Abstract
Using type 1a angiotensin receptor (AT1a) receptor-deficient (Agtr1a−/−) mice and in vivo autoradiography, we tested the hypothesis that intracellular uptake of ANG II in the kidney and adrenal glands is primarily mediated by AT1a receptors and that the response is regulated by prevailing endogenous ANG II. After pretreatment of wild-type (Agtr1a+/+) and Agtr1a−/− mice (n = 6–9 each group) with or without captopril (25 mg·kg−1 ·day−1) or losartan (10 mg·kg−1 ·day−1) for 2 wk, [125I]Val5-ANG II was infused for 60 min. Intracellular uptake of [125I]Val5-ANG II was determined by quantitative in vivo autoradiography after washout of circulating [125I]Val5-ANG II. Basal intracellular ANG II levels were 65% lower in the kidney (P < 0.001), but plasma ANG II levels were threefold higher, in Agtr1a−/− than wild-type mice (P < 0.01). Although plasma [125I]Val5-ANG II levels were similar, urinary excretion of [125I]Val5-ANG II was fourfold higher in Agtr1a−/− mice (P < 0.001). By contrast, intracellular [125I]Val5-ANG II levels were ~80% lower in the kidney and adrenal glands of Agtr1a−/− mice (P < 0.01). Captopril decreased endogenous plasma and renal ANG II levels (P < 0.01) but increased intracellular uptake of [125I]Val5-ANG II in the kidney and adrenal glands of wild-type and Agtr1a−/− mice (P < 0.01). Losartan largely blocked renal and adrenal uptake of [125I]Val5-ANG II in wild-type and Agtr1a−/− mice. Thus 80% of intracellular ANG II uptake in the kidney and adrenal glands is mediated by AT1a receptors, whereas AT1b receptor- and other non-receptor-mediated mechanisms account for 20% of the response. Our results suggest that AT1a receptor-mediated uptake of extracellular ANG II may play a physiological role in the kidney and adrenal glands.
Keywords: AT1 receptor-mediated endocytosis, in vivo autoradiography, losartan
It is well recognized that local ANG II levels are much higher in many target tissues, such as the kidney and adrenal glands, than in the plasma (3, 5, 20, 34). High tissue ANG II levels in tissues is generally thought to be primarily due to increased local formation, because all major components of the renin-angiotensin system, including angiotensinogen, renin, and angiotensin-converting enzyme (ACE), are expressed in these tissues (3, 20, 24). However, it recently became clear that the receptor-mediated uptake of circulating and/or extracellular ANG II may also contribute to high levels of ANG II in tissues (17, 25, 34, 36). We and others have shown that ANG II levels in rat and mouse kidneys were increased by chronic ANG II infusion (17, 34, 36). Because local ANG II formation is physiologically regulated by a negative-feedback mechanism by ANG II via an angiotensin type 1 (AT1) receptor-mediated mechanism, intrarenal ANG II levels are generally expected to fall, rather than increase, during long-term ANG II administration. Furthermore, blockade of ANG II receptors with AT1 receptor antagonists, which increase renin release (and, therefore, ANG II generation) and inhibit ANG II binding to cell surface AT1 receptors, prevents the increases in intrarenal ANG II induced by long-term ANG II infusion (17, 25, 34, 36). These studies strongly suggest that high tissue ANG II levels in the kidney during long-term ANG II administration are likely due to AT1 receptor-mediated endocytosis of extracellular ANG II, rather than increases in local ANG II synthesis. By contrast, whether adrenal glands also take up extracellular ANG II via the AT1 receptor-mediated mechanism is less clear, because inconsistent results have been reported (23, 25, 37).
Two isoforms of AT1 receptors, AT1a and AT1b, are expressed in rodent kidneys and adrenal glands (9, 19, 31). It is not known which AT1 receptor plays a predominant role in mediating ANG II uptake in rodents, nor is it clear whether the AT1b receptor would assume the role of the AT1a receptor when the former is absent. Previous studies in rats or pigs with use of AT1 receptor antagonists were unable to determine the precise role of AT1a and AT1b receptors, because these antagonists block both receptors with similar specificities and affinities (25, 34, 37). Although we recently showed that intracellular ANG II levels were significantly reduced in AT1a receptor-deficient (Agtr1a−/−) mice compared with wild-type mice, it is not certain whether this result was due to reduced intracellular ANG II synthesis or receptor-mediated uptake of extracellular ANG II (17). Moreover, markedly elevated extracellular ANG II due to global deletion of AT1a receptors in these mice may alter the kinetics of AT1b receptor- or other non-receptor-mediated ANG II uptake in the kidney and other tissues.
In the present study, we used quantitative in vivo autoradiography with [125I]Val5-ANG II as a novel tool (30, 31) and Agtr1a−/− mice (17) as a unique animal model to determine the relative contribution of AT1a and AT1b receptors to intracellular uptake of circulating and/or extracellular ANG II in the kidney and adrenal glands. Because Agtr1a−/− mice exhibit markedly elevated circulating and whole kidney ANG II levels due to combined effects of reduced ANG II receptor occupancy and increased renin expression (14, 17, 21, 22), we treated wild-type and Agtr1a−/− mice for 2 wk with the ACE inhibitor captopril to suppress endogenous ANG II production before the experiment was performed. This approach helped us determine whether AT1 receptor-mediated intracellular uptake of ANG II is also modulated by prevailing ANG II levels in the mouse kidney and adrenal glands.
METHODS AND MATERIALS
Animals and genotyping
Adult male wild-type C57BL/6J (Agtr1a+/+) mice (10 wk of age; Jackson Laboratories) were maintained on a normal rodent chow and had free access to tap water. Agtr1a−/− mice were bred in our laboratory from a pair of heterozygous Agtr1a+/− mice initially purchased from Jackson Laboratories (17). These mice were deposited by Drs. Oliver Smithies of the University of North Carolina (B6.129P2-Agtr1tm1Unc/J) (14, 21, 22). Male Agtr1a−/− mice were genotyped according to a standard protocol provided by the vendor (stock no. 002682) (14, 17). All animal protocols were approved by the Institutional Animal Care and Use Committee of the Henry Ford Health System. Genotyping of Agtr1a−/− mice was performed by PCR duplicates on tail DNA samples (14, 17). PCR primers for genotyping the AT1a receptor were purchased from Invitrogen, and their respective sequences are neo generic primers, which amplify a 280-bp DNA product from the bacterial neomycin resistance gene [5′-CTT ggg Tgg AgA ggC TAT TC-3′(oIMR0013) and 5′-Agg TgA gAT gAC Agg AgA TC-3′(oIMR0014)], and Agtr1a wild-type primers, which together amplify a 483-bp DNA product from the wild-type allele [5′-TgA gAA CAC CAA TAT CAC Tg-3′ (0IMR0738) and 5′TTC gTA gAC Agg CTT gAg-3′ (oIMR0739)]. Cycling conditions were set for denaturation at 94°C for 3 min, annealing at 55°C for 45 s, and extension at 72°C for 45 s over 35 cycles (14, 17).
Measurement of systolic blood pressure
Adult male wild-type and Agtr1a−/− mice (~25 g, 10–12 wk old) were trained for systolic blood pressure (SBP) measurements once per day for ·days before the experiment was begun (17, 34). Basal SBP was determined using a computerized tail-cuff method (Visitech, Cary, NC), as described elsewhere (17, 34). Intra-arterial blood pressure was measured in anesthetized wild-type and Agtr1a−/− mice before and after [125I]Val5-ANG II infusion, as described previously for rats (15, 30).
Measurement of basal urine and urinary sodium and potassium excretion
To determine basal urinary excretory responses, wild-type and Agtr1a−/− mice were housed individually in metabolic cages to ensure collection of urine samples for measurements of 24-h urine flow rate and urinary excretion of sodium and potassium (UNaV and UKV), as we described previously for rats (32, 34) and mice (17). Special care was taken to train mice individually in the metabolic cage for ·days before measurement of drinking and collection of urine to minimize the effects of stress to the animals due to these procedures. Twenty-four-hour drinking and urine output were measured gravimetrically and plasma and urinary sodium and potassium concentrations were determined by flame photometry, as previously described (17, 32, 34).
Measurements of intracellular uptake of [125I]Val5-ANG II in the kidney and adrenal glands
After basal SBP and 24-h urine volume were measured in wild-type and Agtr1a−/− mice, the animals were anesthetized with pentobarbital sodium (50 mg/kg ip), and a catheter (PE-10) was inserted into the jugular vein for intravenous infusion of the radioligand [125I]Val5-ANG II (specific activity 2,176 Ci/mmol; provided by Dr. Robert Speth, University of Mississippi) or into the carotid artery for measurement of mean arterial blood pressure and collection of arterial blood samples for measurement of plasma [125I]Val5-ANG II concentrations, as described elsewhere (29, 30). After a 30-min equilibration period, [125I]Val5-ANG II was infused into each mouse at an identical rate of ~10 μCi/25 g body wt (equivalent to ~10 nM radiolabeled Val5-ANG II) for 60 min. With this infusion rate, steady-state plasma levels of [125I]Val5-ANG II were reached by 10 min, whereas steady-state tissue levels of the radioligand were obtained within 30 min, as described previously (25–27, 29, 30). A catheter was also inserted into the urinary bladder for determination of [125I]Val5-ANG II concentrations in urine. At the end of infusion, a blood sample was collected from the carotid catheter and used for measurements of plasma [125I]Val5-ANG II directly by a gamma counter (29, 30) and unlabeled ANG II concentrations by ELISA (16–18, 35). The animals were perfused with an acidic buffer solution in 150 NaCl solution for 5 min to dissociate cell surface receptor-bound [125I]Val5-ANG II and wash out blood and extracellular fluid [125I]Val5-ANG II. The kidneys and adrenal glands were removed, and ANG II was extracted for measurement of [125I]Val5-ANG II activity, expressed as counts per minute (cpm) per gram kidney weight or per adrenal, using a gamma counter (15, 25–27, 29–32). The measured radioactivity represents the intracellular uptake of [125I]Val5-ANG II in the kidney and adrenal glands.
Measurements of endogenous plasma, whole kidney, and intracellular ANG II
Unlabeled plasma, whole kidney, and intracellular ANG II levels were measured by a sensitive ANG II enzyme immunoassay, as described recently (16–18, 35). Briefly, trunk blood samples were collected into chilled tubes containing a mixed-inhibitor cocktail (5 mM EDTA, 10 μM pepstatin A-o-phenanthroline, 10 μM captopril, 10 mg/ml bestatin, and 1 mg/ml aprotinin) for measurement of plasma ANG II, as described previously (15, 17, 34, 35). For measurement of whole kidney ANG II, one-half of each kidney from all mice was sliced into two parts, one of which was saved for autoradiography (see below). The remaining kidney tissues were weighed and homogenized in an ANG II extraction buffer containing 20 mM Tris base, 10 mM EDTA, 5 mM EGTA, 5 mM β-mercaptoethanol, 50 μg/ml phenyl-methylsulfonyl fluoride, and 1 μg/ml aprotinin, as described elsewhere (15, 17, 34, 35). ANG II was extracted in a phenyl-bonded solid-phase peptide extraction column (Bond Elut-C18, Varian), vacuum dried overnight, and reconstituted in ANG II assay buffer before ELISA of ANG II (15, 17, 34, 35).
Effects of ACE inhibition with captopril and AT1 receptor blockade with losartan on intracellular uptake of [125I]Val5-ANG II
Animals and humans treated with AT1 receptor blockers (ARBs) normally exhibit elevated circulating and/or extracellular ANG II levels (4, 17, 25, 34). This response is due to the occupancy of cell surface receptors by the antagonists and increased renin expression following removal of AT1 receptor-mediated negative-feedback regulation by ANG II (14, 22, 29, 30). To exclude the influence of elevated endogenous circulating and extracellular ANG II levels on intracellular uptake of [125I]Val5-ANG II in Agtr1a−/− mice, additional groups of wild-type and Agtr1a−/− mice were treated with the ACE inhibitor captopril (25 mg·kg−1 ·day−1 po) for 2 wk to suppress endogenous ANG II formation (25, 26, 30). To determine whether AT1b receptors play a compensatory role in mediating [125I]Val5-ANG II uptake in the mouse kidney and adrenal glands, wild-type and Agtr1a−/− mice were treated with the ARB losartan (10 mg·kg−1 ·day−1 po) for 2 wk (17). We previously showed that losartan completely blocked AT1 receptors in rat kidneys and adrenal glands (29, 30, 33). After treatment, mice were anesthetized and infused with [125I]Val5-ANG II at the rate described above.
Effects of ANG II or losartan on plasma aldosterone concentrations in wild-type and Agtr1a−/− mice
To determine whether the changes in intracellular uptake of [125I]Val5-ANG II in adrenal glands and UNaV and UKV are associated with the changes in plasma aldosterone concentrations in wild-type and Agtr1a−/− mice, blood samples were collected into tubes containing a protease inhibitor cocktail at the end of the experiment (15, 17, 34). After centrifugation, plasma samples were collected and purified by extraction, as described for ANG II (15, 17, 34). Plasma aldosterone concentrations were measured using a sensitive aldosterone ELISA kit (Cayman). The assay is 100% specific for aldosterone, and the antibody does not cross-react with other adrenocortical hormones or peptides, such as corticosterone, androsterone, testosterone, estrogen, or cortisol.
Autoradiographic mapping of intracellular uptake of [125I]Val5-ANG II in the kidney and adrenal glands
In addition to direct counting of [125I]Val5-ANG II radioactivity after perfusion with an acidic buffer to wash out extracellular radioligands as an index of intracellular ANG II uptake in the kidney and adrenal glands, quantitative in vivo autoradiography was used to visualize and determine intracellular [125I]Val5-ANG II uptake in wild-type and Agtr1a−/− mice. This approach is similar to quantitative in vivo or in vitro autoradiographic mapping of ANG II receptors in the kidney with use of [125I]Sar1, Ile8-ANG II or [125I]ANG II, as we described previously (13, 15, 17, 29, 30, 33). Briefly, immediately after infusion of [125I]Val5-ANG II and perfusion-wash procedures, one-half of each kidney and one adrenal gland from each mouse were snap frozen in isopentane on dry ice at the time the animal was killed. Frozen kidney and adrenal sections (20 μm thick) were cut on a cryostat and dried under reduced pressure overnight and then exposed to X-ray films for 3 days. After exposure, sections were developed, and autoradiographs were visualized and analyzed by microcomputerized densitometry (MCID, Imaging Research, ON, Canada) (13, 15, 17, 29, 30, 33).
Statistical analysis
Values are means ± SE. Differences in the same parameters between groups of wild-type and Agtr1a−/− mice treated with or without captopril or losartan were compared using unpaired t-test or one-way ANOVA followed by post hoc Newman-Keuls multiple comparison test if P < 0.05. Significance was set at P < 0.05.
RESULTS
Basal blood pressure and renal excretory functional phenotypes and their responses to long-term ACE inhibition or AT1 receptor blockade
Table 1 summarizes basal SBP and renal excretory functional phenotypes and their responses to long-term ACE inhibition or AT1 receptor blockade in wild-type and Agtr1a−/− mice. Basal SBP was ~25 mmHg lower in age-and body weight-matched Agtr1a−/− than wild-type mice. Deletion of AT1a receptors in Agtr1a−/− mice was associated with ~4-fold increases in 24-h urine and 1.7-fold increases in UNaV compared with wild-type values. A 2.5-fold increase in 24-h water intake was observed in Agtr1a−/− mice, in parallel with increased 24-h urine output. Conversely, 24-h UKV was significantly lower in Agtr1a−/− mice. Long-term (2 wk) treatment of wild-type mice with captopril decreased SBP by 35 mmHg, which was associated with small, but significantly increased, diuresis and natriuresis (UNaV) responses without altering 24-h drinking or UKV. Losartan also reduced SBP significantly, but the hypotensive effect of losartan was smaller than that of captopril (Table 1). With the exception of a small increase in 24-h urine excretion rate and a decrease in 24-h UKV, losartan also did not alter UNaV or drinking. In Agtr1a−/− mice, captopril further decreased SBP from basal levels without altering 24-h drinking and urine excretion or UNaV (Table 1). Interestingly, losartan also further reduced SBP to a similar extent in Agtr1a−/− mice treated with captopril.
Table 1.
Body weight, SBP, drinking, urinary excretion, UNaV, and UKV responses to captopril and losartan
| Wild-Type
|
Agtr1a−/−
|
|||||
|---|---|---|---|---|---|---|
| Responses | Control | Captopril | Losartan | Control | Captopril | Losartan |
| Body wt, g | 24.4±0.9 | 24.4±0.8 | 24.8±0.6 | 23.7±0.7 | 24.2±0.6 | 26.1±0.8 |
| SBP, mmHg | 118±2 | 83±4b | 108±3b,d | 93±2f | 86±2a | 85±2a,f |
| Drinking, ml/24 h | 3.8±0.3 | 3.4±0.4 | 3.9±0.2 | 9.6±0.8f | 9.7±0.6f | 7.7±0.7a,c,f |
| Urine, ml/24 h | 1.3±0.2 | 1.9±0.3a | 2.0±0.2a | 5.1±0.8f | 6.2±0.6f | 4.6±0.6c,f |
| UNaV, μmol/24 h | 199.0±9.0 | 233.3±8.6a | 188.6±6.2c | 328.7±7.1f | 292.8±15.5f | 285.7±19.5f |
| UKV, μmol/24 h | 517.2±22.4 | 521.8±14.3 | 320.2±10.7a,c | 393.8±11.9e | 322.7±14.6f | 511.1±41.8a,c,f |
Values are means ± SE. Basal systolic blood pressure (SBP), drinking, and renal excretory phenotypes and their responses to 2 wk of treatment with the angiotensin-converting enzyme inhibitor captopril (25 mg · kg−1·day−1 po) and the type 1 angiotensin (AT1) receptor antagonist losartan (10 mg · kg−1 ·day−1 po) were measured in wild-type and AT1a receptor-deficient (Agtr1a−/−) mice. UNaV and UKV, urinary Na+ and K+ excretion. Differences in the same response between different groups in the same strain of mice were analyzed by 1-way ANOVA:
P < 0.05;
P < 0.01 vs. control;
P < 0.05;
P < 0.01 vs. captopril.
Differences in the same response between wild-type and Agtr1a−/− mice were analyzed by unpaired t-test:
P < 0.05;
P < 0.01 vs. wild-type.
Basal plasma and renal intracellular ANG II in wild-type and Agtr1a−/− mice
Global deletion of AT1a receptors was associated with markedly increased plasma and decreased intracellular ANG II levels in the kidney of Agtr1a−/− mice compared with wild-type mice (Fig. 1). Plasma ANG II levels were more than threefold higher in Agtr1a−/− than wild-type mice: 208.9 ± 68.9 vs. 648.7 ± 66.9 pg/ml (P < 0.001). By contrast, intracellular ANG II levels in Agtr1a−/− mice were ~35% of those in wild-type mice: 154.0 ± 35.5 vs. 55.4 ± 10.0 pg/100 mg kidney wt (P < 0.01).
Fig. 1.

Basal plasma and intracellular ANG II levels in kidneys of adult male wild-type and type 1 angiotensin receptor-deficient (Agtr1a−/−) mice (n = 6–9 each group). Intracellular ANG II levels in the kidney were determined after kidneys were perfused with an acidic buffer to clear blood and cell surface receptor-bound ANG II. KW, kidney weight. **P < 0.01 vs. wild-type.
Plasma and urine concentrations and urinary excretion of [125I]Val5-ANG II in wild-type and Agtr1a−/− mice
The concentrations of plasma [125I]Val5-ANG II were statistically similar in wild-type and Agtr1a−/− mice: 20,753 ± 2,247 and 23,714 ± 1,841 cpm/ml × 103, respectively [P = not significant (NS)]. The urinary concentrations of [125I]Val5-ANG II were also similar in wild-type and Agtr1a−/− mice: 70,435 ± 2,742 and 80,917 ± 2,968 cpm/ml × 103, respectively (P = NS). However, since Agtr1a−/− mice excreted three times more urine than wild-type mice, the urinary excretion of [125I]Val5-ANG II was four times higher in Agtr1a−/− than wild-type mice: 26,437 ± 4,348 vs. 6,358 ± 3,160 cpm/min (P < 0.001).
Intracellular uptake of [125I]Val5-ANG II in kidneys of wild-type and Agtr1a−/− mice
Despite similar concentrations of circulating [125I]Val5-ANG II in both strains of mice, the extent of intracellular uptake of infused [125I]Val5-ANG II in wild-type mice was quite different from that in Agtr1a−/− mice. Figure 2 shows quantitative in vivo autoradiographs of intrarenal uptake of [125I]Val5-ANG II in a representative wild-type and Agtr1a−/− mouse kidney and with direct results of gamma counting. Intracellular uptake of [125I]Val5-ANG II was markedly decreased in Agtr1a−/− mouse kidney compared with wild-type control: 1,771.8 ± 246.6 vs. 484.8 ± 83.4 cpm/g kidney wt × 103 (P < 0.001).
Fig. 2.

A and B: visualization of intracellular uptake of [125I]Val5-ANG II in kidneys of wild-type and Agtr1a−/− mice (n = 6–9 each group) by quantitative in vivo autoradiography. Intracellular [125I]Val5-ANG II in the kidney was determined after kidneys were perfused with an acidic buffer to clear blood and cell surface receptor-bound ANG II. Levels of [125I]Val5-ANG II uptake are keyed to the color calibration bar, with red representing the highest (H) and blue the background (L) level of uptake. C: quantitated intracellular [125I]Val5-ANG II. **P < 0.01 vs. wild-type.
Effects of captopril or losartan on intracellular uptake of [125I]Val5-ANG II in kidneys of wild-type and Agtr1a−/− mice
To determine whether endogenous ANG II levels in the circulation and the kidney influence intracellular uptake of [125I]Val5-ANG II in wild-type and Agtr1a−/− mice, all animals were pretreated with captopril (25 mg·kg−1·day−1 po) for 2 wk to suppress endogenous ANG II production before the experiment began. As shown in Fig. 3, captopril markedly decreased unlabeled plasma (18.3 ± 3.3 and 129.6 ± 13.7 pg/ml in wild-type and Agtr1a−/− mice, respectively, P < 0.01) and intracellular ANG II levels in the kidneys (39.2 ± 7.6 and 10.2 ± 2.9 pg/100 mg kidney wt in wild-type and Agtr1a−/− mice, respectively, P < 0.01). By contrast, captopril raised plasma concentrations of [125I]Val5-ANG II in wild-type and Agtr1a−/− mice (Fig. 4, top). Urinary [125I]Val5-ANG II excretion was not altered in wild-type mice but was decreased by captopril in Agtr1a−/− mice (Fig. 4, bottom). The effects of captopril and losartan on intracellular uptake of [125I]Val5-ANG II in wild-type and Agtr1a−/− mouse kidneys are visualized in autoradiographs in Fig. 5, and the respective quantitated data are shown in Fig. 6. As under basal conditions, intracellular uptake of [125I]Val5-ANG II was dominant in the cortex, although the medulla also took up [125I]Val5-ANG II (Fig. 5). Captopril increased intracellular uptake of [125I]Val5-ANG II by ~40% in the kidneys of wild-type mice (2,583 ± 349 cpm/g kidney wt × 103) and by 116% in the kidneys of Agtr1a−/− mice (1,087 ± 387 cpm/g kidney wt × 103, P < 0.01) compared with their respective controls. The kidney-to-plasma [125I]Val5-ANG II concentration ratios in wild-type and Agtr1a−/− mice (Table 2) are consistent with the autoradiographic or direct-counting results. Since Agtr1a−/− mice also express AT1b receptors, we investigated whether AT1b receptors mediate this response in Agtr1a−/− mice by pretreating the animals with losartan for 2 wk. Losartan largely abolished intracellular uptake of [125I]Val5-ANG II in the kidneys of wild-type and Agtr1a−/− mice (Figs. 5 and 6).
Fig. 3.
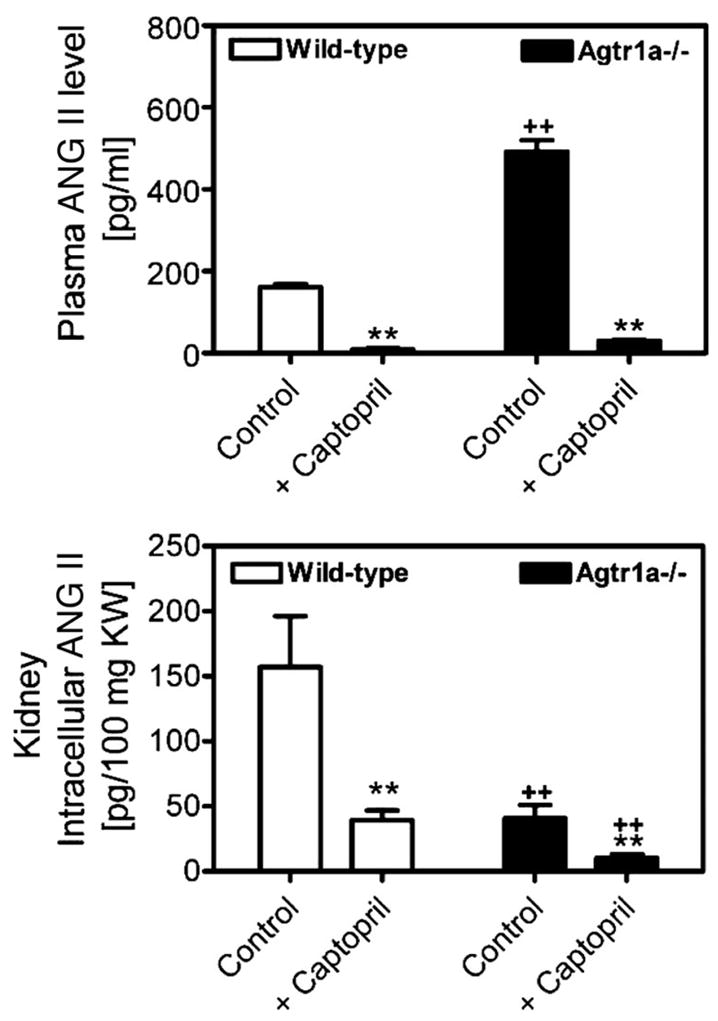
Effects of long-term (2 wk) angiotensin-converting enzyme (ACE) inhibition with captopril (25 mg · kg−1·day−1 po) on endogenous plasma and renal intracellular ANG II levels in wild-type and Agtr1a−/− mice (n = 6–9 each group). Intracellular ANG II in kidneys was determined as described in Fig. 1 legend. **P < 0.01 vs. control in the same strain of mice. ++P < 0.01 vs. corresponding wild-type control or captopril-treated mice.
Fig. 4.

Circulating and urinary concentrations of [125I]Val5-ANG II in wild-type and Agtr1a−/− mice (n = 6–9 each group). **P < 0.01 vs. control in the same strain of mice. ++P < 0.01 vs. corresponding wild-type control or captopril-treated mice.
Fig. 5.

Visualization of intracellular uptake of [125I]Val5-ANG II in kidneys of wild-type and Agtr1a−/− mice (n = 6–9 each group) treated chronically (2 wk) with captopril (25 mg·kg−1·day−1 po) or losartan (10 mg·kg−1·day−1 po) by quantitative in vivo autoradiography. Intracellular [125I]Val5-ANG II in the kidney was determined after kidneys were perfused with an acidic buffer to clear blood and cell surface receptor-bound ANG II. Levels of [125I]-Val5-Ang II uptake are keyed to color calibration bar, as described in Fig. 2 legend.
Fig. 6.

Effects of long-term ACE inhibition with captopril and AT1 receptor blockade with losartan on intracellular uptake of [125I]Val5-ANG II in kidneys of wild-type and Agtr1a−/− mice (n = 6–9 each group). *P < 0.05; **P < 0.01 vs. control mice in the same strain. ##P < 0.01 vs. wild-type or Agtr1a−/− mice treated with captopril. ++P < 0.01 vs. wild-type mice treated with captopril.
Table 2.
Effect of captopril on tissue-to-plasma [125I]Val5-ANG II concentration ratios
| Wild-Type
|
Agtr1a−/−
|
|||
|---|---|---|---|---|
| Tissue | Control | Captopril | Control | Captopril |
| Kidney | 0.88±0.10 | 1.14±0.04* | 0.20±0.02† | 0.42±0.03*† |
| Adrenal | 0.012±0.001 | 0.016±0.001* | 0.002±0.001† | 0.004±0.001*† |
| Urine | 3.40±0.85 | 1.10±0.26* | 3.38±0.73 | 0.70±0.13*† |
Values are means ± SE of 6–9 mice for each group. Captopril-treated mice received captopril (25 mg · kg−1·day−1 po) for 2 wk.
P < 0.01 vs. respective control.
P < 0.01 vs. respective wild-type control or captopril-treated mice.
Effects of captopril or losartan on intracellular uptake of [125I]Val5-ANG II in adrenal glands of wild-type and Agtr1a−/− mice
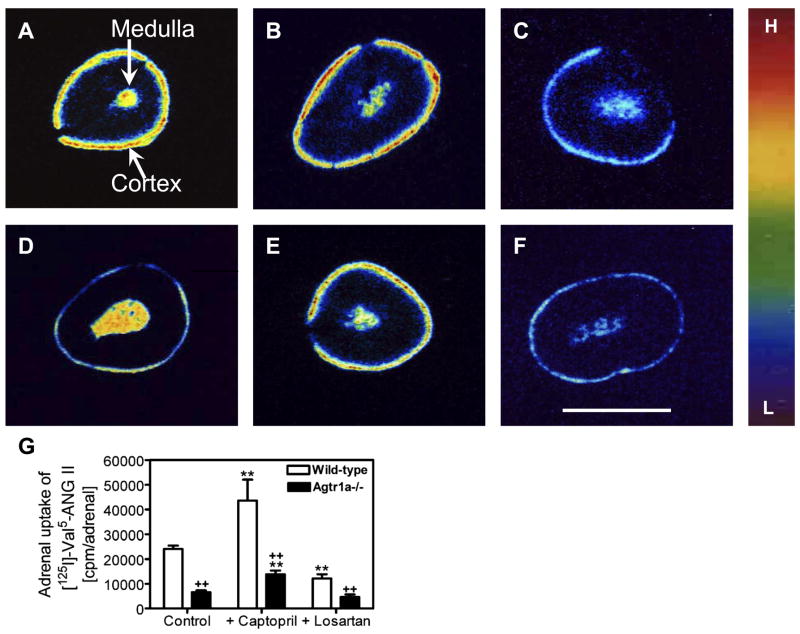
Figure 7 shows [125I]Val5-ANG II uptake in the adrenal cortex and medulla of wild-type and Agtr1a−/− mice and the effects of ACE inhibition with captopril and AT1 receptor blockade with losartan. Basal uptake was higher in the cortex of wild-type (Fig. 7A) than Agtr1a−/− (Fig. 7D) mice. Interestingly, uptake also occurred in the adrenal medulla in wild-type and Agtr1a−/− mice. Uptake of [125I]Val5-ANG II was clearly increased in the adrenal cortex by captopril (Fig. 7, B and E), whereas losartan markedly reduced this uptake in the cortex and medulla of wild-type and Agtr1a−/− mice (Fig. 7, C and F). A direct count of intracellular uptake of [125I]Val5-ANG II in adrenal glands of wild-type and Agtr1a−/− mice is shown in Fig. 7G. Table 2 summarizes adrenal-to-plasma [125I]Val5-ANG II concentration ratios in wild-type and Agtr1a−/− mice. Under basal conditions, [125I]Val5-ANG II uptake was 3.6-fold higher in adrenal glands of wild-type than Agtr1a−/− mice: 24,054 ± 1,373 vs. 6,597 ± 738 cpm/adrenal (P < 0.01). Captopril increased adrenal uptake of [125I]Val5-ANG II by ~80% in wild-type mice (43,593 ± 8,479 cpm/adrenal, P < 0.01 vs. wild-type controls) and by 109% in Agtr1a−/− mice (13,803 ± 1,529 cpm/adrenal, P < 0.01 vs. Agtr1a−/− controls). By contrast, losartan significantly decreased adrenal uptake of [125I]Val5-ANG II in wild-type (12,122 ± 1,683 cpm/adrenal, P < 0.01 vs. wild-type controls) and Agtr1a−/− (4,613 ± 1,080 cpm/adrenal, P < 0.05 vs. Agtr1a−/− controls) mice (Fig. 7G).
Fig. 7.
Visualization of intracellular uptake of [125I]Val5-ANG II in adrenal glands of wild-type and Agtr1a−/− mice treated chronically (2 wk) with captopril (25 mg · kg−1·day−1 po) or losartan (10 mg · kg−1·day−1 po) by quantitative in vivo autoradiography. Intracellular [125I]Val5-ANG II uptake in adrenal glands was determined after the mice were perfused with an acidic buffer to clear blood and cell surface receptor-bound ANG II. A: representative adrenal gland from a wild-type control mouse. B: representative adrenal gland from a wild-type mouse treated with captopril. C: representative adrenal gland from a wild-type mouse treated with losartan. D: representative adrenal gland from a control Agtr1a−/− mouse. E: representative adrenal gland from an Agtr1a−/− mouse treated with captopril as in wild-type mouse. F: representative adrenal gland from an Agtr1a−/− mouse treated with losartan as in wild-type mouse. Scale bar, 1 mm. G: results from direct counting of [125I]-Val5-ANG II radioactivity from each group of 6–9 wild-type or Agtr1a−/− mice. Levels of adrenal [125I]Val5-ANG II uptake are keyed to the color calibration bar, as described in Fig. 2 legend. **P < 0.01 vs. respective wild-type or Agtr1a−/− control. ++P < 0.01 vs. respective wild-type captopril- or losartan-treated mice.
Effects of ANG II and losartan on plasma aldosterone concentrations in wild-type and Agtr1a−/− mice
As shown in Fig. 8, basal plasma aldosterone levels were three times higher in wild-type than Agtr1a−/− mice: 250.3 ± 24.6 vs. 78.8 ± 24.7 pg/ml (P < 0.001). ANG II significantly increased plasma aldosterone levels in wild-type mice by 36% (341.2 ± 18.7 pg/ml, P < 0.05) but had no effect on plasma aldosterone in Agtr1a−/− mice (103.9 ± 13.7 pg/ml, P = NS vs. control). Losartan reduced plasma aldosterone in wild-type mice to a level significantly below control (112.1 ± 28.9 pg/ml, P < 0.001 vs. control or ANG II). However, losartan did not alter plasma aldosterone levels in Agtr1a−/− mice (121.3 ± 26.1 pg/ml, P = NS vs. control or ANG II).
Fig. 8.

Basal plasma aldosterone levels and their responses to ANG II stimulation and/or AT1 receptor antagonist losartan (Los) in wild-type and Agtr1a−/− mice (n = 6–9 each group). *P < 0.05 vs. wild-type control. ##P < 0.01 vs. wild-type treated with ANG II. ++P < 0.01 vs. wild-type control or wild-type treated with ANG II.
DISCUSSION
We and others previously showed that the kidney and adrenal glands are two key organs, among all major ANG II-targeted tissues, that express high levels of AT1 receptors (9, 13, 15, 17, 19, 31). Also, levels of endogenous ANG II are high in the kidney and adrenal glands compared with other tissues (3, 5, 20, 25, 32, 34). High tissue ANG II levels in the kidney and adrenal glands is generally considered to be primarily due to local formation, because all major components of the renin-angiotensin system are expressed in these tissues (3, 20, 24). However, it is also recognized that AT1 receptor-mediated uptake of circulating ANG II may play a significant role in maintaining high tissue ANG II levels in the kidney and adrenal glands (17, 25–27, 34, 37). In support of the role of receptor-mediated uptake or endocytosis of ANG II in the kidney and adrenal glands, most previous studies showed the differences of tissue ANG II levels between control and ANG II-infused animals treated with or without ARBs or demonstrated receptor-mediated endocytosis of ANG II in cultured rat or bovine adrenal glomerulosa cells or adrenal medullary chromaffin cells (2, 25, 27, 28, 34, 36). However, chronic administration of ARBs is known to markedly increase circulating and/or extracellular ANG II levels (4, 17, 34, 37), which may obscure the effects of receptor-mediated intracellular uptake of ANG II or promote non-receptor-mediated uptake of ANG II in the kidney (11). In a recent study, we found that the circulating ANG II levels were more than three times higher in Agtr1a−/− than in wild-type mice because of the global deletion of AT1a receptors (17), and others demonstrated increases in renin expression in the kidney (14, 21, 22). Unexpectedly, whole kidney ANG II levels were also higher, i.e., close to plasma levels, in these mice (17). These findings appear to be at odds with previous findings in rats and pigs that ARBs reduced ANG II uptake in the kidney (25, 27, 34, 36, 37). However, after circulating and/or extracellular ANG II was washed out by perfusion with an acidic buffer to clear all blood/extracellular and cell surface receptor-bound ANG II, intracellular ANG II levels were much lower in the kidneys of Agtr1a−/− than wild-type mice. These results suggest that elevated plasma and extracellular ANG II levels may have confounding effects on receptor-mediated ANG II uptake in tissues of Agtr1a−/− mice. This prompted us to further investigate the role of AT1a receptors and endogenous ANG II in regulating tissue ANG II uptake in the kidney and adrenal glands in the present study.
As a sequel to our recently published work (17), the present study provides strong evidence that AT1a receptors are largely responsible for mediating intracellular ANG II uptake in the mouse kidney and adrenal glands. By contrast, AT1b receptors play only a minor role in mediating receptor-mediated ANG II accumulation in these two tissues. Instead of using RIAs or ELISAs, which could not easily differentiate exogenously infused Val5-ANG II from endogenously produced Ile5-ANG II without, first, a sophisticated HPLC separation, we used quantitative in vivo autoradiography for the first time to visualize intracellular ANG II uptake in mouse kidney and adrenal glands in the present study. We infused [125I]Val5-ANG II into age- and weight-matched wild-type and Agtr1a−/− mice under identical rates and durations of infusion, as well as identical perfusion and processing procedures. The only difference between wild-type and Agtr1a−/− mice was full expression of AT1a receptors in wild-type mice and complete deletion of these receptors in Agtr1a−/− mice. Although plasma concentrations of [125I]Val5-ANG II were not significantly different between wild-type and Agtr1a−/− mice, the intracellular uptake of [125I]Val5-ANG II was markedly decreased, by ~70–80%, in the kidney and adrenal glands of Agtr1a−/− compared with wild-type mice (Figs. 2 and Fig. 7, Table 2). Autoradiographic imaging clearly reveals the differences in intracellular uptake of [125I]Val5-ANG II between wild-type and Agtr1a−/− mice (Figs. 2 and 7). One interesting observation is markedly lower intracellular endogenous ANG II levels in the kidney of Agtr1a−/− mice, which is similar to our recently reported results (Fig. 1) (17). However, we could not determine endogenous ANG II levels in adrenal glands of wild-type and Agtr1a−/− mice, because the mass of the adrenal glands was too small to be processed for meaningful RIA or ELISA measurements of ANG II.
The effects of ACE inhibition or AT1 receptor blockade on the renal uptake of [125I]ANG II have been studied in pig kidneys (25, 27). Van Kat et al. (25, 27) found four times higher [125I]ANG II concentration in the renal cortex than in the plasma, as represented by the renal cortex-to-plasma [125I]ANG II concentration ratios. [125I]ANG II uptake was not significantly altered by captopril but, rather, by the AT1 receptor antagonist eprosartan, suggesting that the AT1 receptors mediate the uptake of [125I]ANG II in pig kidneys and that the uptake was not altered by prevailing endogenous ANG II (25, 27). Interestingly, Van Kats et al. also did not find that captopril altered endogenous ANG II levels in pig kidneys, although eprosartan markedly decreased endogenous ANG II levels. However, the overall effects of eprosartan on renal cortex-to-plasma ratios of [125I]ANG II and endogenous ANG II levels are consistent with our recently published data on intracellular ANG II levels in Agtr1a−/− mice (17). In the present study, we found some differences between pig and mouse kidneys and between the effects of eprosartan in pigs and AT1a receptor deletion in Agtr1a−/− mice. 1) The kidney-to-plasma [125I]Val5-ANG II concentration ratio was much lower in mice than pigs, possibly because we perfused the kidneys with an acidic buffer to clear all plasma/extracellular and cell surface-bound [125I]Val5-ANG II (Table 2). Thus, in contrast to the results of Van Kats et al., which may represent the uptake in the entire kidney tissue (extracellular + intracellular fluid compartments), our results may represent only intracellular uptake of [125I]Val5-ANG II. 2) By comparing the differences in the kidney- and adrenal-to-plasma ratios of [125I]Val5-ANG II in wild-type and Agtr1a−/− mice, we were able to determine the role and extent of AT1a receptors in mediating ~80% of intracellular [125I]Val5-ANG II uptake in the kidney and adrenal glands. Approximately 20% of uptake was likely mediated by AT1b or other non-AT1 receptor-mediated mechanisms, because losartan reduced this uptake in Agtr1a−/− mice. 3) Long-term (2 wk) ACE inhibition with captopril (25 mg·kg−1·day−1 po) significantly increased intra-cellular uptake of the radioligand in wild-type and Agtr1a−/− mice (Table 2). Since captopril significantly lowered plasma and renal intracellular endogenous ANG II levels in wild-type and Agtr1a−/− mice (Fig. 4), our results suggest that prevailing endogenous ANG II may compete with systemically infused [125]Val5-ANG II for binding AT1 receptors or alter the kinetics of AT1b and non-receptor-mediated uptake and, therefore, regulate the AT1 receptor-mediated uptake of [125]Val5-ANG II in the kidney. The alternative explanation is that long-term ACE inhibition may upregulate AT1 receptor expression and, in Agtr1a−/− mice, may increase AT1b receptor expression, which may alter the kinetics of ANG II uptake in the kidney. This possibility needs to be investigated in further studies.
The roles of AT1a vs. AT1b receptors in mediating uptake of systemically infused [125]Val5-ANG II in adrenal glands have not been investigated previously. Whether AT1 receptors mediate the uptake of circulating ANG II in adrenal glands is not well understood. Radioreceptor binding and RT-PCR analyses have shown that the adrenal cortex of rodents expresses AT1a and AT1b receptors to a similar extent, whereas the kidney expresses predominantly AT1a receptors (9, 17, 19, 31). Study of [125]Val5-ANG II uptake in adrenal glands of wild-type and Agtr1a−/− mice would provide novel insights into the relative contribution of these two receptors. Van Kats et al. (25–27) demonstrated that pig adrenal glands took up considerable amounts of circulating [125I]ANG II and that the effects were blocked by ARB. By contrast, Zou et al. (37) showed that rat adrenal glands did not take up ANG II in uninephrectomized rats chronically infused with ANG II. In a recent study by Ortiz et al. (23), ANG II levels were increased in adrenal glands of ANG II-infused rats. Thus there are inconsistent results with respect to intra-adrenal ANG II levels during ANG II infusion. We believe that our present results on the adrenal uptake of [125I]Val5-ANG II in Agtr1a−/− mice via quantitative in vivo autoradiography may help resolve this inconsistency. In the present study, we demonstrated that adrenal glands took up more than four times as much plasma [125I]Val5-ANG II in wild-type as in Agtr1a−/− mice (Table 2, Fig. 7), suggesting a predominant role of AT1a receptors in mediating ANG II uptake in mouse adrenal glands. By contrast, we found that 20% of ANG II uptake was likely mediated by AT1b receptors or other non-receptor-mediated mechanisms in adrenal glands of Agtr1a−/− mice. Long-term ACE inhibition with captopril increased [125I]Val5-ANG II uptake in wild-type and Agtr1a−/− mice. Increased ANG II uptake in adrenal glands of Agtr1a−/− mice indicates that long-term ACE inhibition may increase AT1b receptor expression, which in turn augments ANG II uptake. Indeed, losartan effectively blocked the uptake of [125I]Val5-ANG II in adrenal glands of Agtr1a−/− mice, thus supporting a limited role of AT1b receptors in mediating this uptake.
Although the present study was not designed to directly address whether AT1a receptor-mediated intracellular uptake of ANG II plays a role in renal and adrenal physiology, our overall results on blood pressure, urinary water excretion, UNaV, UKV, and plasma aldosterone levels may be physiologically relevant. In the present study, the decreases in the intracellular uptake of ANG II in the kidney and adrenal glands of Agtr1a−/− mice were associated with a lower SBP, higher urine excretion and UNaV, and lower plasma aldosterone levels and UKV (Table 1, Fig. 8). These responses may be interpreted by two different cellular mechanisms. First, total deletion of AT1a receptors would lead to loss of all known responses to extracellular ANG II that are attributable to cell surface AT1 receptors (6, 7, 14, 17). This mechanism likely plays a predominant role in Agtr1a−/− mice (7, 14). However, intracellular uptake of ANG II was decreased in kidney and adrenal glands of Agtr1a−/− mice by 70–80% (Table 2). These marked differences in intracellular ANG II levels between wild-type and Agtr1a−/− mice suggest that intracellular ANG II may also play a role in the physiological regulation of blood pressure, aldosterone release, urinary excretion of water, UNaV, and UKV. Indeed, we and others previously showed that administration of ANG II directly into single proximal tubule cells, vascular smooth muscle cells, or cardiomyocytes induced intracellular calcium signaling via activation of AT1 receptors (10, 12, 35). Similarly, expression of intracellular ANG II-fusion proteins was found to stimulate cell growth and proliferation in hepatocytes or Chinese hamster ovary cells (1, 8). Whether intracellular ANG II plays a physiological role in adrenal glands is not known. However, the present study showed that basal intracellular ANG II and plasma aldosterone levels were decreased in parallel by ~70–80% in Agtr1a−/− mice (Fig. 8). This finding in part helps explain the higher UNaV and lower UKV under basal conditions in Agtr1a−/− mice, because ANG II is the major stimulus for aldosterone synthesis and release, and the primary action of aldosterone is to conserve sodium and excrete potassium. The fact that losartan did not have further effect on plasma aldosterone levels in Agtr1a−/− mice suggests that AT1b receptors may not play an important role in mediating aldosterone synthesis or release in mice.
In summary, the present study used quantitative in vivo autoradiography for the first time to determine the relative roles of AT1a and AT1b receptors in mediating the uptake of circulating [125I]Val5-ANG II in the kidneys and adrenal glands of wild-type and Agtr1a−/− mice. The uniqueness of our approach was that the extent and localization of AT1 receptor-mediated uptake of circulating [125I]Val5-ANG II could be quantified and visualized by in vivo autoradiography. Furthermore, the combination of Agtr1a−/− mice and/or losartan permitted us to evaluate the relative contribution of AT1a vs. AT1b receptors in mediating [125I]Val5-ANG II uptake in the kidney and adrenal glands. Our results provide direct evidence that AT1a receptors play a dominant role in the regulation of this biological/physiological process (70–80%) and that, in the absence of the AT1a receptor, AT1b receptors and/or other non-receptor-mediated mechanisms may play a limited role in mediating renal and adrenal uptake of circulating ANG II (20–30%). Thus AT1a receptor-mediated uptake of circulating ANG II may contribute to high ANG II levels in the kidney and adrenal glands of rodents under physiological conditions, and this response may be influenced by endogenous ANG II. The decreases in AT1a receptor-mediated uptake of extracellular ANG II in the kidney and adrenal glands may therefore play some role in increases in urinary water excretion and UNaV and decreases in aldosterone release and UKV and hypotension in Agtr1a−/− mice.
Acknowledgments
Portions of this work were presented at the 39th Annual Scientific Meeting and Exposition of the American Society of Nephrology, San Diego, CA, 2006 and the 61st Annual Fall Conferences and Scientific Sessions of the Council for High Blood Pressure Research, American Heart Association, Tucson, AZ, 2007.
GRANTS
This work was supported by a National Institute of Diabetes, Digestive, and Kidney Diseases Grant 5RO1 DK-067299 and in part by an American Heart Association Grant-in-Aid and a National Kidney Foundation of Michigan Grant-in-Aid to J. L. Zhuo. X. C. Li was supported by a National Kidney Foundation of Michigan Grant-in-Aid (2006–2007).
References
- 1.Baker KM, Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol. 2006;291:C995–C1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi C, Gutkowska J, Charbonneau C, Ballak M, Anand-Srivastava MB, De Lean A, Genest J, Cantin M. Internalization and lysosomal association of [125I]angiotensin II in norepinephrine-containing cells of the rat adrenal medulla. Endocrinology. 1986;119:1873–1875. doi: 10.1210/endo-119-4-1873. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell DJ, Krum H, Esler MD. Losartan increases bradykinin levels in hypertensive humans. Circulation. 2005;111:315–320. doi: 10.1161/01.CIR.0000153269.07762.3B. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension. 1995;25:1014–1020. doi: 10.1161/01.hyp.25.5.1014. [DOI] [PubMed] [Google Scholar]
- 6.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 7.Cervenka L, Mitchell KD, Oliverio MI, Coffman TM, Navar LG. Renal function in the AT1A receptor knockout mouse during normal and volume-expanded conditions. Kidney Int. 1999;56:1855–1862. doi: 10.1046/j.1523-1755.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 8.Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol. 2004;36:75–90. doi: 10.1016/j.yjmcc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 9.De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 10.De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32:976–982. doi: 10.1161/01.hyp.32.6.976. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol. 2005;288:F420–F427. doi: 10.1152/ajprenal.00243.2004. [DOI] [PubMed] [Google Scholar]
- 12.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79:765–772. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- 13.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XC, Campbell Ohishi MDJ, Shao Y, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced responses in renal microvasculature and glomerular mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1024–F1033. doi: 10.1152/ajprenal.00221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XC, Carretero OA, Navar LG, Zhuo JL. AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol. 2006;291:F375–F383. doi: 10.1152/ajprenal.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of angiotensin II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2007;293:F586–F593. doi: 10.1152/ajprenal.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol. 2007;293:C367–C378. doi: 10.1152/ajpcell.00463.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994;24:538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- 20.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 21.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in AT1A receptor-deficient mice: a role for AT1B receptors in blood pressure regulation. Am J Physiol Renal Physiol. 1997;272:F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 22.Oliverio MI, Madsen K, Best CF, Ito M, Maeda N, Smithies O, Coffman TM. Renal growth and development in mice lacking AT1A receptors for angiotensin II. Am J Physiol Renal Physiol. 1998;274:F43–F50. doi: 10.1152/ajprenal.1998.274.1.F43. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz RM, Graciano ML, Seth D, Awayda MS, Navar LG. Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am J Physiol Renal Physiol. 2007;293:F139–F147. doi: 10.1152/ajprenal.00504.2006. [DOI] [PubMed] [Google Scholar]
- 24.Tang SS, Jung F, Diamant D, Brown D, Bachinsky D, Hellman P, Ingelfinger JR. Temperature-sensitive SV40 immortalized rat proximal tubule cell line has functional renin-angiotensin system. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F435–F446. doi: 10.1152/ajprenal.1995.268.3.F435. [DOI] [PubMed] [Google Scholar]
- 25.Van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30:42–49. doi: 10.1161/01.hyp.30.1.42. [DOI] [PubMed] [Google Scholar]
- 26.Van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001;60:2311–2317. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens. 2001;19:583–589. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- 28.Wang JM, Llona I, De Potter WP. Receptor-mediated internalization of angiotensin II in bovine adrenal medullary chromaffin cells in primary culture. Regul Pept. 1994;53:77–86. doi: 10.1016/0167-0115(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhuo JL, Alcorn D, Harris PJ, Mendelsohn FA. Localization and properties of angiotensin II receptors in rat kidney. Kidney Int Suppl. 1993;42:S40–S46. [PubMed] [Google Scholar]
- 30.Zhuo JL, Alcorn D, McCausland J, Casley D, Mendelsohn FA. In vivo occupancy of angiotensin II subtype 1 receptors in rat renal medullary interstitial cells. Hypertension. 1994;23:838–843. doi: 10.1161/01.hyp.23.6.838. [DOI] [PubMed] [Google Scholar]
- 31.Zhuo J, Allen AM, Alcorn D, MacGregor D, Aldred GP, Mendelsohn FA. The distribution of angiotensin II receptors. In: Laragh JH, Brenner BM, editors. Hypertension: Pathology, Diagnosis and Management. 2. New York: Raven; 1995. pp. 1739–1762. [Google Scholar]
- 32.Zhuo JL, Ohishi M, Mendelsohn FA. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension. 1999;33:347–353. doi: 10.1161/01.hyp.33.1.347. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo JL, Song K, Abdelrahman A, Mendelsohn FA. Blockade by intravenous losartan of AT1 angiotensin II receptors in rat brain, kidney and adrenals demonstrated by in vitro autoradiography. Clin Exp Pharmacol Physiol. 1994;21:557–567. doi: 10.1111/j.1440-1681.1994.tb02555.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. ANG II accumulation in rat renal endosomes during ANG II-induced hypertension: role of AT1 receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular angiotensin II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 37.Zou LX, Imig JD, von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]