Abstract
Conjunctival goblet cells synthesize and secrete mucins onto the ocular surface to lubricate it and protect it from bacterial infections. Mucin secretion is under neural control, and cholinergic agonists released from parasympathetic nerves are major stimuli of this secretion. The signal transduction pathways these agonists use to stimulate secretion involve activating protein kinase C (PKC) and increasing intracellular [Ca2+] to activate the non-receptor kinases Pyk2 and p60Src (Src) to transactivate the EGF receptor. Transactivation of the EGF receptor activates a kinase cascade culminating in the activation of p42/p44 MAPK (MAPK) and ultimately that leads to secretion of high molecular weight glycocongujates (HMWGC), including mucins. To further examine the roles of PKC and Ca2+ in the activation of MAPK, Pyk2, and Src in mucin secretion, rat conjunctival pieces and cultured goblet cells were incubated with the PKC activator phorbol myristate acid (PMA), the cholinergic agonist carbachol, or the calcium ionophore, ionomycin for varying times. Conjunctival pieces were preincubated with PKC inhibitors 10 mins prior to addition of carbachol (10−4 M) for 10 min. The amount of phosphorylated (activated) MAPK, Pyk2 and Src was determined by western blotting techniques using antibodies specific to the phosphorylated forms of each kinase. PMA significantly increased the activation of MAPK, Pyk2, and Src in a time and concentration-dependent manner. PMA-stimulated MAPK activity was completely inhibited by the EGF receptor inhibitor AG1478 (10−7 M). Carbachol-stimulated MAPK activity was inhibited by three PKC inhibitors, calphostin C, chelethyrine, and staurosporine. Ionomycin (10−6 M)-stimulated MAPK activity was inhibited 66% by AG1478 (10−7 M). Ionomycin also significantly increased Pyk2 and Src in time dependent manner. PKC and ionomycin also activated p42/p44 MAPL, Pyk2, and Src in cultured conjunctival goblet cells. We conclude that PKC and intracellular Ca2+ activate Pyk2 and Src and phosphorylated the EGF receptor leading to stimulation of MAPK in conjunctival goblet cells.
Keywords: goblet cells, signal transduction, MAPK, mucin secretion
Goblet cells of the conjunctiva are responsible for synthesis, storage, and secretion of mucins, which make up the mucous layer of the tear film (Dartt, 2004, Gipson and Argueso, 2003). Mucins serve to lubricate the ocular surface, protect from bacterial infections and provide for a smooth refractive surface. These cells are highly specialized epithelial cells that are interspersed throughout the stratified squamous cells of the conjunctiva either singly or in clusters, depending on the species. A decrease in the quantity of goblet cells or their ability to secrete mucins is deleterious to the ocular surface.
Conjunctival goblet cell mucin secretion, similar to secretion from other tissues, is under neural control. We have shown that parasympathetic and sympathetic nerves surround conjunctival goblet cells (Dartt, et al., 1995). Neurotransmitters released from parasympathetic nerves, namely the cholinergic agonist acetylcholine and vasoactive intestinal peptide (VIP), caused secretion of high molecular weight glycoconjugates (HMWGC), including mucins, from these cells (Dartt, et al., 1996, Rios, et al., 1999). In addition, activating of sensory nerves in the cornea caused goblet cell mucin secretion by activation the efferent parasympathetic and sympathetic nerves (Dartt, et al., 1995, Kessler, et al., 1995).
In the conjunctiva, cholinergic agonists transmit their extracellular signal by binding to the M2 and M3 muscarinic receptors on the conjunctival goblet cells (Kanno, et al., 2003, Rios, et al., 1999). These receptors are G-protein coupled receptors (GPCR) that are present on the plasma membrane of the goblet cells. Upon agonist binding, the receptor is activated which in turn stimulates the hydrolysis of phosphatidylinositolbisphosphate (PIP2) by phospholipase C. Hydrolysis of PIP2 increases the intracellular concentrations of diacylglycerol (DAG) and 1,4,5 inositol trisphsphate (IP3). DAG activates the classical and novel isoforms of protein kinase C (PKC). IP3 releases Ca2+ from intracellular stores to increase intracellular [Ca2+] ([Ca2+]i). Both of these events, PKC activation and the increase in [Ca2+]i, lead to phosphorylation of additional proteins and ultimately to HMWGC secretion.
It is now well established that G-protein coupled receptors, such as muscarinic receptors, can interact with receptor tyrosine kinases such as the EGF receptor (Gschwind, et al., 2001). Activation of the EGF receptor involves phosphorylation of the receptor on specific tyrosine residues resulting in recruitment of adaptor molecules. These adaptor molecules cause the EGF receptor to dimerize and autophosphorylate (Bazley and Gullick, 2005) leading to downstream effects. In conjunctival goblet cells, we previously showed that cholinergic agonists activate the focal adhesion kinase Pyk2 through PKC and Ca2+. Pyk2 binds to and activates the non-receptor tyrosine kinase p60src (Src) (Kanno, et al., 2003). This complex can then transactive the EGF receptor recruiting the adaptor proteins Shc, Grb2, and the Ras guanine nucleotide exchange factor Sos. Sos binds to the low molecular weight GTPase, Ras, causing the exchange of GDP for GTP. Ras then activates a cascade of protein kinases, Raf (MAPK kinase kinase), MEK (MAPK kinase) and p42/p44 MAPK (also known as Erk). p42/p44 MAPK has been implicated in a variety of cellular processes, both long term processes such as gene expression, differentiation, and cell proliferation, and short term processes such as secretion of HMWGC secretion from conjunctival goblet cells (Dartt, et al., 1996, Kanno, et al., 2003, Rios, et al., 1999).
In the current study, we examined the roles of PKC and [Ca2+]i in cholinergic agonist- stimulated p42/p44 MAPK, Pyk2, and p60Src activation that ultimately leads to HMWGC secretion from goblet cells.
MATERIALS AND METHODS
Materials
Monoclonal antibodies to phosphorylated (active) p42/p44 MAPK, total p42 MAPK, and total Pyk2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to Pyk2 phosphorylated at tyrosine 881 and total Src were from Biosource International (Camarillo, CA). The antibody directed against Src phosphorylated on tyrosine 416 was purchased from Cell Signaling Technology (Beverly, MA). Anti-rabbit and anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Ionomycin, phorbol 12-myristate 13-acetate (PMA), staurosporine, calphostin C, and chelerythrine chloride were from LC Labs (Waltham, MA). Keratinocyte basal medium (KBM) was from Clonetics (San Diego, CA). Chemiluminescence reagents were from Pierce (Rockville, IL). All other reagents were from Sigma (St. Louis, MO).
Methods
All experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee. Male Sprague Dawley rats (250–300 g) were obtained from Taconic Farms (Germantown, NY). Rats were anesthetized for 1 min in CO2 and decapitated. The entire conjunctiva was dissected around the limbus of the cornea, placed on filter paper and cut into four pieces per eye. The pieces were preincubated for 60 min in KBM.
Culture of Rat Conjunctival Goblet Cells
Rat conjunctival goblet cells were grown in organ culture as described previously (Shatos, et al., 2001). In brief, the conjunctival pieces were placed in RPMI 1640 media supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C. Goblet cells were identified by the presence of numerous secretory granules and contaminating non-goblet cells were removed with a rubber policeman (Shatos, et al., 2001). Goblet cells were allowed to grow, trypsinized and placed in 6-well culture plates and grown to confluency.
Measurement of MAPK, Pyk2, and c-Src activity
The activation of p42/44 MAPK, Pyk2, and c-Src were examined using western blot techniques. Conjunctival pieces or cultured rat goblet cells were incubated with the cholinergic agonist carbachol (10−4 M), ionomycin (10−6 M) or PMA (10−7 M) for 10 min. Inhibitors were added 10 minutes prior to stimulation. The pieces or cells were removed and homogenized in RIPA buffer (10 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 100 μg/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin, 1 mM Na3VO3). The homogenate was centrifuged at 10,000 x g for 30 minutes at 4 oC. Proteins in the supernatant were separated by SDS-PAGE on an 10% gel, transferred onto nitrocellulose membranes, which were blocked overnight at 4°C in 5% non-fat dried milk in buffer containing 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween-20 (TBST). The blots were then probed with antibodies directed against the non-phosphorylated form of the enzyme (total) or the phosphorylated form of the enzyme (activated) followed by HRP-conjugated secondary antibody. Immunoreactive bands were digitally scanned and analyzed using NIH Image. The amount of phosphorylated enzyme in each sample was standardized to either the amount of total enzyme or total MAPK.
Data presentation and statistical analysis
Data are expressed as fold increase above basal value, which was standardized to 1.0. Results are expressed as mean ± SEM. Data were analysed by Student’s t-test. p<0.05 was considered statistically significant.
RESULTS
Effect of Time and Concentration of PMA on p42/p44 MAPK Activation
We previously showed that the phorbol esters PMA and PdBu increased secretion of HMWGC from conjunctival pieces (Dartt, et al., 2000). In addition, we have shown that activation of p42/p44 MAPK also leads to HMWGC secretion (Kanno, et al., 2003). In order to determine the effects of phorbol esters on p42/p44 MAPK activation, conjunctival pieces were incubated with the phorbol ester PMA 10−8–10−6 M (Figure 1A) for 10 min. PMA caused a concentration dependent-increase in p42/p44 MAPK activity. When 3 independent experiments were analyzed, PMA caused a significant increase in p42/p44 MAPK of 2.0 ± 0.3 fold above basal at 10−7 M (Figure 1B).
Figure 1. Effect of Time and Concentration of Phorbol Ester on p42/p44 MAPK Activation.
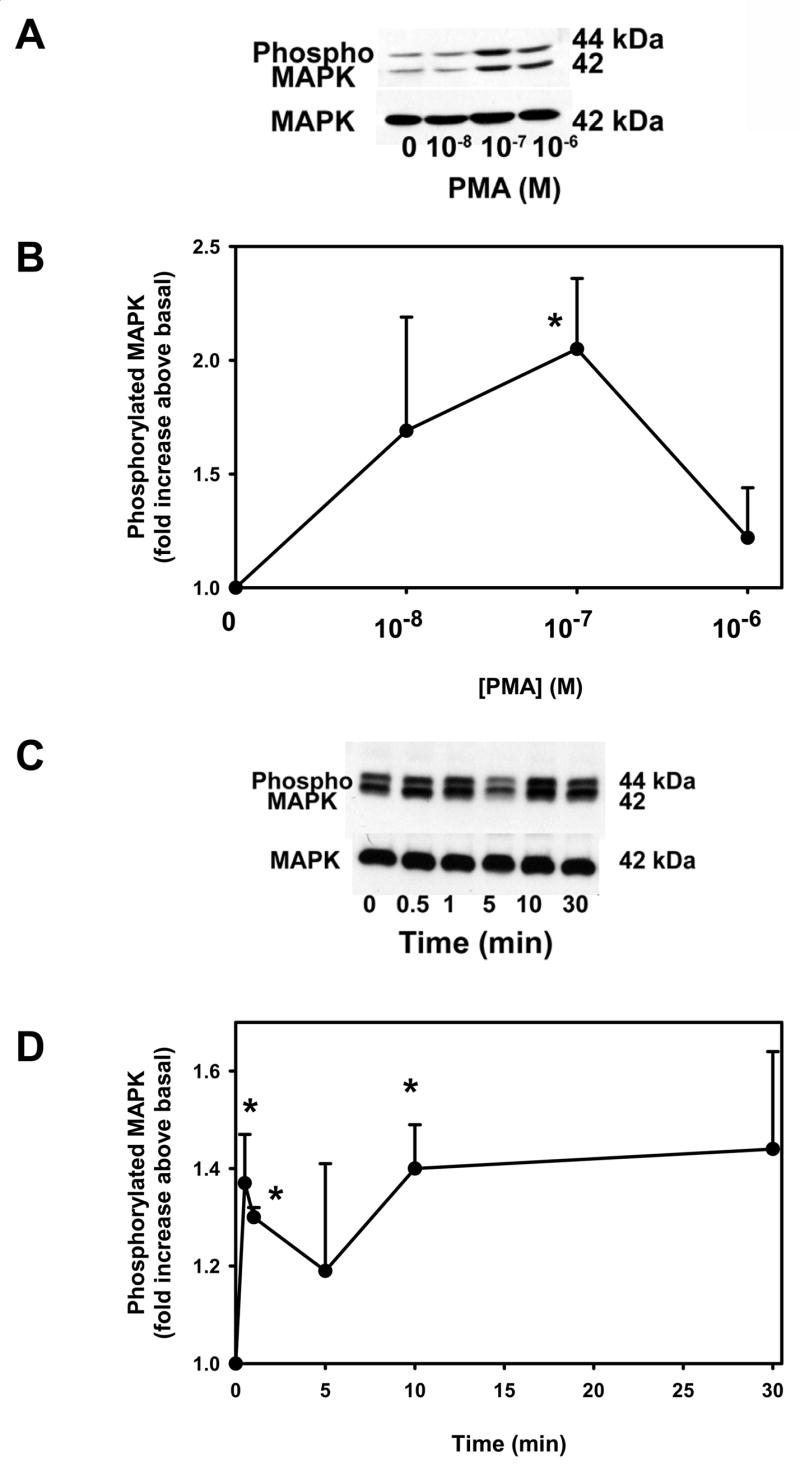
Conjunctival pieces were incubated with increasing concentrations of the PKC activator PMA for 10 min and the amount of phosphorylated p42/p44 MAPK and total p42 MAPK were determined by western blot analysis (A). Three independent experiments were analyzed by densitometry and mean ± SEM are shown in B. Conjunctival pieces were incubated for increasing time of the PKC activator PMA (10−7 M) and the amount of phosphorylated p42/p44 MAPK and total p42 MAPK were determined by western blot analysis (C). Four independent experiments were analyzed by densitometry and mean ± SEM is shown in D. * indicates significant difference from basal.
Conjunctival pieces were also incubated with PMA 10−7 M for 0 – 30 minutes. As shown in Figure 1C, PMA induced a biphasic reaction with a rapid activation of p42/p44 MAPK by 30 s, decreased slightly at 5 min before increasing again by 10 min. When 4 independent experiments were analyzed, PMA caused a significant increase in p42/p44 MAPK activation of 1.4 ± 0.1 fold above basal at 30 s, 1.3 ± 0.02 fold increase at 1 min, 1.4 ± 0.1 fold increase at 10 min.
These data demonstrate that PKC activates p42/p44 MAPK in rat conjunctiva.
Effect of PKC Inhibitors on Cholinergic Agonist-Stimulated p42/p44 MAPK Activation
We previously showed that cholinergic agonists activated p42/p44 MAPK and inhibition of p42/p44 MAPK inhibited cholinergic agonist-stimulated mucin secretion from conjunctival pieces (Kanno, et al., 2003). We also showed that cholinergic agonists activated PKC (Dartt, et al., 2000). However, we were unable to determine a direct role of PKC in mucin secretion as the inhibitors themselves increased secretion. We therefore determined the effects of PKC on cholinergic-agonist stimulated p42/p44 MAPK activation. Rat conjunctival pieces were preincubated with the PKC inhibitors calphostin C (10−8 M), chelethryine chloride (10−7 M) or staurosporine (10−6 M) for 10 min prior to incubation with the cholinergic agonist carbachol (10−4 M) for 10 min. Activation of p42/p44 MAPK was measured as described. As shown in Figure 2A-C, all three inhibitors inhibited carbachol-stimulated p42/p44 MAPK activation. Of the three inhibitors used, only staurosporine increased the basal activation of p42/p44 MAPK. When six experiments were analyzed, staurosporine alone significantly increased p42/p44 MAPK activity 1.5 ± 0.3 fold above basal (data not shown). This is in comparison to calphostin C and chelethryine chloride that activated p42/p44 MAPK by 1.1 ± 0.3 and 0.8 ± 0.2 fold above basal, respectively (n=3, data not shown). All three inhibitors significantly decreased carbachol-stimulated p42/p44 MAPK activation (Figure 2D). Calphostin C decreased carbachol-stimulated MAPK activity by 60 ± 18 %, chelethyrine chloride decreased it by 82 ± 11 %, while staurosporine decreased it by 63 ± 19 %. These experiments demonstrate that cholinergic agonists stimulate PKC to activate p42/p44 MAPK leading to glycoconjugate secretion.
Figure 2. Effect of PKC Inhibitors on Cholinergic Agonist-Stimulated p42/p44 MAPK Activation.
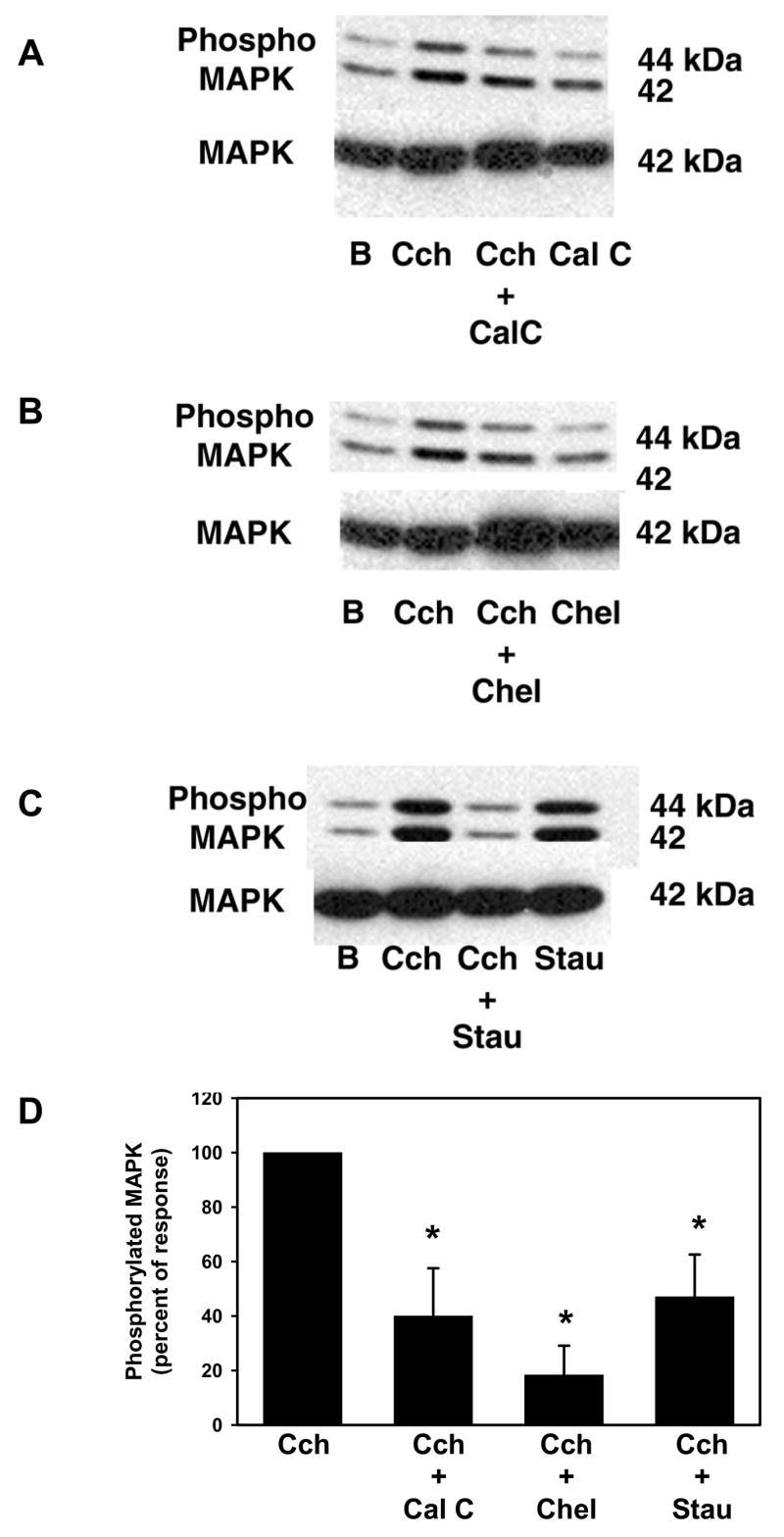
Conjunctival pieces were preincubated with the PKC inhibitors calphostin C (10−8 M, A), chelethryine chloride (10−7 M, B), or staurosporine (10−6 M, C) for 10 min prior to stimulation with carbachol (Cch, 10−4 M) for 10 min. The amount of phosphorylated p42/p44 MAPK and total p42 MAPK were determined by western blot analysis. Six independent experiments were analyzed by densitometry and mean ± SEM is shown in D. * indicates significant difference from carbachol stimulation.
Effect of Inhibition of the EGF Receptor on PMA-stimulated p42/p44 MAPK Activation
We previously showed that cholinergic agonists transactivate the EGF receptor to stimulate mucin secretion (Kanno, et al., 2003). To determine if activation of PKC occurs downstream of this transactivation, we preincubated rat conjunctival pieces with the EGF receptor inhibitor AG1478 (10−7 M) for 10 min before stimulation with PMA (10−7 M) for 10 min. Activation of p42/p44 MAPK was measured as described. AG1478 alone did not have any effect on basal p42/p44 MAPK activity while it inhibited PMA-stimulated p42/p44 MAPK activity (Figure 3A). When 4 independent experiments were analyzed, PMA significantly increased MAPK activity 1.5 ± 0.2 fold above basal. This stimulation was completely inhibited by AG1478 (Figure 3B). These data show that activation of PKC is involved in the transactivation of the EGF receptor, leading to activation of p42/p44 MAPK.
Figure 3. Effect of Inhibition of the EGF receptor on Phorbol Ester-Stimulated p42/p44 MAPK Activation.
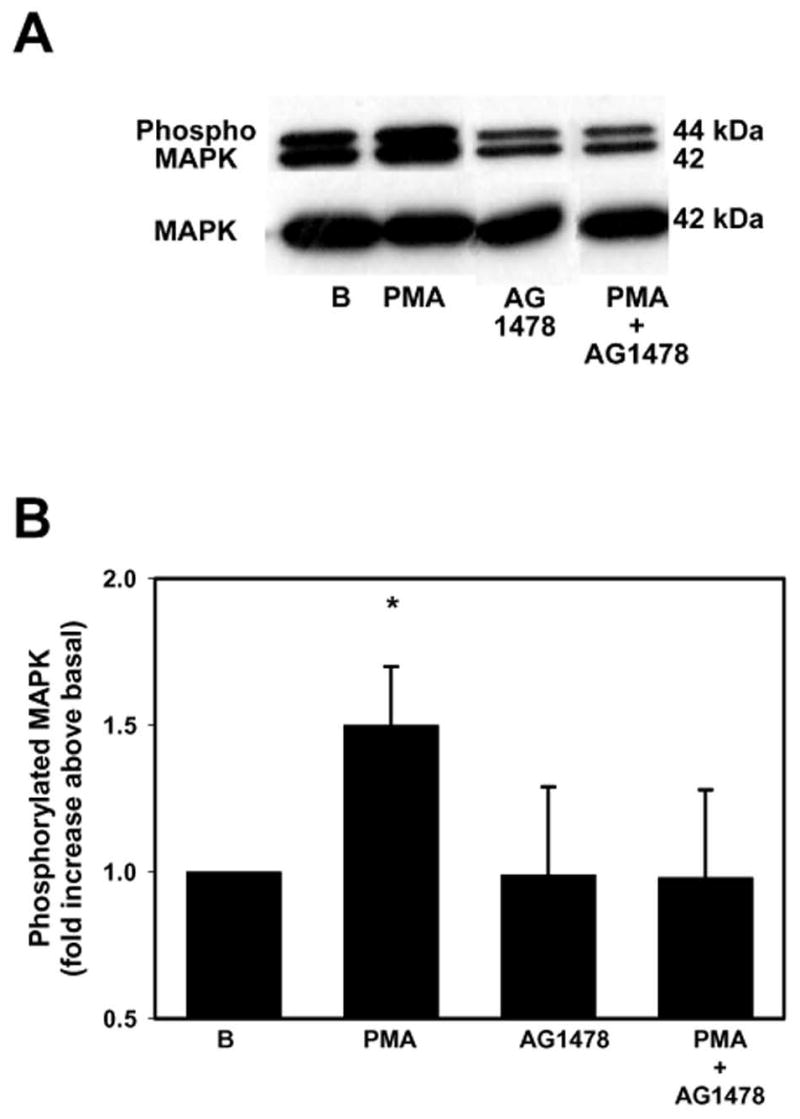
Conjunctival pieces were preincubated with the EGF receptor inhibitor AG1478 (10−7 M) for 10 min prior to stimulation with PMA (10−7 M) for 10 min. The amount of phosphorylated p42/p44 MAPK and total p42 MAPK were determined by western blot analysis and is shown in A. Four independent experiments were analyzed by densitometry and mean ± SEM is shown in B. * indicates significant difference from basal.
Effect of Phorbol Esters on Pyk2 and Src Activation
We previously showed that cholinergic agonists activate Pyk2 and Src to activate p42/p44 MAPK (Kanno, et al., 2003). To determine if these agonists do so by activating PKC, we incubated conjunctival pieces with PMA (10−7 M) for 0 – 30 min and measured the amount of phosphorylated (active) Pyk2 and total Pyk2 determined by western blot analysis. As shown in Figure 4A, the amount of phosphorylated Pyk2 increased by 5 min and declining by 10 and 30 min. When 6 independent experiments were analyzed, PMA induced a time-dependent phosphorylation of Pyk2 with a maximum significant increase at 5 min to 1.9 ± 0.3 fold above basal (Figure 4B). The amount of phosphorylated Pyk2 declined to nearly basal levels by 10 min.
Figure 4. Effect of Phorbol Ester on Pyk2 and Src Activation.
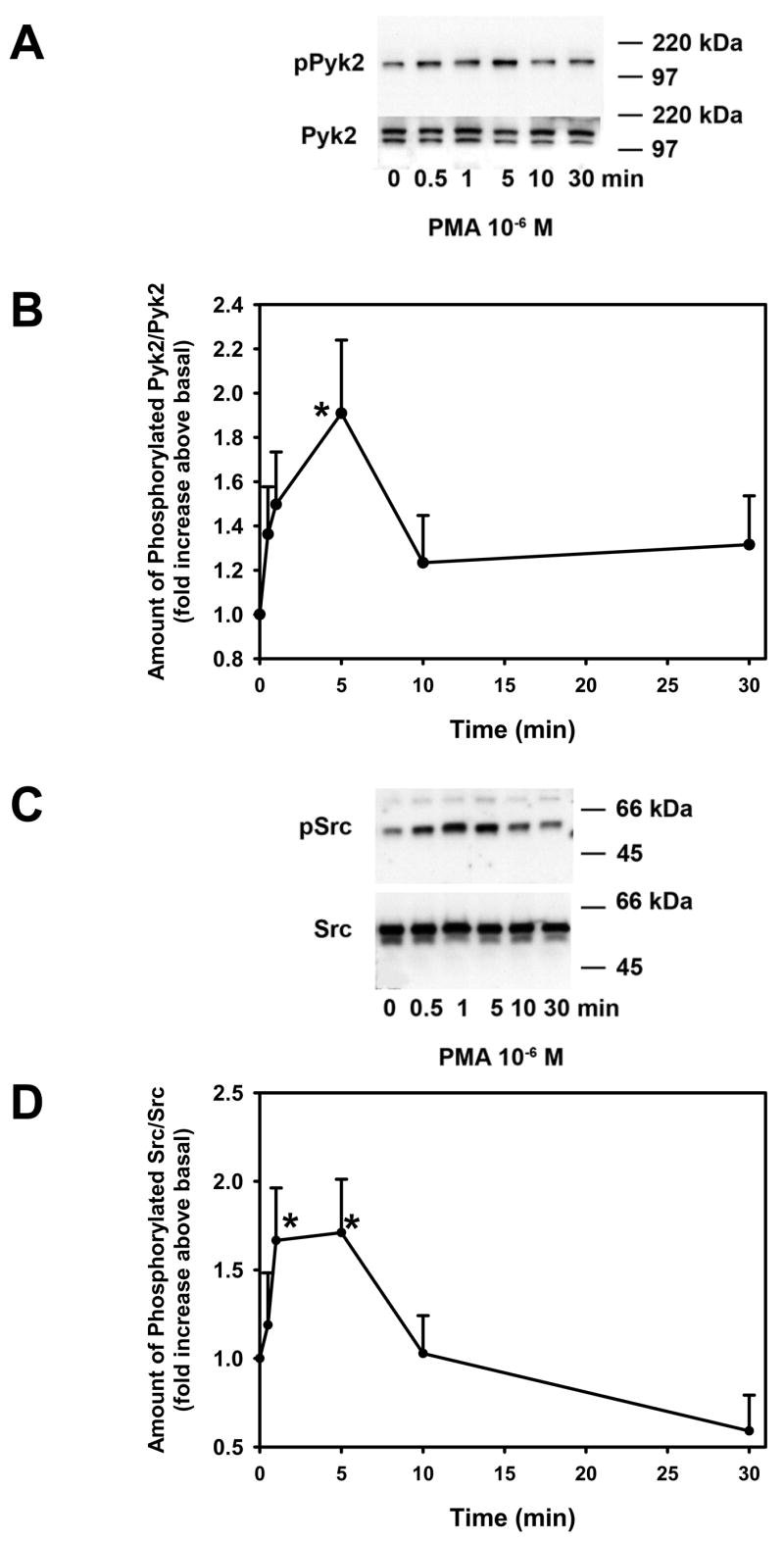
Conjunctival pieces were incubated with the PKC activator PMA for 0 – 30 min and the amount of phosphorylated Pyk2 and total Pyk2 was determined by western blot analysis and shown in A. Six independent experiments were analyzed by densitometry and mean ± SEM are shown in B. Conjunctival pieces were incubated with the PKC activator PMA for 0 – 30 min and the amount of phosphorylated Src and total Src was determined by western blot analysis and shown in C. Six independent experiments were analyzed by densitometry and mean ±SEM is shown in D. * indicates significant difference from basal.
Using the same samples as used to determine Pyk2 activation, we also determined if PMA increased activation of Src using antibodies directed against phosphorylated and total Src. PMA increased activated Src in a time dependent manner over a 30 min incubation time (Figure 4C). This increase was significant at 1 and 5 mins (1.6 ± 0.3 and 1.7 ± 0.3 fold above basal, respectively, Figure 4D). Activation of Src by PMA decreased to basal level by 10 min and decreased further by 30 min.
Effect of Inhibition of the EGF Receptor on Ca2+-stimulated MAPK Activation
We previously showed that increasing intracellular Ca2+ with the calcium ionophore ionomycin stimulated mucin secretion from conjunctival pieces in a time- and concentration-dependent manner (Dartt, et al., 2000). To determine if Ca2+-stimulated secretion was dependent upon activation of the EGF receptor, conjunctival pieces were pretreated with AG1478 (10−7 M) for 10 min before incubation with ionomycin (10−6 M) for 10 min. AG1478 alone did not have an effect on basal MAPK-activation while ionomycin significantly increased MAPK activity 1.5 ± 0.2 fold above basal (Figure 5). AG1478 inhibited ionomycin-stimulated MAPK activation by 66 % to 1.19 ± 0.1 fold above basal. These data indicate that increasing [Ca2+]i is involved in the transactivation of the EGF receptor prior to activation of p42/p44 MAPK.
Figure 5. Effect of Inhibition of the EGF Receptor on Ca2+-stimulated MAPK Activation.
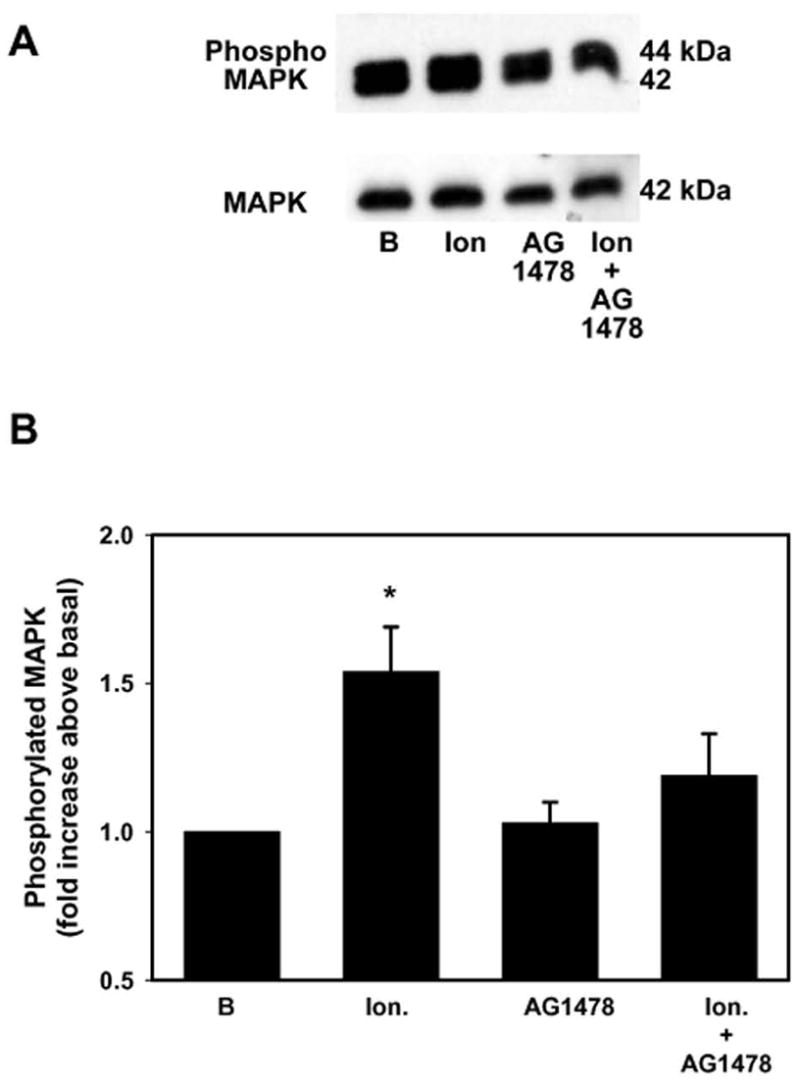
Conjunctival pieces were preincubated with the EGF receptor inhibitor AG1478 (10−7 M) for 10 min prior to stimulation with ionomycin (10−6 M) for 10 min. The amount of phosphorylated p42/p44 MAPK and total p42 MAPK was determined by western blot analysis and is shown in A. Four independent experiments were analyzed by densitometry and mean ± SEM is shown in B. * indicates significant difference from basal.
Effect of Increasing Intracellular Ca2+ on Pyk2 and Src Activation
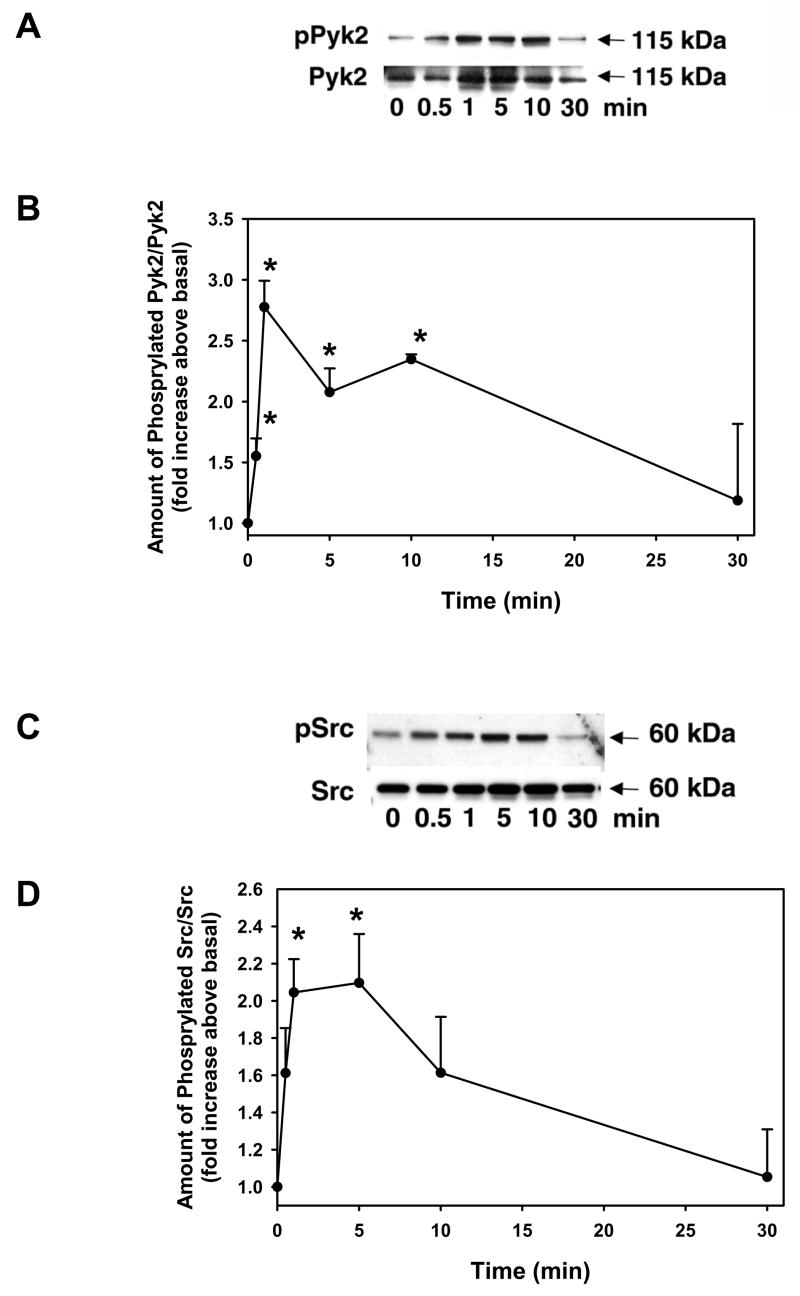
As cholinergic agonists and PKC both activate Pyk2 and Src, we determined if increasing intracellular Ca2+ with ionomycin also activates Pyk2 and Src in conjunctival pieces. The pieces were incubated with ionomycin (10−6) for 0 – 30 min and the amount of phosphorylated Pyk2 (active) was determined by western blot analysis. Ionomycin caused a concentration-dependent increase in phosphorylated Pyk2 (Figure 6A). When 3 independent experiments were analyzed, ionomycin significantly increased the amount of phosphorylated Pyk2 to 2.8 ± 0.2, 2.1 ± 0.2, and 2.4 ± 0.04 fold above basal at 1, 5, and 10 min respectively (Figure 6B). By 30 min, the amount of activated Pyk2 had declined to basal levels.
Figure 6. Effect of Ca2+ on Pyk2 and Src Activation.
Conjunctival pieces were incubated with ionomycin (10−6 M) for 0 – 30 min and the amount of phosphorylated Pyk2 and total Pyk2 was determined by western blot analysis and shown in (A). Three independent experiments were analyzed by densitometry and mean ± SEM are shown in B. Conjunctival pieces were incubated with ionomycin (10−6 M) for 0 – 30 min and the amount of phosphorylated Src and total Src was determined by western blot analysis and shown in C. Three independent experiments were analyzed by densitometry and mean ± SEM is shown in D. * indicates significant difference from basal.
As activation of Pyk2 can precede activation of Src, we used the same samples as measured for Pyk2 phosphorylation to determine if ionomycin increased activity of Src. As shown in Figure 6C, ionomycin (10−6 M) caused a time-dependent increase in Src phosphorylation. When 3 independent experiments were analyzed, ionomycin significantly increased Src activation by 2.0 ± 0.2 and 2.1 ± 0.3 fold increased at 1 and 5 min, respectively before declining to basal levels by 30 min.
These data indicate that increasing intracellular Ca2+ activates Pyk2 and Src in rat conjunctival pieces.
Effect of PKC and Ca2+, and Inhibition of the EGF Receptor on p42/p44 MAPK, Pyk2, and Src Activation in Cultured Rat Goblet Cells
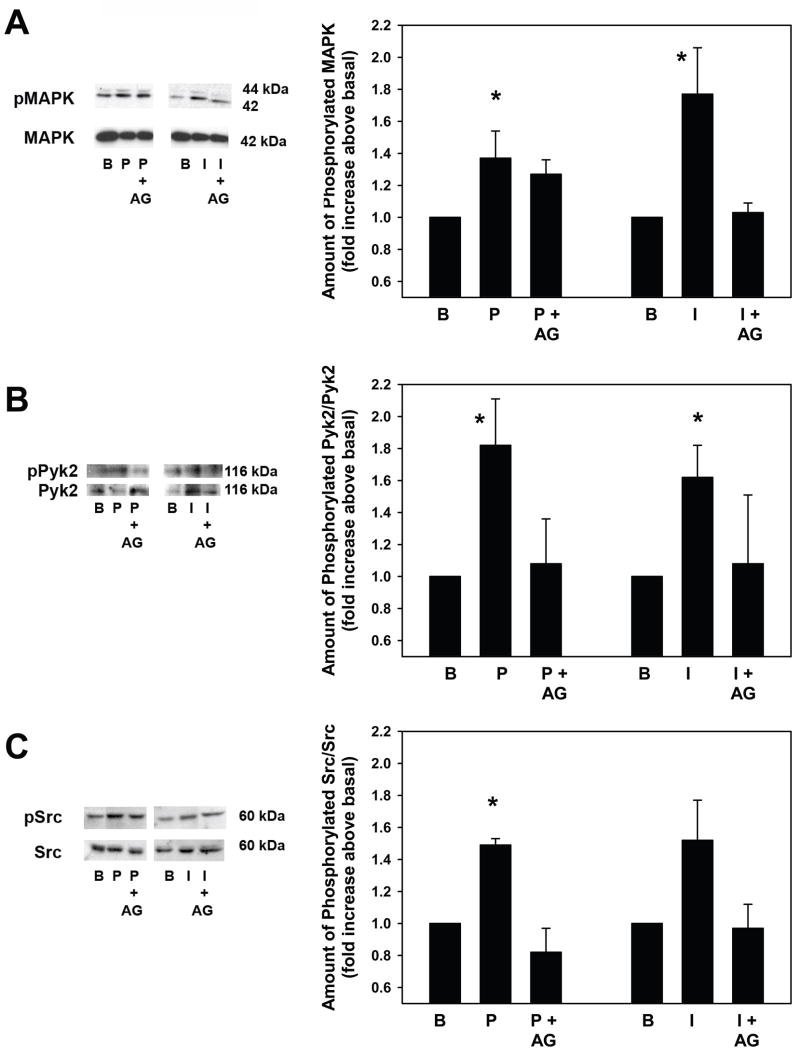
To ensure that the p42/p44 MAPK, Pyk2, and Src pathways are indeed present in goblet cells, we measured the activity of these enzymes upon stimulation with PMA and ionomycin in cultured goblet cells. Goblet cells from rat conjunctiva were grown in culture as described in the Methods. The cells were incubated with or without the EGF receptor inhibitor AG1478 (10−7 M) prior to stimulation with either PMA (10−7 M) or ionomycin (10−7 M) for 5 min. Cells were homogenized and the amount of activated p42/p44 MAPK, Pyk2, and Src was determined by western blot analysis. PMA and ionomycin significantly increased p42/p44 MAPK activity to 1.4 ± 0.1 and 1.8 ± 0.3 fold above basal, respectively (Figure 7A). Incubation with AG1478 decreased PMA-stimulated p42/p44 MAPK activity by 40 ± 7% and ionomycin-stimulated p42/p44 MAPK activity by 96 ± 4% (Figure 7A).
Figure 7. Effect of PKC and Ca2+, and Inhibition of the EGF Receptor on p42/p44 MAPK, Pyk2, and Src Activation in Cultured Rat Goblet Cells.
Cultured rat goblet cells were treated with or without the EGF receptor inhibitor AG1478 (10–7 M) for 10 min prior to stimulation with either PMA (10–7 M) and ionomycin (10–7 M) for 5 min. The amount of phosphorylated p42/p44 MAPK (A), Pyk2 (B), and Src (C) was determined by western blot analysis. Representative blots are shown on left. Three independent experiments were analyzed by densitometry and mean ± SEM is shown in graphs. * indicates significant difference from basal.
The same samples used for p42/p44 MAPK analysis were also assayed for Pyk2 activity. PMA and ionomycin also significantly increased Pyk2 activity to 1.8 ± 0.3 and 1.6 ± 0.2 fold above basal, respectively (Figure 7B). Incubation with AG1478 decreased PMA-stimulated Pyk2 activity by 62 ± 22% and ionomycin-stimulated Pyk2 activity by 60 ± 21% (Figure 7B).
PMA also significantly increased Src activity to 1.5 ± 0.03 fold above basal while ionomycin increased Src activity to 1.5 ± 0.2 fold above basal (Figure 7C). Incubation with AG1478 decreased PMA-stimulated Pyk2 activity by 96 ± 4% and ionomycin-stimulated Src activity by 85 ± 15% (Figure 7C).
DISCUSSION
In the present study we extend our knowledge of the signal transduction pathways utilized by cholinergic agonists to stimulate p42/p44 MAPK activation and ultimately HMWGC secretion in rat conjunctival goblet cells. Cholinergic agonists bind to and activate the M2 and M3 muscarinic receptors to increase hydrolysis of PIP2 (Kanno, et al., 2003). This results in the formation of DAG, to activate PKC, and IP3 to cause an increase in [Ca2+]i. In addition, cholinergic agonists activate Pyk2 and Src to transactivate the EGF receptor. Transactivation of the EGF receptor stimulates p42/p44 MAPK activity to increase HMWGC secretion (Kanno, et al., 2003). In this study we determined that Ca2+ and PKC are responsible for activating Pyk2 and Src to transactivate the EGF receptor to stimulate p42/p44 MAPK.
It is well established that GPCRs can transactivate the EGF receptor through a mechanism known as “triple membrane passing signal” model (Peschon, et al., 1998). In this model, GPCRs activate matrix metalloproteinases (MMPs). MMPs cause the ectodomain shedding of the membrane-bound EGF family member (in either pro- or mature form of the growth factor) thereby releasing the molecule into the extracellular milieu. The EGF family member can then bind to the EGF receptor on the surface of the same cell or an adjacent cell. Ectodomain shedding of EGF has been shown to occur in the lacrimal gland (Chen, et al., 2006) while shedding of TGFα and HB-EGF occurs in many other tissues (Prenzel, et al., 2000). Determining if ectodomain shedding occurs in conjunctival goblet cells in response to cholinergic stimulation and the identification of the specific growth factor shed is under investigation.
As we were previously unable to determine the role of PKC in HMWGC secretion due to an increase in basal secretion by PKC inhibitors themselves (Dartt, et al., 2000), we determined the effects of PKC inhibitors on p42/p44 MAPK activation. The PKC inhibitors calphostin C and chelethyrine chloride did not affect basal MAPK activity but inhibited cholinergic-agonist stimulated p42/p44 MAPK. Another PKC inhibitor, staurosporine, caused an increase in basal p42/p44 MAPK activation. This could be due to the fact that staurosporine has been shown to increase hepatocyte growth factor production in a variety of cell types (Yagi, et al., 2003) and prostaglandin E2 production (Yamaki, et al., 2000). It is not known what effects these compounds have on conjunctival goblet cell secretion or p42/p44 MAPK activity. However, the PKC activator PMA caused a time-dependent increase in Pyk2 and Src activity as well as p42/p44 MAPK activity. PMA-stimulated p42/p44 MAPK activation was prevented by the EGF receptor inhibitor AG1478 indicating that PKC is involved in the transactivation of the EGF receptor. Because p42/p44 MAPK activation is critical to cholinergic-agonist stimulated mucin secretion, these data imply that PKC plays an important role in intracellular pathways leading to this secretion.
PKC is a family of enzymes consisting of at least ten different isoforms. The conjunctiva contains at least 8 of them and the closely related PKD (Dartt, et al., 2000). None of the inhibitors used are specific for a particular isoform so it is not known which isoforms are involved in either HMWGC secretion or p42/p44 MAPK activity. It is interesting to note that activation of p42/p44 MAPK by PMA is biphasic with a first peak occurring at 30 s and a second peak occurring at 10 min. This could be due to different isoforms of PKC becoming active at specific, different times. It is not uncommon for a given stimulus to activate multiple PKC isoforms with different time dependencies. In the lacrimal gland, cholinergic agonists caused differential translocation of four different PKC isoforms with a transient translocation of PKCδ and a sustained translocation of PKCε (Zoukhri, et al., 1997). Differential activation of PKC isoforms by angiotensin II in neuroblastoma X glioma cells has also been reported (Greenland and Mukhopadhyay, 2004).
In the present study we observed that activation of PKC and an increase in [Ca2+]i resulted in phosphorylation of Pyk2 at Tyr881 and Src at Tyr416. Similar to conjunctival goblet cells, activation of Pyk2 by PKC and [Ca2+]i has been reported in PC12 cells (Lev, et al., 1995) and other tissues as well (Hodges, et al., 2006, Schindler, et al., 2007, Wang and Reiser, 2003). Phosphorylation of Src on Tyr416 has been shown up-regulate the activity of Src (Xu, et al., 1999) while Pyk2, upon activation, undergoes autophosphorylation on Tyr402 (Dikic, et al., 1996, Park, et al., 2004). Autophosphorylation causes the recruitment of Src, which then participates in the phosphorylation of Pyk2 on Tyr580 and Tyr881 (Katagiri, et al., 2000, Li, et al., 1999). Thus phosphorylation of Pyk2 on Try881 should occur after the activation of Src. We did not determine phosphorylation of Pyk2 on Tyr402. In the case of PKC stimulation of conjunctival goblet cells, maximum Src activation occurred at 1 min while maximum phosphorylation of Pyk2 on Try881 occurred at 5 min. In contrast, stimulation with ionomycin, which increases [Ca2+]i, caused maximum Pyk2 activation at the same time (1 min) as does maximum Src activation. It is not clear why PKC stimulation of Pyk2 is a slower process than activation of Pyk2 by Ca2+.
Similar to conjunctival goblet cells, cholinergic agonists also transactive the EGF receptor via Pyk2 and Src to activate p42/p44 MAPK in T84 cells (Keely, et al., 2000). This activation, however in contrast to goblet cells, down-regulates Cl− secretion in these cells. We have also found that activation of p42/p44 MAPK attenuates protein secretion in the lacrimal gland (Ota, et al., 2003). This emphasizes the fact that the signal transduction pathways in different cell types are associated with one another in a cell-specific manner.
It should be noted that many of the experiments in this study were performed using pieces of rat conjunctiva. The conjunctiva contains, in addition to goblet cells, epithelial and stromal cells. Thus activation of p42/p44 MAPK, Pyk2, and Src could occur in multiple cell types. However, using conjunctival pieces, we have measured mucin secretion with a lectin specific for goblet cell mucins to confirm that goblet cells utilize these enzymes. We have recently developed a method for culturing purified rat and human conjunctival goblet cells (Shatos, et al., 2003, Shatos, et al., 2001). We have confirmed that muscarinic receptors are present on cultured goblet cells and that cholinergic agonists transactivate the EGF receptor to activate p42/p44 MAPK in cultured goblet cells (Horikawa, et al., 2003). In the present study, we demonstrated that Ca2+ and PKC do activate p42/p44 MAPK, Pyk2, and Src in cultured goblet cells. Therefore, it is likely that the results were obtained in conjunctival pieces are, at least in part, due to the goblet cells present in the pieces.
In summary, we have shown that in conjunctival goblet cells activation of PKC and increasing [Ca2+]i stimulate Pyk2 and Src which play a critical role in the transactivation of the EGF receptor. The activated EGF receptor then increased p42/p44 MAPK activity in these cells.
Acknowledgments
The authors would like to thank Dr. Driss Zoukhri for his critical reading of the manuscript. This study was supported by NIH EY09057.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12(Suppl 1):S17–27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- Chen L, Hodges RR, Funaki C, Zoukhri D, Gaivin RJ, Perez DM, Dartt DA. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am J Physiol Cell Physiol. 2006;291:C946–56. doi: 10.1152/ajpcell.00014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78:173–85. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Dartt DA, Kessler TL, Chung EH, Zieske JD. Vasoactive intestinal peptide-stimulated glycoconjugate secretion from conjunctival goblet cells. Exp Eye Res. 1996;63:27–34. doi: 10.1006/exer.1996.0088. [DOI] [PubMed] [Google Scholar]
- Dartt DA, McCarthy DM, Mercer HJ, Kessler TL, Chung EH, Zieske JD. Localization of nerves adjacent to goblet cells in rat conjunctiva. Curr Eye Res. 1995;14:993–1000. doi: 10.3109/02713689508998520. [DOI] [PubMed] [Google Scholar]
- Dartt DA, Rios JD, Kanno H, Rawe IM, Zieske JD, Ralda N, Hodges RR, Zoukhri D. Regulation of conjunctival goblet cell secretion by Ca(2+)and protein kinase C. Exp Eye Res. 2000;71:619–28. doi: 10.1006/exer.2000.0915. [DOI] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–50. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- Greenland KJ, Mukhopadhyay AK. Selective activation of protein kinase C isoforms by angiotensin II in neuroblastoma X glioma cells. Mol Cell Endocrinol. 2004;213:181–91. doi: 10.1016/j.mce.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Rios JD, Vrouvlianis J, Ota I, Zoukhri D, Dartt DA. Roles of protein kinase C, Ca2+, Pyk2, and c-Src in agonist activation of rat lacrimal gland p42/p44 MAPK. Invest Ophthalmol Vis Sci. 2006;47:3352–9. doi: 10.1167/iovs.06-0028. [DOI] [PubMed] [Google Scholar]
- Horikawa Y, Shatos MA, Hodges RR, Zoukhri D, Rios JD, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2535–44. doi: 10.1167/iovs.02-1117. [DOI] [PubMed] [Google Scholar]
- Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, Dartt DA. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003;284:C988–98. doi: 10.1152/ajpcell.00582.2001. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi T, Sasaki T, Nakamura S, Hattori S. Protein-tyrosine kinase Pyk2 is involved in interleukin-2 production by Jurkat T cells via its tyrosine 402. J Biol Chem. 2000;275:19645–52. doi: 10.1074/jbc.M909828199. [DOI] [PubMed] [Google Scholar]
- Keely SJ, Calandrella SO, Barrett KE. Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular ca(2+), PYK-2, and p60(src) J Biol Chem. 2000;275:12619–25. doi: 10.1074/jbc.275.17.12619. [DOI] [PubMed] [Google Scholar]
- Kessler TL, Mercer HJ, Zieske JD, McCarthy DM, Dartt DA. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr Eye Res. 1995;14:985–92. doi: 10.3109/02713689508998519. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–45. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274:8917–24. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- Ota I, Zoukhri D, Hodges RR, Rios JD, Tepavcevic V, Raddassi I, Chen LL, Dartt DA. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am J Physiol Cell Physiol. 2003;284:C168–78. doi: 10.1152/ajpcell.00151.2002. [DOI] [PubMed] [Google Scholar]
- Park SY, Avraham HK, Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J Biol Chem. 2004;279:33315–22. doi: 10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2:184–90. doi: 10.1186/bcr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–11. [PubMed] [Google Scholar]
- Schindler EM, Baumgartner M, Gribben EM, Li L, Efimova T. The Role of Proline-Rich Protein Tyrosine Kinase 2 in Differentiation-Dependent Signaling in Human Epidermal Keratinocytes. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5700662. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Horikawa Y, Hodges RR, Chang EL, Bernardino CR, Rubin PA, Dartt DA. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477–86. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1455–64. [PubMed] [Google Scholar]
- Wang H, Reiser G. The role of the Ca2+-sensitive tyrosine kinase Pyk2 and Src in thrombin signalling in rat astrocytes. J Neurochem. 2003;84:1349–57. doi: 10.1046/j.1471-4159.2003.01637.x. [DOI] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–38. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- Yagi Y, Sotani T, Nagao T, Horio T, Yamamoto I, Gohda E. Induction by staurosporine of hepatocyte growth factor production in human skin fibroblasts independent of protein kinase inhibition. Biochem Pharmacol. 2003;66:1797–808. doi: 10.1016/s0006-2952(03)00547-1. [DOI] [PubMed] [Google Scholar]
- Yamaki K, Yonezawa T, Ohuchi K. Signal transduction cascade in staurosporine-induced prostaglandin E(2) production by rat peritoneal macrophages. J Pharmacol Exp Ther. 2000;293:206–13. [PubMed] [Google Scholar]
- Zoukhri D, Hodges RR, Willert S, Dartt DA. Immunolocalization of lacrimal gland PKC isoforms. Effect of phorbol esters and cholinergic agonists on their cellular distribution. J Membr Biol. 1997;157:169–75. doi: 10.1007/s002329900226. [DOI] [PubMed] [Google Scholar]