Abstract
In the present study, high levels of peptidylglycine α-amidating monooxygenase (PAM), which catalyzes the two-step formation of bioactive α-amidated peptides from their glycine-extended precursors, have been found in the uterus. Expression of PAM was evaluated in the uterus of intact cycling adult female rats and after experimental manipulation of the estrogen status of the rats. During the estrous cycle, PAM mRNA levels exhibited striking changes inversely related to the physiological variations of plasma estrogen levels. The levels of PAM transcripts changed markedly during the estrous cycle, reaching the highest levels at metestrus. There was a 15-fold increase in the abundance of PAM mRNA between metestrus and proestrus. Chronic treatment of ovariectomized rats with 17β-estradiol decreased PAM mRNA levels to values comparable with those found in intact rats at proestrus. Progesterone was without effect on PAM mRNA levels, indicating that the effect was specific for estradiol. In situ hybridization studies were conducted to determine the tissue disposition and cell types expressing PAM. High levels of PAM mRNA were localized in the endometrium at the level of luminal and glandular cells. A weak signal was observed in stromal cells, and the myometrium cells were negative. 17β-Estradiol treatment induced an overall decrease of the hybridization signal, as compared with ovariectomized rats. These results demonstrate the presence of high levels of PAM in the uterus and indicate that estrogens are involved in regulating the expression of the enzyme in this tissue. However, the present study provides no information regarding whether this regulation takes place at the level of transcription or influences mRNA stability.
Small bioactive peptides are derived from larger precursor proteins following a series of post-translational cleavage and modification steps (1, 2). For many of these peptides, full biological activity depends on α-amidation of the carboxyl-terminal amino acid. The two-step α-amidation reaction is catalyzed by the bifunctional enzyme peptidylglycine α-amidating monooxygenase (PAM; EC 1.14.17.3). The PAM precursor protein executes both of the enzymatic activities involved in peptide amidation (3, 4). Soluble and membrane-associated PAM activities have been identified, and their distribution is tissue specific (5). Alternative splicing of the single-copy PAM gene and post-translational processing of the PAM proteins both contribute to the generation of tissue-specific forms of PAM (6–9).
In their studies on the distribution of soluble and membrane-associated PAM activity and PAM mRNA in different rat tissues, Braas et al. (5) showed that the uterus contained extremely low levels of PAM. Recently, we showed that estrogen status is involved in the regulation of PAM expression in the rat anterior pituitary gland (10). We demonstrated that PAM mRNA levels showed changes inversely related to the physiological variations of plasma estrogen levels during the estrous cycle (10). Chronic treatment of ovariectomized (OVX) rats with 17β-estradiol decreased PAM mRNA levels to values comparable with those found in intact rats at proestrus (10).
The uterus is composed of various cell types that respond differentially to estrogen and progesterone. In the adult OVX rodent uterus, estrogen stimulates the proliferation of luminal and glandular epithelia, whereas in the stroma this process requires progesterone and is potentiated by estrogen (11, 12).
Because PAM expression in the anterior pituitary gland is regulated by the estrogen status and the uterus is one of the tissues most sensitive to estrogen regulation, we began studies aimed at examining the presence and the functional role of the enzyme in uterine tissue.
The studies described here were conducted to identify PAM expression and to examine the effects of estrogen status on PAM in the rat uterus. Levels of PAM mRNA were assessed by Northern blotting analysis, and changes in PAM mRNA forms were investigated by using reverse transcription–PCR. Tissue levels of PAM activity were measured. PAM protein forms were examined by Western blot analysis. In situ hybridization studies were conducted to determine the cell type expressing the enzyme and whether changes in estrogen status altered PAM mRNA levels in all or only a fraction of the cell population of the uterus.
MATERIALS AND METHODS
Animals and Treatments.
Ten-week-old female Sprague–Dawley rats (200–250 g; Dépré, Lyon, France) were maintained under standard laboratory conditions with a 14-h light, 10-h dark schedule, with food and water provided ad libitum. At least three animals were used for each stage of the estrous cycle and for each experimental group (ovariectomy with or without hormone replacement). Estrous cyclicity was monitored by cytological examination of vaginal smears taken between 0800 and 1000 hours. Only those females who exhibited at least four consecutive 4-day estrous cycle were selected for study. The complete study of the estrous cycle was repeated three times.
For studies on hormone replacement, rats were OVX and then rested for 1 week. To examine the effect of estradiol or progesterone on PAM expression, OVX animals received for 1 week daily s.c. injections of 17β-estradiol (E2, 4 μg per day; Merck), or progesterone (P, 1 mg per rat; Sigma), diluted in 100 μl of sesame oil, alone or in a combination of both. Control animals were injected with sesame oil.
At the end of each experiment, the animals were killed by decapitation and trunk blood was collected for serum luteinizing hormone (LH) and estradiol measurements. Uteri were rapidly removed for determination of PAM activity, preparation of total RNA, or in situ hybridization.
LH RIA.
Plasma levels of LH were measured in duplicate in a single assay by a double-antibody RIA and expressed as ng/ml of National Institute of Diabetes and Digestive and Kidney Diseases rat LH-RP 2. Anti-rat LH serum CSU 120 was provided by G. D. Niswender (Colorado State University, Fort Collins). The sensitivity of the assay was 0.25 ng/ml of plasma. The intra- and interassay coefficients of variation were below 10%.
Estradiol RIA.
Plasma estradiol levels were measured by using a radioimmunoassay (125I-Estradiol COATRIA; BioMerieux, Lyon, France). The cross-reactivity of the estradiol antiserum with corticosterone was less than 0.017%. The limit of sensitivity of the assay was 12 pg/ml of plasma. The intra- and interassay coefficients of variation were 6% and 7%, respectively.
RNA Analysis.
Total uterine RNA was prepared (13), fractionated on 1% agarose gels containing formaldehyde, transferred to Hybond-N membrane (Amersham), UV cross-linked, and hybridized to α-32P-labeled full-length PAM cDNA (3.8 kb) (7). Filters were prehybridized, hybridized, and washed as previously described (14). To correct for the actual amount of RNA in each lane, blots were stripped and hybridized to cDNA probes derived from ribosomal protein S26 mRNA. S26 mRNA is an invariant control for gene regulation experiments in eukaryotic cells and tissues (15). The autoradiograms were analyzed by measurement of optical density by scanner-densitometer using nih image 1.54 software (National Institutes of Health). The results are expressed as optical density (OD) of PAM mRNA/OD of S26 mRNA, with the bars representing the mean ± SEM for each group of rats.
Combined Reverse Transcription–PCR.
Total uterine RNA (2 μg) from OVX and OVX rats treated with 17β-estradiol, was reverse transcribed into cDNA by using reverse transcriptase encoded by Moloney murine leukemia virus, as described by the supplier (Life Technologies, Paris). Amplification of cDNA derived from 200 ng of total RNA was performed in a 50-μl reaction volume with a buffer consisting of 20 mM Tris⋅HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 0.1% Triton X-100 in the presence of 200 μM each dNTP, 1 μM each primer, and 2.5 units of Extrapol I DNA polymerase (Eurobio, les Ulis, Paris). Thirty-five cycles of annealing at 52°C for 50 sec, elongation at 72°C for 2 min, and denaturation at 94°C for 40 sec were performed on a Biometra trio-thermoblock cycler (Biometra, Göttingen, Germany). Samples were fractionated on 1.8% agarose gels in 45 mM Tris/borate/1 mM EDTA, pH 8, and visualized by staining with ethidium bromide.
Preparation of Tissue Extracts.
Uterine tissue from different groups of animals was homogenized at 4°C (in a Teflon/glass homogenizer, 10 strokes) in 20 mM sodium Tes [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid], pH 7.4/10 mM mannitol, containing 2 μg/ml leupeptin, 16 μg/ml benzamidine, and 300 μg/ml phenylmethylsulfonyl fluoride (PMSF). The homogenates were centrifuged at 2,000 × g for 10 min to yield a pellet containing nuclei, unbroken cells, and connective tissue. The supernatants were either submitted to three consecutive freeze-thaw cycles or sonicated for 15 sec, and then centrifuged for 10 min at 2,000 × g. The supernatants were stored at −70°C until assayed for α-amidation activity (PAM assay) and immunoactive PAM (Western blot). Protein concentrations were determined by using the bicinchoninic acid protein assay reagent (Pierce) and BSA as standard.
Amidation Assays.
Amidation assays were performed in duplicate essentially as described previously (10, 14). Unless indicated otherwise, assay tubes contained 25,000–35,000 cpm of mono-125I-dTyr-Val-Gly, 0.5 μM dTyr-Val-Gly, 500 μM ascorbate, 10 μM Cu2SO4, catalase (100 μg/ml), and 2–6 μg of protein in 150 mM NaTes buffer, pH 8.5. Reaction velocities are generally expressed as picomoles of product formed per microgram of protein per hour (specific activity). The variation between duplicate samples was less than 5%. Reaction velocities reported are initial velocities, measured at a concentration of substrate about 1/10 of the Km of the enzyme for peptide substrate. In general, no more than 10% of the substrate was converted into product in the assay.
Western Blot Analysis.
Samples from uterine and anterior pituitary tissues were prepared for electrophoresis by making them 2% in SDS and 5% in 2-mercaptoethanol and boiling for 5 min. Samples were fractionated by SDS/PAGE and subjected to Western blot analysis (10) using a 1:2000 dilution of rabbit antiserum raised against purified bacterially expressed PHM domain of PAM [Ab1764 to rPAM-(37–382); kindly provided by B. A. Eipper, Johns Hopkins University] and an enhanced chemiluminescence kit (Amersham).
In Situ Hybridization.
In situ hybridization using 35S-labeled riboprobes was performed as previously described (16, 17). Cryostat sections (10 μm) were mounted onto gelatin-coated slides. Labeled probes were prepared by using [α-35S]thio-UTP (New England Nuclear/DuPont) and T3 or T7 RNA polymerase (Stratagene) to synthesize sense and antisense RNA transcripts, respectively, from the rPAM-1 cDNA cloned into Bluescript (7). Slide preparation, hybridization, and washes were carried out as described (10). Slides were exposed to x-ray film (Kodak X-Omat AR) for 3 days. For higher resolution analysis, slides were dipped in Ilford K5 photographic emulsion. The slides were stored for 2 weeks at room temperature, developed, and lightly counterstained with hematoxylin.
Statistical Analysis.
All results are expressed as the mean values ± SEM. Statistical analysis was performed by a one-way analysis of variance followed by Fisher’s PLSD (protected least significant difference) test (ANOVA, Statview 512; Brain Power, Calabasas, CA).
RESULTS
Identification of PAM Expression in Rat Uterus During the Estrous Cycle.
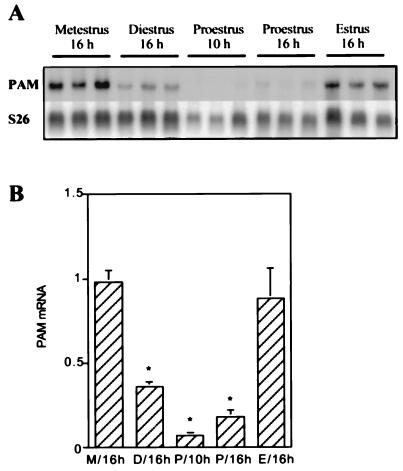
With the demonstration of a tissue-specific regulation of pituitary PAM expression by thyroid hormone (18) and estrogen (10), a reexamination of the levels of PAM in various tissues in the adult female rat was undertaken. PAM activity and PAM mRNA were clearly shown to be present in the uterus. PAM mRNA expression was examined during the estrous cycle in intact cycling rats. PAM mRNA levels in the uterus, at different periods of the estrous cycle, were assessed by Northern blotting analysis. Total RNA extracted from the uteri of individual rats was fractionated on denaturing agarose gels and transferred to Hybond-N. A radiolabeled rPAM-1 probe spanning the entire rat PAM cDNA was used to visualize PAM mRNA (Fig. 1A). Uterine PAM mRNA was 4.2–3.6 kb in size (data not shown). The amount of ribosomal protein S26 RNA present in each sample was determined by hybridization to a cDNA probe for rat ribosomal protein S26 mRNA (Fig. 1A). The amount of PAM mRNA in each sample was then normalized to the amount of S26 mRNA (Fig. 1B). High levels of PAM mRNA were detected in rat uterus. There was a significant change in uterus PAM mRNA levels during the different periods in cycling females. Uterine PAM mRNA levels at proestrus and diestrus were significantly lower than at metestrus and estrus. PAM mRNA levels at proestrus (1000 hours) were (15 ± 0.5)-fold lower than at metestrus and estrus (n = 3; mean ± SEM, Fig. 1B). The regularity of the estrous cycle was verified by measurement of circulating LH and estradiol levels (Table 1). As expected, mean concentrations of LH were greater (P < 0.0001) in rats at 1600 hours during the proestrus period and were not different among the other periods of estrous cycle. Similarly, estradiol levels were highest at 1600 hours during the proestrus period (Table 1). The changes in uterine PAM mRNA expression observed in normal cycling females may be related to the changes of estrogen status during the cycle.
Figure 1.
Northern analysis of PAM mRNA at different period of the estrous cycle in uterus. (A) Total RNA (10 μg) from the uterus of an individual rat at a specific stage of the estrous cycle was fractionated on a denaturing agarose gel as described (14). After hybridization with the rPAM-1 cDNA probe and exposure to film (16 h, −70°C with intensifying screen), the filter was stripped and reprobed with the ribosomal protein S26 mRNA probe. (B) For densitometric analysis of PAM mRNA levels, the amount of PAM mRNA was normalized to the amount of S26 mRNA; this arbitrary ratio was used to express relative tissue PAM mRNA levels. Error bars indicate the SEM. The asterisk indicates that the values for diestrus and proestrus are significantly different from the values for metestrus and estrus (∗, P < 0.003). Similar data were obtained in three independent experiments.
Table 1.
LH and estradiol levels in cycling female rats across the estrous cycle
| Stage of estrous cycle | Plasma LH, ng/ml | Plasma estradiol, pg/ml |
|---|---|---|
| Metestrus (16 h) | 0.66 ± 0.1 | <12 |
| Diestrus (16 h) | 0.53 ± 0.08 | <12 |
| Proestrus (10 h) | 0.66 ± 0.24 | 12 ± 0.33 |
| Proestrus (16 h) | 10.20 ± 0.07∗ | 34 ± 0.35 |
| Estrus (16 h) | 0.38 ± 0.02 | <12 |
, P < 0.0001; proestrus (16 h) vs. all other times.
Effect of Estrogen Status on PAM Expression.
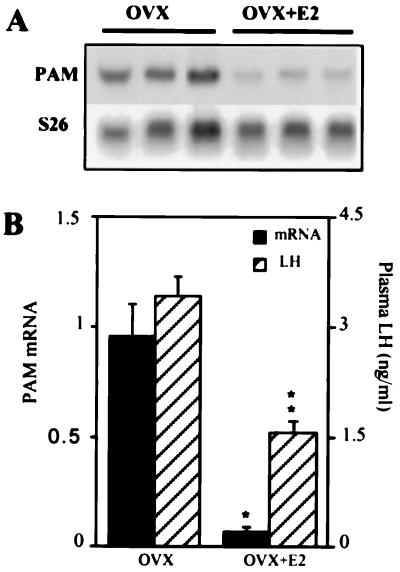
Adult female rats were OVX, and the effect of 17β-estradiol replacement (4 μg/day for 1 week) on uterine PAM expression was examined. The efficiency of surgical ovariectomy and estradiol treatment was verified by measurement of plasma LH levels (Fig. 2B). 17β-Estradiol treatment decreased plasma LH levels from 3.41 ± 0.28 ng/ml to 1.55 ± 0.15 ng/ml in OVX rats (P < 0.0001). Uterine PAM expression in OVX and OVX–17β-estradiol-treated rats (OVX+E2) was assessed by Northern blotting analysis to examine PAM mRNA levels and forms, and by measurement of PAM activity. PAM mRNA levels in total uterine RNA prepared from individual animals were analyzed by Northern blotting as described above (Fig. 2A). The amount of PAM mRNA in each sample was then normalized to the amount of S26 mRNA (Fig. 2B). Uterine PAM mRNA levels in OVX+E2 rats were decreased (12.5 ± 0.9)-fold below OVX values (n = 3; mean ± SEM). This result is in agreement with the data shown in Fig. 1. No alteration in the molecular size distribution of PAM mRNA could be detected.
Figure 2.
Effect of estrogen status on uterus PAM mRNA expression. (A) Total RNA (10 μg) from individual animals in each treatment group was subjected to Northern analysis. The blot was hybridized first with the PAM cDNA probe and then stripped and hybridized with the ribosomal protein S26 mRNA probe. (B) Quantitative analysis of the blots shown in A was performed as described for Fig. 1B (▪). Each bar represents the mean ± SEM of three independent experiments. Plasma LH levels (▨) in individual (n = 9) animals were measured as described in the text. The asterisks indicate a significant difference from OVX value (∗, P < 0.0002; ∗∗, P < 0.0001).
PCR Analysis of PAM mRNAs in the Uterus.
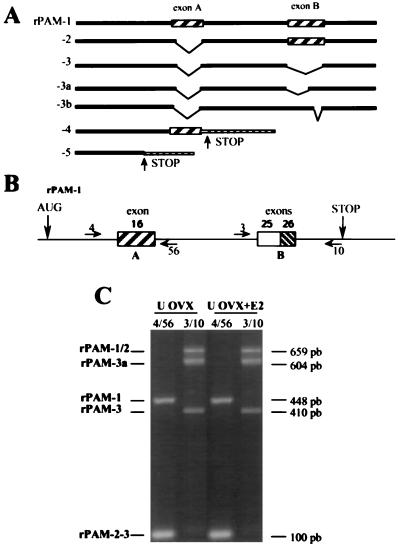
In the rat, alternative splicing of a single-copy PAM gene generates multiple forms of PAM mRNA (Fig. 3A) (6, 8, 9). To determine whether estrogen status altered the forms of PAM mRNA present in the uterus, a qualitative measure of the forms of PAM mRNA present was obtained by carrying out PCR amplification using pairs of oligonucleotide primers that distinguish among the different alternatively spliced forms of PAM mRNA (Fig. 3B). The primer pair (4/56) spanning exon A (315 nt) distinguishes between mRNA of the rPAM-1 type and mRNAs of the rPAM-2, -3, -3a, or -3b types (Fig. 3A); mRNAs of the rPAM-4 and -5 types are not amplified by this primer pair. The data demonstrated that rPAM-1, -2, and -3 are present in the uterus.
Figure 3.
PCR analysis of forms of PAM mRNA in uterus of OVX and OVX+E2 rats. (A) Schematic representation of seven forms of rat PAM mRNA (2, 6, 8, 9). (B) Schematic diagram of rPAM-1 cDNA shows the positions and orientations of oligonucleotide primers used for amplification. The initiation (AUG) and termination (STOP) codons as well as the positions of exon 16 (optional exon A) and exons 25 and 26 (optional exon B) are shown. (C) Total RNA was extracted and 2 μg was subjected to reverse transcription; PCR amplification was carried out with a primer pair (BE4/BE56) that spans exon A (315 nt) or a primer pair (BE3/BE10) that spans exon B (258 nt). The amplified products were fractionated on 1.8% agarose gels and stained with ethidium bromide. The number of base pairs present in the amplified products derived from appropriate plasmid controls is indicated on the right; the forms of PAM to which these bands correspond are indicated on the left. The data presented are representative of three individual experiments.
The primer pair (3/10) spanning the region referred to as exon B (258 nt) distinguishes between mRNAs of the rPAM-1, -2, -3b, -3a, and -3 types (Fig. 3A). Elucidation of the exon/intron structure of the gene encoding rat PAM indicates that exon B is composed of two exons (exons 25 and 26); exon 25 (204 nt) encodes the transmembrane domain of PAM and is followed by the 54-nt exon 26. Alternative splicing can exclude either both or neither of these exons to generate the forms of PAM mRNA observed. The data showed that the predominant splicing pattern induced both of these exons; rPAM-1 or 2, 3b, and 3 were observed. No mRNAs encoding rPAM-3a could be detected. On the basis of amplification using these two sets of primers, we found no effect of estrogen status on the alternatively spliced forms of PAM mRNA in the uterus; this observation is consistent with the results of Northern blotting analysis, which failed to demonstrate an alteration in the molecular weight pattern of PAM mRNA after manipulation of the estrogen status. rPAM-4 and rPAM-5 mRNAs were barely detectable by reverse transcription followed by PCR amplification with form-specific primers, and the results demonstrated that estrogen status had no effect on these two forms (data not shown).
Because estrogen status had a dramatic effect on PAM mRNA levels, the effect of estrogen replacement on the uterine PAM specific activity was also assessed (Table 2). The specific PAM activity was found to be 0.09 pmol/h per μg of protein in OVX+E2 rats and 0.21 pmol/h per μg in OVX rats. The data demonstrated that estradiol treatment resulted in a (2.4 ± 0.2)-fold (n = 3, mean ± SEM) decrease in total PAM specific activity in the uterus compared with OVX rats (Table 2).
Table 2.
Effect of estrogen status on PAM activity in uterus
| Status | PAM activity
|
|
|---|---|---|
| Specific, pmol/h per μg | Total, pmol/h | |
| OVX | 0.21 (9) | 105 (9) |
| OVX+E2 | 0.9∗ (9) | 45∗ (9) |
Total PAM activity was calculated by taking into account the amount of protein in the uterine samples for each group. Results are expressed as the mean of three determinations. The asterisk indicates that the value is significantly different between the two groups (∗, P < 0.05). The number of animals per determination is given in parentheses.
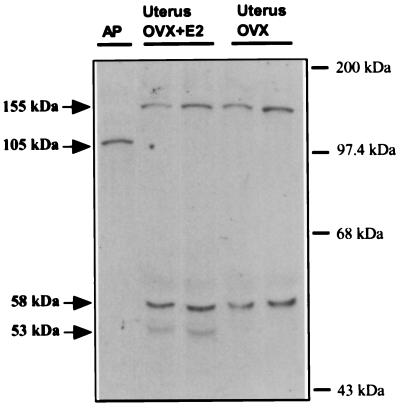
The PAM proteins in any tissue reflect both the forms of PAM mRNA present and the co- or post-translational modifications that occur (6). To determine the forms of PAM protein present in the uterus, and whether PAM proteins were affected by the estrogen status, equal amounts of protein (50 μg) prepared from the tissue extracts from OVX and OVX+E2 rats were subjected to Western blotting analysis (Fig. 4). The PAM protein in particulate fractions from rat anterior pituitary (20 μg) was used for comparison. Antibody 1764 specific to the PHM domain was used to identify PAM proteins. As expected, the major PAM protein in anterior pituitary membranes had a mass of 105 ± 3 kDa (Fig. 4) and is thought to represent rPAM-2 and rPAM-3b (6). Two major PAM proteins at 155 ± 3 kDa and 58 ± 2 kDa were identified in uterine extracts from both groups of rats. In preparations from OVX+E2 rats, a 53-kDa protein was also present. The 58-kDa PAM protein may derive from rPAM-1 by endoproteolytic cleavage or it may be intact rPAM-4 (6). The 53-kDa protein also may be a product of endoproteolytic cleavage of the precursor form of rPAM-1. Thus the post-translational processing of the PAM precursor in the uterus appears to be sensitive to estrogen status.
Figure 4.
Western blotting analysis. Uterus samples (50 μg of protein) prepared from the OVX and OVX+E2 animals and particulate fractions from rat anterior pituitary (AP; 20 μg) were fractionated on SDS/10% polyacrylamide gels and transferred to Hybond-C membrane. PAM proteins were visualized with antiserum to the PHM domain (amino acid residues 37–382) and a chemiluminescence kit. The locations of molecular mass markers analyzed in a separate lane are indicated on the right; apparent molecular masses of the various PAM proteins are indicated on the left of the blot. Similar data were obtained in three additional independent experiments.
Effect of Ovariectomy and Progesterone Replacement on PAM Expression.
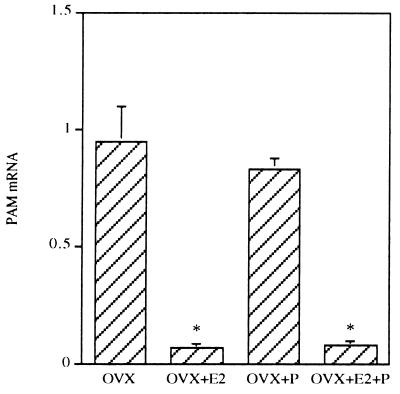
Adult rats were OVX and the effect of 7-day treatment with progesterone (1 mg per animal) alone and in combination with 17β-estradiol (4 μg per animal) was investigated. The data showed that estradiol treatment sharply decreased PAM mRNA levels, whereas daily administration of progesterone to OVX rats failed to reverse the ovariectomy-induced PAM mRNA rise or to interfere with estrogen-induced decrease of PAM mRNA concentrations (Fig. 5). These data confirmed that PAM mRNA expression in uterus is regulated only by estrogens during the estrous cycle.
Figure 5.
Effect of sex steroid treatment on uterus PAM mRNA content in OVX rats. Total RNA (10 μg) from individual animals in each treatment group (n = 3) was subjected to Northern analysis. The histogram shows optical density of PAM mRNA signal after normalization with S26 mRNA signal. Results shown are the mean ± SEM of three independent experiments. The asterisk indicates that the values for OVX+E2 and OVX+E2+P rats are significantly different from those for OVX and OVX+P rats (∗, P < 0.0002).
In Situ Hybridization.
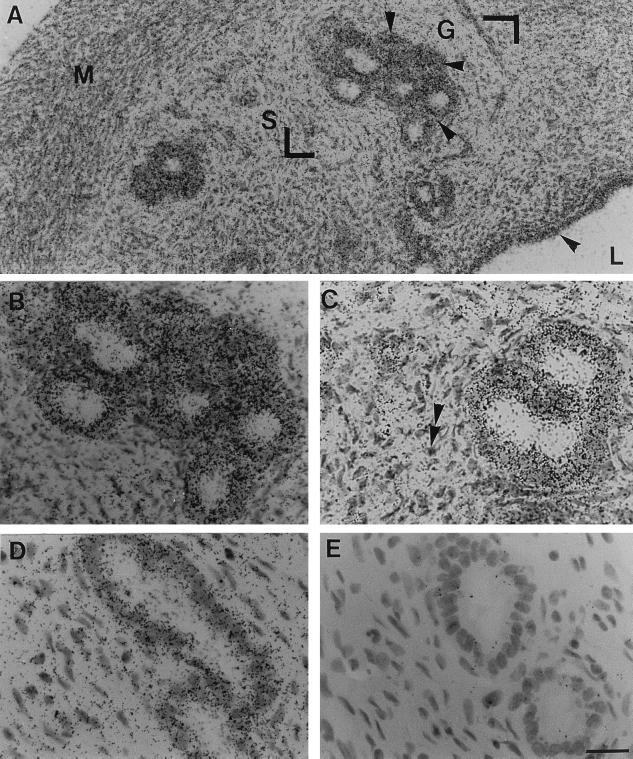
Because the above findings suggested that the rat uterus has the capacity to synthesize PAM, we attempted to identify the uterine histological compartment where PAM expression might occur. PAM expression was then examined by in situ hybridization. The riboprobe used in this study corresponds to the full-length rat PAM cDNA and was selected for its ability to recognize all of the known forms of PAM mRNA (8). When radiolabeled antisense PAM RNA probes were used on cryosections of uterine tissue in OVX rat, a majority of the cells in endometrium were found to contain autoradiographic grains (Fig. 6A). Luminal and glandular cells were heavily labeled (see arrowheads in Fig. 6A). A weak labeling was observed in stromal cells (double arrowhead in Fig. 6C). Myometrium cells were negative (Fig. 6A). 17β-Estradiol treatment of OVX rats induced an overall decrease of the signal (Fig. 6D). Nonspecific background labeling using the sense probe was negligible (Fig. 6E).
Figure 6.
In situ hybridization for uterus PAM mRNA in OVX or OVX+E2 rats. Cryostat uterus sections (10 μm) were hybridized to 35S-labeled antisense PAM riboprobe. Cryosections were dipped in photographic emulsion for histological resolution of autoradiographic grains. After 2 weeks of exposure, the tissues were processed and stained with hematoxylin. In situ hybridization signal in the uterus of the adult OVX rat (A) was observed in luminal (L) and glandular (G) cells (arrowheads); myometrium cells (M) were negative. The area indicated by the two right angles was magnified and shown in B. Stroma (S) cells appeared to be devoid of grains (see double arrowheads in C). (D) Compared with the OVX rats, labeling was generally less prominent in glandular cells of estrogen-treated OVX rats. (E) Bright-field micrograph of a section hybridized to sense PAM riboprobe shown for comparison. (The scale bar in E represents 25 μm for A and 50 μm for B–E.)
DISCUSSION
The uterus was found to contain unexpectedly manifest levels of PAM activity, mRNA, and protein. The enzymatic properties of PAM activity in the uterus are similar to those described for PAM in other tissues (2). However, the apparent molecular mass of PAM in the uterine tissue is 155,000 Da on a Western blot, whereas membrane-associated PAM in the anterior pituitary has a mass of 105,000 Da (Fig. 4). The increase in size may reflect the fact that PAM proteins in the uterus are glycosylated. In fact, there are two potential N-glycosylation sites in rPAM-1 and only one in rPAM-2. Glycosylation may therefore contribute to some extent to the difference between the apparent molecular masses and those predicted for pro-rPAM-1 (106 kDa) and pro-rPAM-2 (94 kDa). Despite the presence of similar forms of PAM mRNA (data not shown) the PAM proteins found in the anterior pituitary and uterus differ. Thus post-translational processing contribute to the tissue-specific production of proteins derived from precursor PAM.
The results of the present studies clearly demonstrate that PAM expression in the uterine tissue is regulated by estrogen status. PAM activity (data not shown) as well as PAM mRNA levels followed a synchronized cyclic pattern, being higher at metestrus and lower at proestrus. Furthermore, PAM expression in the uterus was reduced when OVX rats were chronically treated with estradiol. The (15 ± 0.5)-fold decrease in PAM mRNA levels observed after estrogen treatment of OVX rats resulted in only a (2.4 ± 0.2)-fold decrease in total specific activity. The amount of PAM activity in uterine extracts represents a balance among synthesis, storage, inactivation, and secretion of the enzyme. This dissociation between levels of PAM mRNA and enzyme activity may reflect variation in the forms of PAM present and/or altered translational efficiency for PAM mRNA. In situ hybridization studies indicated that endometrium cells were the major cellular source of PAM.
All these data indicate that PAM synthesis in the uterus shows a reciprocal relationship with the estrogen status, suggesting that uterine PAM is regulated mainly by either estrogen itself or an estrogen-dependent factor. Estradiol action on PAM mRNA levels and activity appears to be specific, because progesterone has no effect on either parameter. The molecular mechanism by which steroids modulate the expression of PAM mRNA is not clear. On the basis of the size of the single-copy PAM gene spanning at least 240 kb of genomic DNA with 31 exons (ref. 8 and unpublished data) and the post-transcriptional regulation by estrogen of PAM expression in the anterior pituitary gland, plus the cyclicity of this regulation during the estrous cycle, we suggest that uterine PAM regulation by estrogen occurs at the post-transcriptional level. Further experiments are necessary to elucidate this mechanism.
During the estrous cycle the uterus is subjected to changes in cell proliferation and differentiation. The response of the uterus to estrogen is a complex phenomenon involving numerous biochemical events (19). It is customary to divide the response of the uterus to estrogen into two major temporal phases. In the early phase, within the first 4 h after estrogen administration, there is an increase in uterine wet weight (20), glucose metabolism (21), histamine depletion (22), and RNA polymerase activity (23). One of the early events that is likely to be important in the proliferative response of the uterus to estrogen is activation of protooncogenes that encode nuclear regulatory proteins (24), the enhanced expression of growth factors and their receptors (25), and cAMP-independent protein kinase (26). The intermediate and late phase (4–48 h after estrogen) includes increases in protein and RNA synthesis and an increase in uterine dry weight and DNA content (27). One emerging concept is that estrogens might stimulate proliferation of cells indirectly through a mitogenic polypeptide or protein.
The physiological significance of uterine PAM and its regulation by estrogen is not clear yet. PAM expression in uterine tissue suggests that PAM contributes to the synthesis of some potential α-amidated peptides. The production of bioactive α-amidated peptide requires the concerted action of several different proteases, whose activities are modulated by complex mechanisms (28, 29). Several earlier studies have shown that estrogens can enhance proteolytic activity in the rodent uterus or in cultured uterine cells (30–33). In uterine tissue, we can hypothesize that some protein precursors [for example, growth factor precursors such as the precursor for insulin-like growth factor (IGF) and preproneuropeptides] undergo a cascade of activating proteolytic reactions whose end result is the release of different peptides, some of which could be potential PAM substrates. Such processing could result in the production of novel α-amidated peptides that could be active. Recently, Whitley et al. (34) have demonstrated that gastrin-releasing peptide (GRP), an amidated peptide, is synthesized, stored, and secreted in the pregnant human uteroplacental unit. The observation that GRP is synthesized in the uterus suggests that this peptide plays an important role during pregnancy in women and may have autocrine, paracrine, and endocrine functions. These could include the regulation of the growth, motility, and secretion of the uterus.
The finding of high PAM expression in heart tissue (14) with the lack of substrate(s) and the same finding now in the uterus open the possibility that PAM may have functions other than that linked to its well known amidating activity. Recently, a role for PAM in the biosynthesis of fatty acid primary amides, recognized as biological signaling molecules (35), has been suggested (36).
In summary, the results reported in this study demonstrate the overt expression of PAM in the uterus and its regulation by estrogen. Our current efforts are directed to understanding the role of this key processing enzyme in the uterus and the analysis of the molecular mechanisms concerning its regulation by estrogens.
Acknowledgments
We thank F. Youssouf, J. C. Orsoni, and H. Gerard for their technical help, Dr. C. Somma-Delpers for determination of plasma estradiol levels, and Dr. A. J. Zamora for helpful discussions. Reagents for LH RIA were provided by the Hormone Distribution Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
ABBREVIATIONS
- PAM
peptidylglycine α-amidating monooxygenase
- OVX
ovariectomized
- OVX+E2
ovariectomized and treated with 17β-estradiol
- LH
luteinizing hormone
References
- 1.Bradbury A F, Smyth D G. Trends Biochem Sci. 1991;16:112–115. doi: 10.1016/0968-0004(91)90044-v. [DOI] [PubMed] [Google Scholar]
- 2.Eipper B A, Stoffers D A, Mains R E. Annu Rev Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 3.Kato I, Yonekura H, Tajima M, Yanagi M, Yamamoto H, Okamoto H. Biochem Biophys Res Commun. 1990;172:197–203. doi: 10.1016/s0006-291x(05)80193-7. [DOI] [PubMed] [Google Scholar]
- 4.Perkins S N, Husten E J, Mains R E, Eipper B A. Endocrinology. 1990;127:2771–2778. doi: 10.1210/endo-127-6-2771. [DOI] [PubMed] [Google Scholar]
- 5.Braas D M, Stoffers D A, Eipper B A, May V. Mol Endocrinol. 1989;3:1387–1398. doi: 10.1210/mend-3-9-1387. [DOI] [PubMed] [Google Scholar]
- 6.Eipper B A, Green C B-R, Campbell T, Stoffers D A, Keutmann H T, Mains R E, Ouafik L’H. J Biol Chem. 1992;267:4008–4015. [PubMed] [Google Scholar]
- 7.Stoffers D A, Green C B-R, Eipper B A. Proc Natl Acad Sci USA. 1989;86:735–739. doi: 10.1073/pnas.86.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouafik L’H, Stoffers D A, Campbell T A, Johnson R C, Bloomquist B I, Mains R E, Eipper B A. Mol Endocrinol. 1992;6:1571–1584. doi: 10.1210/mend.6.10.1448112. [DOI] [PubMed] [Google Scholar]
- 9.Stoffers D A, Ouafik L’H, Eipper B A. J Biol Chem. 1991;266:1701–1707. [PubMed] [Google Scholar]
- 10.El Meskini R, Delfino C, Boudouresque F, Hery M, Oliver C, Ouafik L’H. Endocrinology. 1997;138:379–388. doi: 10.1210/endo.138.1.4895. [DOI] [PubMed] [Google Scholar]
- 11.Martin L, Finn C A. J Endocrinol. 1968;41:363–371. doi: 10.1677/joe.0.0410363. [DOI] [PubMed] [Google Scholar]
- 12.Huet Y M, Andrews G K, Dey S K. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Ouafik L’H, May V, Keutmann H T, Eipper B A. J Biol Chem. 1989;264:5839–5845. [PubMed] [Google Scholar]
- 15.Vincent S, Marty L, Fort P. Nucleic Acids Res. 1993;21:1498. doi: 10.1093/nar/21.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal D, Shilo B Z. Mol Cell Biol. 1986;6:2241–2248. doi: 10.1128/mcb.6.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouafik L’H, May V, Saffen D W, Eipper B A. Mol Endocrinol. 1990;4:1497–1505. doi: 10.1210/mend-4-10-1497. [DOI] [PubMed] [Google Scholar]
- 18.Fraboulet S, Boudouresque F, Delfino C, Fina F, Oliver C, Ouafik L’H. Endocrinology. 1996;137:5493–5501. doi: 10.1210/endo.137.12.8940376. [DOI] [PubMed] [Google Scholar]
- 19.Szego C M, Roberts S. Recent Prog Horm Res. 1953;8:419–469. [Google Scholar]
- 20.Meuller G C, Herranen A, Jervell K. Recent Prog Horm Res. 1958;14:95–139. [PubMed] [Google Scholar]
- 21.Meier D A, Garner C W. Endocrinology. 1987;121:1366–1374. doi: 10.1210/endo-121-4-1366. [DOI] [PubMed] [Google Scholar]
- 22.Szego C M. Fed Proc. 1959;24:1343–1352. [PubMed] [Google Scholar]
- 23.Aziz S, Balmain A, Knowler J T. Eur J Biochem. 1979;100:95–100. doi: 10.1111/j.1432-1033.1979.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 24.Murphy L J, Murphy L C, Friesen H G. Clin Res. 1986;34:714A. (abstr.). [Google Scholar]
- 25.Travers M T, Knowler J T. FEBS Lett. 1987;211:27–30. doi: 10.1016/0014-5793(87)81267-x. [DOI] [PubMed] [Google Scholar]
- 26.Loose-Mitchell D S, Chiappetta C, Stancel G M. Mol Endocrinol. 1988;2:946–951. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- 27.Mukku V R, Stancel G M. J Biol Chem. 1985;260:9820–9824. [PubMed] [Google Scholar]
- 28.Mignatti P, Rifkin D B. Physiol Rev. 1993;73:161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 29.Barr P J. Cell. 1991;66:1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- 30.Peltz S W, Katzenellenbogen B S, Kneiffel M A, Mangel W F. Endocrinology. 1983;112:890–897. doi: 10.1210/endo-112-3-890. [DOI] [PubMed] [Google Scholar]
- 31.Pietras R J, Szego C M. J Cell Biol. 1979;81:649–663. doi: 10.1083/jcb.81.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz J, Troll W, Levy M, Filkins K, Russo J, Levitz M. Arch Biochem Biophys. 1976;173:347–354. doi: 10.1016/0003-9861(76)90269-1. [DOI] [PubMed] [Google Scholar]
- 33.Henrikson K P, Dickerman H W. Mol Cell Endocrinol. 1983;32:143–156. doi: 10.1016/0303-7207(83)90078-3. [DOI] [PubMed] [Google Scholar]
- 34.Whitley J C, Giraud A S, Shulkes A. J Clin Endocrinol Metab. 1996;81:3944–3950. doi: 10.1210/jcem.81.11.8923842. [DOI] [PubMed] [Google Scholar]
- 35.Cravatt B F, Prospero-Garcia O, Siuzdak G, Gilula N B, Henriksen S J, Bolger D L, Lerner R A. Science. 1996;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- 36.Merkler D J, Merkler K A, Stern W, Fleming F F. Arch Biochem Biophys. 1996;330:430–434. doi: 10.1006/abbi.1996.0272. [DOI] [PubMed] [Google Scholar]