Abstract
The development of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands for the treatment of diseases including metabolic syndrome, diabetes and obesity has been hampered due to contradictory findings on their potential safety. For example, while some reports show that ligand activation of PPARβ/δ promotes the induction of terminal differentiation and inhibition of cell growth, other reports suggest that PPARβ/δ ligands potentiate tumorigenesis by increasing cell proliferation. Some of the contradictory findings could be due in part to differences in the ligand examined, the presence or absence of serum in cell cultures, differences in cell lines, or differences in the method used to quantify cell growth. For these reasons, this study examined the effect of ligand activation of PPARβ/δ on cell growth of two human cancer cell lines, MCF7 (breast cancer) and UACC903 (melanoma) in the presence or absence of serum using two highly specific PPARβ/δ ligands, GW0742 or GW501516. Culturing cells in the presence of either GW0742 or GW501516 caused upregulation of the known PPARβ/δ target gene angiopoetin-like protein 4 (ANGPTL4). Inhibition of cell growth was observed in both cell lines cultured in the presence of either GW0742 or GW501516, and the presence or absence of serum had little influence on this inhibition. Results from the present studies demonstrate that ligand activation of PPARβ/δ inhibits the growth of both MCF7 and UACC903 cell lines and provide further evidence that PPARβ/δ ligands are not mitogenic in human cancer cell lines.
Keywords: peroxisome proliferator-activated receptor, melanoma, breast cancer, nuclear receptor, cell proliferation
Introduction
Ligands for peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) have great potential for treating metabolic syndrome, diabetes and obesity. This is due to their ability to increase serum HDL cholesterol (Leibowitz et al. 2000; Oliver et al. 2001; Sprecher et al. 2006; van der Veen et al. 2005; Wallace et al. 2005), increase fatty acid catabolism in skeletal muscle, improve insulin resistance and inhibit inflammation (reviewed in (Barish et al. 2006)). However, there is considerable controversy regarding the safety of PPARβ/δ ligands due to contradictory reports in the literature, in particular those describing effects in cancer models. For example, ligand activation of PPARβ/δ is reported to increase proliferation of human liver, cholangiocarcinoma, breast and prostate cancer cell lines (Stephen et al. 2004; Xu et al. 2006a; Xu et al. 2006b). In contrast, colon cancer cell lines fail to exhibit an increase in cell growth by PPARβ/δ in either the presence or absence of serum (Shimada et al. 2002; Stephen et al. 2004). Additionally, more recently it was shown that two different PPARβ/δ ligands inhibit cell growth of human liver and colon cancer cell lines, independent of culture medium serum (Hollingshead et al. 2007a).
While some reports suggest that PPARβ/δ ligands promote the growth of cancer cell lines, there are many observations inconsistent with this hypothesis. Anti-inflammatory activity of PPARβ/δ and/or PPARβ/δ ligands has been shown in a number of different models including immune cells, colon epithelium, macrophages, cardiomyocytes, keratinocytes, myoblasts, endothelial cells, nerve tissue and hepatocytes (Ding et al. 2006; Graham et al. 2005; Hollingshead et al. 2007b; Jakobsen et al. 2006; Kim et al. 2006; Nagasawa et al. 2006; Peters et al. 2000; Polak et al. 2005; Rival et al. 2002; Schmuth et al. 2004; Welch et al. 2003; Woo et al. 2006). There is also strong evidence that ligand activation of PPARβ/δ promotes terminal differentiation in intestinal epithelium, breast and colon cancer cell lines, trophoblasts and primary keratinocytes (Aung et al. 2006; Burdick et al. 2007; Kim et al. 2006; Marin et al. 2006; Nadra et al. 2006; Schmuth et al. 2004; Tan et al. 2001; Varnat et al. 2006; Westergaard et al. 2001). Evidence from a large number of independent laboratories also shows that cell growth is inhibited by PPARβ/δ and its ligands in colonocytes, keratincytes, cardiomyocytes, fibroblasts, endothelial cells and a variety of cancer cell lines (Ali et al. 2005; Aung et al. 2006; Burdick et al. 2007; Fukumoto et al. 2005; Hollingshead et al. 2007a; Kim et al. 2004; Kim et al. 2006; Kim et al. 2005; Man et al. 2007; Marin et al. 2006; Martinasso et al. 2006; Matthiessen et al. 2005; Michalik et al. 2001; Müller-Brüsselbach et al. 2007; Nadra et al. 2006; Ou et al. 2007; Peters et al. 2000; Planavila et al. 2005; Schmuth et al. 2004; Tan et al. 2001; Teunissen et al. 2007; Varnat et al. 2006; Westergaard et al. 2001). Given the potential of PPARβ/δ ligands as therapeutic agents, it is of great importance to determine the effect of ligand activation of PPARβ/δ on cell growth in vitro and in vivo.
There are a number of possible explanations for the reported differences described for the effect of PPARβ/δ ligands on cell growth including differences between high affinity PPARβ/δ ligands (Berger et al. 1999; Sznaidman et al. 2003) and differences due to the presence or absence of serum. The present studies evaluated the possible influence of these variables using two different PPARβ/δ ligands (GW0742 and GW501516), and comparing the effects of these ligands in two human cancer cell lines in the presence or absence of serum.
1. Experimental procedures
1.1 Chemicals
GW0742 was synthesized by GlaxoSmithKline. GW501516 was synthesized according to procedures previously described by others (Sznaidman et al. 2003; Wei and Kozikowski 2003). It was characterized using 1H-NMR (DMSO-d6) and MS, and determined to be 99% pure based on HPLC analysis.
1.2 Cell culture
MCF7 cells were obtained from ATCC in 2006. UACC903 cells were provided by Dr. Gavin Robertson. Cells were maintained in DMEM (Invitrogen, Carlsbad, CA) with 10% FBS at 37°C and 5% CO2. Cells were plated on 6-well dishes at a density of 10,000 – 30,000 cells per well 24 hours prior to determining plating efficiency with a Z1 coulter particle counter® at time 0 (Beckman Counter, Inc., Hialeah, FL). Cells were then either serum starved for 24 hours, or not, prior to ligand treatment. After this 24 hour period, cells were maintained in respective culture medium with or without serum and treated with either GW0742 or GW501516 for 24, 48 and 72 hours at concentrations of 0 (DMSO control), 0.1 μM, 1 μM, or 10 μM. These concentrations of ligand were used because concentrations ranging from 0.1 μM to 1 μM are known to specifically activate PPARβ/δ (Kim et al. 2006). Cells were quantified every 24 hours with a Z1 coulter particle counter® (Beckman Counter, Inc., Hialeah, FL). Triplicate samples for each treatment were used for each timepoint for every treatment, and each replicate was counted three times.
1.3 RNA analysis
Total RNA was isolated from cells using Trizol reagent and the manufacturer’s recommended procedures. The mRNA encoding angiogenin-related protein-like 4 (ANGPTL4), was quantified using real-time PCR analysis. The cDNA was generated using 2.5 μg total RNA with MultiScribe Reverse Transcriptase kit (Applied Biosystems, Foster City, CA). Primers were designed for real-time PCR using the Primer Express software (Applied Biosystems, Foster City, CA). Real-time PCR reactions were carried out using SYBR green PCR master mix (Finnzymes, Espoo, Finland) in the iCycler and detected using the MyiQ Realtime PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The following conditions were used for PCR: 95 °C for 15 sec, 94 °C for 10 sec, 60 °C for 30 sec, and 72 °C for 30 sec, and repeated for 45 cycles. The PCR included a no template control reaction to control for contamination and/or genomic amplification. All reactions had >90% efficiency. Relative expression levels of mRNA were normalized to GAPDH.
2. Results
2.1 Expression of PPARβ/δ and activation by GW0742 and GW501516 in human breast cancer (MCF7) and melanoma (UACC903) cell lines
To verify that the MCF7 and UACC903 cells expressed a functional PPARβ/δ, mRNAs encoding PPARβ/δ and the known PPARβ/δ target gene (Ge et al. 2005), ANGPTL4, were quantified. As compared to mouse primary keratinocytes, expression of mRNA encoding PPARβ/δ was significantly less, but detectable in both human cancer cell lines (Fig. 1A). In response to either PPARβ/δ ligand, expression of ANGPTL4 was increased in both cancer cell lines (Fig. 1B, C). These results demonstrate that MCF7 and UACC903 express a functional PPARβ/δ and respond to both GW0742 and GW501516.
Fig. 1.
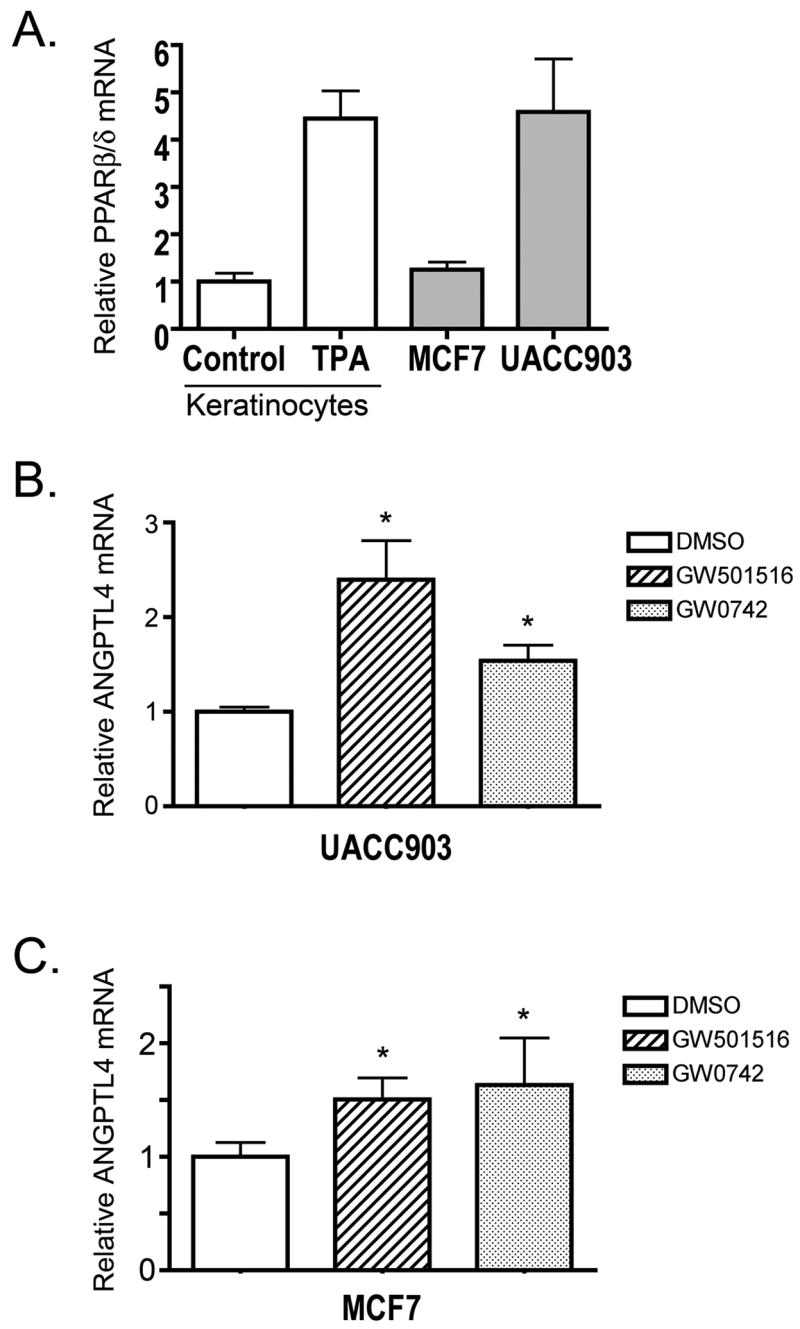
Expression of PPARβ/δ and ligand activation of target genes in MCF7 and UACC903 cells. A. Expression of mRNA encoding PPARβ/δ was quantified in MCF7 and UACC903 cells by quantitative realtime PCR. For comparison, expression of mRNA encoding PPARβ/δ was quantified in control and phorbol ester (TPA)-treated mouse primary keratinocytes. Expression of mRNA encoding the known PPARβ/δ target gene ANGPTL4 was quantified in (B) UACC903 and (C) MCF7 cells by quantitative realtime PCR. *Significantly different from control, P ≤ 0.05.
2.2 GW501516 and GW0742 inhibit growth of human breast cancer (MCF7) and melanoma (UACC903) cell lines
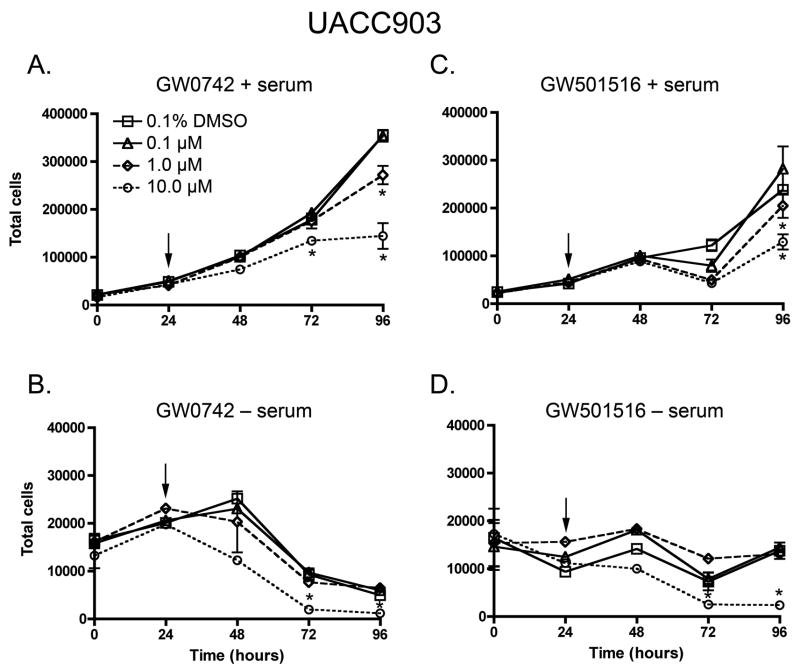
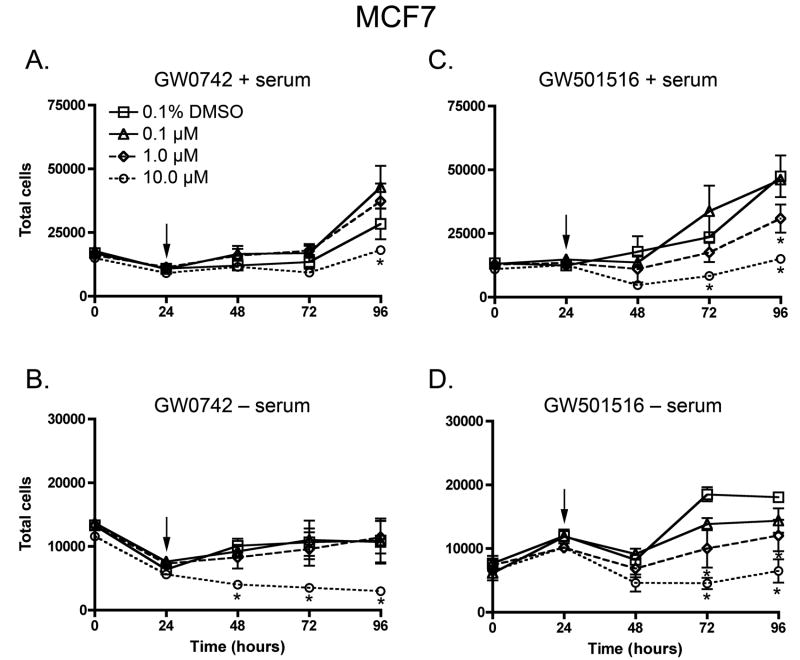
To examine the effect of PPARβ/δ ligands on cell growth of human breast cancer and melanoma cell lines, cell proliferation was quantified in the presence of either GW0742 or GW501516, in the presence or absence of serum. Inhibition of cell growth was observed by both GW0742 and GW501516, and this effect was typically observed only in cells cultured in the presence of 1–10 μM ligand (Figs. 2, 3). No significant increase in cell proliferation was observed in either of the human cancer cell lines with either potent PPARβ/δ ligand (Figs. 2, 3). In the presence of serum, cell growth was faster in both MCF7 and UACC903 cells as compared to that found in the absence of serum (Figs. 2, 3).
Fig. 2.
Effect of GW0742 and GW501516 on cell proliferation in the UACC903 melanoma cell line, in the presence (A, C) or absence (B, D) of culture medium serum. Cells were treated with the indicated concentration of ligand (arrow) and cell number quantified using Coulter counting as described in Materials and Methods. Values represent the mean ± S.E.M. *Significantly different than DMSO control, P ≤ 0.05.
Fig. 3.
Effect of GW0742 and GW501516 on cell proliferation in the MCF7 breast cancer cell line, in the presence or absence of culture medium serum. Cells were treated with the indicated concentration of ligand (arrow) and cell number quantified using Coulter counting as described in Materials and Methods. Values represent the mean ± S.E.M. *Significantly different than DMSO control, P ≤ 0.05.
3. Discussion
Results from the present study demonstrate with the use of two highly specific PPARβ/δ ligands and the most reliable method for quantifying cell number, that ligand activation of PPARβ/δ modestly inhibits cell growth in either MCF7 or UACC903 cells. This work is consistent with past studies showing that ligand activation of PPARβ/δ inhibits cell growth in human liver and colon cancer cell lines (Hollingshead et al. 2007a). Because of different culture conditions used by various laboratories, it is very difficult to draw firm conclusions on the role of PPARβ/δ in cell growth. For example, in some cases, serum is withheld from cells to synchronize the cell cycle while in other cases the cells are cultured in the presence of serum. Additionally, quantification of cell numbers using assays linked to enzyme activity has been used, which is flawed since PPAR agonists are known to increase activity of these enzymes (Conway et al. 1989; Tanaka et al. 2003). For this reason, in the present study, the effect of the presence or absence of serum was evaluated using Coulter counting, which accurately determines the number of cells over time. Using this approach, it is important to note that the only differences observed between treatment was that cells cultured in the presence of serum proliferated faster, consistent with past findings, and that the PPARβ/δ ligands only inhibited, rather than increased, cell growth of both MCF7 and UACC903 cells. It is important to note that measures of apoptosis were not evaluated in these studies. However, it was recently shown that human liver and colon cancer lines do not exhibit changes in poly ADP ribose polymerase cleavage (Hollingshead et al. 2007a) using similar culture conditions used for the present studies (e.g. with and without serum ± GW0742 or GW501516). Coupled with the observed lack of increase in the actual number of cells over time, these observations suggest that apoptosis is likely unaffected by ligand activation of PPARβ/δ in these models, but should be examined further to confirm this idea.
To date, only a few papers have examined the association between PPARβ/δ and cell growth in human breast cancer cell lines. Previous work by others suggests that ligand activation of PPARβ/δ increases the growth of MCF7 cells (Stephen et al. 2004). This increase in cell growth occurred in cells that were cultured only at low confluency and the serum used in these studies was stripped with charcoal. Additionally, cell growth was only assessed after seven or twelve days of culture using one concentration of ligand, rather than monitoring over time with increasing does of ligand as performed for the present studies. It remains possible that the previously reported increase in cell growth was influenced in part by charcoal stripping the medium. Additionally, since no dose response was examined, a mitogenic effect was not conclusively demonstrated (Stephen et al. 2004). In contrast to these findings, others have demonstrated an association between PPARβ/δ expression and differentiation in MCF7 cells (Aung et al. 2006). These studies suggest that expression of PPARβ/δ is likely involved in the induction of differentiation, which is consistent with the present findings indicating that ligand activation of PPARβ/δ inhibits cell growth. Given the relatively small number of studies that have examined the effect of ligand activation of PPARβ/δ on breast cancer cell growth, further studies are necessary before firm conclusions can be drawn.
The effect of ligand activation of PPARβ/δ on melanoma cell growth has not been examined to date. Thus, this is the first report to demonstrate that ligand activation of PPARβ/δ inhibits the growth of UACC903 cells. Since the removal of serum did not further influence this interpretation, serum deprivation does not appear to modulate the effect of ligand activation of PPARβ/δ on cell growth of UACC903 cells. It will be of interest to determine the mechanisms underlying the observed inhibition of cell proliferation in UACC903 cells, and could include the induction of terminal differentiation that may or may not be dependent on concomitant cell growth inhibition and/or anti-inflammatory activity of PPARβ/δ.
Collectively, results from the present study demonstrate that ligand activation of PPARβ/δ modestly inhibits cell growth of a human breast cancer cell line (MCF7) and a human melanoma cell line (UACC903). These observations are consistent with recent findings made in other human cancer cell lines (Hollingshead et al. 2007a), as well as numerous reports linking PPARβ/δ with inducing terminal differentiation and/or inhibiting cell growth (Ali et al. 2005; Aung et al. 2006; Burdick et al. 2007; Fukumoto et al. 2005; Hollingshead et al. 2007a; Kim et al. 2004; Kim et al. 2006; Kim et al. 2005; Man et al. 2007; Marin et al. 2006; Martinasso et al. 2006; Matthiessen et al. 2005; Michalik et al. 2001; Müller-Brüsselbach et al. 2007; Nadra et al. 2006; Ou et al. 2007; Peters et al. 2000; Planavila et al. 2005; Schmuth et al. 2004; Tan et al. 2001; Teunissen et al. 2007; Varnat et al. 2006; Westergaard et al. 2001). The relatively modest activation of PPARβ/δ in both UACC903 and MCF7 cells as shown by increased mRNA encoding ANGPTL4 shows that these cells are not highly responsive to PPARβ/δ ligands as compared to other cells such as keratinocytes. However, this could also be due to the fact that these studies were performed in cell culture, which are not influenced by variables resulting from other cell types not found in an in vitro model. While the concentration of the PPARβ/δ ligands used for these studies are within the range that will specifically activate PPARβ/δ, the findings were not evaluated using gene knockdown. Thus, it also remains possible that the inhibition of cell growth observed could be due to off target events. Further studies are required to examine these possibilities.
Acknowledgments
Supported in part by the National Institutes of Health grants CA97999 and CA124533 (J.M.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali FY, Egan K, Fitzgerald GA, Desvergne B, Wahli W, Bishop-Bailey D, Warner TD, Mitchell JA. Role of Prostacyclin Receptor Versus PPAR{beta} with Treprostinil Sodium on Lung Fibroblast Proliferation. Am J Respir Cell Mol Biol. 2005;34:242–246. doi: 10.1165/rcmb.2005-0289OC. [DOI] [PubMed] [Google Scholar]
- Aung CS, Faddy HM, Lister EJ, Monteith GR, Roberts-Thomson SJ. Isoform specific changes in PPARalpha and beta in colon and breast cancer with differentiation. Biochem Biophys Res Commun. 2006;340:656–660. doi: 10.1016/j.bbrc.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Leibowitz MD, Doebber TW, Elbrecht A, Zhang B, Zhou G, Biswas C, Cullinan CA, Hayes NS, Li Y, Tanen M, Ventre J, Wu MS, Berger GD, Mosley R, Marquis R, Santini C, Sahoo SP, Tolman RL, Smith RG, Moller DE. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J Biol Chem. 1999;274:6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-beta/delta(PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JG, Tomaszewski KE, Olson MJ, Cattley RC, Marsman DS, Popp JA. Relationship of oxidative damage to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl)phthalate and Wy-14,643. Carcinogenesis. 1989;10:513–519. doi: 10.1093/carcin/10.3.513. [DOI] [PubMed] [Google Scholar]
- Ding G, Cheng L, Qin Q, Frontin S, Yang Q. PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes. J Mol Cell Cardiol. 2006;40:821–828. doi: 10.1016/j.yjmcc.2006.03.422. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Yano Y, Virgona N, Hagiwara H, Sato H, Senba H, Suzuki K, Asano R, Yamada K, Yano T. Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005;579:3829–3836. doi: 10.1016/j.febslet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ge H, Cha JY, Gopal H, Harp C, Yu X, Repa JJ, Li C. Differential regulation and properties of angiopoietin-like proteins 3 and 4. J Lipid Res. 2005;46:1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPAR[delta] agonist GW0742X reduces atherosclerosis in LDLR-/- mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Hollingshead HE, Killins RL, Girroir EE, Billin AN, Willson TM, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007a doi: 10.1093/carcin/bg,183. [DOI] [PubMed] [Google Scholar]
- Hollingshead HE, Morimura K, Adachi M, Kennett MJ, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARbeta/delta Protects Against Experimental Colitis Through a Ligand-Independent Mechanism. Dig Dis Sci. 2007b;52:2912–2919. doi: 10.1007/s10620-006-9644-9. [DOI] [PubMed] [Google Scholar]
- Jakobsen MA, Petersen RK, Kristiansen K, Lange M, Lillevang ST. Peroxisome proliferator-activated receptor alpha, delta, gamma1 and gamma2 expressions are present in human monocyte-derived dendritic cells and modulate dendritic cell maturation by addition of subtype-specific ligands. Scand J Immunol. 2006;63:330–337. doi: 10.1111/j.1365-3083.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Akiyama TE, Harman FS, Burns AM, Shan W, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor beta (delta)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J Biol Chem. 2004;279:23719–23727. doi: 10.1074/jbc.M312063200. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARb/d selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Murray IA, Burns AM, Gonzalez FJ, Perdew GH, Peters JM. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits epidermal cell proliferation by down-regulation of kinase activity. J Biol Chem. 2005;280:9519–9527. doi: 10.1074/jbc.M413808200. [DOI] [PubMed] [Google Scholar]
- Leibowitz MD, Fievet C, Hennuyer N, Peinado-Onsurbe J, Duez H, Bergera J, Cullinan CA, Sparrow CP, Baffic J, Berger GD, Santini C, Marquis RW, Tolman RL, Smith RG, Moller DE, Auwerx J. Activation of PPARdelta alters lipid metabolism in db/db mice. FEBS Lett. 2000;473:333–336. doi: 10.1016/s0014-5793(00)01554-4. [DOI] [PubMed] [Google Scholar]
- Man MQ, Barish GD, Schmuth M, Crumrine D, Barak Y, Chang S, Jiang Y, Evans RM, Elias PM, Feingold KR. Deficiency of PPARbeta/delta in the Epidermis Results in Defective Cutaneous Permeability Barrier Homeostasis and Increased Inflammation. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701026. [DOI] [PubMed] [Google Scholar]
- Marin HE, Peraza MA, Billin AN, Willson TM, Ward JM, Kennett MJ, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) inhibits colon carcinogenesis. Cancer Res. 2006;66:4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- Martinasso G, Maggiora M, Trombetta A, Canuto RA, Muzio G. Effects of di(2-ethylhexyl) phthalate, a widely used peroxisome proliferator and plasticizer, on cell growth in the human keratinocyte cell line NCTC 2544. J Toxicol Environ Health A. 2006;69:353–365. doi: 10.1080/15287390500227522. [DOI] [PubMed] [Google Scholar]
- Matthiessen MW, Pedersen G, Albrektsen T, Adamsen S, Fleckner J, Brynskov J. Peroxisome proliferator-activated receptor expression and activation in normal human colonic epithelial cells and tubular adenomas. Scand J Gastroenterol. 2005;40:198–205. doi: 10.1080/00365520410009573. [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ, Zakany J, Metzger D, Chambon P, Duboule D, Wahli W. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Brüsselbach S, Kömhoff M, Rieck M, Meissner W, Kaddatz K, Adamkiewicz J, Keil B, Klose KJ, Moll R, Burdick AD, Peters JM, Müller R. Deregulation of tumor angiogenesis and blockade of tumor growth in PPARβ-deficient mice. Embo J. 2007;26:3686–3698. doi: 10.1038/sj.emboj.7601803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadra K, Anghel SI, Joye E, Tan NS, Basu-Modak S, Trono D, Wahli W, Desvergne B. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol Cell Biol. 2006;26:3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou YC, Yang CR, Cheng CL, Raung SL, Hung YY, Chen CJ. Indomethacin induces apoptosis in 786-O renal cell carcinoma cells by activating mitogen-activated protein kinases and AKT. Eur J Pharmacol. 2007;563:49–60. doi: 10.1016/j.ejphar.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Peters JM, Lee SST, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adipose, brain and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ) Molecular and Cellular Biology. 2000;20:5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planavila A, Rodriguez-Calvo R, Jove M, Michalik L, Wahli W, Laguna JC, Vazquez-Carrera M. Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;65:832–841. doi: 10.1016/j.cardiores.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Polak PE, Kalinin S, Dello Russo C, Gavrilyuk V, Sharp A, Peters JM, Richardson J, Willson TM, Weinberg G, Feinstein DL. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2005;168:65–75. doi: 10.1016/j.jneuroim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Rival Y, Beneteau N, Taillandier T, Pezet M, Dupont-Passelaigue E, Patoiseau JF, Junquero D, Colpaert FC, Delhon A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002;435:143–151. doi: 10.1016/s0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, Lau P, Fowler AJ, Chuang G, Moser AH, Brown BE, Mao-Qiang M, Uchida Y, Schoonjans K, Auwerx J, Chambon P, Willson TM, Elias PM, Feingold KR. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kojima K, Yoshiura K, Hiraishi H, Terano A. Characteristics of the peroxisome proliferator activated receptor gamma (PPARgamma) ligand induced apoptosis in colon cancer cells. Gut. 2002;50:658–664. doi: 10.1136/gut.50.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher DL, Massien C, Pearce G, Billin AN, Perlstein I, Willson TM, Hassall DG, Ancellin N, Patterson SD, Lobe DC, Johnson TG. Triglyceride:High-Density Lipoprotein Cholesterol Effects in Healthy Subjects Administered a Peroxisome Proliferator Activated Receptor {delta} Agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004;64:3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, Goreham D, Sierra ML, LeGrumelec C, Xu HE, Montana VG, Lambert MH, Willson TM, Oliver WR, Sternbach DD. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)-synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. doi: 10.1016/s0960-894x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Fluhmann B, Desvergne B, Wahli W. Critical roles of PPARbeta/delta in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen BE, Smeets PJ, Willemsen PH, De Windt LJ, Van der Vusse GJ, Van Bilsen M. Activation of PPARdelta inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc Res. 2007;75:519–529. doi: 10.1016/j.cardiores.2007.04.026. [DOI] [PubMed] [Google Scholar]
- van der Veen JN, Kruit JK, Havinga R, Baller JF, Chimini G, Lestavel S, Staels B, Groot PH, Groen AK, Kuipers F. Reduced cholesterol absorption upon PPARdelta activation coincides with decreased intestinal expression of NPC1L1. J Lipid Res. 2005;46:526–534. doi: 10.1194/jlr.M400400-JLR200. [DOI] [PubMed] [Google Scholar]
- Varnat F, Heggeler BB, Grisel P, Boucard N, Corthesy-Theulaz I, Wahli W, Desvergne B. PPARbeta/delta regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006;131:538–553. doi: 10.1053/j.gastro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Schwarz M, Coward P, Houze J, Sawyer JK, Kelley KL, Chai A, Rudel LL. Effects of peroxisome proliferator-activated receptor {alpha}/{delta} agonists on HDL-cholesterol in vervet monkeys. J Lipid Res. 2005;46:1009–1016. doi: 10.1194/jlr.M500002-JLR200. [DOI] [PubMed] [Google Scholar]
- Wei ZL, Kozikowski AP. A short and efficient synthesis of the pharmacological research tool GW501516 for the peroxisome proliferator-activated receptor delta. The Journal of organic chemistry. 2003;68:9116–9118. doi: 10.1021/jo035140g. [DOI] [PubMed] [Google Scholar]
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard M, Henningsen J, Svendsen ML, Johansen C, Jensen UB, Schroder HD, Kratchmarova I, Berge RK, Iversen L, Bolund L, Kragballe K, Kristiansen K. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J Invest Dermatol. 2001;116:702–712. doi: 10.1046/j.1523-1747.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, Che W, Vulapalli SR, Yan C, Abe JI. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (PPARdelta ) stimulation. J Biol Chem. 2006;281:32164–32174. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]
- Xu L, Han C, Lim K, Wu T. Cross-talk between Peroxisome Proliferator-Activated Receptor {delta} and Cytosolic Phospholipase A2{alpha}/Cyclooxygenase-2/Prostaglandin E2 Signaling Pathways in Human Hepatocellular Carcinoma Cells. Cancer Res. 2006a;66:11859–11868. doi: 10.1158/0008-5472.CAN-06-1445. [DOI] [PubMed] [Google Scholar]
- Xu L, Han C, Wu T. A novel positive feedback loop between PPARdelta and PGE2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006b;281:33982–33996. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]