Abstract
In murine models of systemic lupus erythematosus (SLE), administration of either prolactin or estradiol (E2) increases autoimmunity, and there is evidence that elevated prolactin in response to E2 administration may contribute substantially to E2 effects. Hormonal influence on SLE can extend to environmental agents, as demonstrated by the ability of estrogenic organochlorine pesticides such as chlordecone to accelerate the development of lupus in female (NZB x NZW)F1 mice. In order to evaluate a potential role for prolactin in chlordecone effects on SLE, it was necessary to first determine whether treatment with chlordecone, like E2, results in elevated prolactin levels. Ovariectomized (NZB x NZW)F1 mice were treated for 5–6 weeks with chlordecone or E2 in doses shown previously to significantly shorten the time to onset of SLE. At the end of the treatment period, serum prolactin levels were increased 10- to 20-fold in E2-treated mice compared to untreated controls, but decreased in an apparent dose-dependent manner in mice treated with chlordecone. Prolactin receptor in purified B and CD4 T cells from treated animals, assessed through measurement of mRNA using quantitative real-time PCR, was increased by E2 treatment but unchanged in response to chlordecone. These observations suggest that the role of prolactin in eliciting autoimmunity in E2-treated animals is absent in the case of chlordecone, and by implication, that chlordecone possesses other actions that can replace the contribution of prolactin to development of SLE.
Keywords: prolactin, estradiol, systemic lupus erythematosus, chlordecone, organochlorine pesticides
INTRODUCTION
The single-chain polypeptide hormone prolactin is synthesized and secreted primarily by the anterior pituitary gland, but it can also be produced in many extrapituitary sites, including cells of the immune system, such as lymphocytes [1–3]. While the main function of prolactin is to regulate the growth and differentiation of the mammary gland and the ovary [2], it also acts as an important connection between the endocrine and immune systems [4]. Prolactin receptors, which can be found in many cell types including monocytes and lymphocytes, mediate the signal transduction of prolactin [5–8]. The initial signaling events include prolactin ligand binding, receptor dimerization, and recruitment of cytoplasmic molecules, such as JAK2 and STAT family members, to bind to receptors [9].
Accumulated evidence clearly suggests that prolactin is an important immunoregulator with its roles in modulating lymphoproliferation, cytokine production, antibody production, and cell survival [10]. Animal studies and clinical trials also suggest that prolactin plays a role in the pathogenesis of systemic lupus erythematosus (SLE). In (NZB x NZW)F1 and R4A-IgG2b anti-dsDNA BALB/c transgenic mouse models, elevated serum prolactin concentrations are associated with accelerated autoimmune disease, evidenced by increased titers of anti-dsDNA antibody, enhanced glomerular IgG deposition, and increased mortality [11,12]. Although a straightforward correlation of elevated prolactin and SLE development is difficult to establish in humans, several clinical observations have suggested that prolactin stimulates SLE activity. Hyperprolactinemia is observed in about 20% of SLE patients [13–15]. Lymphocytes and peripheral blood mononuclear cells (PBMC) from SLE patients secreted higher levels of prolactin than cells from control subjects [16,17]. Treatment with low doses of bromocriptine, a dopamine receptor agonist that can block the secretion of prolactin from anterior pituitary, has shown beneficial effects in SLE patients, including a reduction of fatigue and lupus headaches, and a decrease of anti-DNA antibodies [18,19].
Prolactin and estradiol (E2) have a reciprocal endocrinologic relationship. E2 stimulates prolactin secretion from the anterior pituitary by inhibition of dopaminergic suppression, while high concentrations of prolactin suppresses E2 production [20]. In the immune system, both hormones modulate autoimmunity. Although both E2 and prolactin appear to stimulate humoral immunity, E2 appears to suppress cell-mediated immunity while prolactin augments it [10]. Recent studies showed that the immunostimulatory effects of E2 are directly associated with the presence of prolactin. Treatment with the dopamine agonist bromocriptine, which blocks prolactin secretion, prevented exogenous E2 from breaking tolerance and accelerating autoimmunity in murine models [21,22]. Further, high prolactin and low E2 states have been reported to increase autoimmunity, while high E2 and low prolactin states did not [21], providing additional support for the postulate that prolactin plays a critical role in E2 effects in accelerating autoimmunity in mice.
We have recently shown that treatment of ovariectomized (NZB x NZW)F1 mice with chlordecone, an organochlorine pesticide with estrogenic effects, accelerated the rate of development of SLE [23]. A similar effect was produced by E2 treatment, leading to speculation that chlordecone affects autoimmunity through its estrogenicity. We hypothesized that like E2, chlordecone increases circulating prolactin levels, and that prolactin might be an important mediator of chlordecone effects on autoimmunity. As a critical first test of this hypothesis, we measured prolactin levels in ovariectomized (NZB x NZW)F1 mice given doses of chlordecone shown previously to hasten the development of SLE. Mice treated with a relevant dose of E2 were also included as a positive control.
EXPERIMENTAL PROCEDURES
Mice
Female (NZB x NZW)F1 mice (6–8 weeks old) were purchased from The Jackson Laboratories (Bar Harbor, ME) and housed in a climate-controlled facility under specific pathogen-free (SPF) conditions. Mice were allowed free access to food and water at all times during the study. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida.
Test Materials and Treatments
Custom formulated 60-day sustained-release pellets containing either 1 or 5 mg chlordecone each were prepared by Innovative Research of America (Sarasota, FL). Sixty-day release pellets containing 0.05 mg 17-β estradiol (E2) or only matrix (for controls) were obtained from the same source. At least two weeks prior to E2 or chlordecone treatment, mice were surgically ovariectomized as described previously [23]. To begin treatment, a single pellet containing chlordecone, E2, or matrix only was implanted subcutaneously in each animal under light methoxyflurane anesthesia. Mice tolerated the ovariectomy and pellet implantation procedures without complications. Five to six weeks after pellet implantation, mice were euthanized and serum and spleen were removed.
Determination of serum prolactin level
Serum was obtained at sacrifice and stored frozen at −80 °C until analysis. Prolactin radioimmunoassay (RIA) used mouse PRL (mPRL) reference preparation AFP6476C, mPRL AFP1077D for iodination, and anti-mPRL antiserum AFP131078 (provided by the National Hormone and Peptide Program, Harbor–UCLA Medical Center, Torrance, California, USA). The assay was performed by the National Hormone and Peptide Program. Briefly, 50 μl of serum from each sample was used in the RIA, and approximately 20,000 cpm was added to each tube. Quantitative limits for the assays ranged from 3–6 ng/mL with intra- and interassay coefficients of variation <15%. Samples were analyzed in duplicate at multiple dilutions.
RNA and cDNA preparation
Mouse B and CD4 T cell isolation kits (Miltenyi Biotec, Auburn, CA) were used to enrich splenic B and CD4 T cell preparations by negative selection. RNA from purified B and CD4 T cells was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA), and the RNA concentration was measured spectrophotometrically. RNA (1 μg) was then treated with DNase I (Invitrogen) to remove genomic DNA and reverse transcribed to cDNA using the Superscript II First-Strand Synthesis System (Invitrogen) for real-time PCR.
Real-time PCR
Gene expression was determined by quantitative real-time PCR using SYBR green (Applied Biosystems, Foster City, CA). Primers were designed as follows: Prolactin receptor forward TGATCCTCAGTTTGGTGCAG, reverse TTCAGGATAGGCCTGGCTAA; β-actin forward CGGCCTAGCTCTGAGACAAT, reverse GTCACCATCCTTTTGCCAGTT. Procedures for real-time PCR were identical to those described previously (24). Transcripts were quantified using the comparative (2 −ΔΔCt) method.
Statistical analysis
Statistical analyses were conducted using the GraphPad Prism software, version 4.00 (GraphPad, San Diego, CA). Groups were compared using Dunnett’s procedure for the one-way analysis of variance (ANOVA). All comparisons with p < 0.05 were considered to be statistically significant.
RESULTS
Serum prolactin was measured in mice treated with implanted, sustained-release pellets containing chlordecone, E2 or matrix only (controls) for 5–6 weeks (Figure 1). The manner of administration and chlordecone and E2 doses were the same as has been found previously to accelerate the development of SLE in this mouse strain [23]. As expected, E2 treatment caused a dramatic 10- to 20-fold increase in serum prolactin levels, with an average concentration of 500 ng/ml. In marked contrast, chlordecone-treated mice showed a significant and dose-dependent decrease in prolactin levels compared to the control-treated group.
Figure 1. Comparison of chlordecone and E2 effects on serum prolactin level.
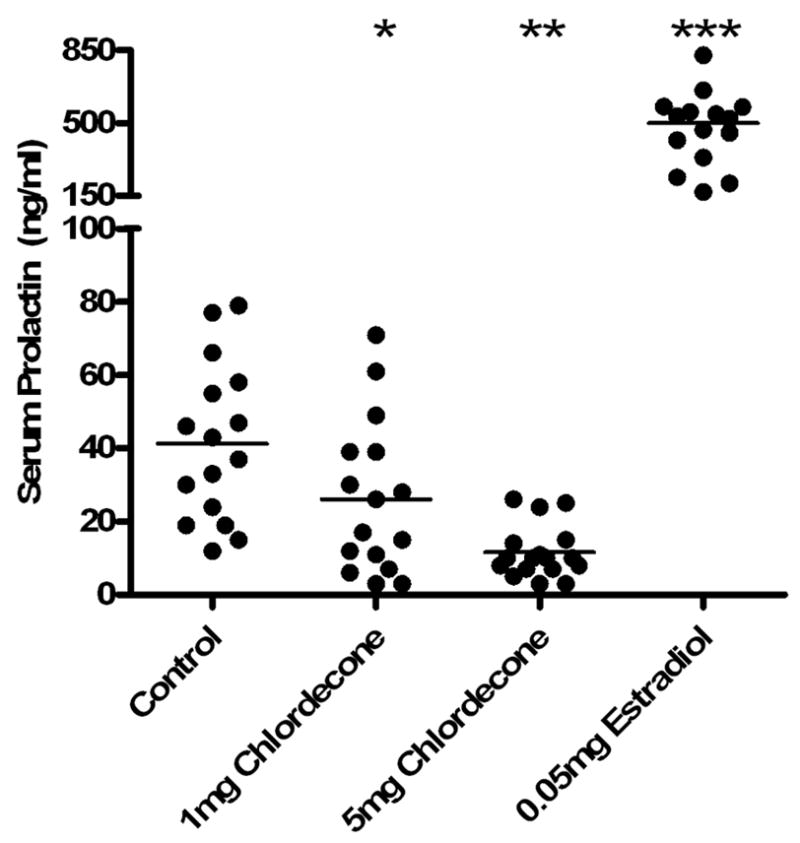
Mice were treated with 60-day sustained-release pellets containing E2 or chlordecone in the indicated amounts and euthanized 5–6 weeks later. Each point in the figure represents the results from an individual subject. Serum prolactin concentrations were significantly increased by E2 treatment, and significantly decreased by chlordecone. Results from chlordecone-treated mice showed a dose-related trend. *, p < 0.05; **, p<0.01; ***, p<0.001.
Although serum prolactin levels were decreased in the chlordecone-treated group, it was still possible that the prolactin pathway was being induced in cells of the immune system, perhaps through upregulation of the prolactin receptor and autocrine or paracrine secretion of prolactin. For this reason, we also tested the level of expression of prolactin receptor on lymphocytes by quantitative real-time PCR. The primer that we designed for the experiment covered a gene fragment between exon 6 and exon 7, and is common to all four isoforms of the prolactin receptor protein (25,26). E2 exposure in vivo produced a significant increase in prolactin receptor mRNA in B (Fig. 2A) and CD4 T cells (Fig. 2B) compared with the control group. The chlordecone-treated group showed no change in prolactin receptor mRNA levels in either population (Fig. 2).
Figure 2. Comparison of chlordecone and E2 effects on prolactin receptor gene expression in B and CD4 T cells.
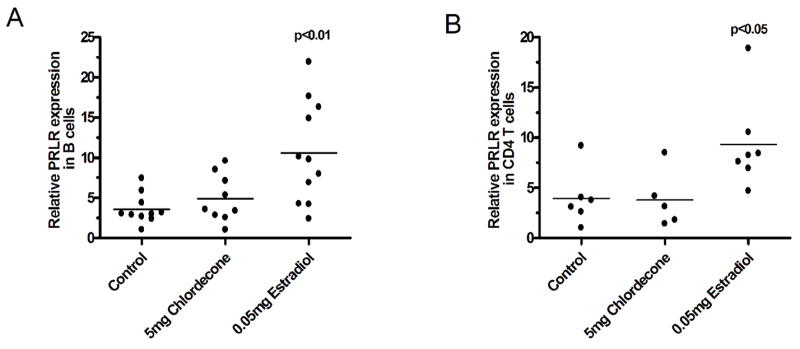
Mice were treated with 60-day sustained-release pellets containing E2 or chlordecone in the indicated amounts and euthanized 5–6 weeks later. Each point in the figure represents the results from an individual subject. Prolactin receptor mRNA was significantly increased in E2-treated mice in both B (Panel A) and CD4 T (Panel B) cells. Prolactin receptor mRNA in chlordecone-treated mice was not significantly different from controls.
DISCUSSION
E2 can markedly stimulate prolactin secretion, which has been shown in both the mouse model and humans [20]. In the study presented here, E2 significantly increased serum prolactin levels, as expected. Because of the reported estrogenic effects of chlordecone [27,28], it was anticipated that chlordecone at doses that affect autoimmunity would also increase serum prolactin levels. It was thus surprising that chlordecone caused a dose-dependent decrease in serum prolactin. Whether chlordecone decreased prolactin levels in serum by inhibiting its secretion or accelerating degradation remains unknown.
As prolactin effects on lymphocytes are mainly through the prolactin receptor [2,9], it was important to determine whether chlordecone might produce an increased prolactin effect in the face of diminished serum concentrations by upregulating receptors. Previous studies have reported that E2 can increase prolactin receptor expression in both mice and humans in cells outside the immune system [29–31]. Results from Figure 2 show that E2 treatment increases prolactin receptor expression in cells of the immune system as well, as both B and CD4 T cells showed significant increases. In contrast, chlordecone exposure at dose levels that can clearly accelerate autoimmunity had no effect on B cell and CD4 T cell prolactin receptor expression at the mRNA level. There are a few caveats to interpretation of these observations that should be acknowledged. One is that mRNA might not accurately reflect the number of functioning receptors if, for example, chlordecone were to possess a receptor stabilizing effect (i.e., an effect to slow receptor turnover). A second is the possibility that chlordecone could upregulate one prolactin receptor isoform while down-regulating another. In this situation, a critical receptor could be increased, even though the overall receptor levels are unchanged. We consider both possibilities to be unlikely, but acknowledge that understanding of prolactin receptor isoforms and their turnover in immunocytes is extremely limited at present.
Both E2 and chlordecone are equally effective in accelerating the rate of development of SLE in the (NZB x NZW)F1 mouse [23]. The apparent decreased, rather than increased, presence of prolactin in chlordecone-treated mice has interesting implications in terms of the comparative mechanisms by which these two agents affect autoimmunity. For example, it has been shown that without the help of prolactin, E2 can still mediate the survival of autoreactive B cells, but loses its ability to activate these anergic B cells [22]. We reported earlier that both chlordecone and E2 share the same features in reducing apoptosis of B cells in germinal center, a place where negative selection of autoreactive B cells occurs [24]. Therefore, it is possible that E2 and chlordecone share the same pathway in helping the survival of autoreactive B cells, while chlordecone has its own prolactin-like mechanism to break the tolerance of these B cells to produce autoantibodies. Direct prolactin-like effects of chlordecone could also explain decreased prolactin secretion through negative regulatory feedback, although there is currently no experimental evidence to support or refute this hypothesis. Additional study comparing immune responses to chlordecone, E2, and prolactin in murine lupus models could provide valuable insights into the potential roles of both endogenous and environmental endocrine-active agents in the pathogenesis of SLE.
Acknowledgments
This research was supported in part by a grant from the Superfund Basic Research Program, National Institute for Environmental Health Sciences, ES07375.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 2.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 3.Hiestand PC, Mekler P, Nordmann R, Grieder A, Permmongkol C. Prolactin as a modulator of lymphocyte responsiveness provides a possible mechanism of action for cyclosporine. Proc Natl Acad Sci U S A. 1986;83:2599–2603. doi: 10.1073/pnas.83.8.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeva E, Venkatesh J, Michael D, Diamond B. Prolactin as a modulator of B cell function: implications for SLE. Biomed Pharmacother. 2004;58:310–319. doi: 10.1016/j.biopha.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Matera L, Muccioli G, Cesano A, Bellussi G, Genazzani E. Prolactin receptors on large granular lymphocytes: dual regulation by cyclosporin A. Brain Behav Immun. 1988;2:1–10. doi: 10.1016/0889-1591(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 6.Matera L, Cutufia M, Geuna M, Contarini M, Buttiglieri S, Galin S, et al. Prolactin is an autocrine growth factor for the Jurkat human T-leukemic cell line. J Neuroimmunol. 1997;79:12–21. doi: 10.1016/s0165-5728(97)00096-9. [DOI] [PubMed] [Google Scholar]
- 7.Matera L, Geuna M, Pastore C, Buttiglieri S, Gaidano G, Savarino A, et al. Expression of prolactin and prolactin receptors by non-Hodgkin’s lymphoma cells. Int J Cancer. 2000;85:124–130. doi: 10.1002/(sici)1097-0215(20000101)85:1<124::aid-ijc22>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery DW, Shen GK, Ulrich ED, Steiner LL, Parrish PR, Zukoski CF. Human thymocytes express a prolactin-like messenger ribonucleic acid and synthesize bioactive prolactin-like proteins. Endocrinology. 1992;131:3019–3026. doi: 10.1210/endo.131.6.1446637. [DOI] [PubMed] [Google Scholar]
- 9.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Ann Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 10.McMurray RW. Estrogen, prolactin, and autoimmunity: actions and interactions. Int Immunopharmacol. 2001;6:995–1008. doi: 10.1016/s1567-5769(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.McMurray R, Keisler D, Kanuckel K, Izui S, Walker SE. Prolactin influences autoimmune disease activity in the female B/W mouse. J Immunol. 1991;147:3780–3787. [PubMed] [Google Scholar]
- 12.Peeva E, Michael D, Cleary J, Rice J, Chen X, Diamond B. Prolactin modulates the naive B cell repertoire. J Clin Invest. 2003;111:275–283. doi: 10.1172/JCI16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker SE, McMurray RW, Houri JM, Allen SH, Keisler D, Sharp GC, et al. Effects of prolactin in stimulating disease activity in systemic lupus erythematosus. Ann N Y Acad Sci. 1998;840:762–772. doi: 10.1111/j.1749-6632.1998.tb09615.x. [DOI] [PubMed] [Google Scholar]
- 14.McMurray RW. Prolactin and systemic lupus erythematosus. Ann Med Interne. 1996;147:253–258. [PubMed] [Google Scholar]
- 15.Allen SH, Sharp GC, Wang G, Conley C, Takeda Y, Conroy SE, et al. Prolactin levels and antinuclear antibody profiles in women tested for connective tissue disease. Lupus. 1996;5:30–37. doi: 10.1177/096120339600500107. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez MA, Molina JF, Jara LJ, Cuellar ML, Garcia C, Gutierrez-Urena S, et al. Prolactin and systemic lupus erythematosus: prolactin secretion by SLE lymphocytes and proliferative (autocrine) activity. Lupus. 1995;4:348–352. doi: 10.1177/096120339500400504. [DOI] [PubMed] [Google Scholar]
- 17.Larrea F, Martinez-Castillo A, Cabrera V, Alcocer-Varela J, Queipo G, Carino C, et al. A bioactive 60-kilodalton prolactin species is preferentially secreted in cultures of mitogen-stimulated and nonstimulated peripheral blood mononuclear cells from subjects with systemic lupus erythematosus. J Clin Endocrinol Metab. 1997;82:3664–3669. doi: 10.1210/jcem.82.11.4356. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Nemegyei J, Cobarrubias-Cobos A, Escalante-Triay F, Sosa-Munoz J, Miranda JM, Jara LJ. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus. 1998;7:414–419. doi: 10.1191/096120398678920334. [DOI] [PubMed] [Google Scholar]
- 19.McMurray RW, Weidensaul D, Allen SH, Walker SE. Efficacy of bromocriptine in an open label therapeutic trial for systemic lupus erythematosus. J Rheumatol. 1995;22:2084–2091. [PubMed] [Google Scholar]
- 20.Wilson J, Foster D. Textbook of Endocrinology. 8. Philadelphia (PA): WB Saunders; 1992. [Google Scholar]
- 21.Elbourne KB, Keisler D, McMurray RW. Differential effects of estrogen and prolactin on autoimmune disease in the NZB/NZW F1 mouse model of systemic lupus erythematosus. Lupus. 1998;7:420–427. doi: 10.1191/096120398678920352. [DOI] [PubMed] [Google Scholar]
- 22.Peeva E, Grimaldi C, Spatz L, Diamond B. Bromocriptine restores tolerance in estrogen-treated mice. J Clin Invest. 2000;106:1373–1379. doi: 10.1172/JCI10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organochlorine pesticides in (NZBxNZW)F1 mice. Environ Health Perspect. 2005;113:323–328. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Roberts SM, Butfiloski EJ, Morel L, Sobel ES. Acceleration of autoimmunity by organochlorine pesticides: a comparison of splenic B-cell effects of chlordecone and estradiol in (NZBxNZW)F1 mice. Toxicol Sci. 2007 doi: 10.1093/toxsci/kfm137. in press. [DOI] [PubMed] [Google Scholar]
- 25.Davis JA, Linzer DI. Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol. 1989;3:674–680. doi: 10.1210/mend-3-4-674. [DOI] [PubMed] [Google Scholar]
- 26.Clarke DL, Linzer DI. Changes in prolactin receptor expression during pregnancy in the mouse ovary. Endocrinology. 1993;133:224–232. doi: 10.1210/endo.133.1.8319571. [DOI] [PubMed] [Google Scholar]
- 27.Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000;54:355–364. doi: 10.1093/toxsci/54.2.355. [DOI] [PubMed] [Google Scholar]
- 28.Okubo T, Yokoyama Y, Kano K, Soya Y, Kano I. Estimation of estrogenic and antiestrogenic activities of selected pesticides by MCF-7 cell proliferation assay. Arch Environ Contam Toxicol. 2004;46:445–453. doi: 10.1007/s00244-003-3017-6. [DOI] [PubMed] [Google Scholar]
- 29.Mizoguchi Y, Kim JY, Enami J, Sakai S. The regulation of the prolactin receptor gene expression in the mammary gland of early pregnant mouse. Endocr J. 1997;44:53–58. doi: 10.1507/endocrj.44.53. [DOI] [PubMed] [Google Scholar]
- 30.Tseng L, Zhu HH. Progestin, estrogen, and insulin-like growth factor-I stimulate the prolactin receptor mRNA in human endometrial stromal cells. J Soc Gynecol Investig. 1998;5:149–155. doi: 10.1016/s1071-5576(97)00116-0. [DOI] [PubMed] [Google Scholar]
- 31.Leondires MP, Hu ZZ, Dong J, Tsai-Morris CH, Dufau ML. Estradiol stimulates expression of two human prolactin receptor isoforms with alternative exons-1 in T47D breast cancer cells. J Steroid Biochem Mol Biol. 2002;82:263–268. doi: 10.1016/s0960-0760(02)00184-x. [DOI] [PubMed] [Google Scholar]


