Abstract
Sema4D (CD100), a member of the neuro-semaphorin family of proteins, has recently been shown to play a role in modulating certain immune responses. We tested the requirement of Sema4D expression on T cells in the induction of T cell allo-immune responses. Sema4D−/− Tcells showed reduced expansion in vitro upon stimulation with allogeneic antigen presenting cells (APCs) when compared to wild type (wt) Tcells. Similar in vitroresults were observed using anti-Sema4D mAbs. Further studies demonstrated that the reduced proliferation was not due to intrinsic T cell defects and that the cytotoxic functions were preserved. After allogeneic bone marrow transplant (BMT), recipients of Sema4D−/− Tcells showed reduced mortality and graft-versus-host disease (GVHD) target organ damage. Allogeneic dendritic cells (DCs) co-cultured with Sema4D−/− responder T cells secreted less TNFα and IL-12p70 compared to wt Tcells. Similar reduction of DC function was observed with anti-Sema4D mAbs. Given the preservation of CTL function we evaluated graft-versus-leukemia (GVL) responses. When BALB/c recipient mice were challenged with the P815 murine mastocytoma cell line (H2d) the recipients of allogeneic Sema4D−/− B6 T cells showed a significant improvement in tumor free survival when compared to syngeneic recipients thus demonstrating preservation of GVL, albeit of a lesser magnitude than allogeneic wt T cells. In summary, Sema4D plays a significant role in mediating in vitro and in vivo allogeneic responses by modulating T cell - APC interactions.
Keywords: CD100, T cells, cytokines, GVHD, BMT
INTRODUCTION
Sema4D (CD100), a 150-KDa transmembrane protein, belongs to the family of semaphorins (1-4), which are chemorepulsive factors needed for the guidance of axons to target cells to establish neuronal synapses. Sema4D is highly expressed in secondary lymphoid organs such as the lymph nodes and spleen and is constitutively expressed on naïve T cells. Sema4D has recently been shown to have immuno-modulatory functions in auto-immune diseases (5-11). CD72, a C type lectin, is the ligand for Sema4D in the immune system (5, 12). It is expressed on the surface of antigen presenting cells (APCs) such as B cells and DCs, suggesting that Sema4D-CD72 interaction modulates immune responses. Absence of Sema4D does not cause differences in the surface phenotype, numbers and ratios of T and B cells between Sema4D−/− and wild type (wt) mice (11). However, Sema4D deficiency has been shown to cause dysregulated B cell responses while Sema4D transgenic animals show enhanced B cell activation and antibody production (13). Kumanogoh and colleagues also showed impaired priming of Sema4D−/− T cells in the context of experimental autoimmune encephalomyelitis (EAE)(10). In these studies Sema4D deficient animals were resistant to EAE while wt mice succumbed to the disease. These observations suggested that Sema4D might be important role in regulating T cell function. However, the role of sema4D in regulating T cell allo-responses is not known. We therefore utilized a well characterized murine model of graft-versus-host (GVHD) and graft-versus-leukemia (GVL) to evaluate the role of Sema4D in modulating allo-responses.
Acute GVHD is a major complication in allogeneic bone marrow transplantation (BMT) that is caused by donor T cells (14-16). Donor T cells encounter with alloantigen on APCs (16) is critical for the induction of GVHD. The secretion of pro-inflammatory mediators such as TNFα and IL-12 by the APCs further enhances the allo-responses of donor T cells (17) and aggravates GVHD. We evaluated the role of Sema4D expression by donor T cells on the severity of GVHD in a [B6→BALB/c] MHC mismatched model of allogeneic BMT. Because GVHD is tightly linked to the beneficial GVL effects after allo-BMT, we also analyzed the impact of Sema4D expression on donor T cells in the anti-tumor responses after allo-BMT. We demonstrate that the absence or blockade of Sema4D a) regulated allo-proliferative responses in vitro (b) reduced GVHD in vivo (c) did not impair the cytolytic T-cell responses and (d) preserved GVL effects to a lesser magnitude.
STUDY DESIGN
Mice
BALB/c (H-2d), C57BL/6 (H-2b), were purchased from Jackson Laboratories (Bar Harbor, ME). Sema4D−/− B6 (H-2b) (10) (courtesy Dr. Kumanogoh) homozygous animals were bred and maintained in a specific free environment in compliance with the University of Michigan animal use and care protocols. Recipients used for BMT were between the ages of 12 and 20 wk and weigh at least 18 grams. All experiments were approved by the University of Michigan Committee on the Use and Care of Animals.
Bone Marrow Transplantation
Bone marrow (BM) was harvested from the femurs and tibias of donor mice. Cell mixtures of 5 × 106 BM cells supplemented with 0.50 × 106 isolated splenic T cells from either wt B6 or Sema4D−/− with CD90 magnetic beads (Miltenyi-Biotec automated cell sorter) were resuspended in Leibovitz's L-15 medium (Life Technologies, Grand Island, NY) and transplanted into B6 or BALB/c recipients via tail vein infusion (0.25 ml total volume). Before transplant, host mice received 8 Gy of total body irradiation (137Cs source) delivered in two fractions separated by 3 h to reduce gastrointestinal toxicity. Mice were subsequently housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated water for the first 3 wk after BMT and filtered water thereafter.
Systemic and histopathologic assessment of GVHD
The degree of systemic GVHD was assessed by a standard scoring system as described previously (18-20). Transplanted mice were ear punched, and individual weights were obtained and recorded on day 0 and weekly thereafter. A clinical index was generated weekly by summation of five criteria scores: percentage of weight change, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10). Animals that received a score of 6.5 or higher were humanely euthanized.
Acute GVHD was also assessed by detailed histopathologic analysis of the small (ileum) and large (ascending) intestine. Specimens were harvested from animals on days +14 and +60, placed in 10% buffered formalin, embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin for histological examination. Slides were coded without reference to mouse type or prior treatment status and examined systematically by a single pathologist (C. Liu) using a semiquantitative scoring system. Specific parameters scored included villous blunting, crypt regeneration, crypt epithelial cell apoptosis, crypt loss, luminal sloughing of cellular debris, lamina propria inflammatory cell infiltrate, and mucosal ulceration in the small bowel, crypt regeneration, crypt epithelial cell apoptosis, crypt loss, surface colonocyte vacuolization, surface colonocyte attenuation, lamina propria inflammatory cell infiltrate, and mucosal ulceration in the large bowel and portal tract expansion by an inflammatory cell infiltrate, lymphocytic infiltrate of bile ducts, bile duct epithelial cell apoptosis, bile duct epithelial cell sloughing, vascular endotheliitis, parenchymal apoptosis, parenchymal microabscesses, parenchymal mitotic figures, hepatocellular cholestasis, and hepatocellular steatosis in the liver. The scoring system for each parameter that evaluated both the extent and severity of tissue damage denoted 0 as normal, 0.5 as focal and rare, 1 as focal and mild, 2 as diffuse and mild, 3 as diffuse and moderate, and 4 as diffuse and severe.
Mixed leukocyte reactions and DC cultures
All culture media reagents were purchased from Gibco BRL (Gaithersburg, MD). For analysis of proliferative response and IFN-γ production, splenocytes were harvested from naive BALB/c, B6 or Sema4D−/− mice. Anti-Sema4D mAbs (courtesy of Dr. Kumanogoh) were utilized in the cultures at 1μg/mL (21). Cells were suspended in 5% FCS/RPMI supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acid, 0.02 mM β-mercaptoethanol, and 10 mM HEPES (pH 7.75). These cells (0.5 × 105 to 2 × 105) were cultured in flat-bottomed 96-well Falcon plates (Lincoln Park, NJ) in the presence of irradiated (2,000 Rad) splenocytes from naive BALB/c animals at 37°C in a humidified incubator supplemented with 5% CO2. Supernatants were collected at 48 h for IL-2 and IFN-γ analysis by ELISA, and proliferative response to host antigen was measured by a 1205 Betaplate reader (Wallac, Turku, Finland) after 72-96 h by incorporation of [3H]thymidine (1 μCi) for the last 18-24 h of incubation.
For the DC cultures BM was isolated from BALB/c femurs and tibias (as described previously) and cultured for 7 days with GMCSF and 10% DMEM. CD11c+ DCs were purified using CD11c magnetic beads (Miltenyi-Biotec automated cell sorter) and co-cultured for 10-72 hours with CD3+ T cells from BALB/c, wt B6 +/− antiCD100mAbs or CD100−/− animals. TNFα and IL-12p70 supernatants were harvested and measured with sandwich ELISA (below).
Cytokine ELISA
Concentrations of TNF-α, IL-12p70, IL-2 and IFN-γ were measured from serum and cell culture supernatants in triplicate by sandwich ELISA by using specific anti-murine MoAbs for capture and detection and the appropriate standards: IFN-γ, IL-2 and IL-12p70 (BD Pharmingen, San Diego, CA) and TNF-α (R&D Systems, Minneapolis, MN). All of the in vitro assays were performed more than twice, the in vivo analyses were from seven different mice / group and run according to the manufacturer's protocol. Samples were diluted 1:2 to 1:5. ELISA plates were read at 450 nm by using a microplate reader (Bio-Rad Labs, Hercules, CA).
Flow Cytometric analysis
A flow cytometric analysis was performed using FITC, PE or allyphycocyanin-conjugated monoclonal antibodies (mAbs) to mouse CD3, CD8, CD4, H2b (Pharmigen) (22). To analyze cell surface phenotype, splenocytes from naive or transplanted mice were resuspended in PBS and stained as described in manufacturer's protocol. In brief, cells (0.5 × 106) were incubated for 20 min at 4°C with mAb 2.4G2 to block nonspecific staining by Fc receptors and then with the appropriate conjugated mAbs for 30 min at 4°C. They were subsequently washed twice with PBS/0.2% BSA and fixed in 1% paraformaldehyde. Two or three-color flow cytometric analysis was performed by using FACS Vantage or FACS Diva SE cell sorter (Becton Dickinson, San Jose, CA).
Luciferase+ P815 cell line
P815 leukemia cells were purchased from ATCC (Virginia) and were transduced with a third generation lentivirus co-expressing GFP and firefly luciferase (Luc) (23, 24). Briefly, 0.5 × 106 fresh P815 cells were suspended in 1 mL of 10% DMEM and 8μg/mL of polybrene in a 6 well plate. Cells were centrifuged at 2000 rpms at 32 °C for 30 minutes. Cells were then cultured for 7 hours at 37 °C . 1mL of fresh media (total 2mL) mas added for overnight incubation. On day two, P815 cells were washed with fresh 10% DMEM and allowed to expand for 24 hours. Stably transduced cells were sorted (FACS Diva) for high expression of GFP and re-cultured in vitro for 2-4 days with 10% DMEM.
Bioluminescence imaging
Bioluminescence imaging (25) was performed with a cryogenically cooled CCD camera (IVIS, Xenogen). Acquisition and analysis of images were performed as previously described (26, 27). All animals were imaged 10 minutes after being injected IP with 100μL (40mg/mL) of firefly D-luciferin (Biosyth, Switzerland). Animals were imaged 5 minutes to 30 seconds depending on the signal strength. All animals were maintained under isoflurane anesthesia and in a 37 °C heated environment.
Induction of Leukemia
The P815 leukemia model was used for the graft-versus-leukemia (GVL) experiments (28). P815 is a mastocytoma (H2d) cell line derived from DBA/2 mouse. On day 0, 400 P815 cells were injected to each recipient along with syngeneic (BALB/c) or allogeneic (B6) BM and either BALB/c, CD100−/− or B6 (wt) CD90+ splenic T cells. As few as 100 P815 cells can cause fatal leukemia in syngeneic hosts. P815 cells invade particularly the liver, the lymph nodes, bone marrow and spleen. Animals were monitored twice daily for survival and the cause of death determined by postmortem gross pathology examination. Tumor burden was indirectly assessed via in vivo bioluminescence to document disease progression over time.
Chromium release assays
Tumor targets, 2×106 P815 (H-2d) or EL4 (H-2b), were labeled with 100 µCi of 51Cr sodium salt (NEN Life Sciences Products) for 2 h. After washing labeled targets were resuspended and the 51Cr-release assays was performed as described previously (28). Allogeneic splenocytes were harvested and normalized for donor CD8 (CD45.1+, CD8+) cells on day +14. These preparations were added to quadruplicate wells at varying effector-to-target ratios and incubated for 4 h. Maximal and background release was determined by the addition of Triton-X (Sigma) or media alone to targets, respectively. 51Cr activity in supernatants taken 5 h later was determined in an autogamma counter (Packard) as described (28).
Statistical analysis
Satistical analysis was performed as described earlier (22).The Mann Whitney U test was used for the statistical analysis of clinical scores, histopathology scores, and cytometric analysis. The Wilcoxon rank test was used to analyze survival data. p<0.05 was considered statistically significant.
RESULTS
Deficiency of Sema4D causes less T cell alloreactivity
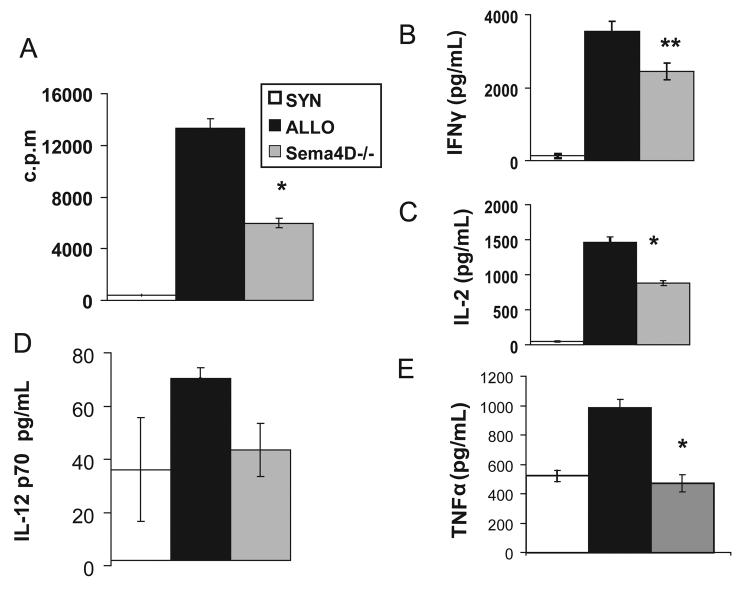
We first tested the role of Sema4D expression on T cells in modulating in vitro allo-responses. We used a genetic approach by utilizing Sema4D deficient T cells as alloresponders in a mixed lymphocyte reaction (MLR) using allogeneic BALB/c stimulators. Sema4D−/− T cells expanded two fold less compared to the wt Tcells (p<0.01) (Fig 1A). Consistent with the decreased proliferation, T cell cytokines IFNγ (Fig 1B) and IL-2 (Fig 1C) production was also significantly decreased. We next cultured B6 wt Tcells with anti-Sema4D monoclonal antibodies (mAbs) and irradiated BALB/c splenocytes. Allogeneic T cells expanded 32% less in the presence of anti-Sema4D mAbs when compared to controlled cells (p<0.01, Table 1). Taken in the context of previous observations that Sema4D−/− T cells proliferated normally to anti-CD3 and concanavilin A stimulation(10), these results demonstrate diminished responses by T cells to allo-stimulation but not to nominal antigens in the presence of monoclonal antibody or with lack of Sema4D expression on T cells.
FIGURE 1. Deficiency of Sema4D decreases cytokine production of Tcells and DCs and lowers T cell expansion in an allogeneic in vitro setting.
BALB/c stimulators and T cells from either B6 wt (black bars, allogeneic), Sema4D −/− (grey bars, allogeneic), or BALB/c Tc (white bars, syngeneic) were used., Fig 1a-c, BALB/c splenocytes irradiated and co-cultured with either BALB/c, B6 wt, or Sema4D −/− Tcells at a 2/1 stimulator/responder ratio. (a)- proliferation (72 hours), (b)- IL-2 production (48 hours), (c)- IFNγ (48 hours). (d-e) TNFα and IL12p70 levels measured from supernatants in 18-24 hours co-cultures with non-irradiated CD11c+ BALB/c DCs and BALB/c T cells (white bar), B6 wt T cells (black bars), Sema4D −/− Tcells (gray bar) at a 2:1 ratio. *p<0.01 **p<0.02
TABLE 1. Anti-Sema4D monoclonal antibody effects in allogeneic responses.
BALB/c stimulators and either BALB/c or wtB6 T cells +/− anti-Sema4D monoclonal antibody (1μg/mL). BALB/c splenocytes irradiated and co-cultured with T cells at a 2/1 stimulator/responder ratio. Non-irradiated CD11c+ BALB/c DCs and either BALB/c T cells or B6 wt T cells +/− anti-Sema4D mAbs at a 2:1 ratio where co-cultured for the analysis of TNFα and IL-12p70. Cytokine levels measured from supernatants 18-24 hours later.
| SYN | WT (allo control) |
WT (anti-Sema4DmAbs) |
|
|---|---|---|---|
| cpm | 824+/− 445 | 10614+/−900 | 7229+/−808* |
| IL-12p70 | 275+/−60 | 377+/−54 | 233+/−39 |
| TNFα | 50+/−40 | 654+/−57 | 326+/−29* |
P<0.03
Sema4D expression on T cells modulates allogeneic dendritic cell function
Next, we investigated the mechanism of decreased alloproliferative responses by the Sema4D−/− Tcells. APC function is critical for modulating adaptive T cell responses (29). Because Sema4D−/− T cells do not have any cell intrinsic defect upon TCR stimulation we hypothesized that Sema4D expression on Tcells modulates T cell responses indirectly. To test our hypothesis we performed co-culture experiments of DCs, the most potent APCs (30, 31), either with wt or Sema4D−/− T cells. The DC function was determined by secretion of pro-inflammatory cytokines (32) as described in materials and methods. BALB/c DCs that were cultured with B6 Sema4D−/− T cells showed a significant reduction in the secretion of TNFα and IL12p70 (Fig 1D, 1E) when compared to DCs cultured with wt Tcells. To rule out non-specific and/or compensatory effects of Sema4D−/− T cells we performed similar experiments in the presence of anti-Sema4D mAbs. Similar reduction in DC cytokine secretion was observed in the presence of anti-Sema4D mAbs (Table 1). We also assessed for the expression of co-stimulatory molecules (CD80, CD86, CD40) on the surface of the DCs (30, 33) in these cultures and observed no statistically significant differences between the groups (data not shown). Collectively these data show that expression of Sema4D on allo-T cells enhances APC function as determined by cytokine secretion without significantly altering the phenotype of APCs.
Effect of Sema4D on T cell cytotoxicity
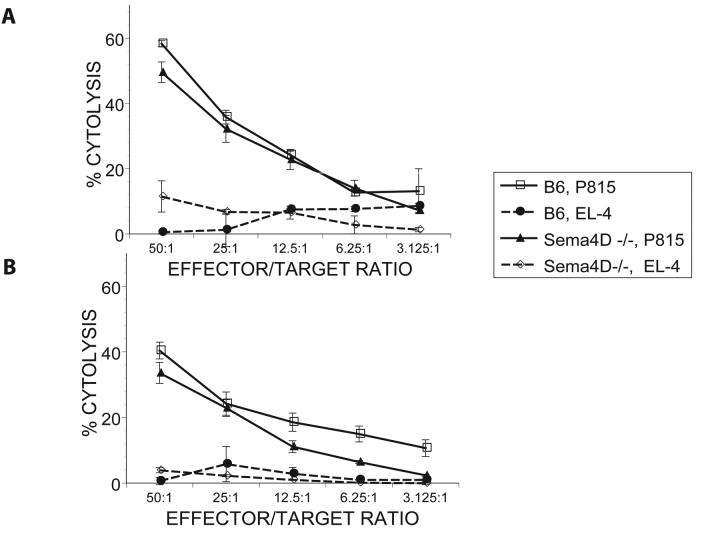
We next assessed the effects of Sema4D deficiency on T cell mediated cytotoxic responses. We examined the allo-specific cytolytic functions of Sema4D−/− and wt T cells after in vitro priming. Wt and Sema4D−/− Tcells were stimulated with irradiated BALB/c splenocytes for 5 days. The cells were then harvested and evaluated for their CTL function in a chromium release assay against allogeneic (H2d) P815 target cells. As shown in Figure 2A, Sema4D−/− cytolytic effects were preserved and were comparable to B6 wt T cells. Lack of significant lysis of syngeneic targets demonstrated that was no indiscriminate killing of target cells.
FIGURE 2. Maintenance of CTL by Sema4D−/− T cells.
B6 wt (allogeneic H2b) or Sema4D−/− (allogeneic H2b) CD90+ T cells cells were cultured in vitro for 5 days with irradiated BALB/c (H2d) splenocytes (a) or transplanted into irradiated BALB/c mice (b) as described in materials and methods. Splenocytes were harvested from the cultures on day +5-6 in the in vitro priming experiments (a) or from recipients (n=5/group) on day +14 after BMT. CTL assays, normalized for total donor (H2b) CD8+ cells, and used in a 51Cr-release assay. Cytotoxic T lymphocyte activity against allo targets (P815) in wt allo-control (□) vs. Sema4D−/− allo (▲) groups. Lysis in allogeneic groups was similar, whereas no significant lysis of syngeneic targets (EL-4) observed in either of wt (●) or Sema4D−/− (◇)groups . Data from one of two similar experiments shown.
Sema4D−/− donor T cells induce less severe GVHD morbidity and mortality
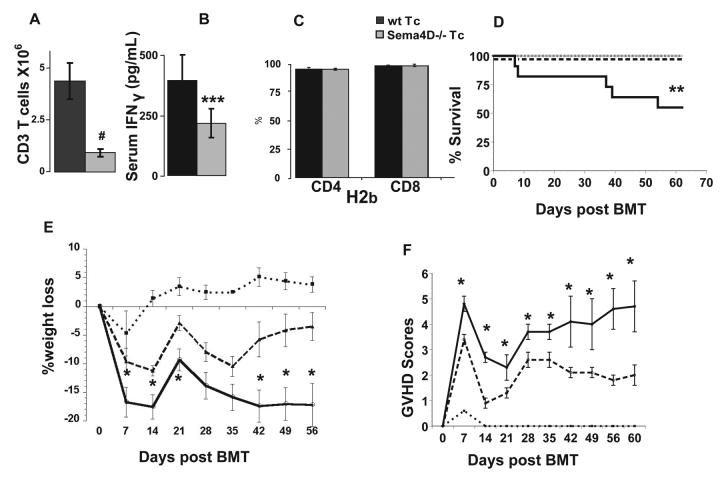
We determined the in vivo relevance of Sema4D in modulating allo-responses. To that end, we used GVHD as a read-out for T cell alloreactivity in MHC mismatched model of allogeneic BMT. Based on the in vitro observations and the reductions in T cell expansion, we hypothesized that Sema4D deficiency would reduce GVHD. BALB/c animals were transplanted with B6 wt bone marrow (BM) along with purified splenic T cells from wt or Sema4D−/− B6 donors following lethal irradiation with 800cGy. Consistent with the in vitro data, Sema4D−/− donor Tcells showed a significant reduction in expansion on day +14 after BMT (Fig 3A). Serum IFNγ was significantly reduced in recipients of Sema4D−/− T cells compared to animals injected with allogeneic wt T cells (Fig 3B). Allogeneic animals that received B6 Sema4D−/− T cells exhibited significantly less weigh loss (Fig 3E), less severe GVHD (Fig 3F), and mortality (p<0.02) (Fig 3D) when compared to the recipients of wt T cells. As expected, all syngeneic recipients of wt T cells were alive by day 60. Furthermore, syngeneic control animals that received Sema4D−/− T cells also demonstrated 100% survival showing that homeostatic expansion of Sema4D−/− T cells did not affect overall survival.
FIGURE 3. Sema4D −/− Tcell recipients have improved survival and clinical GVHD scores.
(a-f) BALB/c (allogeneic) or B6 (syngeneic) recipients were lethally irradiated (800cGy) and intravenously given 5.0×106 B6 wt BM and either 5.0×105 wt B6 or Sema4D −/− T cells. (a-c) On day +14 mice were humanely euthanized and (a) spleens of recipient animals were analyzed for total H2b CD3+ Tcells (b) serum IFNγ levels (c) CD4+ and CD8+ donor derived (H2b) chimerism. (d) survival (e) % weight loss and (f) GVHD scores compared to d+0. (d-f) B6 (solid line), Sema4D −/− Tcells (dashed line) and BALB/c (dotted line). # p<0.01 *p<0.05 **p<0.02 ***p<0.06. Results are representative of one of three similar experiments with a total of n =18 / allogeneic recipients of the control and sema4−/− donors.
Sema4D−/− T cells reduce target organ damage and improve immune reconstitution after allogeneic BMT
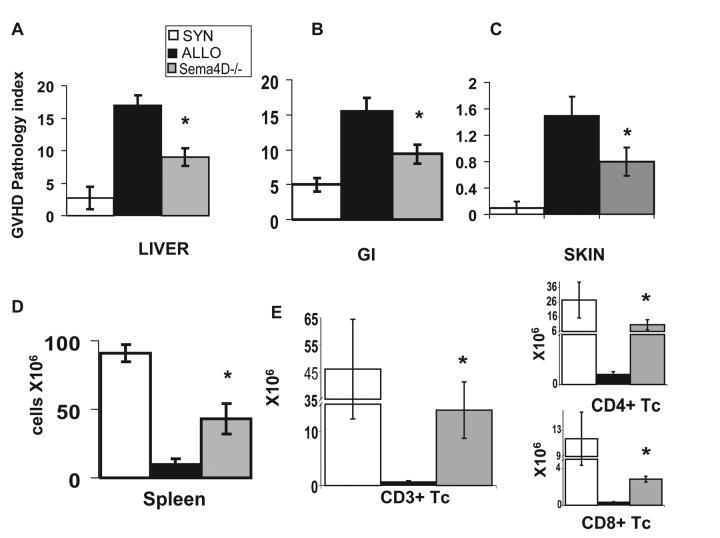
Next, we confirmed the reduction of GVHD by histopathologic analysis of the GVHD target organs, liver, skin and intestines, on day +60 post BMT. As shown in Figure 4A-C, allogeneic recipients of Sema4D−/− T cells showed reduced GVHD target organ damage when compared to recipients of wt T cells. Similar reductions were also observed on day +14 (data not shown). On day +14 wt and Sema4D−/− allo-T cell recipients demonstrated comparable donor T cell chimerism thus indicating that alterations in the degree of mixed chimerism was not a cause for the reduction in GVHD (Fig 3C).
FIGURE 4. Sema4D−/− T cell recipients have improved immune reconstitution and decreased GVHD target organ pathology.
(a-e) BALB/c (allogeneic) or B6 (syngeneic) recipients were irradiated with 800cGy and intravenously given 5.0×106 B6 wt BM and either 5.0×105 wt B6 or Sema4D−/− T cells. n=4syn, n=6 allogeneic control, n=6 Sema4D−/− (a-c) Pathology scores of GVHD target organs on day +60 post BMT as described in materials and methods of (a) liver (b) small and large intestine, and (c) skin. (d) Splenic cellularity on d+60 post BMT. (e) Donor (H2b) CD3+, CD4+ and CD8+ from recipient spleens on d+60. Syngeneic recipients (white bars), allogeneic B6 wt Tc (black bars) and allogeneic recipients of Sema4D −/− B6 Tc recipients (gray bars). One of two similar experiments. *p<0.03
We next assessed the degree of immune reconstitution after allo-BMT. We analyzed T cell and other donor cell lineages in the spleen of recipient mice at the end of the observation period as a marker for immune reconstitution. Consistent with reduced GVHD results, the overall splenic cellularity (Fig 4D), the numbers of donor CD3+ T cells and CD4, and CD8 T cell subsets (FIG 4 E) was significantly better in the allo-recipients of Sema4D−/− T cells. These data demonstrate that allo-recipients of Sema4D−/− T cells show an improvement in immune reconstitution when compared to recipients of wt T cells and less GVHD target organ damage.
Sema4D−/− T cells and graft-versus-leukemia effects
Preservation of in vivo donor T cell CTL is critical for maintenance of GVL after allo-BMT (22, 34). We therefore first determined whether T cell CTL were preserved in vivo post allo-BMT. T cells were harvested from the spleens of animals 14 days post allogeneic BMT. T cells from animals that received allo B6 wt or CD100−/− Tcells (Fig 2B) demonstrated similar cytolytic function towards P815 targets.
We further assessed whether the preservation of CTL functions held true despite the reduction in proliferation in the absence of Sema4D on donor T cells and would lead to maintenance of GVL. BALB/c (H2d) recipients where lethally irradiated with 800cGy and injected with 400 syngeneic P815 (H2d) mastocytoma cells along with allogeneic wt BM and T cells from either wt or Sema4D−/− donors at time of transplant (given on day +0 at the same time of the BM inoculum). BALB/c animals that received 5×106 BM cells and 0.5×106 BALB/c T cells along with P815 cells served as syngeneic controls. Prior to the in vivo infusion, the P815 cells were transduced with a third generation lentivirus as described in materials and methods section, which contained a GFP and Luciferase (Luc+) construct to facilitate for in vivo monitoring of the tumor burden in the same animals and at different time points by using bioluminescence imaging (BLI) (FIG 5A, 5B). Mice with leukemia and a BLI photon flux of 1.0 ×1010/ animal died within 24 hours. This corresponded to a tumor burden of 12 billion total cells/ mouse. All of the syngeneic recipients that did not receive tumor survived while all of the syngeneic recipients that were injected with tumor died from the tumor without signs of GVHD by day 12. By contrast, animals that received the allogeneic T cells died by day 30 with signs of GVHD (FIG 5C). The median survival time (MST) of tumor bearing syngeneic animals was 10 days, while MST for the allogeneic recipients was 25 days (B6 wt T cell recipients) and 24 days (Sema4D −/− T cell recipients). However, allogeneic recipients showed evidence of tumor as detected by BLI, but did so at a lesser intensity with a significantly slower kinetics, thus demonstrating better anti-tumor responses than syngeneic controls. We further evaluated the magnitude of CTL responses by increasing the tumor cell dose to 1000 cells. Sema4D−/− T cell recipients demonstrated equivalent median tumor free survival (MST d+21 wt vs. d+22 Sema4D−/−) as the wt T cell recipients in the higher tumor cell challenge (Figure 5D).
FIGURE 5. Sema4D−/− GVL effects.
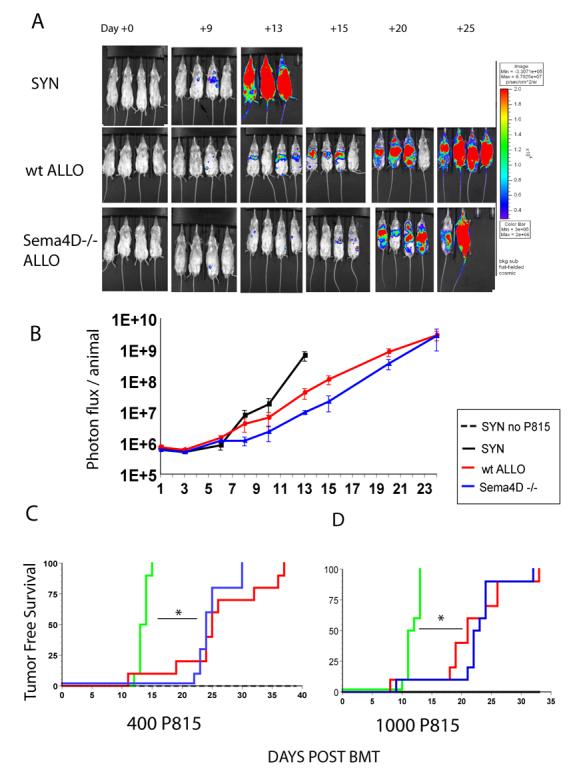
BALB/c (syngeneic H2d), B6 wt (allogeneic H2b) or Sema4D−/− (allogeneic H2b) recipients were irradiated with 800cGy and intravenously given 5.0×106 BM cells from BALB/c (syngeneic) or wt B6 (allogeneic groups) mice, plus either 5.0×105 wt B6 (allogeneic control), Sema4D−/− T cells (experimental) or BALB/c T cells (syngeneic control). 400 P815 tumor cells were added into the BM inoculum of all groups. A syngeneic [BALB/c→BALB/c] control group did not receive tumor.
A) BLI images show progression of tumor growth systemically over 24 days n=4/group. Tumor burden in Sema4D−/− T cell recipients is similar to B6 wt T cell recipients. (B) Mean values ± SEM for photon flux/animal (n = 4 mice). (C and D) Tumor free survival n=6 syngeneic without tumor, n=10 syngeneic + P815, n=10 allo B6 wt T cell recipients + P815 and n=10 allo Sema4D−/− T cell recipients + 400 P815(C) or 1000 P815 (D). Improved tumor free survival in allogeneic T cell recipients compared to the syngeneic recipients in both tumor challenges. Sema4D−/− allogeneic T cell recipients had a similar GVL effect as wt T cell control allogeneic recipients. *p<0.01
DISCUSSION
Our data demonstrate a novel role for Sema4D in modulating in vitro and in vivo allogeneic responses. We show that in the absence of Sema4D, T cells show decreased proliferation to allostimulation but not to nominal antigens, decreased cytokine secretion and maintenance of CTL. Our data extend the previous observations demonstrating an important immuno-modulatory role of Sema4D in autoimmune models (10, 35). In those studies, modulation of humoral immune responses were key to the observed effects of Sema4D (35). By contrast, our experiments focused primarily on T cell mediated allo-immune processes.
In our studies Sema4D−/− Tcells in an allogeneic setting expanded less when compared with wt T cells. These results were further confirmed by anti-Sema4D mAbs experiments. These MLR differences were associated with decreased production of the T cell cytokines IL-2 and IFNγ. These results are in line with previous observations that Sema4D−/− OVA TCR transgenic (Tg) animals produced less IFNγ compared with wt OVA TCR Tg animals. Furthermore, crosslinking the T cell receptor with the lectin concanavilin A (10), demonstrated that Sema4D−/− T cell proliferation capacity was unaffected (data not shown). In addition, consistent with previous observations there were no significant differences in CD4/CD8 ratios between the WT and the sema4D−/− animals (11). Furthermore, there were no differences in the absolute numbers of T cells or CD4/CD8 ratios in the purified T cells from the Sema4D−/− mice and the WT animals (CD4+ 64 +/− 7 % vs. 68 +/−4%, P = NS) (Supplemental Figure 1). All these findings support the concept that the intrinsic proliferative responses of Sema4D−/− are not altered, at least, when exposed to strong stimulatory signals.
These in vitro responses were further supported by our in vivo studies demonstrating reduced Sema4D−/− T cell numbers in the spleen of recipients 14 days post BMT compared to recipients that received wt T cells. This demonstrates that that Sema4D deficiency does not completely abrogate the capacity of the T cells to expand and secrete IFNγ upon in vivo alloactivation. Nonetheless, the reduction of proliferation was still significant enough to confer a survival benefit from GVHD. However, when the T cell dose was increased, consistent with lack of complete abrogation of alloproliferative responses, all of the recipient animals succumbed to GVHD (data not shown).
We also observed that Sema4D−/− T cell had comparable cytolytic effects with wt T cells. Although our CTL assays were normalized for CD8+ Tcells, these experiments included both CD4 and CD8 T cells and it is therefore conceivable that part of the preservation in cytolytic responses were mediated through CD4 help (22, 36-38). These studies nontheless support the notion that CD8 T cell CTL function was not affected by CD100 deficiency, which possibly may have allowed for the GVL effects to remain intact. In the GVL studies most animals, however, eventually succumbed to the leukemia despite small tumor doses. This is likely due to the small numbers of T cells that were utilized in our in vivo studies. We maintained similar transplant conditions to evaluate GVL responses at the T cell dose that best produced a reduction in GVHD specific mortality. In any case, both Sema4D−/− and wt T cells demonstrated a clear GVL response as determined by the BLI and tumor free survival when compared to syngeneic recipients. In order to further support that CD8 T cell responses were not affected, we performed a minor mismatch CD8+ T cell driven BMT [B6→ C3H.sw] (39). In this primarily CD8+ driven system, the survival advantage of Sema4D deficiency was not observed (data not shown). In summary, the GVL / CTL experiments together demonstrate that Sema4D−/− CD8+ T cells show no cytolytic deficiencies when primed in vivo or in vitro.
It has been shown that Sema4D is required for the maturation of DCs (10). DC maturation may be an important factor for explaining the differences in alloresponses observed by our Sema4D−/− T cells. CD72, the receptor for Sema4D in the immune system, modulates APC activation by switching off CD72 induced negative signals (5). When we assessed DC cytokine functional responses, we observed a significant reduction in TNFα and IL12p70 secretion after in vitro cultures with allogeneic sema4D−/− T cells than allogeneic wt T cells. The differences were further confirmed by utilizing anti-Sema4D mAbs. The differences in the DC function as determined by the secretion of the pro-inflammatory cytokines were also maintained after treatment with LPS (data not shown). We nonetheless did not observe significant differences in DC phenotype as determined by CD80/CD86 co-stimulatory molecule expression. However, DC phenotype does not always correlate with DC immune functions (40, 41). Alternatively it is possible that Sema4D-CD72 interaction is sufficient for altering cytokine secretion of DCs but not for co-stimulatory expression in the context of a strong allo-stimulus. Moreover previous in vitro studies with CD40 antibody induced DC stimulation showed that deficiency of CD100 impaired the production of IL-12 and prevented expression of CD80(10). Furthermore, addition of soluble Sema4D enhanced the activation and function of CD40 antibody treated DCs (10). Our observations suggest that Sema4D expression on T cells is necessary for the full activation and maturation of allogeneic DCs, which in return further activate the T cell responses.
Our data thus extend previous observations and suggest a novel role for Sema4D in the activation and maturation of APCs and in the modulation of in vivo and in vitro allo-responses. The modulation is significant enough to decrease GVHD and preserve CTL and GVL. Thus targeting Sema4D might facilitate in modulating allo-responses enough to reduce GVHD but preserve CTL dependent GVL.
Supplementary Material
Wt and Sema4D−/− spleens from unexperimented animals were analyzed for CD4 and CD8 phenotype ratios. The splenocytes were gated for whole cell light scatter and βTCR. Adjusted percentages background substracted. Similar CD4 and CD8 ratios can be observed in a representative Sema4D−/− and wt mouse.
Acknowledgements
The author would like to thank the husbandry staff for the care of the animal colonies and the flow core from the University of Michigan's Comprehensive Cancer Center.
Funding: NIH/NCRR T32-RR07008-21A1 (R.D.S), NIH 5 K08 AI052863-04 (P.R.), National Marrow Donor Program Alaina J. Enlow Amy Strelzer Manasevit Research Agreement 13998 (P.R.), NIH 5 P01 CA039542-20 (J.L.M.F.)
Abbreviations used
- DC
dendritic cells
- BMT
bone marrow transplantation
- GVHD
graft-versus-host disease
- MHC
major histocompatibility antigen
- mAbs
monoclonal antibodies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
RDS designed and performed experiments and wrote the paper, PR, JLMF intellectual contribution and wrote the paper, CL pathology analysis, SC, statistical analysis, GL,AK, KL, BW, IT, performed experiments, and CR FACS analysis.
All authors concur with the submission and do not have any conflicting financial interests pertaining to this work.
References
- 1.Bougeret C, Mansur IG, Dastot H, et al. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. 1992:318–323. [PubMed] [Google Scholar]
- 2.Hall KT, Boumsell L, Schultze JL, et al. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. 1996:11780–11785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaire S, Elhabazi A, Bensussan A, Boumsell L. CD100 is a leukocyte semaphorin. Cell Mol Life Sci. 1998;54:1265–1276. doi: 10.1007/s000180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spriggs MK. Shared resources between the neural and immune systems: semaphorins join the ranks. Current Opinion in Immunology. 1999;11:387–391. doi: 10.1016/S0952-7915(99)80065-X. [DOI] [PubMed] [Google Scholar]
- 5.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nature reviews. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 6.Kumanogoh A, Kikutani H. The CD100-CD72 interaction: a novel mechanism of immune regulation. Trends in immunology. 2001;22:670–676. doi: 10.1016/s1471-4906(01)02087-7. [DOI] [PubMed] [Google Scholar]
- 7.Kumanogoh A, Kikutani H. Immune semaphorins: a new area of semaphorin research. Journal of cell science. 2003;116:3463–3470. doi: 10.1242/jcs.00674. [DOI] [PubMed] [Google Scholar]
- 8.Kumanogoh A, Kikutani H. Roles of the semaphorin family in immune regulation. Advances in immunology. 2003;81:173–198. doi: 10.1016/s0065-2776(03)81005-2. [DOI] [PubMed] [Google Scholar]
- 9.Kumanogoh A, Kikutani H. Biological functions and signaling of a transmembrane semaphorin, CD100/Sema4D. Cell Mol Life Sci. 2004;61:292–300. doi: 10.1007/s00018-003-3257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumanogoh A, Suzuki K, Ch'ng E, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J Immunol. 2002;169:1175–1181. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- 11.Shi W, Kumanogoh A, Watanabe C, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 12.Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe C, Kumanogoh A, Shi W, et al. Enhanced immune responses in transgenic mice expressing a truncated form of the lymphocyte semaphorin CD100. J Immunol. 2001;167:4321–4328. doi: 10.4049/jimmunol.167.8.4321. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara JL, Cooke KR, Pan L, Krenger W. The immunopathophysiology of acute graft-versus-host-disease. Stem cells (Dayton, Ohio) 1996;14:473–489. doi: 10.1002/stem.140473. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Seminars in hematology. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara JL, Krenger W. Graft-versus-host disease: the influence of type 1 and type 2 T cell cytokines. Transfusion medicine reviews. 1998;12:1–17. doi: 10.1016/s0887-7963(98)80085-0. [DOI] [PubMed] [Google Scholar]
- 18.Cooke K, Hill G, Crawford J, et al. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft versus host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda Y, Reddy P, Lowler KP, Liu C, Bishop DK, Ferrara JL. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood. 2005;106:749–755. doi: 10.1182/blood-2004-10-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 21.Kumanogoh A, Watanabe C, Lee I, et al. Identification of CD72 as a Lymphocyte Receptor for the Class IV Semaphorin CD100: A Novel Mechanism for Regulating B Cell Signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 22.Maeda Y, Levy RB, Reddy P, et al. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood. 2005;105:2023–2027. doi: 10.1182/blood-2004-08-3036. [DOI] [PubMed] [Google Scholar]
- 23.Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer research. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 24.Contag CH, Jenkins D, Contag PR, Negrin RS. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia (New York, NY. 2000;2:41–52. doi: 10.1038/sj.neo.7900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nature reviews. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 26.Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. Journal of virology. 2003;77:11082–11093. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luker GD, Bardill JP, Prior JL, Pica CM, Piwnica-Worms D, Leib DA. Noninvasive bioluminescence imaging of herpes simplex virus type 1 infection and therapy in living mice. Journal of virology. 2002;76:12149–12161. doi: 10.1128/JVI.76.23.12149-12161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annual review of immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau J, Briere F, Caux C, et al. Immunobiology of Dendritic Cells. 2000:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara JL. The cytokine modulation of acute graft-versus-host disease. Bone marrow transplantation. 1998;21(Suppl 3):S13–15. [PubMed] [Google Scholar]
- 33.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 34.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 35.Li M, O'Sullivan KM, Jones LK, et al. CD100 enhances dendritic cell and CD4+ cell activation leading to pathogenetic humoral responses and immune complex glomerulonephritis. J Immunol. 2006;177:3406–3412. doi: 10.4049/jimmunol.177.5.3406. [DOI] [PubMed] [Google Scholar]
- 36.Giralt SA, Kolb HJ. Donor lymphocyte infusions. Curr Opin Oncol. 1996;8:96–102. doi: 10.1097/00001622-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kolb HJ, Holler E. Adoptive immunotherapy with donor lymphocyte transfusions. Curr Opin Oncol. 1997;9:139–145. doi: 10.1097/00001622-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kolb HJ, Mittermuller J, Holler E, Thalmeier K, Bartram CR. Graft-versus-host reaction spares normal stem cells in chronic myelogenous leukemia. Bone Marrow Transplant. 1996;17:449–452. [PubMed] [Google Scholar]
- 39.Baker MB, Altman NH, Podack ER, Levy RB. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. The Journal of experimental medicine. 1996;183:2645–2656. doi: 10.1084/jem.183.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nature immunology. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 41.Menges M, Rossner S, Voigtlander C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. The Journal of experimental medicine. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wt and Sema4D−/− spleens from unexperimented animals were analyzed for CD4 and CD8 phenotype ratios. The splenocytes were gated for whole cell light scatter and βTCR. Adjusted percentages background substracted. Similar CD4 and CD8 ratios can be observed in a representative Sema4D−/− and wt mouse.