SUMMARY
This review, primarily for general readers, briefly presents experimental approaches to therapeutics of cancer, HIV/AIDS and various other diseases based on advances in glycobiology and glycochemistry. Experimental cancer and HIV/AIDS vaccines are being developed in attempts to overcome weak immunological responses to carbohydrate – rich surface antigens using carriers, adjuvants and novel carbohydrate antigen constructs. Current carbohydrate – based vaccines are used for typhus, pneumonia, meningitis and vaccines for anthrax, malaria and leishmaniasis are under development. The link between O– linked β-N-acetylglucosamine glycosylation and protein phosphorylation in diseases including diabetes and Alzheimer's disease is also explored. Carbohydrate – associated drugs that are in current use or under development such as heparan sulphate binders, lectins, acarbose, aminoglycosides, tamiflu, and heparin, and technologies using carbohydrate and lectin microarrays, that offer improved diagnostic and drug development possibilities, are described. Advances in carbohydrate synthesis, analysis, and manipulation through the emerging fields of glycochemistry and glycobiology, are providing new approaches to disease therapeutics.
Keywords: Carbohydrate – based experimental drugs and vaccines, cancer, HIV/AIDS, diabetes, Alzheimer's disease, microarray diagnostics
INTRODUCTION
The cell surface consists of proteins, lipids and carbohydrates. The carbohydrates of glycoproteins and glycolipids usually reach furthest from the surfaces of cells and therefore are often involved in initial interactions with other cells and substrates. Thus, carbohydrates play a key role in cellular interactions ( reviews in Oppenheimer, 2004; 2006,). Until recent years, little attention has been given to these molecules, primarily because they are so diverse and so much more difficult to study than, for example, proteins that are directly encoded by genes. It is only fairly recently that carbohydrates have been given more attention because of overwhelming evidence that they are extensively involved in human health and disease and because of advances that have been made in their synthesis, analysis and manipulation (Finkelstein, 2007).
This brief review is not intended to cover the chemistry of carbohydrates. It treats selected topics on carbohydrate involvement in various human diseases such as cancer and HIV/AIDS and how approaches based on glycobiology and glycochemistry are providing new avenues for study. A series of reviews for specialists has been published recently (Finkelstein, 2007) ; it is highly recommended for those who wish more in–depth coverage, and helped form the foundation for this review for general readers. The term therapeutics is used here in its broad sense as the application of remedies to diseases whether they be vaccines or treatment modalities. Histochemistry has been widely used to identify many of the carbohydrate antigens described in the review.
CANCER
Certain carbohydrates on cancer cell surfaces offer an approach for prevention and treatment (Feize, 1985; Galonic and Gin, 2007). Many attempts have been made to develop vaccines and cancer treatment strategies based on differences between cancer and non-cancer cell surface carbohydrates (Slovin et al. 2005).
CANCER VACCINES
Some carbohydrate – containing tumor associated cell surface antigens include TN, T and sialyl TN (STN)5 (glycoproteins with N-acetylgalactosamine core oligosaccharide), GM2, Globo – H, GD2, GD3 (glycolipids, where the carbohydrate is bound to the cell surface via a lipid link) (Slovin et al., 2005; Galonic and Gin, 2007). These antigens have been used in attempts to produce cancer vaccines, but generally have been found to induce only weak immune responses (Galonic and Gin, 2007). However, when some of these antigens were conjugated to a carrier such as keyhole limpet haemocyanin (KLH), a more powerful immune response was induced (Helling et al., 1994). Immunological adjuvants such as QS – 21A (a plant – derived complex saponin from the tree Quillaja saponaria molina) have also been used in combination with carbohydrate antigens and potentiators to improve immune responses (Helling et al., 1995).
Some carbohydrate antigen – based vaccines are now in development or in clinical trials (Ragupathi, et al., 2003; Slovin et al., 2003; Ragupathi et al., 2005; Livingston and Ragupathi, 2006) and some early results look promising. For example, melanoma patients exhibited a consistent antibody response against ganglioside GD2 using a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21 (Ragupathi, et al., 2003). One of the major problems in using carbohydrate antigens, potentiators and adjuvants in the development of anti-cancer vaccines has been inability to isolate sufficient quantities of some of these homogeneous compounds. Major developments have occurred in glycochemistry technologies that allow for the synthesis of tumor associated carbohydrate antigens, potentiators and adjuvants. The cell surface glycosphingolipid Globo – H, sialyated gangliosides such as GM2, GD2 and GD3 and mucin associated glycans, are tumor associated antigens that have been synthesized, making sufficient quantity available for the development of cancer vaccines (Ouerfelli et al., 2005). Clustered carbohydrate antigen displays have also been synthesized by constructing amino acid carbohydrate units that are typically present on cancer cell surfaces. Some of these peptide – antigen conjugates that have been prepared include TN antigen constructs, STN antigen glycopeptides and 2,3- and 2, 6- ST conjugates (Elofsson et al., 1997; Satz and Kunz, 1995, Galonic and Gin, 2007). The synthesis of QS-21A adjuvant mentioned earlier has also been successfully accomplished (Kim, et al., 2006). Advances in glycochemisty and glycobiology are not only producing sufficient quantities of tumor antigens and adjuvants but are also leading to the development of novel carbohydrates antigen constructs and displays that may be better immunogens than naturally occurring displays (Galonic and Gin, 2007).
CANCER TREATMENT APPROACHES
Heparan sulphate proteoglycans are present on the surfaces of animal cells and in extracellular matrices and play important roles in many aspects of animal physiology (Bishop et al., 2007). These molecules consist of core protein and heparan sulphate glycosaminoglycan. The latter is composed of linear polysaccharides with alternating N – acetlyated or N – sulphated glucosamine units and uronic acids. In cancer, heparan sulphate proteoglycans allow primary tumor growth and angiogenesis through growth-factor-dependent signaling (Fuster and Esko, 2005, Bishop et al., 2007). It might be possible to target tumor cells by developing agents that bind heparan sulphate or influence its synthesis.
Lectins that are carbohydrate binding proteins, have been tested for their toxicity towards a variety of cancer cells (Valentiner et al., 2003; Heinrich et al., 2005; Welty et al., 2006) and may be useful as anti-cancer drugs. For example, different lectins exerted different effects at specific concentrations on various breast cancer cell lines (Valentiner et al., 2003). The results suggested that certain dietary lectins can inhibit growth of breast cancer cells (Valentiner et al., 2003). Mistletoe lectin has been used directly as an anti – cancer drug in complementary therapy programs and Fritz et al. (2004) have found that this lectin binds to breast cancer cells . But no studies, so far, have provided reliable evidence that this lectin or any other lectin , has been successfully used clinically as an effective anti-cancer drug. Some lectins have been used as carrier molecules that can be conjugated to chemotherapy drugs (Minko, 2004; Bies et al., 2004; Mody et al., 1995). For example, the reported studies showed that lectins could differentially bind to diseased colon and could be potentially used to specifically deliver anti-cancer agents directly to the site of the disease. But, as yet, no lectin-coupled anti-cancer agent is being widely used clinically. As some lectins are mitogenic, they might be coupled with anti – cancer drugs to synergistically potentiate drug effectiveness (Petrossian et al., 2007). In addition to studying human cancers, work with simple model systems, such as the U.S. National Institutes of Health designated sea urchin embryo model, can uncover principles of carbohydrate-associated cellular interactions that are more difficult to study in complex human systems (Khurrum et al., 2004; Latham et al., 1995a; 1995b; 1998, 1999; Coyle – Thompson et al., 2005; Sajadi et al., 2007).
HIV/AIDS
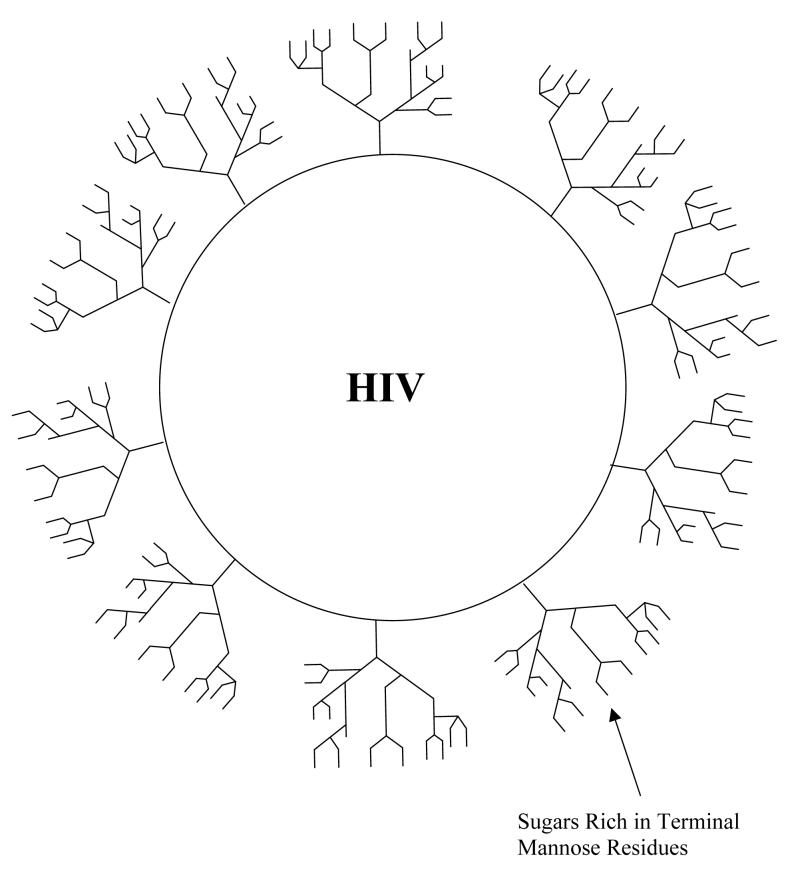
One of the main reasons for difficulties in producing a vaccine against HIV/AIDS is that the virus surface is covered with coatings of carbohydrates that mask underlying viral antigens that might be good targets in the production of vaccines (Scanlan et al., 2007). The glycans on the viral surface are produced by the host cell making the virus appear as “self” and therefore not attacked by the host immune system. Much of the viral coat carbohydrates are rich in mannose residues (Scanlan et al., 2007). A possible target for prevention of HIV infection is the fact that the sugars in the HIV coat are unusually dense and loaded with terminal mannose groups (see Figure 1). Lectins such as cyanovirin, isolated from bacteria, preferentially bind to α 1-2 linked mannose residues. Such lectins are being investigated as possible therapeutic tools (Tsai et al., 2004). But it is well-known that lectins are often toxic, may be considered as foreign by the host immune system, and they may bind to host carbohydrates damaging host cells.
Figure 1.
HIV surrounded by mannose-rich carbohydrate.
Not intended to be structurally accurate. The figure is intended to show that therapeutic approaches to this disease must contend with a carbohydrate coat consisting of host glycans.
One antibody isolated from HIV infected individuals binds effectively to the virus envelope, mannosylated glycoprotein gp 120. This antibody, IgG1 2G12 binds to Mannose α 1-2 Mannose residues of gp120 in the mannose6-9 N-acetylglucosamine2 glycans (Scanlan et al., 2002, Scanlan et al., 2007). The antibody appears to recognize these glycans because although they are host sugars, they are clustered in a non-self manner (Scanlan et al., 2002; Scanlan et al., 2007). Recall that antibodies are being developed against self glycolipids and glycoproteins (such as GM2, GM3 Globo-H, TN, sialyl-TN (STN), and sialyl – lewis A) that are presented as clusters in cancer cells, in attempts to produce effective anti-cancer vaccines (Galonic and Gin, 2007). We also described studies using immune enhancing adjuvants ,carrier peptides such as keyhole limpet hemocyanin and altered glycan structure constructs that enhance immune recognition in cancer vaccine development (Galonic and Gin, 2007). These same strategies are being used in development of possible HIV vaccines. It has been clearly shown that these sorts of strategies can result in antibodies against self carbohydrates that are arranged slightly differently on cancer cells and HIV (Galonic and Gin, 2007). We also described studies using immune response-enhancing adjuvants , carrier peptides such as keyhole limpet hemocyanin and altered glycan structure constructs that enhance immune recognition in cancer vaccine development (Galonic and Gin, 2007). These same strategies are being used in development of possible HIV vaccines. It has been clearly shown that these sorts of strategies can result in development of antibodies against self-carbohydrates that are arranged slightly differently on cancer cells and HIV-infected cells in comparison to normal cells (Galonic and Gin, 2007). These approaches have not as yet led to clinically effective vaccines, but it is clear that antibodies that strongly bind to carbohydrate antigens on, for example, prostate cancer cells, have been generated (Slovin et al., 2003) and this appears to be a highly promising approach..
A variety of drugs inhibit synthesis of carbohydrates (Scanlan et al., 2007). At concentrations needed for antiviral activity, however, they are often quite toxic. N-butyldeoxynorjirimycin (NB-DNU) is one such drug that causes structural alterations in gp120 in viral envelopes that can prevent binding to host cells membranes (Fischer et al., 1995; Fischl et al., 1994). Further exploration of this group of drugs is another approach to HIV infection that is based on the rich carbohydrate coat of the virus. Strategies based on glycobiology and glycochemisty are providing new approaches that may lead to improved prevention and treatment modalities in cancer and HIV/AIDS.
DIABETES AND ALZHEIMER'S DISEASE
Altered glycosylation of proteins by o-linked β-N-acetylglucosamine is associated with diabetes and neurodegeneration (Hart et al., 2007). Nutrient excess and/or stress can cause such altered glycosylation of proteins. This glycosylation is associated with glucose toxicity and appears to be involved with insulin resistance in diabetes (Hart et al., 2007). Such altered glycosylation is associated with diabetes, vascular disease, erectile disfunction, retinopathy, glomerular sclerosis, cardiomyopathy and Alzheimer's disease (Hart et al., 2007). In the case of Alzheimer's disease, such glycosylation is reduced in brain tissue. Such reduced glycosylation appears to alter neuronal function possibly by resulting in hyperphosphorylation of specific proteins such as tau. Hyperphosphorylation of tau appears to cause tau to aggregate resulting in neurofibril tangles that are characteristic of Alzheimer's disease (Liu et al., 2004).
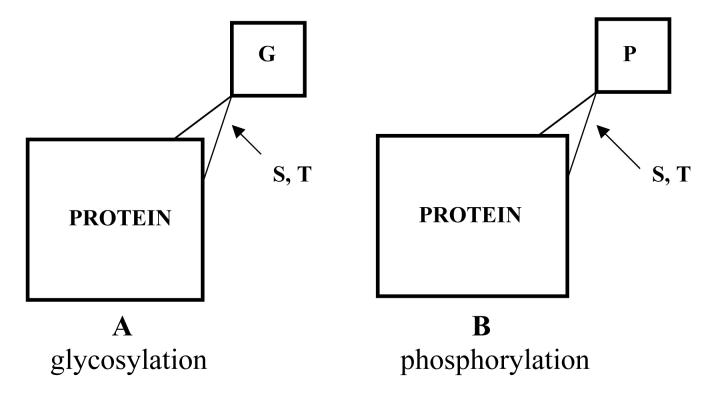
More than 500 proteins have been shown to be N-acetylglucosamine glycosylated (Love and Hanover, 2005; Zachara and Hart, 2006). These proteins are involved in nearly all aspects of cellular function. Both hyperglycosylation and hypoglycosylation have been associated with diseased states. Only a few highlights of the role of N-acetylglucosamine glycosylation have been summarized here. A better understanding of how altered glycosylation leads to insulin resistance, glucose toxicity, altered transcription and signalling could lead to new approaches in the treatment and diagnosis of diabetes and other diseases. In summary hypoglycosylation leaves certain key proteins subject to hyperphosphorylation that can cause diseased states. On the other hand, hyperglycosylation of proteins can also lead to diseased states. Alzheimer's disease is an example of the former, while diabetes and diabetic pathologies are examples of the latter. Drugs may be developed that can alter such glycosylation and therefore affect phosphorylation of proteins that may be involved in these diseases (Marx, 2007). Drugs that are effective in treating diabetes or Alzheimer's disease, based on these principles have not yet been developed, but the glycosylation/phosphorylation relationship associated with these diseases is an exciting avenue for further study. This is illustrated in Figure 2.
Figure 2.
A.Glycosylation (G) versus B. phosphorylation (P) that can occur on some serine (S) and threonine (T) residues of the proteins.
OTHER CONDITIONS
Mutated genes coding for proteoglycans (such as heparan sulphate proteoglycan) have implicated these carbohydrate – rich molecules in many conditions including: hyperglyceridaemia, impared hippocampal function, cerebral hypoplasia, neural – tube closure defects, eye and lens defects, defective neuromuscular junction and synapse formation, cartilage defects, rib malformations, craniofacial defects, altered ossification, short body length, postaxial polydactyly, poorly organized epidermis, impaired wound healing, impaired fetal vessels in placenta, enhanced infection, and virulence by Pseudomonas aeruginosa, defective secretory granules in mast cells, mast cell deficiency, and lung inflammation (Bishop et al., 2007).
Vaccines made from the carbohydrate – rich coats of various pathogenic bacteria have been widely used to control infections by: Salmonella typhi, Streptococcus pneumoniae, Neisseria meningitides, Haemophilus influenza type b (Seeberger and Werz., 2007). Carbohydrate vaccines are being developed against Bacillus anthracis ,the anthrax causing pathogen (Seeberger and Werz, 2007). Malaria, caused by the protozoan Plasmodium falciparum, kills over 2 million people each year. Carbohydrate vaccines using Plasmodium surface glycolipids are under development. Leishmaniasis also caused by a protozoan parasite Leishmania, affects millions of people each year worldwide. Vaccines using synthetic versions of surface phosphoglycolipid antigens are being developed (Seeberger and Werz, 2007). In the case of Leismaniasis, for example, cell surface oligosaccharide was synthesized and coupled to a viral particle to improve the immune response. These vaccines were able to specifically recognize Leishmania infected livers (Seeberger and Werz, 2007).
MICROARRAYS
Carbohydrate and lectin microarrays have been constructed by attaching glycans or lectins to glass slides or other substrates (Seeberger and Werz, 2007). Whole cells or proteins or antibodies that are labeled with fluorescent or other types of tags are then incubated with the glycan or lectin microarray. In this way the glycans or lectins to which the labeled cell, protein or antibody binds are rapidly identified, illustrated in Figure 3. Carbohydrate and lectin microarrays are being used for diagnostic purposes to, for example, identify specific bacteria in blood (Disney and Seeberger, 2004). Microarrays are also being used to identify the carbohydrate binding preference of human pathogens in the development of carbohydrate-based drugs (Seeberger and Werz, 2007). Carbohydrate microarrays are being used to screen human sera for antibodies against malaria toxin glycoslyphosphatidylinositol (GPI) anchor and for correlating the presence of specific antibodies with resistance to malaria (Schofield et al., 2002). Serological markers have been identified for Crohn's disease using microarrays (Dotan et al., 2006). In addition to microarrays, carbohydrate and lectin derivatized microbeads have been used as model systems for development of carbohydrate-based drugs and diagnostic tests (Zem et al., 2006).
Figure 3.
Carbohydrate microarray. Protein, cell or antibody ( X ) coupled to a label ( W ) is incubated with the carbohydrate microarray that contains sugars ( A-H ) covalently attached ( Y ) to a glass slide or other substrate ( Z ) to determine to which sugar(s) the labeled entity binds.
CARBOHYDRATE – BASED DRUGS
Various carbohydrate-based drugs and diagnostics are currently in use (Seeberger and Werz, 2007). Tamiflu (oseltamivir phosphate) is a monosaccharide-based drug that is used for treating influenza (De Clercq, 2006). It is believed to work by inhibiting viral neuraminidase that alters virus particle aggregation and release. Aminoglycosides are used to treat various infections by gram negative bacteria, as they inhibit protein synthesis by binding to bacterial ribosomes (Weizman and Thor, 2003). Heparin, consisting of a mixture of polysaccharides, has been used for decades as an anti-clotting agent (Capila and Linhardt, 2002). Anticoagulants have been used experimentally in reducing clots that may facilitate cancer cell spread (Lemoine, 2005; Varki 2002; Borsig et al., 2001). Acarbose is a glycosidase inhibitor that is used to treat type-2-diabetes by regulating carbohydrate digestion, intestinal absorption and carbohydrate metabolism (Truscheit et al., 1981). It has been shown to cause a significant decrease in plasma glucose in patients with non-insulin dependent diabetes (Chiasson et al., 1994).
Recent advances in glycochemistry have facilitated the synthesis of many carbohydrate constructs that should substantially enhance the development of effective carbohydrate-based vaccines and drugs. Microarray technology is also leading to advances in the diagnosis of human diseases and in the development of carbohydrate based therapeutic agents. Some of the highlights of this review are summarized in Table 1.
Table 1.
Some Carbohydrate-Based Experimental and Currently Used Therapeutics
| CANCER |
| Vaccines (Galonic and Gin, 2007) |
| Enhanced immune response to carbohydrate-containing tumor-associated antigens using: |
| Carriers (e.g., keyhole limpet hemocyanin) |
| Adjuvants (e.g., QS-21A) |
| Novel carbohydrate antigen constructs (e.g. TN antigen constructs, STN antigen glycopeptide, 2,3 – and 2,6 – ST conjugates) |
| PotentialTreatments (Bishop et al., 2007) |
| Heparan sulphate binders and synthesis alteration agents may block tumor growth and angiogenesis; lectins that inhibit cancer cell growth (e.g. mistletoe lectin) or serve as carriers for anticancer drugs. |
| HIV/AIDS |
| Vaccines (Scanlan et al., 2007) |
| IgG1 2G12 antibodies that bind to clustered mannose alpha 1-2 mannose residues of gp120 in HIV envelope; adjuvants, carriers and altered glycan constructs as used in cancer vaccine development |
| Potential Treatments (Scanlan et al., 2007) |
| Lectins (e.g. cyanovirin that binds alpha 1-2 linked mannose residues); carbohydrate synthesis inhibitors (e.g., N-butyldeoxynorjirimycin, NB-DNJ) |
| DIABETES AND ALZHEIMER'S DISEASE |
| (Seeberger and Werz, 2007) |
| Acarbose, a glycosidase inhibitor that regulates carbohydrate digestion, intestinal absorption and carbohydrate metabolism, currently in-use for diabetes treatment |
| (Hart et al., 2007) |
| Protein glycosylation by o-linked beta-N-acetylglucosamine alters phosphorylation of protein associated with diabetes and Alzheimer's disease (tau protein). Protein glycosylation and phosphorylation could be targets for drug design. |
| VARIOUS INFECTIOUS DISEASES AND OTHER CONDITIONS |
| (Seeberger and Werz, 2007) |
| Salmonella typhi- Typhus. Vaccine made from coat carbohydrates |
| Sreptococcus pneumoniae- Pneumonia. Vaccine made from coat carbohydrates |
| Neisseria meningitidis- Bacterial Meningitis. Vaccine made from coat carbohydrates |
| Haemophilus influenza type b- Influenza, Vaccine made from coat carbohydrates |
| Bacillus anthracis- Anthrax, Vaccine under development |
| Plasmodium falciparum- Malaria. Vaccines using surface glycolipid under development |
|
Leishmania- Leishmaniasis. Vaccines using synthetic version of surface phosphoglycolipid antigens under development |
| Influenza- Tamiflu (oseltamivir phosphate), a monosaccharide based drug currently in-use |
| Infections, gram-negative bacteria. Aminoglycosides, protein synthesis inhibitors that bind to bacterial ribosomes, currently in-use |
| Diseases involving blood clotting, Heparin, an anti-clotting agent, is a mixture of polysaccharides, in-use for decades |
References are comprehensive reviews that include many scores of individual references for each category listed.
ACKNOWLEDGEMENTS
This work was supported by NIH NIGMS SCORE (S0648680), NIH MBRS RISE, NIH MARC, and the Joseph Drown Foundation. SBO is a Participating Investigator in the Consortium for Functional Glycomics.. We thank William Krohmer for expert assistance in generating the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56:425–35. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–7. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH, Wolever TMS. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus: a multicenter controlled clinical trial. Ann Intern Med. 1994;121:928–935. doi: 10.7326/0003-4819-121-12-199412150-00004. [DOI] [PubMed] [Google Scholar]
- Coyle-Thompson C, Oppenheimer SB. A novel approach to study adhesion mechanisms by isolation of the interacting system. Acta Histochem. 2005;107:243–251. doi: 10.1016/j.acthis.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Antiviral agents active against influenza A viruses. Nat Rev Drug Discov. 2006;5:1015–25. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem Biol. 2004;11:1701–7. doi: 10.1016/j.chembiol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Dotan I, Fishman S, Dgani Y, Schwartz M, Karban A, Lerner A, Weishauss O, Spector L, Shtevi A, Altstock RT, Dotan N, Halpern Z. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology. 2006;131:366–78. doi: 10.1053/j.gastro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Elofsson M, Salvador LA, Kihlberg J. Preparation of Tn and sialyl Tn building blocks used in Fmoc solid-phase synthesis of glycopeptides fragments from HIV gp120. Tetrahedron. 1997;53:369–90. [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314:53–7. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein J, editor. Glycochemistry and Glycobiology. Nature. 2007;446:999–1051. [Google Scholar]
- Fischer PB, Collin M, Karlsson GB, James W, Butters TD, Davis SJ, Gordon S, Dwek RA, Platt FM. The alpha-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J Virol. 1995;69:5791–7. doi: 10.1128/jvi.69.9.5791-5797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl MA, Resnick L, Coombs R, Kremer AB, Pottage JC, Jr, Fass RJ, Fife KH, Powderly WG, Collier AC, Aspinall RL, Smith SL, Kowalski KG, Wallemark CB. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200-500 CD4 cells/mm3. J Acquir Immune Defic Syndr. 1994;7:139–47. [PubMed] [Google Scholar]
- Fritz P, Dippon J, Kierschke T, Siegle I, Mohring A, Moisa A, Murdter TE. Impact of mistletoe lectin binding in breast cancer. Anticancer Res. 2004;24:1187–92. [PubMed] [Google Scholar]
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–42. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- Galonic DP, Gin DY. Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines. Nature. 2007;446:1000–7. doi: 10.1038/nature05813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Heinrich EL, Welty LAY, Banner LR, Oppenheimer SB. Direct targeting of cancer cells: a multiparameter approach. Acta Histochem. 2005;107:335–44. doi: 10.1016/j.acthis.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling F, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HF, Livingston PO. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- Helling F, Zhang S, Shang A, Adluri S, Calves M, Koganty R, Longenecker BM, Yao TJ, Oettgen HF, Livingston PO. GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res. 1995;55:2783–8. [PubMed] [Google Scholar]
- Kim YJ, Wang P, Navarro-Villalobos M, Rohde BD, Derryberry J, Gin DY. Synthetic studies of complex immunostimulants from Quillaja saponaria: synthesis of the potent clinical immunoadjuvant QS-21Aapi. J Am Chem Soc. 2006;128:11906–15. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurrum M, Hernandez A, Eskalaei M, Badali O. Coyle-Thompson C, Oppenheimer SB. Carbohydrate involvement in cellular interactions in sea urchin gastrulation. Acta Histochem. 2004;106:97–106. doi: 10.1016/j.acthis.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Latham VH, Ducut JL, Rostamiani K, Chun HH, Lopez ME, Herrera S, Oppenheimer SB. A rapid lectin receptor binding assay: comparative evaluation of sea urchin embryo cell surface lectin receptors. Acta Histochem. 1995a;97:89–97. doi: 10.1016/S0065-1281(11)80209-6. [DOI] [PubMed] [Google Scholar]
- Latham VH, Herrera S, Rostamiani K, Chun HH, Oppenheimer SB. Rapid identification of lectin receptors and their possible function in sea urchin cell systems. Acta Histochem. 1995b;97:373–82. doi: 10.1016/S0065-1281(11)80062-0. [DOI] [PubMed] [Google Scholar]
- Latham V, Martinez A, Cazares L, Hamburger H, Tully MJ, Oppenheimer SB. Accessing the embryo interior without microinjection. Acta Histochem. 1998;100:193–200. doi: 10.1016/S0065-1281(98)80027-5. [DOI] [PubMed] [Google Scholar]
- Latham V, Tully M, Oppenheimer SB. A putative role for carbohydrates in sea urchin gastrulation. Acta Histochem. 1999;101:293–303. doi: 10.1016/S0065-1281(99)80030-0. [DOI] [PubMed] [Google Scholar]
- Lemoine NR. Antithrombotic therapy in cancer. J Clin Oncol. 2005;23:2119–20. doi: 10.1200/JCO.2005.10.975. [DOI] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:10804–9. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PO, Ragupathi G. Cancer vaccines targeting carbohydrate antigens. Hum Vaccin. 2006;2:137–43. doi: 10.4161/hv.2941. [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005:re13. doi: 10.1126/stke.3122005re13. 2005. [DOI] [PubMed] [Google Scholar]
- Marx J. Alzheimer's disease. A new take on tau. Science. 2007;316:1416–7. doi: 10.1126/science.316.5830.1416. [DOI] [PubMed] [Google Scholar]
- Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv Drug Deliv Rev. 2004;56:491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Mody R, Joshi S, Chaney W. Use of lectins as diagnostic and therapeutic tools for cancer. J Pharm Tox Methods. 1995;33:1–10. doi: 10.1016/1056-8719(94)00052-6. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SB. Cancer a biological and clinical introduction. Pearson; Boston: 2004. pp. 39–70. chapters 4,5. [Google Scholar]
- Oppenheimer SB. Cellular basis of cancer metastasis: A review of fundamentals and new advances. Acta Histochem. 2006;108:327–34. doi: 10.1016/j.acthis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Synthetic carbohydrate-based antitumor vaccines: challenges and opportunities. Expert Rev Vaccines. 2005;4:677–85. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- Petrossian K, Banner LR, Oppenheimer SB. Lectin binding and effects in culture on human cancer and non-cancer cell lines: examination of issues of interest in drug design strategies. Acta Histochem. 2007 doi: 10.1016/j.acthis.2007.05.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragupathi G, Gathuru J, Livingston P. Antibody inducing polyvalent cancer vaccines. Cancer Treat Res. 2005;123:157–80. doi: 10.1007/0-387-27545-2_7. [DOI] [PubMed] [Google Scholar]
- Ragupathi G, Livingston PO, Hood C, Gathuru J, Krown SE, Chapman PB, Wolchok JD, Williams LJ, Oldfield RC, Hwu WJ. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9:5214–20. [PubMed] [Google Scholar]
- Sajadi S, Rojas P, Oppenheimer SB. Cyclodextrin, a probe for studying adhesive interactions. Acta Histochem. 2007 doi: 10.1016/j.acthis.2007.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Offer J, Zitzmann N, Dwek RA. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature. 2007;446:1038–45. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L, Hewitt MC, Evans K, Siomos MA, Seeberger PH. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature. 2002;418:785–9. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- Seeberger PH, Werz DB. Synthesis and medical applications of oligosaccharides. Nature. 2007;446:1046–51. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- Seitz O, Kunz H. A novel allylic anchor for solid-phase synthesis-synthesis of protected and unprotected O-glycosylated mucin-type glycopeptides. Angew Chem Int Ed. 1995;34:803–5. [Google Scholar]
- Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–28. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO, Scher HI. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–8. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- Truscheit E, Frommer W, Junge B, Müller L, Schmidt DD, Wingender W. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angew Chem Int.Ed Engl. 1981;20:744–61. [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–8. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- Valentiner U, Fabian S, Schumacher U, Leathem AJ. The influence of dietary lectins on the cell proliferation of human breast cancer cell lines in vitro. Anticancer Res. 2003;23:1197–206. [PubMed] [Google Scholar]
- Varki A. Six blind men and the elephant-the many faces of heparan sulfate. Proc Natl Acad Sci USA. 2002;99:543–5. doi: 10.1073/pnas.022649499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman H, Thor Y. In: Carbohydrate-Based Drug Discovery. Wong CH, editor. Wiley-VCH; Weinheim: 2003. pp. 661–684. [Google Scholar]
- Welty LAY, Heinrich EL, Garcia K, Banner LR, Summers ML, Baresi L, Metzenberg S, Coyle-Thompson C, Oppenheimer SB. Analysis of unconventional approaches for the rapid detection of surface lectin binding ligands on human cell lines. Acta Histochem. 2006;107:411–20. doi: 10.1016/j.acthis.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Zem GC, Badali O, Gaytan M, Hekmatjou H, Alvarez M, Nnoli J, Katus E, Oppenheimer SB. Microbead analysis of cell binding to immobilized lectin: an alternative to microarrays in the development of carbohydrate drugs and diagnostic tests. Acta Histochem. 2006;108:311–7. doi: 10.1016/j.acthis.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]