (50−200 × 103cell/insert) were resuspended in 0.3 ml of serum free RPMI 1640 medium (no-phenol red) and layered in the upper compartment of a transwell on a BD falcon Fluoroblock cell culture insert (8 μm pore size). The outer chamber was filled with 0.6 ml of RPMI 10 % SFB (no-phenol red). Following incubation for the times indicated, cells that migrated to the undersurface of the membrane were detected and quantified with a fluorescence plate reader (1420 Multilabel counter, PerkinElmer Life Science).
Statistical Analyses
Mann-Whitney or Student's t tests were performed between the samples indicated. A p value of 0.05 or less is considered as statistically significant.
Results
Plasma membrane translocation and decrease in phosphorylation of β-catenin in response to confluence
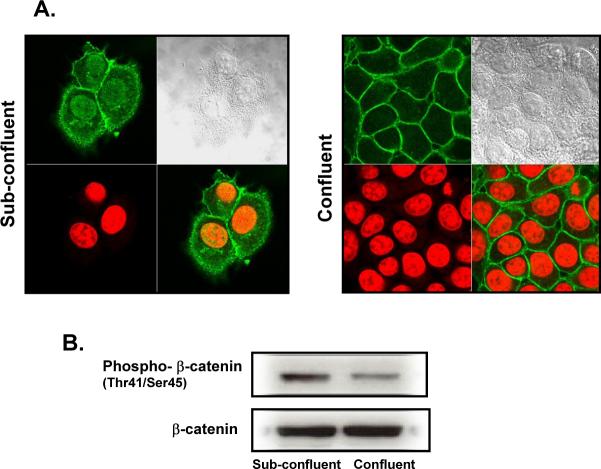
To determine if β-catenin localization and/or phosphorylation are regulated by cell density, MCF7 cells were seeded to 50% confluence and cultured for 4 days. After 2 days of growth, the cell number did not significantly increase (data not shown), confirming confluence-induced growth arrest and suggesting that MCF7 cells are regulated by contact-dependent growth inhibition. Confocal microscopy studies with anti-β-catenin antibodies revealed that β-catenin was located mainly in the nucleus and cytosol in sub-confluent cells (Fig. 1A). In contrast, during confluence, β-catenin became located at the PM, and nuclear β-catenin decreased markedly (Fig. 1A). Western blot analysis for phospho-β-catenin phosphorylated at threonine41/serine45 and for total β-catenin showed that β-catenin levels were not substantially changed in sub-confluent versus confluent cells. However, β-catenin phosphorylation was higher in sub-confluent cells (Fig. 1B). These results suggested that the decrease in phosphorylation of β-catenin during confluence may contribute to the localization of β-catenin to the PM and regulate contact-dependent growth inhibition in MCF7 cells.
Figure 1.
Confluence-induced translocation β-catenin and decrease in phosphorylation of phospho-β-catenin (Thr41/Ser45). (A) Low density MCF7 cells were seeded and cultured as described in Materials and Methods. Immunofluorescence was performed at 48 hr (sub-confluent) and 96 hr (confluent) of growth with anti-β-catenin rabbit polyclonal antibody and Alexa Fluor 488 goat anti-rabbit secondary antibody. Nuclei were visualized via DRAQ-5 nuclear staining (red). Results are representative of four independent experiments. (B) Cells were plated at low cell density and collected at 48 hr (sub-confluent) and 96 hr (confluent), whole cell lysates were normalized to total protein, and 80 μg was separated by SDS-PAGE. Phospho-β-catenin (Thr41/Ser45) and total β-catenin levels were analyzed by Western blotting. Results shown are representative of three independent experiments.
Role for nSMase2 in confluence dependent regulation of β-catenin
In a previous report, we showed that nSMase2 is up-regulated and becomes localized at the sites of cell-cell contact during confluence [8] whilst other studies have disclosed important connections between sphingolipids and β-catenin [21]. To determine if nSMase2 regulated the phosphorylation status of β-catenin during confluence, the effects of down-regulating nSMase2 on β-catenin were investigated. Western blot analysis of total and phospho-β-catenin (Thr41/Ser45) revealed that downregulation of nSMase2 with siRNA (Fig. 2A) reverted the decrease in phosphorylation of β-catenin and the increase in ceramide observed at high confluence (data not shown) without any changes in total β-catenin levels (Figs. 2B and C). This effect was specific for nSMase2 as acid sphingomyelinase (A-SMase) siRNA had no effect on the phosphorylation of β-catenin (Fig. 2D). These results therefore show a role for nSMase2 in mediating the decrease in phosphorylation of β-catenin at threonine41/serine45 during confluence.
Figure 2.
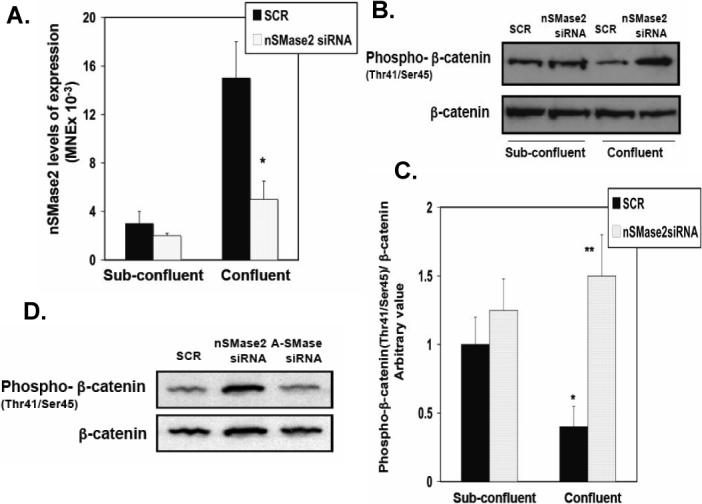
Effects of downregulation of nSMase2 on confluence-dependent regulation of phospho-β-catenin (Thr41/Ser45). (A) Low-density cells were treated the following day with SCR siRNA, or hnSMase2 siRNA and collected at 24 hr (sub-confluent) or 72 hr of growth (confluent). Real time RT-PCR analysis of nSMase2 levels of expression during cell was measured in MCF7 cells as described under Material and Methods. Results are representative of three independent experiments. Data are averages ± SD of a representative experiment performed in triplicate. Statistical significance was calculated with respect to scrambled siRNA at confluence (*, p < 0.01) (B) Cells were treated as in (A) and total β-catenin and phospho-β-catenin (Thr41/Ser45) were analyzed by Western blotting (B) and quantified by densitometry (C). The data were normalized for total β-catenin and were plotted as -fold increase with respect to the scrambled control at sub-confluence. Results in (B) are representative of three independent experiments. Data in (C) are averages ± SD of a representative experiment performed in triplicate. Statistical significance was calculated with respect to scrambled siRNA at sub-confluence (*, p < 0.025) and scrambled siRNA at confluence (**, p < 0.01). (D) Low-density cells were treated the following day with SCR siRNA, hnSMase2 siRNA or A-SMase siRNA and collected 72 hr of growth (confluent). Eighty μg of protein was separated by SDS-PAGE and phospho-β-catenin (Thr41/Ser45) and total β-catenin levels were analyzed by Western blotting. Results shown are representative of three independent experiments.
Effects of ceramide on β-catenin translocation and phosphorylation
To determine if ceramide was sufficient for regulating the localization and/or phosphorylation of β-catenin at threonine41/serine45 during confluence, sub-confluent MCF7 cells were treated with exogenous ceramide. As shown in Figure 3A, C6-ceramide (10μM) and C24:1-ceramides (1μM) induced translocation of β-catenin to the PM after 1 and 2 hr incubation, respectively. Importantly, Western blot analysis showed that exogenous C6-ceramide and very long chain C24:1-ceramide induced a significant decrease in the phosphorylation of β-catenin in a concentration-dependent manner (Fig. 3B). These results suggest that ceramide generated during confluence is necessary and sufficient for decreased phospho-β-catenin levels.
Figure 3.
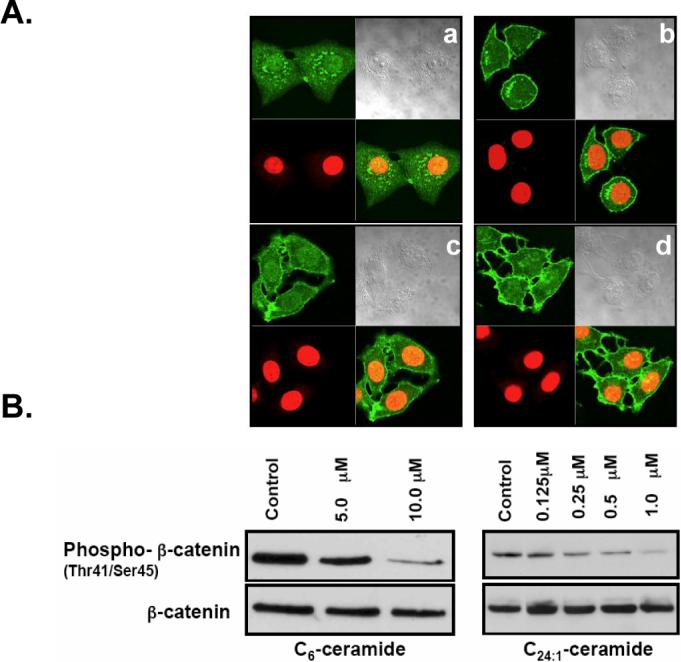
Effects of exogenous ceramide on translocation of β-catenin and regulation of phospho-β-catenin (Trh41/Ser45) levels. (A) Cells were plated at low density and treated with vehicle control (a, c) or 10 μM C6-ceramide (1 hr) (b) and 1 μM C24:1-ceramide (2 hr) (d). Immunofluorescence was performed with anti-β-catenin-antibody and Alexa Fluor 488 goat anti-rabbit secondary antibody. Nuclei were visualized via DRAQ-5 nuclear staining (red). (B) Cells were treated with C6-ceramide or C24:1-ceramide at the concentrations indicated for 1 hr. Whole-cell lysate was normalized to total protein, and 80 μg of protein was separated by SDS-PAGE. Phospho-β-catenin (Thr41/Ser45) and total β-catenin levels were analyzed by Western blotting. Results shown are representative of three independent experiments.
Regulation of β-catenin by PP1cγ
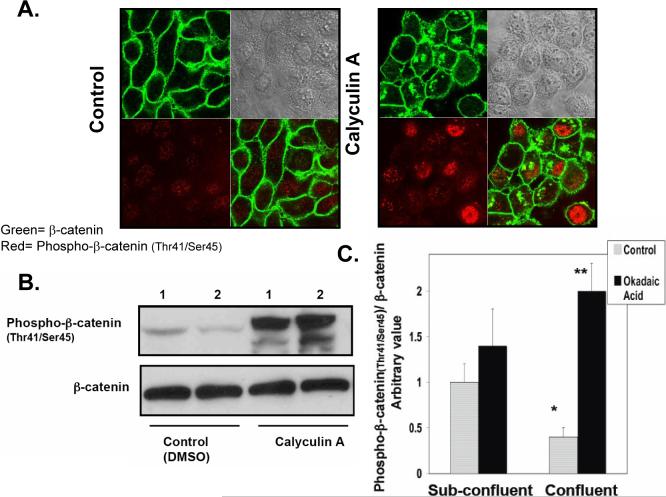
The results from the studies described above suggested that ceramide generated during confluence may mediate the dephosphorylation of β-catenin at threonine41/serine45 through activation of PP1 or PP2A, two serine/threonine phosphatases known to be activated by ceramide in vitro [25]. Therefore, the effects of the serine/threonine phosphatase inhibitors, calyculin A and okadaic acid, on dephosphorylation of β-catenin were evaluated in confluent cells. Figure 4A shows that after 2 hr of incubation with 1 nM of calyculin A, there was an increase in cytosolic β-catenin and phospho-β-catenin (Thr41/Ser45), compared to control cells. Western blot analysis indicated that incubation with 1 nM calyculin A or 50 nM okadaic acid for 2 hr inhibited/reversed the decrease in phosphorylation of β-catenin observed during confluence (Figs. 4B and C). There were no changes in total β-catenin levels with the phosphates inhibitors; however, low molecular weight bands were detected with the phospho-β-catenin (Thr41/Ser45) antibody, indicating probably some degradation products of β-catenin (Fig 4B). Since okadaic acid can inhibit both PP1 and PP2A and calyculin A shows some selectivity towards PP1, these results suggest a role for PP1/PP2A in regulating dephosphorylation of β-catenin with possible preference for a role for PP1. To corroborate and extend these results, we employed protein phosphatase siRNAs (Fig. 5A) and evaluated their effects on dephosphorylation of β-catenin. Figures 5B and C shows that only downregulation of PP1cγ increased phospho-β-catenin (Thr41/Ser45) levels during confluence. Moreover, as shown in Figure 5C, total β-catenin decreased, suggesting that this phospho-β-catenin pool may have access to the ubiquitination and degradation pathway known to act on phosphorylated β-catenin [15]. These results suggest that the dephosphorylation of β-catenin during confluence may be mediated by the activation of PP1cγ.
Figure 4.
Effects of calyculin A and okadaic acid on regulation of β-catenin and phospho-β-catenin (Thr41/Ser45) levels and localization. (A) Cells plated at low density were grown to confluence on confocal dishes for 96 hr and incubated 2 hr with calyculin A 1 nM or solvent control (DMSO). Cells were fixed and stained with anti-phospho-β-catenin (Thr41/Ser45) and anti-β-catenin polyclonal and monoclonal antibodies, respectively. Primary antibodies were detected with Alexa Fluor 488 anti-mouse or Alexa Fluor 633 goat anti-rabbit secondary antibodies. Results shown are representative of four independent experiments. (B) Cells were plated at low cell density and growht to confluence (96 hr) prior to incubation with calyculin A (1 nM) or solvent control 1 hr (Lane 1) or 2 hr (Lane 2). Total cell lysate (100 μg) was separated by SDS-PAGE, and phospho-β-catenin (Thr41/Ser45) and β-catenin were analyzed by Western blotting as described in Materials and Methods. Results shown are representative of three independent experiments. (C) Cells were plated as in (B) and treated with okadaic acid (50 nM) during 2 hr. Total β-catenin and phospho-β-catenin (Thr41/Ser45) were analyzed by Western blotting and quantified by densitometry. The data were normalized for total β-catenin and were plotted as -fold increase with respect to the control at sub-confluence. Results are representative of three independent experiments. Data are averages ± SD of a representative experiment performed in triplicate. Statistical significance was calculated with respect to control at sub-confluence (*, p<0.01) and control at confluence (**, p<0.005).
Figure 5.
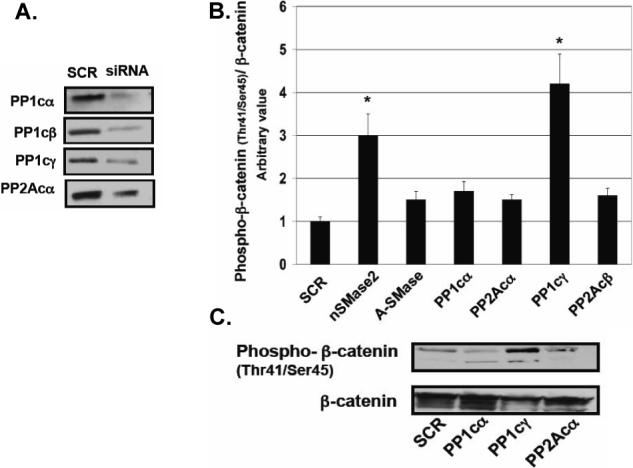
Effects of downregulation of protein phosphatases on dephosphorylation of β-catenin. (A) Cells plated at high cell density were treated with SCR or protein phosphatases, siRNAs (25 nM) for 48 hr. Whole-cell lysates were normalized to total protein, and 20 μg of each was separated by SDS-PAGE. The downregulation of protein phosphatases was analyzed by Western blotting as described in Materials and Methods. Results shown are representative of three independent experiments. (B) Cells were treated with the specific siRNAs as in (A) and total β-catenin and phospho-β-catenin (Thr41/Ser45) were analyzed by Western blotting (C) and quantified by densitometry (B) as in Figure 2B and C. The data were normalized for total β-catenin and were plotted as -fold increase with respect to the scrambled control. Results are representative of three independent experiments. Data are averages ± SD of a representative experiment performed in triplicate. Statistical significance was calculated with respect to scrambled (*, p<0.005).
PP1cγ translocation at confluence
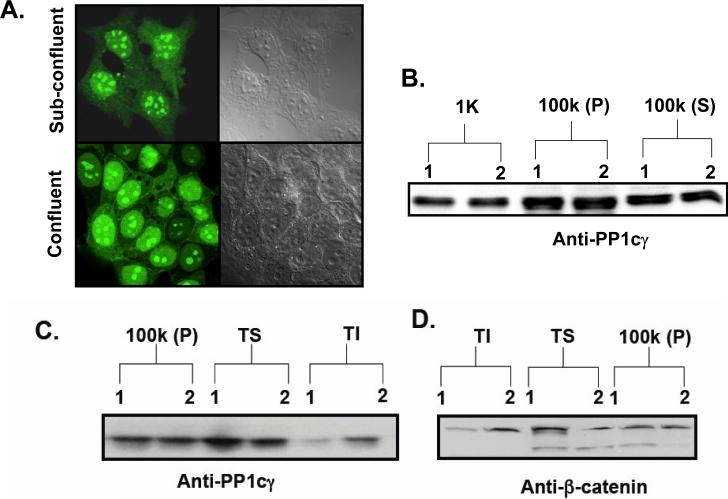
To determine if confluence regulates PP1cγ, we evaluated the sub-cellular localization of GFP-PP1cγ in sub-confluent and confluent cells. Results showed that GFP-PP1cγ was distributed all over the cell in sub-confluent cells, but in confluent cells it showed noticeable plasma membrane translocation (Fig. 6A). Sub-cellular fractionation of the total cell lysate (TL) of sub-confluent and confluent cells showed no significant difference in the distribution of PP1cγ in the 100000 × g membrane (P) and the cytosolic fraction (S) (Fig. 6B). However, Western blot analysis of the Triton X-100 soluble (TS) and insoluble fractions (TI) revealed that there was a significant increase of PP1cγ and β-catenin associated with the cytoskeletal fraction during confluence (Figs. 6C and D). Hence, there was an enhanced recruitment of PP1cγ and β-catenin to the cytoskeleton during confluence.
Figure 6.
Confluence-induced translocation of GFP-PP1cγ. (A) MCF7 cells plated at low or high confluence were transfected with GFP-PP1cγ. After 24 hr, translocation of GFP-PP1cγ was analyzed by confocal microscopy. Results are representative of four independent experiments. (B) Cells plated at low density were collected at 48 hr (Lane 1, sub-confluent) or 96 hr (Lane 2, confluent) of growth. Cell fractionation was performed as described in Materials and Methods. Protein (20 μg) was separated by SDS-PAGE, and endogenous PP1cγ was analyzed by Western blotting. (C, D) P-100 000 × g post-nuclear membrane fraction was processed as described in Materials and Methods to obtain the Triton X-100 soluble and insoluble fractions. S-cytosolic fraction; TS-Triton X-100 soluble fraction; TI-Triton X-100 insoluble fraction. Protein (20 μg) was separated by SDS-PAGE, and endogenous PP1cγ (C) and β-catenin (D) were analyzed by Western blotting. Results shown are representative of three independent experiments.
Regulation of PP1cγ translocation to the plasma membrane by ceramide
To determine if the membrane translocation of PP1cγ during confluence is regulated by the increase in ceramide levels, the ability of very short, short, and very long chain ceramides to induce PP1cγ translocation was determined in sub-confluent cells. Figure 7 shows that incubation with 10 μM C2- and C6-ceramides (30 min) and 1 μM C24:1-ceramide (1 hr) induced translocation of PP1cγ to the PM. To determine the stereospecificity of PP1cγ translocation by ceramide, the four stereoisomers of C6-ceramide were employed in confocal microscopy studies. As shown in Figure 8, at 30 min incubation with 10 μM C6-ceramide only D-erythro-C6-ceramide (Fig. 8) induced translocation of PP1cγ to the PM whereas D-threo-C6-ceramide, L-threo-C6-ceramide, and L-erythro-C6-ceramide had no effect (Fig. 8). Collectively, these results show that exogenous ceramide was sufficient to induce the translocation of PP1cγ to the PM.
Figure 7.
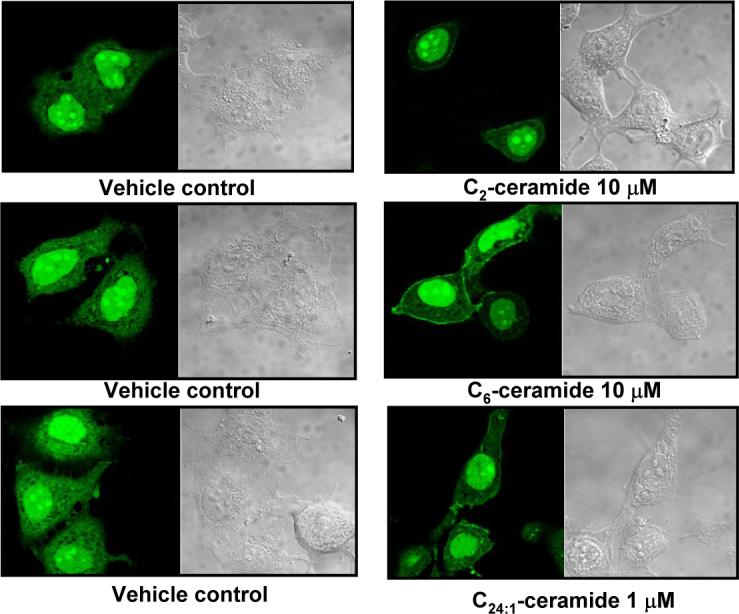
Translocation of GFP-PP1cγ in response to exogenous very short, short ,and very long chain ceramide. MCF7 cells were transfected with GFP-PP1cγ at low confluence, and after 24 hr, cells were treated with solvent control or C2- and C6-ceramide 30 min or C24:1-ceramide 1 hr at the concentrations indicated. Translocation of GFP-PP1cγ was analyzed by confocal microscopy. Results are representative of four independent experiments.
Figure 8.
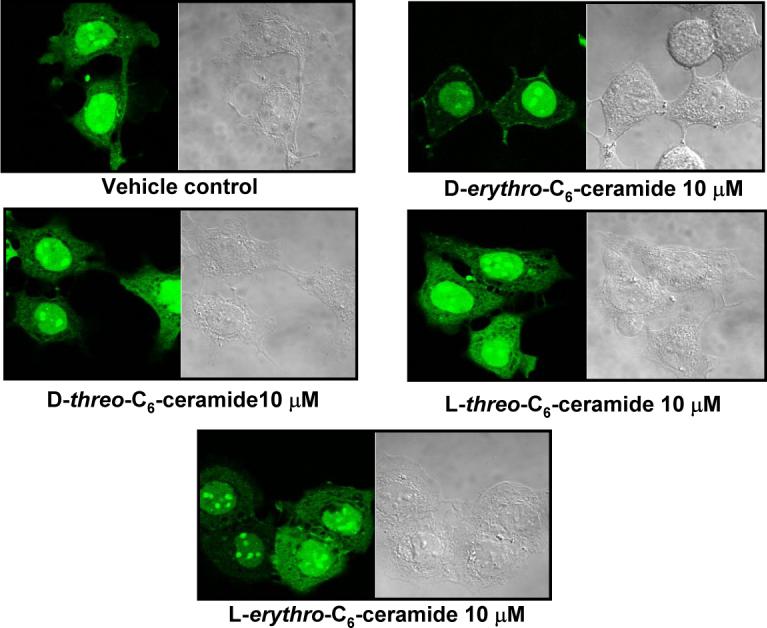
Effects of ceramide stereoisomers on GFP-PP1cγ translocation. Cells plated at low cell density were transfected with GFP-PP1cγ, and after 24 hr, cells were incubated with solvent control or C6-ceramide stereoisomers at 10 μM during 30 min. Translocation of GFP-PP1cγ was analyzed by confocal microscopy. Data are representative of at least three separate experiments.
Downregulation of PP1cγ increases cell migration
The results above suggested that a ceramide-activated PP1cγ regulates the translocation and dephosphorylation of β-catenin during confluence. β-catenin translocation to the plasma membrane is associated with the formation of mature and cytoskeleton-connected junctions and with decreased cell migration. Therefore, we evaluated if PP1cγ could affect cell migration in MCF7 cells. Sub-confluent MCF7 cells treated with SCR or PP1cγ siRNA (25 nM) during 72 hr were serum starved during for 4 hr and plated at varying cell densities on transwell filters. Fetal bovine serum was then added to the lower chamber, and the cells allowed to migrate towards the chemotactic stimulus for 12−24 hours. As shown in Figure 9A, an increase in the percentage of the cells that migrated was observed in the cells pretreated with the PP1cγ siRNA compared to the SCR control. These results suggested that activation of PP1cγ during confluence decreased cell migration. Figure 9B shown that the levels of the endogenous PP1cγ are downregulated previous and after the migration assay.
Figure 9.
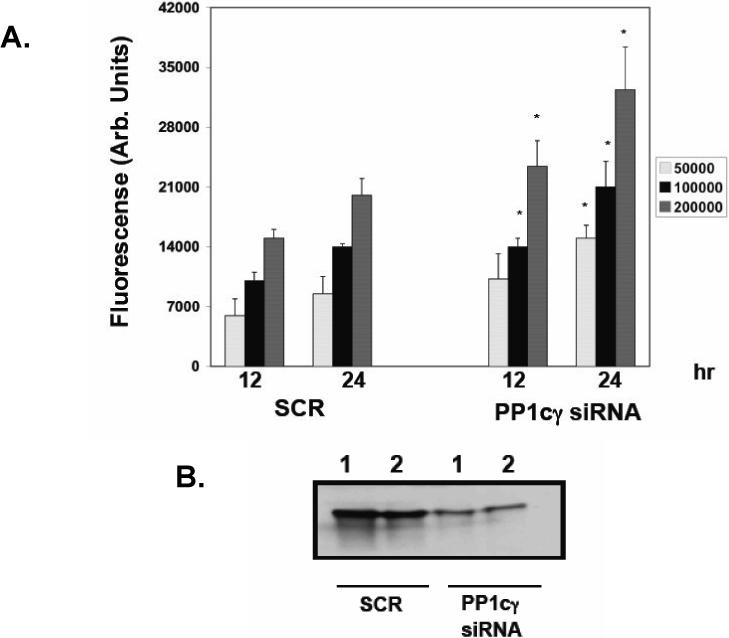
Effects of downregulation of PP1cγ on cell migration. (A) Sub-confluent MCF7 cells treated with SCR or PP1cγ siRNA (25 nM) during 72 hr were collected at confluence and treated as indicated in Materials and Methods. Cells were counted and seeded at the indicated cell density in transwell plates for the indicated times. The migrating cells were quantitated by fluorescence as described in Materials and Methods. Results are the average ± SD of three different experiments. Statistical significance was calculated with respect to the SCR control for each time and cell density using Student's t test. A p value of 0.05 or less is considered as statistically significant and marked in the figures with an asterisk (B) Sub-confluent MCF7 cells treated with SCR or PP1cγ siRNA (25 nM) during 72 hr were collected as in (A) and lysed (Lane 1) or plated and lysed after 24 hrs of further growth in the absence of the siRNA (Lane 2). Protein (20 μg) was separated by SDS-PAGE, and endogenous PP1cγ was analyzed by Western blotting. Results shown are representative of two independent experiments.
Discussion
The current results provide evidence for a cell regulation pathway that involves ceramide and ceramide-activated protein phosphatases in the dephosphorylation of phospho-β-catenin phosphorylated at threonine41/serine45 resulting in decreased cell migration. Specifically, the results demonstrate that up-regulation of nSMase2 during confluence is involved in the ceramide-mediated dephosphorylation of phospho-β-catenin (Thr41/Ser45) through the activation of PP1. Results also clearly implicate ceramide as an in vivo regulator of PP1cγ.
Previous results showed that confluence induced up-regulation of nSMase2, and indeed, nSMase2 was initially cloned as CCA1 (for cell confluence associated gene) in a study that found this gene to be significantly induced in growth-arrested confluent 3Y1 rat cells [26]. In a previous study, we showed that nSMase2 also increased upon confluence of MCF7 cells, and this resulted in specific increase in the levels of very long chain ceramides. Moreover, confluence caused translocation of nSMase2 to sites of cell-cell contact [8] where it colocalizes with β-catenin (Marchesini, N. and Hannun, Y.A, unpublished results). β-catenin plays an important role at the sites of cell contact by interacting with adhesion proteins, suggesting that β-catenin may regulate the levels of protein available for cell contact interaction [11, 18, 27].
The results from this study show that in MCF7 cells, the levels of phospho-β-catenin (Thr41/Ser45) were decreased when the cells reached confluence, and this was accompanied with an increase of β-catenin associated at cell–cell contact sites. Total β-catenin levels were not changed during confluence, suggesting that dephosphorylated β-catenin localized to the PM is protected from degradation. Mechanistically, siRNA-mediated downregulation of nSMase2 prevented the decrease in phospho-β-catenin levels. Moreover, the role of ceramide, in the regulation of phospho-β-catenin (Thr41/Ser45) and PM translocation of β-catenin was extended by demonstrating that the addition of exogenous ceramide to sub-confluent cells is able to cause decrease in phospho-β-catenin levels and PM translocation of β-catenin. In a previous report, it was shown that sphingolipids may regulate β-catenin by demonstrating that sphingolipid feeding in vivo reduced the number of colon tumors and induced the distribution of β-catenin to intercellular junctions between intestinal epithelial cells. In addition, in the same report it was shown that the treatment of two human colon cancer cell lines in culture (SW480 and T84) with sphingosine or natural long-chain ceramide reduced cytosolic and nuclear beta-catenin, inhibited growth, and induced cell death [21].
As ceramide has been reported to activate serine/threonine protein phosphatases [25], results from this study suggest a role of serine/threonine phosphatases in the ceramide-mediated decrease in phospho-β-catenin (Thr41/Ser45) levels in MCF7 cells. This was demonstrated with the serine/threonine phosphatase inhibitor okadaic acid (50 nM) and calyculin A (1 nM) at a concentration that inhibits both PP1 and PP2A activities [28, 29]. The specific phosphatase involved in the dephosphorylation of β-catenin was established using siRNA directed at each of the major serine/threonine phosphatases. Cells depleted of PP1cγ showed increased phospho-β-catenin (Thr41/Ser45) and a decrease in total β-catenin suggesting that this phospho-β-catenin pool may be available for degradation through the ubiquitination pathway. Interestingly and in agreement with our results, a role of PP1 in the regulation of phospho-β-catenin levels has been recently suggested in the rat fibroblast cell line, 3Y1 [19]. However, it should not be discarded that inactivation of casein kinase I (CKI), responsible for Ser45 phosphorylation of β-catenin could also occur during confluence.
The regulation of PP1cγ by confluence was studied in GFP-PP1cγ transfected cells. In sub-confluent cells, GFP-PP1cγ was distributed throughout the cell but upon confluence, PP1cγ translocated to the sites of cell-cell contact and became detectable in the TI fraction, indicating that PP1cγ functions as a downstream target of ceramide. Previous studies have shown that PP1 and PP2A are stereospecifically activated in vitro by short and long chain ceramides [25, 30]. Moreover, the present confocal microscopy data with exogenous ceramide demonstrate that very short (C2), short (C6), and very long chain (C24:1) ceramides can stimulate PP1cγ translocation to the membranes and that this translocation is induced in a stereospecific manner such that only D-erythro-C6-ceramide induced PP1cγ translocation. Interestingly, no significant translocation of PP1cγ was observed with C16-ceramide or bacterial SMase treatment, which increased mainly C16-ceramide (data not shown). These results raise the possibility that activation of PP1cγ may be more specific for very long chain ceramide which are the ones that increase during confluence. Taken together, these results suggest a pathway in which confluence-induced increases of ceramide is now implicated in activating PP1cγ, leading to the dephosphorylation of β-catenin.
The regulation of PP1 by ceramide is supported by other studies that have demonstrated the activation of PP1 via ceramide generated by the novo pathway after TNF-α stimulation [31] and Fas activation [32] or via the protein kinase C-dependent salvage pathway [23]. Cellular substrates for ceramide-mediated activation of PP1 include, serine/arginine-rich (SR) proteins [32], retinoblastoma proteins [28], and p38 MAPK [23]. On the other hand, other phospho-substrates such as Akt [33], PKCα and Bcl-2 [34, 35] appear to be regulated by ceramide-mediated activation of PP2A.
The physiological effect of activation of PP1cγ during confluence was demonstrated in a cell migration assay. Indeed, downregulation of PP1cγ in confluent cells increased cell migration with respect to the control treated with scrambled siRNA. These results also suggested that regulation of phospho-β-catenin (Thr41/Ser45) and β-catenin levels during confluence is an important regulatory mechanism of cell contacts interaction in cancer cells.
In conclusion, the results show for first time a role of the ceramide/PP1 pathway in the regulation of the β-catenin pathway through the dephosphorylation of β-catenin during confluence, and they suggest a novel mechanism by which ceramide-activated PP1 may be regulated through nSMase2 up-regulation leading to decreased cell migration at confluence.
Acknowledgements
We thank Mr. Russell Jenkins for the collaboration with the PP1cγ cell fractionation studies and Drs. Besim Ogretmen, and Jolanta Idkowiak-Baldys for critical review of this paper. We also thank the Hollings Cancer Center Molecular Imaging Facility for use of the confocal microscope and the Lipidomics Core at the Medical University of South Carolina for providing the short chain ceramides and stereoisomers. We are particularly grateful to Dr. Jennifer Schnellman for critical review and editorial assistance.
This work was supported in part by a National Institutes of Health Grant GM 43825 and CA87584
Abbreviations
- A-SMase
acid sphingomyelinase
- nSMase2
neutral sphingomyelinase 2
- PM
plasma membrane
- PP1
protein phosphatase 1
- PP1cγ
γ isoform of the catalytic subunit of protein phosphatase 1
- Phospho-β-catenin (Thr41/Ser45)
phosphorylated-β-catenin at threonine41/serine45
- PP2A
protein phosphatase 2A
- SCR
scrambled
- siRNA
small interference RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. The Journal of biological chemistry. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 3.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 4.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 5.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J Biol Chem. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344:900–905. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karakashian AA, Giltiay NV, Smith GM, Nikolova-Karakashian MN. Expression of neutral sphingomyelinase-2 (NSMase-2) in primary rat hepatocytes modulates IL-beta-induced JNK activation. Faseb J. 2004;18:968–970. doi: 10.1096/fj.03-0875fje. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. The Journal of biological chemistry. 2004;279:25101–25111. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 9.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 10.Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol. 1994;126:247–258. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich C, Scherwat J, Faust D, Oesch F. Subcellular localization of beta-catenin is regulated by cell density. Biochem Biophys Res Commun. 2002;292:195–199. doi: 10.1006/bbrc.2002.6625. [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 13.Polakis P. More than one way to skin a catenin. Cell. 2001;105:563–566. doi: 10.1016/s0092-8674(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 14.Luo W, Lin SC. Axin: a master scaffold for multiple signaling pathways. Neuro-Signals. 2004;13:99–113. doi: 10.1159/000076563. [DOI] [PubMed] [Google Scholar]
- 15.Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. The Journal of biological chemistry. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- 16.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 17.Sadot E, Conacci-Sorrell M, Zhurinsky J, Shnizer D, Lando Z, Zharhary D, Kam Z, Ben-Ze'ev A, Geiger B. Regulation of S33/S37 phosphorylated beta-catenin in normal and transformed cells. Journal of cell science. 2002;115:2771–2780. doi: 10.1242/jcs.115.13.2771. [DOI] [PubMed] [Google Scholar]
- 18.Serres M, Grangeasse C, Haftek M, Durocher Y, Duclos B, Schmitt D. Hyperphosphorylation of beta-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp Cell Res. 1997;231:163–172. doi: 10.1006/excr.1996.3443. [DOI] [PubMed] [Google Scholar]
- 19.Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. The EMBO journal. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotz J, Probst A, Mistl C, Nitsch RM, Ehler E. Distinct role of protein phosphatase 2A subunit Calpha in the regulation of E-cadherin and beta-catenin during development. Mechanisms of development. 2000;93:83–93. doi: 10.1016/s0925-4773(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 21.Schmelz EM, Roberts PC, Kustin EM, Lemonnier LA, Sullards MC, Dillehay DL, Merrill AH., Jr. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001;61:6723–6729. [PubMed] [Google Scholar]
- 22.Zeidan YH, Hannun YA. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J Biol Chem. 2007;282:11549–11561. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- 23.Kitatani K, Idkowiak-Baldys J, Bielawski J, Taha TA, Jenkins RW, Senkal CE, Ogretmen B, Obeid LM, Hannun YA. Protein kinase C-induced activation of a ceramide/protein phosphatase 1 pathway leading to dephosphorylation of p38 MAPK. The Journal of biological chemistry. 2006;281:36793–36802. doi: 10.1074/jbc.M608137200. [DOI] [PubMed] [Google Scholar]
- 24.Jones JA, Rawles R, Hannun YA. Identification of a novel phosphatidic acid binding domain in protein phosphatase-1. Biochemistry. 2005;44:13235–13245. doi: 10.1021/bi0505159. [DOI] [PubMed] [Google Scholar]
- 25.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi Y, Kiyono T, Fujita M, Ishibashi M. cca1 is required for formation of growth-arrested confluent monolayer of rat 3Y1 cells. J Biol Chem. 1997;272:18082–18086. doi: 10.1074/jbc.272.29.18082. [DOI] [PubMed] [Google Scholar]
- 27.Peifer M. Cell adhesion and signal transduction: the Armadillo connection. Trends Cell Biol. 1995;5:224–229. doi: 10.1016/s0962-8924(00)89015-7. [DOI] [PubMed] [Google Scholar]
- 28.Kishikawa K, Chalfant CE, Perry DK, Bielawska A, Hannun YA. Phosphatidic acid is a potent and selective inhibitor of protein phosphatase 1 and an inhibitor of ceramide-mediated responses. J Biol Chem. 1999;274:21335–21341. doi: 10.1074/jbc.274.30.21335. [DOI] [PubMed] [Google Scholar]
- 29.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 30.Law B, Rossie S. The dimeric and catalytic subunit forms of protein phosphatase 2A from rat brain are stimulated by C2-ceramide. The Journal of biological chemistry. 1995;270:12808–12813. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh N, Patel N, Jiang K, Watson JE, Cheng J, Chalfant CE, Cooper DR. Ceramide-activated protein phosphatase involvement in insulin resistance via Akt, serine/arginine-rich protein 40, and ribonucleic acid splicing in L6 skeletal muscle cells. Endocrinology. 2007;148:1359–1366. doi: 10.1210/en.2006-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalfant CE, Ogretmen B, Galadari S, Kroesen BJ, Pettus BJ, Hannun YA. FAS activation induces dephosphorylation of SR proteins; dependence on the de novo generation of ceramide and activation of protein phosphatase 1. The Journal of biological chemistry. 2001;276:44848–44855. doi: 10.1074/jbc.M106291200. [DOI] [PubMed] [Google Scholar]
- 33.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 34.Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. The Journal of biological chemistry. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- 35.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. The Journal of biological chemistry. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]